Abstract
Plasma-activated water (PAW) is an eco-friendly technology with potential to improve seed germination and nutritional quality in microgreens. This study investigated the effects of PAW on three cultivars of kangkong (Ipomoea aquatica Forssk.). PAW activated for 10 min (PAW10) significantly enhanced seed germination and vigor, with effects comparable to those of a 15-min treatment. PAW10 treatment not only improved the accumulation of bioactive compounds—including total phenolics, flavonoids, ascorbic acid, chlorophylls, and carotenoids—but also enhanced antioxidant activity. These improvements were accompanied by elevated hydrogen peroxide (H2O2) levels and increased enzymatic activities, specifically catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX). Principal component analysis revealed cultivar-specific responses to PAW10. The Senafore 20 (SF) cultivar showed the most pronounced increases in antioxidant and antiglycation activities, as well as key bioactive compounds. The Phai-ngern (PN) cultivar exhibited elevated SOD activity and fiber content, while the Senee 20 (SN) cultivar showed minimal changes. These findings suggest that PAW10 effectively promotes germination and antioxidant-related biochemical responses in kangkong microgreens, with varying responses depending on cultivar. This study highlights PAW treatment as a promising approach to improve microgreen production and antioxidant capacity, supporting sustainable agriculture.
1. Introduction
The increasing global demand for safe, high-quality food, coupled with the urgent need to reduce the environmental impact of agriculture, has driven the development of sustainable and chemical-free cultivation practices. One such innovation is plasma technology, a non-thermal, eco-friendly method that operates at room temperature [1]. This technology generates reactive oxygen and nitrogen species (RONS), such as hydrogen peroxide (H2O2), ozone (O3), hydroxyl radicals (•OH), nitric oxide (NO), nitrite (NO2−), and nitrate (NO3−), along with electrons, ions, ultraviolet radiation, and mild heat [1,2]. The generated RONS act as effective elicitors that promote seed germination, plant growth, development, and the accumulation of bioactive compounds [3,4,5,6]. Among plasma-based technologies, plasma-activated water (PAW) has emerged as a promising indirect application. PAW is produced by exposing water to plasma discharge, allowing RONS to dissolve into the water [7]. These reactive species alter the water’s physicochemical properties—lowering pH, increasing electrical conductivity (EC), and elevating NO2− and NO3− levels—thereby transforming water into a biologically active medium [8,9,10]. Unlike direct plasma, PAW can be easily stored, transported, and applied to seeds or plants without requiring specialized equipment [6]. In addition to modifying physicochemical properties, PAW has demonstrated significant biological effects by enhancing seed germination [11,12,13,14], promoting seedling vigor [15,16,17], and stimulating the accumulation of bioactive compounds such as antioxidants and phenolics [11,16,18]. These effects are attributed to the RONS acting as elicitors that trigger physiological and molecular responses in plants [5]. Moreover, PAW offers a sustainable and safe alternative to conventional chemical treatments, reducing the environmental burden associated with agrochemical use [6]. Its versatility has been explored in a variety of crops including lentil [19], soybean [12], lettuce [20], and tomato [21], with promising results for improving crop quality and yield. However, the effectiveness of PAW depends on factors such as plasma activation duration, storage conditions, and crop species, highlighting the need for optimization in practical applications.
Despite the growing interest in PAW applications, its effects on leafy vegetables with high nutritional and functional properties—such as kangkong (Ipomoea aquatica Forssk.)—have not been extensively investigated. Kangkong, a widely consumed leafy vegetable in Southeast Asia, is well known for its adaptability to hot climates and rapid growth [22]. It is frequently used in a variety of Asiatic dishes, including stir-fries, soups, and salads, particularly in countries such as Thailand, Vietnam, Malaysia, and the Philippines, where it holds culinary and cultural importance [23]. Kangkong leaves are rich in bioactive compounds, including flavonoids, phenolics, carotenoids, and vitamins, which have been associated with antioxidant, anti-inflammatory, and anti-diabetic properties [24,25,26]. Recently, growing kangkong as microgreens has become increasingly popular due to its superior nutritional content and rapid growth cycle. These characteristics make kangkong a suitable candidate for sustainable microgreen production systems [27]. While our previous research has demonstrated that direct dielectric barrier discharge (DBD) plasma treatment can enhance seed germination and vigor, as well as promote the accumulation of bioactive compounds and antioxidant activity in kangkong microgreens [28,29], studies specifically examining the effects of PAW on kangkong—particularly with respect to germination performance and antioxidant-related biochemical responses during the microgreen stage—remain limited. Although the effects of plasma activation duration on the physicochemical properties of PAW have been extensively studied [6,21,30,31], the downstream implications of these changes for seed germination and biochemical responses in different kangkong cultivars remain largely uninvestigated. We hypothesize that plasma activation duration significantly alters the properties of PAW, thereby differentially affecting seed germination, early growth, and biochemical responses among kangkong cultivars. Therefore, this study aimed to investigate the effects of PAW produced at different activation durations on seed germination and vigor in three kangkong cultivars. Based on germination and vigor performance, the most effective PAW treatment was selected for subsequent microgreen cultivation. During this phase, physiological traits, antioxidant activity, and biochemical responses were further evaluated. These findings will contribute to a better understanding of the bio-chemical effects of PAW and inform its optimal application for the sustainable, chemical-free production of high-quality kangkong microgreens.
2. Materials and Methods
2.1. PAW Generation
PAW was generated using direct arc plasma applied to the water surface. The direct arc plasma device consisted of four tungsten needles, each with 150 mm long with a diameter of 3.7 mm, sharply tapered at the nozzle, and enclosed in a nylon outer casing. A high-voltage fly back transformer delivering 15.8 kV (peak-to-peak) at 10 kHz AC pulses was applied to a quadruple metallic external electrode (Figure 1). The generated plasma was then directed toward 500 mL of distilled water for 5, 10, and 15 min to produce PAW, designated as PAW5, PAW10, and PAW15.
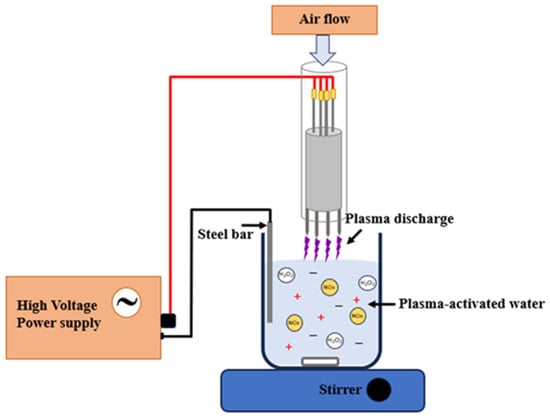
Figure 1.
Schematic diagram of a direct arc plasma and PAW generation.
2.2. Determination of Physicochemical Properties of PAW
EC was measured using a conductivity meter (Hanna HI98311, Hanna Instruments, Șura Mică, Sibiu County, Romania), and pH was measured using a pH meter (SI Analytics Lab855, SI Analytics GmbH, Mainz, Germany).
H2O2 in PAW was assessed following Hou et al. [32]. PAW was mixed with 0.2% o-phenylenediamine dihydrochloride and incubated at 45 °C for 10 min. Then, 10 N H2SO4 was added, and the mixture was incubated at 45 °C for 2 h. Absorbance was measured at 490 nm using a spectrophotometer (Shimadzu Europe UV-1208, Established Shimadzu Aerotech Manufacturing, Inc., Kyoto, Japan). H2O2 content was expressed as milligrams per liter (mg/L).
The concentration of NO2− was determined using the method described by Hou et al. [32]. PAW was mixed with 0.5% sulfanilic acid and 2 M H2SO4, followed by incubation at 4 °C for 5 min. Subsequently, 2 M NaOH was added, and the mixture was incubated at 45 °C for 2 h. Absorbance was then measured at 538 nm using a spectrophotometer. NO2− content was expressed as milligrams per liter (mg/L).
For NO3− concentration, the NO3− test kit (HI3874-0, HANNA Instruments Inc., Smithfield, RI, USA), containing cadmium, potassium disulfate, and sulfanilic acid, was used as described by Chuea-uan et al. [33]. The NO3− concentration was measured at 372 nm using a spectrophotometer. NO3− content was expressed as milligrams per liter (mg/L).
