1. Introduction
In recent years, heavy metal elements have accumulated in soil, water, and the atmosphere, seriously affecting the cultivation and safe production of crops. Cadmium (Cd), as one of the main heavy metal elements contributing to soil pollution, has the characteristics of wide range, wide distribution, non-degradability, strong toxicity, extremely strong mobility, and easy absorption and accumulation by plants, and it can also disrupt plant metabolism and damage plant growth [
1]. When Cd absorbed by plants has accumulated beyond the safety threshold of the plants themselves, it will cause varying degrees of toxic effects to plants at the morphological, physiological, and molecular levels [
2]. The Cd toxicity response in plants is mainly manifested as inhibited growth, damaged root systems, curled and yellowed leaves, and even leaf drop [
2]. Excessive accumulation of Cd in plants induces the production of a large number of reactive oxygen species, causing lipid peroxidation of cell membranes, degradation of chloroplasts, and severe damage to the photosynthetic reaction center of plants, and seriously inhibits the growth and development of plants [
3]. Therefore, plants must quickly take a series of protective measures—mainly by enhancing the activity of the antioxidant enzyme system and promoting the production of osmotic adjustment substances—to ensure the rapid removal of accumulated reactive oxygen species in cells. Studies have shown that Cd markedly increases ROS levels in tomato. In response, tomato plants present enhance antioxidant enzyme activities to alleviate Cd phytotoxicity [
4].
Plant hormones (such as auxin, gibberellin, cytokinin, abscisic acid, and ethylene) are trace organic substances synthesized within plants, which regulate growth and development as well as adaption to environmental changes. They can influence plant life activities through either synergistic or antagonistic effects [
5,
6,
7,
8,
9]. Studies have shown that plant hormones assist in integrating endogenous and exogenous signals, helping plants cope with abiotic stresses, such as Cd stress [
10,
11,
12]. Gibberellins, abscisic acid, auxin, jasmonic acid, cytokinins, ethylene, salicylic acid, brassinosteroids, and polyamines have garnered attention from botanical researchers as sustainable phytohormones that can induce tolerance in Cd-stressed plants [
13]. 4-Benzoylphenylboronic acid (PPBa) is a specific and effective inhibitor of flavin monooxygenase (YUCCA) enzymes, which inhibit the synthesis of IAA [
14]. The YUCCA family plays a vital role in the synthesis pathway of IPA. If the activity of these enzymes is inhibited, it will affect the secretion of IAA, thereby regulating the accumulation of Cd [
15]. Cd affects the normal growth of plants by inhibiting photosynthesis, influencing antioxidant enzyme activities, and regulating the synthesis and transportation of phytohormone [
13]. At the molecular level, Cd stress also induces the expression of a series of genes, such as genes encoding for plant chelators, heavy metal ATPases, YSL transport proteins, ABC transport proteins, antioxidant system activity and oxidative stress response, and chlorophyll synthesis or degradation [
13].