2.3. Effect of PAW on Germination and Vigor of Kangkong Seeds
2.3.1. Seed Materials and Experimental Design
Three kangkong cultivars were used in this study: Phai-ngern (PN) and Senee 20 (SN), which belong to the narrow-leaf group, and Senafore 20 (SF), which belongs to the broad-leaf group. PN was sourced from East-West Seed Co., Ltd. in Nonthaburi, Thailand, while SN and SF were obtained from Chia Seng Heng Agriculture Co., Ltd. in Ang Thong, Thailand.
The experiment was arranged as a 3 × 4 factorial in a completely randomized design (CRD) with three replications. Factor A consisted of three kangkong cultivars (PN, SN, and SF), while Factor B comprised PAW treatments, including seed soaking and irrigation with PAW5, PAW10, and PAW15. Seeds soaked and irrigated with distilled water served as the control.
2.3.2. Seed Germination and Vigor
Fifty seeds were soaked in 20 mL of either PAW or distilled water for 12 h, then sown in plastic boxes (25 × 17 × 8 cm) containing moist, sterilized sand. Germination was carried out under controlled conditions: 30 °C ambient temperature, 12-h light/12-h dark photoperiod, 70% relative humidity, and fluorescent lighting with a photosynthetically active radiation intensity of 13.92 µmol/m2/s. Each box was irrigated daily with 25 mL of the corresponding solution (PAW or distilled water). Germination was monitored daily by counting the number of normal seedlings exhibiting fully expanded cotyledons. Seed germination was evaluated over a period of 10 days. The germination percentage, first count percentage (recorded 4 days after sowing), mean germination time (MGT), and germination index (GI) were calculated in accordance with the guidelines of the International Seed Testing Association [34].
2.4. Effect of PAW on Growth and Antioxidant-Related Biochemical Responses in Kangkong Microgreens
2.4.1. Experimental Design
According to Section 2.3, the PAW10 treatment was found to be optimal for enhancing the germination and vigor of kangkong seeds. Therefore, PAW10 was selected for further investigation. In this experiment, a 3 × 2 factorial in a CRD with three replications was used. Factor A consisted of three kangkong cultivars (PN, SN, and SF), while Factor B included two treatments: PAW10 and the control.
2.4.2. Growth Measurement of Microgreens
Sixteen grams of seeds were soaked in 150 mL of either PAW or distilled water for 12 h, then sown in plastic boxes (25 × 17 × 8 cm) filled with 4 cm of moist peat moss (REKYVA Peat Substrate Professional, Šiauliai, Lithuania). The boxes were maintained under the environmental conditions described in Section 2.3.2 and irrigated daily with 75 mL of the corresponding solution (PAW10 or distilled water) per box. Microgreens were harvested 10 days after sowing. Fresh and dry weights (expressed in kg/m2 and g/m2, respectively) were determined according to the procedure described by Ongrak et al. [28]. Dry weight samples were oven-dried at 70 °C for 72 h. Freeze-dried powders for extraction and biochemical analyses were prepared as previously reported by Ongrak et al. [28].
2.4.3. Determination of Photosynthetic Pigments
Chlorophyll and carotenoid contents were determined following the method of Gunathilake and Ranaweera [35]. Briefly, one gram of dried sample powder was extracted with 5 mL of 95% ethanol, and the absorbance of the supernatant was measured at 470, 649, and 665 nm using a spectrophotometer. Pigment concentrations were calculated using the equations of Lichtenthaler and Wellburn [36] and expressed as micrograms per gram of dry weight (µg/g DW).
2.4.4. Determination of Hydrogen Peroxide (H2O2), Nitric Oxide (NO), and Malondialdehyde (MDA) Contents
H2O2 content was determined following the method described by Billah et al. [37]. Briefly, 0.5 g of dried sample powder was extracted with 0.1% trichloroacetic acid (TCA), and the supernatant was reacted with potassium iodide and phosphate buffer (pH 7.0). The absorbance was measured at 390 nm using a spectrophotometer, and the results were expressed as millimoles per gram of dry weight (mmol/g DW).
NO content was determined following the method described by Hasanuzzaman et al. [38] with slight modifications. Briefly, 0.2 g of dried sample powder was extracted with 2 mL of 50 mM acetic acid buffer (pH 3.6), and 500 µL of the supernatant was reacted with 500 µL of Griess reagent. The absorbance was measured at 540 nm using a spectrophotometer. NO concentration was calculated using a sodium nitrite standard curve and expressed as micromoles per gram of dry weight (µmol/g DW).
MDA content was determined following the method described by da Silva et al. [39]. Briefly, 200 mg of dried sample powder was extracted with 0.1% TCA and reacted with thiobarbituric acid solution. The mixture was incubated in boiling water for 30 min, cooled, and centrifuged. Absorbance was measured at 532 and 600 nm using a spectrophotometer. MDA content was calculated using an extinction coefficient of 155 mM−1·cm−1 and expressed as nanomoles per gram of dry weight (nmol/g DW).
2.4.5. Determination of Antioxidant Enzyme Activity
Antioxidant enzymes were extracted from dried sample powder using a modified procedure based on Ongrak et al. [28]. For the determination of CAT and APX activities, 0.2 g of dried sample powder was homogenized in 0.5 mL of 50 mM sodium phosphate buffer (pH 7.0) containing 0.4 µg of polyvinylpyrrolidone (PVP), with the extraction performed on ice to minimize enzymatic degradation. The homogenate was centrifuged at 15,000× g for 15 min at 4 °C, and the supernatant was collected for enzymatic assays. For SOD activity, 0.5 g of dried sample powder was extracted using 0.5 mL of 100 mM sodium phosphate buffer (pH 7.8) with 0.4 µg of PVP, followed by centrifugation under identical conditions. The activity of CAT was determined according to the method described by Önder et al. [40], while APX and SOD activities were analyzed using the protocol of Heshmati et al. [41]. Enzyme activities were expressed as units per milligram of protein (Unit/mg protein).
2.4.6. Determination of Antioxidant Enzyme Gene Copy Number
Genomic DNA was extracted from fresh plant samples using the conventional cetyl trimethylammonium bromide (CTAB) method, following the protocol described by Aboul-Maaty and Oraby [42]. DNA concentration was measured using a spectrophotometer and adjusted to 50 ng/μL prior to further analysis. Diagnostic polymerase chain reaction (PCR) was performed to detect antioxidant enzyme genes, including CAT, APX, and SOD. The specific primers and expected fragment sizes for each gene are listed in Table 1. PCR products were visualized on a 1% agarose gel stained with SYBR Safe DNA Gel Stain (Invitrogen). The amplicons were then purified and quantified using a Nanodrop spectrophotometer (NanoDrop Lite; Thermo Scientific, Waltham, MA, USA). A standard curve was generated from five serial dilutions ranging from 107 to 103 copies to calculate the copy number of each target gene. Quantitative PCR (qPCR) was conducted using the Applied Biosystems StepOnePlus™ real-time PCR system (Applied Biosystems, Foster City, CA, USA) following the protocol described by Talaat et al. [43]. The 20 µL qPCR reaction mixture consisted of 10 µL of 2X SYBR Green Master Mix, 3 µL of DNA template, and 1 pmol each of forward and reverse primers. Thermal cycling conditions for CAT and SOD included an initial denaturation at 94 °C for 10 min, followed by 40 cycles of 94 °C for 60 s, 55 °C for 90 s, and 72 °C for 120 s. For APX, the cycling conditions were similar except that the annealing temperature was 37 °C instead of 55 °C. All qPCR reactions were performed in triplicate, with negative controls lacking DNA included in each run. The copy numbers of CAT, SOD, and APX genes in unknown samples were quantified by comparing their cycle threshold (Ct) values to those obtained from the standard curve.

Table 1.
Primer sequences for quantification of antioxidant enzyme gene copy number.
2.4.7. Determination of Bioactive Compounds and Phenolic Profile
Briefly, the dried sample powders were processed following the method described by Ongrak et al. [29]. One gram of sample was extracted with 75 mL of 70% ethanol. The resulting crude extracts were used to evaluate bioactive compound content, as well as antioxidant and antiglycation activities.
Total phenolic content was determined using the Folin–Ciocalteu method, following Rithichai et al. [44]. Briefly, the extract was mixed with Folin–Ciocalteu reagent and 7.5% sodium carbonate, then incubated in the dark at room temperature for 30 min. The absorbance was measured at 765 nm using a 96-well microplate reader (BioTek PowerWave XS, Marshall Scientific LLC, Hampton, NH, USA). The phenolic content was calculated using a gallic acid standard curve and expressed as milligrams of gallic acid equivalents per gram of dry extract (mg GAE/g DE).
Total flavonoid content was determined using the aluminum chloride colorimetric assay, following Rithichai et al. [44]. Briefly, the extract was sequentially mixed with 5% sodium nitrite, 10% aluminum chloride, and 1 M sodium hydroxide. After 15 min of incubation, the absorbance was measured at 510 nm using the same microplate reader. The flavonoid content was calculated using a quercetin standard curve and expressed as milligrams of quercetin equivalents per gram of dry extract (mg QE/g DE).
Total ascorbic acid content was determined following the method described by Kammapana et al. [45]. Briefly, 0.1 g of the crude extract was dissolved in 0.2 mL of 5% metaphosphoric acid and mixed with 0.02% indophenol solution. After 3 min of incubation, 2% thiourea and 2% 2,4-dinitrophenylhydrazine were added. The mixture was then incubated in a water bath at 50 °C for 70 min, followed by the addition of 85% sulfuric acid and incubation at room temperature for 30 min. Absorbance was measured at 540 nm using a spectrophotometer. Ascorbic acid content was calculated using a standard curve of ascorbic acid and expressed as milligrams per gram of dry extract (mg/g DE).
Phenolic profiling was performed using HPLC following the method of Kubola and Siriamornpun [46] with slight modification. The method is briefly summarized as follows: Ethanol extracts were filtered through a 0.45 µm syringe filter prior to injection. Analysis was carried out using a reversed-phase HPLC system (Shimadzu LC-20AC, Shimadzu Co., Ltd., Tokyo, Japan) equipped with a diode array detector (SPD-M20A) and an Acclaim C18 column (250 × 4.6 mm, 5 µm particle size; GL Sciences Inc., Tokyo, Japan). The mobile phase consisted of solvent A (acetonitrile) and solvent B (ultrapure water adjusted to pH 2.0 with orthophosphoric acid). A gradient elution was applied, starting from 5% to 70% solvent A over 0–53 min, then returning to 5% from 52–60 min. The flow rate was 1.0 mL/min, the column temperature was maintained at 38 °C, and the injection volume was 20 µL. UV detection was performed at 280 nm for chlorogenic acid and coumaric acid, and at 360 nm for rutin, ferulic acid, and vanillic acid. Phenolic compounds were identified by comparing retention times and UV spectra with authentic external standards. Results were expressed as milligrams per gram of dry extract (mg/g DE).
2.4.8. Determination of Antioxidant and Antiglycation Activities
Antioxidant activity was evaluated using three assays—the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, and the ferric reducing antioxidant power (FRAP) assay—following the method described by Jirakiattikul et al. [47], Puccinelli et al. [48], and Alrifai et al. [49], respectively.
DPPH Radical Scavenging Assay: The extract was mixed with DPPH solution and incubated in the dark at room temperature for 30 min. Absorbance was measured at 517 nm using a 96-well microplate reader, and radical scavenging activity was calculated relative to a control.
ABTS Radical Cation Decolorization Assay: ABTS radical cation (ABTS•+) was generated by reacting ABTS solution with potassium persulfate and incubating the mixture in the dark for 12–16 h. The solution was diluted to an absorbance of 0.68–0.72 at 734 nm before use. The extract was then mixed with the ABTS•+ solution, incubated for 6 min at room temperature, and absorbance was measured at 734 nm using a microplate reader. Antioxidant activity was expressed as percentage inhibition relative to the control.
FRAP Assay: The extract was mixed with FRAP reagent and incubated for 8 min at room temperature. Absorbance was measured at 595 nm using a microplate reader. Ascorbic Acid Standard Curve: A standard curve was prepared using known concentrations of ascorbic acid. The antioxidant activity of the sample was calculated based on this curve and expressed as micromoles of ascorbic acid equivalents per milligram of dry extract (µmol AsAE/mg DE).
Antiglycation Activity: Antiglycation activity was assessed using a bovine serum albumin (BSA)-glucose assay adapted from Rahbar et al. [50] and Wu and Yen [51]. The control solution contained BSA (50 mg/mL) and D-glucose (0.8 M) dissolved in phosphate buffer (1.5 M, pH 7.4). Test mixtures included crude extract or positive controls (aminoguanidine and rutin, 1 mg/mL each) prepared in the same phosphate buffer supplemented with 0.3 g/L sodium azide to inhibit microbial growth. All samples were incubated at 37 °C for 7 days. After incubation, advanced glycation end-products (AGEs) formation was quantified by measuring fluorescence intensity using a fluorescence spectrophotometer (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA) at excitation/emission wavelengths of 330/410 nm. Percentage inhibition of AGE formation was calculated relative to the control without inhibitors.
2.4.9. Determination of Nutritional Contents
Protein content was analyzed by the Kjeldahl method according to Ndamitso et al. [52]. One gram of dried sample powder was digested with concentrated H2SO4 and catalyst at 420 °C, then distilled with 4% boric acid and 4% NaOH using a BUCHI KjelFlex K-360 distillation unit (Büchi Labortechnik AG, Flawil, Switzerland). The distillate was titrated with 0.1 N H2SO4, and nitrogen content was multiplied by 6.25 to estimate crude protein percentage.
Crude lipid content was analyzed following Ndamitso et al. [52] using an Ankom XT15 extractor (ANKOM Technology, Macedon, NY, USA). One gram of dried sample powder was placed in a filter bag, incubated at 100 °C for 3 h, and extracted with petroleum ether. Crude fat was calculated based on the weight difference before and after extraction.
Crude fiber was measured via acid and alkaline digestion using an Ankom A2000 fiber analyzer (ANKOM Technology, Macedon, NY, USA) according to Ndamitso et al. [52]. Samples were pretreated with petroleum ether and acetone to remove lipids, then dried and incinerated at 600 °C for 2 h. Crude fiber content was calculated from weight loss before and after incineration.
Ash content was determined by incinerating 1 g of dried powdered sample in a muffle furnace (Nabertherm LHT series, Nabertherm GmbH, Lilienthal, Germany) at 600 °C for 8 h. The remaining ash was weighed and expressed as a percentage of the dry sample weight [52].
Carbohydrate percentage was calculated using the formula provided by Ndamitso et al. [52].
Carbohydrate (%) = 100 − [Protein (%) + Moisture (%) + Ash (%) + Fiber (%) + Fat (%)]
2.5. Statistical Analysis
Two-way analysis of variance (ANOVA) was conducted, and mean separation was performed using Tukey’s Honestly Significant Difference (HSD) test at a significance level of p < 0.05, using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA). Principal component analysis (PCA) was carried out using JMP statistical software (trial version, JMP®, SAS Institute Inc., Cary, NC, USA). The effect size for each principal component (PC) was calculated according to the method described by Ongrak et al. [29]. Bootstrap resampling was applied to generate 95% confidence intervals (CIs) for the variance explained by PC1 and PC2. Pearson correlation coefficients between 33 variables and either PC1 or PC2 were computed using MATLAB, version R2025a (individual license, MathWorks, Natick, MA, USA).
3. Results
3.1. Physicochemical Properties of PAW
The pH of PAWs decreased as plasma activation time increased, with PAW15 exhibiting the lowest pH. However, the difference in pH between PAW15 and PAW10 was not significant. In contrast, the EC of PAWs was significantly higher than that of the control and increased with longer plasma activation times, with PAW15 showing the highest EC. PAWs also exhibited higher H2O2 levels than the control, although no significant differences were observed among the different activation times. Regarding NO2− and NO3− contents, their concentrations were higher in PAWs than in the control and increased progressively with activation time, with PAW15 showing the highest levels (Table 2).

Table 2.
Physicochemical properties of PAW, including pH, electrical conductivity (EC), and the concentrations of hydrogen peroxide (H2O2), nitrite (NO2−), and nitrate (NO3−).
3.2. Germination and Vigor of Kangkong Seeds After PAW Treatment
There was no significant interaction between cultivar and PAW treatment with respect to germination percentage, first count, MGT, and GI, as shown in Table 3. The SF cultivar exhibited the highest germination percentage, first count, and GI, along with the lowest MGT. Although the differences were not statistically significant, PAW treatments increased germination percentage (by 4.90–11.98%), first count (by 19.37–32.45%), and GI (by 22.42–26.60%) compared to the control. The lowest MGT was observed under the PAW15 treatment; however, it was not significantly different from that under PAW10. Based on these results, PAW10 was selected for subsequent investigation of its effects on the biochemical quality of microgreens, as it demonstrated comparable effectiveness to PAW15 while requiring a shorter plasma activation time. This made it a more practical candidate for further analysis.

Table 3.
Germination and vigor of kangkong seeds (cultivars PN, SN, and SF) under PAW treatments.
3.3. Effect of PAW on Growth and Antioxidant-Related Biochemical Responses in Kangkong Microgreens
3.3.1. Growth of Microgreens
There was no significant interaction between cultivar and PAW treatment with respect to shoot length, fresh weight, and dry weight. Shoot length did not differ significantly among the cultivars; however, the highest fresh and dry weights were observed in PN cultivar. The PAW10 treatment enhanced microgreen growth, leading to significant increases in shoot length, fresh weight, and dry weight by 9.74%, 5.84%, and 8.75%, respectively, compared to the control (Table 4).

Table 4.
Shoot length (SL), fresh weight (FW), and dry weight (DW) of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
3.3.2. Photosynthetic Pigments
There was no significant interaction between cultivar and PAW treatment with respect to chlorophyll and carotenoid contents. The SF cultivar exhibited the highest levels of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids. Under the PAW10 treatment, the contents of chlorophyll b, total chlorophyll, and carotenoids increased significantly by 15.88%, 10.98%, and 11.40%, respectively, compared to the control (Table 5).

Table 5.
Chlorophyll a (ChA), chlorophyll b (ChB), total chlorophyll (TCh), and carotenoid (CAR) contents of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
3.3.3. H2O2, NO, and MDA Contents
There was no significant interaction between cultivar and PAW treatment with respect to H2O2, NO, and MDA contents (Table 6). The levels of H2O2, NO, and MDA did not differ significantly among the cultivars. However, H2O2 content increased significantly by 50.36% under the PAW10 treatment compared to the control, while NO and MDA levels showed no significant differences between the PAW10 and control treatments.

Table 6.
Hydrogen peroxide (H2O2), nitric oxide (NO), malondialdehyde (MDA) contents of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
3.3.4. Antioxidant Enzyme Activities
There was no significant interaction between cultivar and PAW treatment with respect to CAT, APX, and SOD activities (Table 7). The highest CAT and SOD activities were observed in the PN cultivar, while APX activity did not differ significantly among the cultivars. Under the PAW10 treatment, CAT, APX, and SOD activities increased significantly by 42.11%, 66.16%, and 29.13%, respectively, compared to the control.

Table 7.
Catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD) activities of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
3.3.5. Antioxidant Enzyme Gene Copy Number
There was no significant interaction between cultivar and PAW treatment with respect to antioxidant enzyme gene copy number, except for APX (Table 8). The copy numbers of CAT and SOD did not differ significantly among the cultivars. However, under the PAW10 treatment, CAT and SOD copy numbers increased by 10.60% and 93.55%, respectively, compared to the control. In the case of APX, SN and SF cultivars under PAW10 treatment exhibited higher gene copy numbers than those under control (Figure 2).

Table 8.
Copy number of CAT, APX, and SOD genes in kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
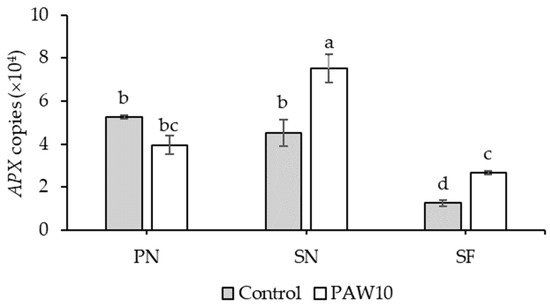
Figure 2.
Interaction effect between cultivar and PAW treatment on the copy number of the APX gene in kangkong microgreens. PN: Phai-Ngern; SN: Senee 20; SF: Senafore 20. Control: seeds were soaked and irrigated with distilled water; PAW10: seeds were soaked and irrigated with plasma-activated water activated for 10 min. Values are means ± SD (n = 3). Treatments sharing the same lowercase letters are not significantly different according to Tukey’s HSD test (p < 0.05).
3.3.6. Bioactive Compounds
There was no significant interaction between cultivar and PAW treatment with respect to total phenolic and flavonoid contents (Table 9). The SN and SF cultivars exhibited the highest total phenolic and flavonoid contents, respectively. The PAW10 treatment significantly increased total phenolic and flavonoid contents by 17.95% and 12.73%, respectively, compared to the control. A significant interaction between cultivar and PAW treatment was observed for total ascorbic acid content (Table 9). In the PN cultivar, total ascorbic acid content increased significantly by 26.24% under the PAW10 treatment compared to the control, whereas the SN and SF cultivars showed no significant differences across treatments (Figure 3).

Table 9.
Total phenolic content (TPC), total flavonoid content (TFC), and total ascorbic acid content (AsA) of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
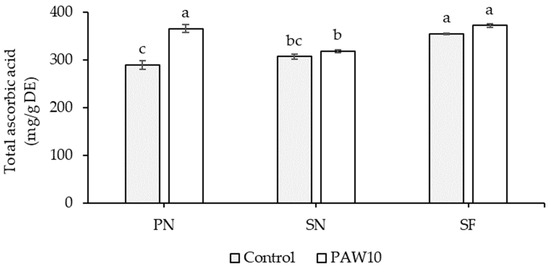
Figure 3.
Interaction effect between cultivar and PAW treatment on total ascorbic content in kangkong microgreens. PN: Phai-Ngern; SN: Senee 20; SF: Senafore 20. Control: seeds were soaked and irrigated with distilled water; PAW10: seeds were soaked and irrigated with plasma-activated water activated for 10 min. Values are means ± SD (n = 3). Treatments sharing the same lowercase letters are not significantly different according to Tukey’s HSD test (p < 0.05).
There was no significant interaction between cultivar and PAW treatment with respect to phenolic profile, except for ferulic acid and vanillic acid (Table 10). The highest content of chlorogenic acid and coumaric acid was observed in SF cultivar, while the highest content of rutin was found in SN cultivar. However, no significant differences were observed in the contents of chlorogenic acid, coumaric acid, and rutin between the PAW10 and control treatments. The ferulic acid content in the SN cultivar increased significantly by 10.08% under the PAW10 treatment compared to the control, while no significant differences were observed in the PN and SF cultivars across treatments (Figure 4a). In contrast, the vanillic acid content in the PN and SN cultivars decreased by 13.12% and 9.78%, respectively, under the PAW10 treatment compared to the control, whereas the SF cultivar showed no significant differences across treatments (Figure 4b).

Table 10.
Chlorogenic acid (CHL), coumaric acid (COU), rutin (RUT), ferulic acid (FER), and vanillic acid (VAN) contents of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
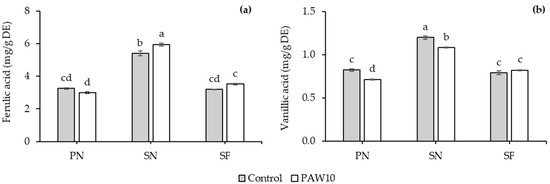
Figure 4.
Interaction effect between cultivar and PAW treatment on ferulic acid (a) and vanillic acid (b) contents in kangkong microgreens. PN: Phai-Ngern; SN: Senee 20; SF: Senafore 20. Control: seeds were soaked and irrigated with distilled water; PAW10: seeds were soaked and irrigated with plasma-activated water activated for 10 min. Values are means ± SD (n = 3). Treatments sharing the same lowercase letters are not significantly different according to Tukey’s HSD test (p < 0.05).
3.3.7. Antioxidant and Antiglycation Activities
There was no significant interaction between cultivar and PAW treatment with respect to antioxidant and antiglycation activities (Table 11). Antioxidant activities assessed by the DPPH and FRAP assays showed no significant differences among the cultivars, while the strongest antioxidant activity, as measured by the ABTS assay, was found in SF cultivar. The PAW10 treatment resulted in stronger antioxidant activities across all three assays compared to the control. In terms of antiglycation activities, no significant differences were observed across cultivar and treatment.

Table 11.
Antioxidant activities using DPPH, FRAP, and ABTS assays and antiglycation activities (AG) of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
3.3.8. Nutritional Contents
There was no significant interaction between cultivar and PAW treatment with respect to nutritional contents, except for protein (Table 12). The SF cultivar under the PAW10 treatment exhibited the highest protein content, whereas the other treatments showed lower protein levels, with no significant differences among them (Figure 5). The SF cultivar showed the highest lipid content, which was not significantly different from that of PN. The PAW10 treatment increased lipid content by 9.37% compared to the control. No significant differences were observed in fiber, ash, or carbohydrate contents among the treatments.

Table 12.
Nutritional contents of kangkong microgreens (cultivars PN, SN, and SF) under PAW treatments.
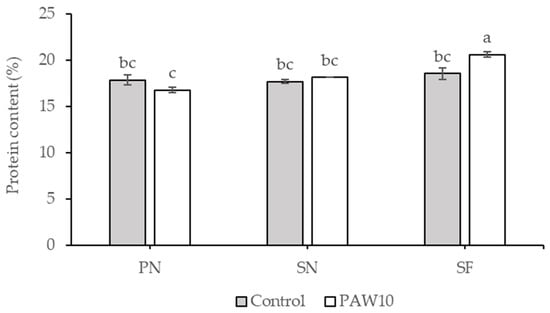
Figure 5.
Interaction effect between cultivar and PAW treatment on protein content in kangkong microgreens. PN: Phai-Ngern; SN: Senee 20; SF: Senafore 20. Control: seeds were soaked and irrigated with distilled water; PAW10: seeds were soaked and irrigated with plasma-activated water activated for 10 min. Values are means ± SD (n = 3). Treatments sharing the same lowercase letters are not significantly different according to Tukey’s HSD test (p < 0.05).
3.3.9. Principal Component Analysis (PCA)
PCA was performed on 33 dependent variables to explore relationships among growth parameters, photosynthetic pigments, biochemical markers, antioxidant enzyme activities and gene copy numbers, bioactive compounds, antioxidant and antiglycation activities, and nutritional contents in three kangkong microgreen cultivars subjected to PAW10 and control treatments. The first two principal components (PC1 and PC2) together explained 54.0% of the total variance (Figure 6), with PC1 accounting for 33.4% (95% CI: 29.69–50.29) and PC2 for 20.6% (95% CI: 16.01–30.04). The effect size of PC1, as indicated by its proportion of explained variance, suggests a substantial contribution to the overall variability, although the relatively wide confidence interval reflects greater uncertainty. In contrast, PC2 shows a moderate effect size with a narrower confidence interval, indicating greater precision. Collectively, these components provide a meaningful summary of the underlying data structure.
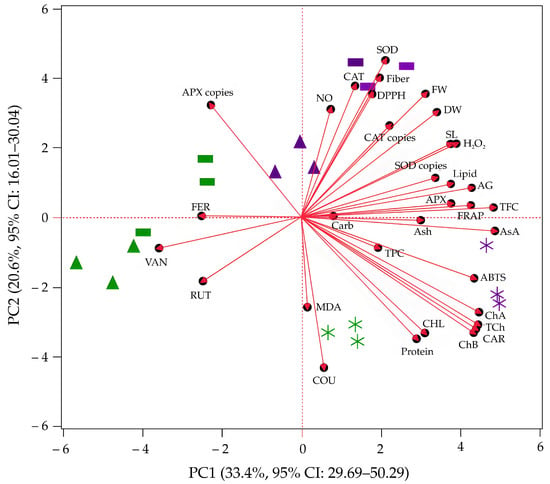
Figure 6.
PCA score and loading plots of physiological, biochemical, and nutritional traits of kangkong microgreens. Cultivars: PN (Phai-Ngern), SN (Senee 20), and SF (Senafore 20). Treatments: Control (seeds soaked and irrigated with distilled water) and PAW10 (seeds soaked and irrigated with plasma-activated water activated for 10 min). Rectangles, triangles, and stars represent PN, SN, and SF, respectively. Purple and green symbols indicate PAW10 and control treatments, respectively.
According to the Pearson correlation coefficients (Table 13), total ascorbic acid (r = 0.86), total flavonoids (r = 0.85), chlorophyll a (r = 0.79), total chlorophyll (r = 0.78), carotenoids (r = 0.77), ABTS (r = 0.77), chlorophyll b (r = 0.76), antiglycation activity (r = 0.76), and FRAP (r = 0.75) exhibited strong positive correlations with PC1. These variables contribute substantially to the variance captured by the first principal component. For PC2, SOD activity (r = 0.79) and fiber content (r = 0.71) showed strong positive correlations, while coumaric acid (r = –0.76) showed a strong negative correlation. These variables contribute to a distinct and independent dimension of variability represented by the second principal component, potentially associated with physiological or compositional attributes not represented by PC1.

Table 13.
Pearson correlation coefficients between original variables and principal components, based on the combined data of all kangkong microgreens cultivars under PAW treatments.
Based on the score plot, the SF cultivar under PAW10 treatment was positioned on the positive side of PC1, corresponding to elevated levels of total ascorbic acid, total flavonoids, chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, ABTS, FRAP, and antiglycation activity, variables strongly associated with PC1. Meanwhile, the PN cultivar under PAW10 treatment was located on the positive side of PC2, reflecting high SOD activity and fiber content, alongside low coumaric acid levels, which were strongly correlated with PC2. The SN cultivar under PAW10 treatment was located near the center of the score plot, indicating that its characteristics are close to the overall average with respect to the variables represented by the principal components. Under control conditions, the SN and PN cultivars were positioned on the negative side of PC1, indicating associations with low levels of bioactive compounds, antioxidant activity, and antiglycation activity. The SF cultivar under control conditions was located on the negative side of PC2, suggesting an association with high coumaric acid content as well as low SOD activity and fiber content.
4. Discussion
Plasma activation altered the physicochemical properties of PAW by decreasing pH and increasing EC as activation time increased. These changes were primarily attributed to the accumulation of RONS, particularly NO2− and NO3−. Although H2O2 levels were elevated in all PAWs activated for different durations, the differences among activation times were not significant. The formation of H2O2 results from energetic electrons dissociating water molecules (e− + H2O → •H + •OH + e−), followed by the recombination of hydroxyl radicals (•OH + •OH → H2O2). Meanwhile, NO2− and NO3− ions are generated at the gas–liquid interface through the dissociation of nitrogen and oxygen molecules in air plasma [53]. These RONS contribute to a decrease in pH and an increase in EC of the PAW by forming nitric and nitrous acids, along with hydronium ions (H3O+) [21]. Additionally, NO and NO2− can be further converted into inorganic acids such as HNO2 and HNO3 through reactions with H+ [30].
The physicochemical properties of PAW vary depending on factors such as plasma device type, activation time, working gas, and treatment method. In this study, PAW was generated using a direct arc plasma device with ambient air as the working gas, with activation times of 5, 10, and 15 min. Key parameters—including pH, EC, and concentrations of RONS (H2O2, NO2−, NO3−)—were analyzed to characterize the treatment effect. While some measured values differed from previous reports, the overall trends of decreasing pH and increasing EC and RONS concentrations with longer activation times were consistent with established plasma–water interaction mechanisms [8,9,10,21,30,31]. These results support the reproducibility and standardization of the treatment protocol, providing clearly defined parameters suitable for future research.
PAW treatment enhanced the germination and vigor of kangkong seeds, with PAW10 and PAW15 exhibiting the most pronounced effects. The acidification resulting from the accumulation of RONS remained within the crop tolerance range and caused no visible tissue damage. This may be attributed to the intrinsic buffering mechanisms of the plant, such as root exudation and vacuolar pH regulation [54]. RONS play vital signaling roles during seed germination [55]. Specifically, reactive oxygen species (ROS) present in PAW enhance oxygen availability and mitochondrial respiration, thereby improving germination rate and uniformity [10]. In addition, ROS such as H2O2 play a crucial signaling role in activating antioxidant defenses and metabolic pathways essential for seed germination [56]. Meanwhile, reactive nitrogen species (RNS) such as NO2− and NO3− serve dual roles as signaling molecules and nitrogen sources. NO3− contributes to nitrogen metabolism via the glutamine synthetase–glutamate synthase (GS–GOGAT) pathway and influences seed dormancy by interacting with phytohormones, including abscisic acid (ABA) and gibberellin (GA) [57,58]. Our results align with previous studies demonstrating the beneficial effects of PAW on seed germination [11,12,13,59]. Although both PAW10 and PAW15 resulted in similar improvements in seed germination and vigor, PAW10 was selected for further investigation due to its shorter plasma activation time and consistent performance across multiple parameters. This choice enabled a more efficient yet effective approach to evaluate its impact on microgreen quality.
In addition to its promotive effects on seed germination, PAW10 treatment significantly improved the early growth performance of kangkong microgreens, as evidenced by increased shoot length and biomass accumulation. This improvement is partly attributed to nitrogenous species such as NO2− and NO3− present in PAW, which serve as both essential nutrients and signaling molecules regulating amino acid and protein biosynthesis [7,58]. Additionally, PAW modulated the phytohormonal balance by reducing ABA and improving GA levels, thereby stimulating cell division and elongation [10]. The PAW10 treatment also increased photosynthetic pigment accumulation, including chlorophylls and carotenoids, likely through redox signaling induced by RONS [10]. These signals activate genes in the tetrapyrrole and isoprenoid biosynthetic pathways responsible for chlorophyll and carotenoid production, respectively [60]. Additionally, PAW alters seed and root membrane permeability, improving the uptake of essential minerals such as magnesium, which is crucial for chlorophyll synthesis [59]. Moreover, NO3− acts both as a nutrient and a signaling molecule, promoting pigment synthesis and overall plant growth [58]. These findings are consistent with previous studies reporting enhanced plant growth and pigment accumulation following PAW treatment. For example, increased seedling vigor and shoot biomass have been observed in mung bean [11], lettuce [15,53], tomato [17], and buckwheat [16]. In terms of pigment content, species-dependent responses have been reported: chlorophyll b and carotenoids increased in maize [61] and lettuce [18], whereas chlorophyll a decreased but chlorophyll b increased in rocket salad [62]. Taken together, these reports indicate that the effects of PAW vary depending on plant species, application methods, and treatment duration.
Beyond enhancing growth and pigment synthesis, PAW10 treatment was also found to significantly increase H2O2 levels in kangkong microgreens. This accumulation likely results from both exogenous sources, namely H2O2 present in PAW itself [63], and endogenous production catalyzed by SOD, which converts superoxide into H2O2, a central molecule in plant redox signaling [64]. Notably, we observed significant increases in the activities of major antioxidant enzymes—SOD, CAT, and APX—accompanied by elevated gene copy numbers for these enzymes, suggesting that gene amplification may partially regulate the enzymatic response [65]. This concurrent upregulation highlights a robust redox adaptation mechanism in kangkong microgreens under PAW10 treatment. Importantly, despite elevated H2O2 levels, the concentrations of MDA—a biomarker of oxidative stress—remained unchanged compared to the controls. This suggests that PAW10 enhances redox signaling and antioxidant preparedness without causing cellular damage or lipid peroxidation, consistent with the hormesis concept observed in plants [66]. This response may also represent a priming effect, where mild oxidative stress induced by PAW prepares the plant for enhanced defense upon subsequent stress exposure [21]. This low-dose stimulation aligns with the hormesis model, in which sub-lethal stress levels promote adaptive responses and resilience in plants [66]. The lack of significant NO increase following PAW treatment suggests that NO may not be involved at this stage, or it could have been rapidly consumed in downstream signaling processes. Overall, these findings suggest a finely tuned balance between ROS generation and detoxification in response to PAW10, representing a favorable oxidative priming state. Furthermore, redox-related responses to PAW treatment have been reported across multiple plant species. For example, Sajib et al. [30] observed elevated H2O2 and CAT activity in black gram roots derived from PAW-treated seeds, while SOD and APX activities remained unchanged, suggesting a selective antioxidant activation pattern. Similarly, Adhikari et al. [21] reported increased H2O2 and NOx levels in tomato seedlings irrigated with PAW activated for 15 to 60 min. Notably, MDA levels remained unchanged at shorter activation durations (15–30 min), consistent with our findings that moderate PAW10 treatment enhances redox signaling without inducing oxidative damage. Yemeli et al. [61] further demonstrated that maize and barley responded to PAW irrigation with increased CAT but decreased SOD activity, suggesting species-specific regulation of redox enzymes. In contrast, our findings indicate that kangkong microgreens exhibit a broader antioxidant response to PAW, characterized by elevated activities of all three enzymes and increased gene copy numbers. This may reflect greater genomic plasticity or heightened sensitivity to oxidative stimuli in this species.
PAW10 treatment enhanced the overall quality of kangkong microgreens by selectively increasing key phytochemicals and antioxidant capacity, with effects differing between cultivars. In particular, the contents of total ascorbic acid, ferulic acid, and vanillic acid were influenced by both cultivar and PAW10 treatment. PAW10 consistently promoted the accumulation of total phenolics and flavonoids, whereas levels of chlorogenic acid, coumaric acid, and rutin remained unaffected. This selective modulation of the phenolic profile suggests a possible regulatory effect of PAW10 on the biosynthesis of specific bioactive compounds in kangkong microgreens. The accumulation of antioxidant compounds under PAW10 treatment appears to be driven by a controlled oxidative stress response, primarily involving reactive species such as H2O2, NO2−, and NO3−. These molecules function as signaling agents, triggering defense pathways and enhancing the biosynthesis of phenolic compounds and flavonoids through the shikimate and phenylpropanoid pathways. These biosynthetic processes are tightly regulated by stress-responsive genes and enzymes [5,67]. Moreover, PAW10 treatment significantly increased the antioxidant activities of kangkong microgreens, as measured by DPPH, FRAP, and ABTS assays. This enhancement is likely due to ROS generated by PAW, which act as signals to stimulate the biosynthesis of antioxidants such as phenolics and flavonoids [68]. Elevated antioxidant activity is a well-established adaptive response to mild oxidative stress, facilitating the accumulation of bioactive compounds that effectively neutralize free radicals and protect cells from oxidative damage [69]. Importantly, this increase occurred without significant changes in oxidative damage markers, suggesting that PAW10 induces a priming effect rather than causing cellular stress. Elevated levels of total phenolics, flavonoids, and antioxidant activities have been consistently reported in various plant species following PAW treatment. For example, PAW activated for 20 min enhanced these compounds in water spinach and buckwheat sprouts [16,68], while activation for just 20 s had similar effects in mung bean sprouts [11]. Short-duration PAW (5 min) also improved antioxidant capacity in wheat seedlings [13]. In tomato, longer PAW treatments (30–60 min) increased ascorbic acid levels [21]. However, no significant effects were observed in mung bean after a 30-min PAW treatment [70], or in pea sprouts [59], suggesting that the effectiveness of PAW varies depending on both activation time and plant species.
Despite the observed improvements in antioxidant bioactive compounds and phenolic profiles, PAW10 treatment did not significantly enhance the antiglycation activity of kangkong microgreens in this study. Although plasma-induced oxidative stress is often associated with the formation of AGEs, and antiglycation compounds are recognized as promising agents for managing diabetic complications [71], the lack of effect observed here may be attributed to insufficient concentration or an unfavorable composition of RONS in PAW10, limiting activation of antiglycation-related biochemical pathways. Overall, these findings suggest that under the current treatment conditions, PAW10 does not significantly influence the antiglycation activity of kangkong microgreens. Conversely, direct plasma treatment using DBD has been shown to enhance antiglycation activity in this species [29]. Collectively, these observations indicate that indirect (PAW10) and direct plasma treatments exert distinct effects on the antiglycation properties of kangkong microgreens, possibly due to differences in reactive species composition and their modes of action.
Regarding nutritional composition, PAW10 treatment significantly increased protein content in kangkong microgreens, but only in the SF cultivar. This cultivar-specific effect is likely due to enhanced nitrogen availability in the form of NO3− and NO2−, which facilitate nitrogen assimilation and protein biosynthesis in plants [72], whereas the other two cultivars (PN and SN) did not show significant changes in protein content compared to the control. This finding aligns with observations in other species; for example, Ji et al. [73] reported elevated protein and amino acid levels in soybean sprouts following cold plasma and PAW treatments, highlighting the potential role of RONS in modulating metabolic pathways associated with nutrient accumulation. In addition to protein, the increase in lipid content under PAW10 treatment may result from mild oxidative stress caused by reactive species, which can stimulate lipid biosynthesis as a protective response [74]. Although the nutritional effects of plasma treatments vary depending on plant species and treatment conditions, similar biochemical modulations have been documented. For instance, tomato seedlings grown from seeds exposed to intermittent DBD plasma for 2 min showed the highest carbohydrate content, while protein content was doubled in seedlings treated for 1 min compared to untreated controls [75]. In contrast, wheat seedlings (cv. Dacic) derived from seeds treated with DBD plasma for 5 min exhibited no significant changes in protein levels and showed reduced fiber content, but had a higher ash content than the control group [76]. Overall, these findings emphasize that plasma treatment effects on nutritional composition depend on multiple factors, such as plant species, treatment duration, and nutrient type, underscoring the complexity of plasma–plant interactions.
PCA revealed distinct cultivar-specific metabolic responses to PAW10 treatment. The SF cultivar exhibited the most pronounced shift, moving from the negative side of PC2 (control) to the positive side of PC1 (PAW10), correlating with significant increases in antioxidant and antiglycation activities, as well as key bioactive compounds. This indicates that PAW10 effectively enhances health-promoting phytochemicals in SF, supporting previous reports of PAW-induced phytochemical accumulation and antioxidant activity in plants [11,16,21,68]. In contrast, the PN cultivar under PAW10 aligned with elevated SOD activity and fiber content along PC2, suggesting a distinct antioxidant response involving enzyme-mediated ROS detoxification [64]. The SN cultivar showed minimal changes, indicating limited responsiveness to PAW10. These findings highlight genotype-dependent metabolic variations in response to elicitor treatments [29] and emphasize the potential of SF for nutritionally enhanced microgreen production. Selecting appropriate cultivars is crucial for targeted metabolic improvements in functional food development [77].
5. Conclusions
This study demonstrates that both PAW10 and PAW15 treatments significantly improved seed germination and vigor in three kangkong cultivars, with germination percentages increasing by 5.22–11.98% and germination indices improving by 25.35–26.60%. Moreover, PAW10 treatment markedly enhanced phytochemical accumulation and antioxidant activity, as evidenced by increases in total phenolic content (17.95%), flavonoids (12.73%), carotenoids (11.40%), and FRAP antioxidant capacity (18.56%). PCA revealed clear cultivar-specific responses to PAW10 treatment. The SF cultivar showed the greatest enhancement in bioactive compounds, antioxidant, and antiglycation activities, while the PN cultivar exhibited moderate responses, including elevated SOD activity and fiber content. In contrast, the SN cultivar displayed minimal responsiveness. These findings support the potential of PAW10 as a sustainable and effective elicitor for improving seedling vigor and functional quality in kangkong microgreens. Further studies are recommended to clarify the underlying mechanisms and optimize treatment conditions for broader applications.
Author Contributions
Conceptualization and methodology, P.R., N.P. and Y.J.; investigation, P.R., and P.O.; data curation, P.R., Y.J., B.H. and P.O.; writing—original draft preparation, P.R. and P.O.; writing—review and editing, P.R. and Y.J.; funding acquisition, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Thailand Science Research and Innovation Fundamental Fund (Project No. 68468), a Ph.D. scholarship granted by Thammasat University (1/2022), and the Research Promotion Fund for International and Educational Excellence (Grant No. 8/2564).
Data Availability Statement
The data presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We gratefully acknowledge the Faculty of Science and Technology, Thammasat University, and the Center of Advanced Nuclear Technology, Thailand Institute of Nuclear Technology, for providing the research facilities. We also extend our sincere thanks to Jureemart Wangkeeree for her valuable assistance in determining the antioxidant enzyme gene copy number.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waskow, A.; Howling, A.; Furno, I. Mechanism of plasma-seed treatment as a potential seed processing technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Darmanin, M.; Fröhling, A.; Bußler, S.; Durek, J.; Neugart, S.; Schreiner, M.; Blundell, R.; Gatt, R.; Schlüter, O.; Valdramidis, V.P. Aqueous and gaseous plasma applications for the treatment of mung bean seeds. Sci. Rep. 2021, 11, 19681. [Google Scholar] [CrossRef] [PubMed]
- Iranbakhsh, A.; Ghoranneviss, M.; Ardebili, Z.O.; Ardebili, N.O.; Tackallou, S.H.; Nikmaram, H. Non-thermal plasma modified growth and physiology in Triticum aestivum via generated signaling molecules and UV radiation. Biol. Plant. 2017, 61, 702–708. [Google Scholar] [CrossRef]
- Sharma, H.P.; Patel, A.H.; Pal, M. Effect of plasma-activated water (PAW) on fruits and vegetables. Am. J. Food Nutr. 2021, 9, 60–68. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current advancements in the molecular mechanism of plasma treatment for seed germination and plant growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-activated water: Physicochemical properties, generation techniques, and applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annopure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Guragain, R.P.; Pradhan, S.P.; Baniya, H.B.; Pandey, B.P.; Basnet, N.; Sedhai, B.; Dhungana, S.; Chhetri, G.K.; Joshi, U.M.; Subedi, D.P. Impact of plasma-activated water (PAW) on seed germination of soybean. J. Chem. 2021, 2021, 7517052. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.H.; Sun, D.W. Enhancement of wheat seed germination, seedling growth and nutritional properties of wheat plantlet juice by plasma activated water. Plant Growth Regul. 2023, 42, 2006–2022. [Google Scholar] [CrossRef]
- Asghari, A.; Sabbaghtazeh, E.; Milani, N.R.; Kouhi, M.; Maralani, A.A.; Gharbani, P.; Khiaban, A.S. Effects of plasma-activated water on germination and initial seedling growth of wheat. PLoS ONE 2025, 20, e0312008. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Bácovčinová, M.; Hensel, K. Effect of plasma activated water, hydrogen peroxide, and nitrates on lettuce growth and its physiological parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Z.; Ma, T. The effects of plasma-activated water treatment on the growth of tartary buckwheat sprouts. Front. Nutr. 2022, 9, 849615. [Google Scholar] [CrossRef]
- Vichiansan, N.; Chatmaniwat, K.; Sungkorn, M.; Leksakul, K.; Chaopaisarn, P.; Boonyawan, D. Effect of plasma-activated water generated using plasma jet on tomato (Solanum lycopersicum L. var. cerasiforme) seedling growth. J. Plant Growth Regul. 2023, 42, 935–945. [Google Scholar] [CrossRef]
- Carmassi, G.; Cela, F.; Trivellini, A.; Gambineri, F.; Cursi, L.; Cecchi, A.; Pardossi, A.; Incrocci, L. Effects of nonthermal plasma (NTP) on the growth and quality of baby leaf lettuce (Lactuca sativa var. acephala Alef.) cultivated in an indoor hydroponic growing system. Horticulturae 2022, 8, 251. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244. [Google Scholar] [CrossRef]
- Abbaszadeh, R.; Nia, K.P.; Fattahi, M.; Maezdashti, H.G. The effects of three plasma-activated water generation systems on lettuce seed germination. Res. Agric. Eng. 2021, 67, 131–137. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, Z.; Han, X.; Chen, X.; Wang-pruski, G. Physiological and transcriptomic responses of water spinach (Ipomoea aquatica) to prolonged heat stress. BMC Genom. 2020, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Rubatzky, V.E.; Yamaguchi, M. World Vegetables: Principles, Production, and Nutritive Values, 2nd ed.; Chapman & Hall: New York, NY, USA, 1996; pp. 711–713. [Google Scholar]
- Malakar, C.; Choudhury, P.P.N. Pharmacological potentiality and medicinal uses of Ipomoea aquatica Forsk: A review. Asian J. Pharm. Clin. Res. 2015, 8, 60–63. [Google Scholar]
- Sajak, A.A.B.; Abas, F.; Ismail, A.; Khatib, A. Effect of different drying treatment and solvent ratios on phytochemical of Ipomoea aquatica and correlation with α-glucosidase inhibitory activity. Int. J. Food Prop. 2016, 19, 2817–2831. [Google Scholar] [CrossRef]
- El-Sawi, N.; Gad, M.H.; Al-Seeni, M.N.; Younes, S.; El-Ghadban, E.M.; Ali, S.S. Evaluation of antidiabetic activity of Ipomoea aquatica fractions in streptozotocin induced diabetic in male rat mode. Sohag J. Sci. 2017, 2, 9–17. [Google Scholar] [CrossRef]
- Harakotr, B.; Charoensup, L.; Rithichai, P.; Jirakiattikul, Y.; Suthamwong, P. Yield, bioactive compounds, and antioxidant potential of twenty-three diverse microgreen species grown under controlled conditions. Resources 2025, 14, 71. [Google Scholar] [CrossRef]
- Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Saidarasamoot, K.; Jirakiattikul, Y.; Rithichai, P. Germination, physicochemical properties, and antioxidant enzyme activities in kangkong (Ipomoea aquatica Forssk.) seeds as affected by dielectric barrier discharge plasma. Horticulturae 2023, 9, 1269. [Google Scholar] [CrossRef]
- Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Harakotr, B.; Jirakiattikul, Y.; Rithichai, P. Enhancing the growth, bioactive compounds, and antioxidant activity of kangkong (Ipomoea aquatica Forssk.) microgreens using dielectric barrier discharge plasma. Resources 2025, 14, 72. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Ma, T. Properties of plasma-activated water with different activation time and its effects on the quality of button mushrooms (Agaricus bisporus). LWT 2021, 147, 111633. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The effects of plasma-activated water on heavy metals accumulation in water spinach. Appl. Sci. 2021, 11, 5304. [Google Scholar] [CrossRef]
- Chuea-uan, S.; Boonyawan, D.; Sawanratg, C.; Thanapornpoonpong, S. Using plasma-activated water generated by an air gliding arc as a nitrogen source for rice seed germination. Agronomy 2024, 14, 15. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2012; pp. 5-1–5-78. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Antioxidative properties of 34 green leafy vegetables. J. Funct. Foods 2016, 26, 176–186. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, R. Determinations of total carotenoids and chlorophyll a and b of leaf extract in different solvents. Biochem. Soc. Trans. 1983, 11, 519–592. [Google Scholar] [CrossRef]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric oxide regulates plant growth, physiology, antioxidant defense, and ion homeostasis to confer salt tolerance in the mangrove species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.J.; Dias, D.C.F.D.S.; Sekita, M.C.; Finger, F.L. Lipid peroxidation and antioxidant enzymes of Jatropha curcas L. seeds stored at different maturity stages. Acta. Sci. Agron. 2018, 40, e34978. [Google Scholar] [CrossRef]
- Önder, S.; Önder, D.G.; Tonguç, M. Determination of hydrogen peroxide content and antioxidant enzyme activities in safflower (Carthamus tinctorius L.) seeds after accelerated aging test. J. Nat. Appl. Sci. 2020, 24, 681–688. [Google Scholar] [CrossRef]
- Heshmati, S.; Dehaghi, M.A.; Farooq, M.; Wojtyla, Ł.; Maleki, K.; Heshmati, S. Role of melatonin seed priming on antioxidant enzymes and biochemical responses of Carthamus tinctorius L. under drought stress conditions. Plant Stress 2021, 2, 100023. [Google Scholar] [CrossRef]
- Aboul-Maaty, N.A.F.; Oraby, H.A.S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef]
- Talaat, N.B.; Ibrahim, A.S.; Shawky, B.T. Enhancement of the expression of ZmBZR1 and ZmBES1 regulatory genes and antioxidant defense genes triggers water stress mitigation in maize (Zea mays L.) plants treated with 24-epibrassinolide in combination with spermine. Agronomy 2022, 12, 2517. [Google Scholar] [CrossRef]
- Rithichai, P.; Jirakiattikul, Y.; Singhawiboon, M.; Poolyarat, N. Enhancement of seed quality and bioactive compound accumulation in sunflower sprouts by dielectric barrier discharge plasma treatment. ScienceAsia 2021, 47, 441–448. [Google Scholar] [CrossRef]
- Kammapana, L.; Mulalin, S.; Tangteerawattana, S. Effects of exogenous methyl jasmonate treatment with polyethylene bag storage on decay reduction and enhanced total ascorbic acid, total phenolic, and antioxidant activities in ‘Kamphaeng Saen 42’ mulberry fruit. Trends Sci. 2022, 19, 3069. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of plumbagin production through elicitation in in vitro-regenerated shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Puccinelli, M.; Maggini, R.; Angelini, L.G.; Santin, M.; Landi, M.; Tavarini, S.; Castagna, A.; Incrocci, L. Can light spectrum composition increase growth and nutritional quality of Linum usitatissimum L. sprouts and microgreens? Horticulturae 2022, 8, 98. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. Amber, red and blue LEDs modulate phenolic contents and antioxidant activities in eight Cruciferous microgreens. J. Food Bioact. 2020, 11, 95–109. [Google Scholar] [CrossRef]
- Rahbar, S.; Natarajan, R.; Yerneni, K.; Scott, S.; Gonzales, N.; Nadler, J.L. Evidence that pioglitazone, metformin and pent-oxifylline are inhibitors of glycation. Clin. Chim. Acta. 2000, 301, 65–77. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Ndamitso, M.M.; Etsuyankpa, M.B.; Jacob, J.O.; Mathew, J.T.; Shab, E.Y.; Olisedeme, K.C. The nutritional values and functional properties of wild Ipomoea aquatica (water spinach) found in the Fadama areas of Minna, Niger state, Nigeria. Acad. Res. Int. 2015, 6, 1–8. Available online: https://www.europub.co.uk/articles/-A-106811 (accessed on 7 July 2023).
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Gámez-Arjona, F.M.; Sánchez-Rodríguez, C.; Montesinos, J.C. The root apoplastic pH as an integrator of plant signaling. Front. Plant Sci. 2022, 13, 931979. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.P.; Kamiya, Y.; Nambara, E.; Truong, H.N. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Gone, S.S.J.; Singh, R.K. Enhancing pea seed sprouting and quality with plasma-activated water. Food Bioprocess Technol. 2025. [Google Scholar] [CrossRef]
- Sun, T.; Wang, P.; Rao, S.; Zhou, X.; Wrightstone, E.; Lu, S.; Yuan, H.; Yang, Y.; Fish, T.; Thannhauser, T.; et al. Co-chaperoning of chlorophyll and carotenoid biosynthesis by ORANGE family proteins in plants. Mol. Plant 2023, 16, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Yemeli, G.B.N.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2020, 18, e2000205. [Google Scholar] [CrossRef]
- Abouelenein, D.; Angeloni, S.; Caprioli, G.; Genovese, J.; Mustafa, A.M.; Nzekoue, F.K.; Petrelli, R.; Rocculi, P.; Sagratini, G.; Tappi, S.; et al. Effect of plasma activated water on selected chemical compounds of rocket-salad (Eruca sativa Mill.) leaves. Molecules 2021, 26, 7691. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, J.; Zhu, Y.; Mo, Y.; Yao, X.-F.; Wang, R.; Ku, W.; Huang, Z.; Xia, S.; Tong, J.; et al. The copy number variation of OsMTD1 regulates rice plant architecture. Front. Plant Sci. 2021, 11, 620282. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Kong, T.-K.; Chen, C.-Y.; Chen, H.-L. Plasma-activated water affects the antioxidant contents in water spinach. Appl. Sci. 2023, 13, 3341. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Jeong, G.H.; Jeong, Y.H.; Kim, T.H. Identification of sesamol byproducts produced by plasma treatment with inhibition of advanced glycation endproducts formation and ONOO− scavenging activities. Food Chem. 2020, 314, 126196. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, F.; Crawford, N.M.; Wang, Y. Molecular regulation of nitrate responses in plants. Int. J. Mol. Sci. 2018, 19, 2039. [Google Scholar] [CrossRef]
- Ji, W.; Li, M.; Yang, T.; Li, H.; Li, W.; Wang, J.; Ma, M. Effect of cold plasma on physical–biochemical properties and nutritional components of soybean sprouts. Food Res. Int. 2022, 161, 111766. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold plasma processing on fruits and fruit juices: A Review on the effects of plasma on nutritional quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Sultan, S.M.E.; Yousef, A.F.; Ali, W.M.; Mohamed, A.A.A.; Ahmed, A.R.; Shalaby, M.E.; Teiba, I.I.; Hassan, A.M.; Younes, N.A.; Kotb, E.F. Cold atmospheric plasma enhances morphological and biochemical attributes of tomato seedlings. BMC Plant Biol. 2024, 24, 420. [Google Scholar] [CrossRef] [PubMed]
- Burducea, I.; Burducea, C.; Mereuta, P.E.; Sirbu, S.R.; Iancu, D.A.; Istrati, M.B.; Straticiuc, M.; Lungoci, C.; Stoleru, V.; Teliban, G.C.; et al. Helium atmospheric pressure plasma jet effects on two cultivars of Triticum aestivum L. Foods 2023, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).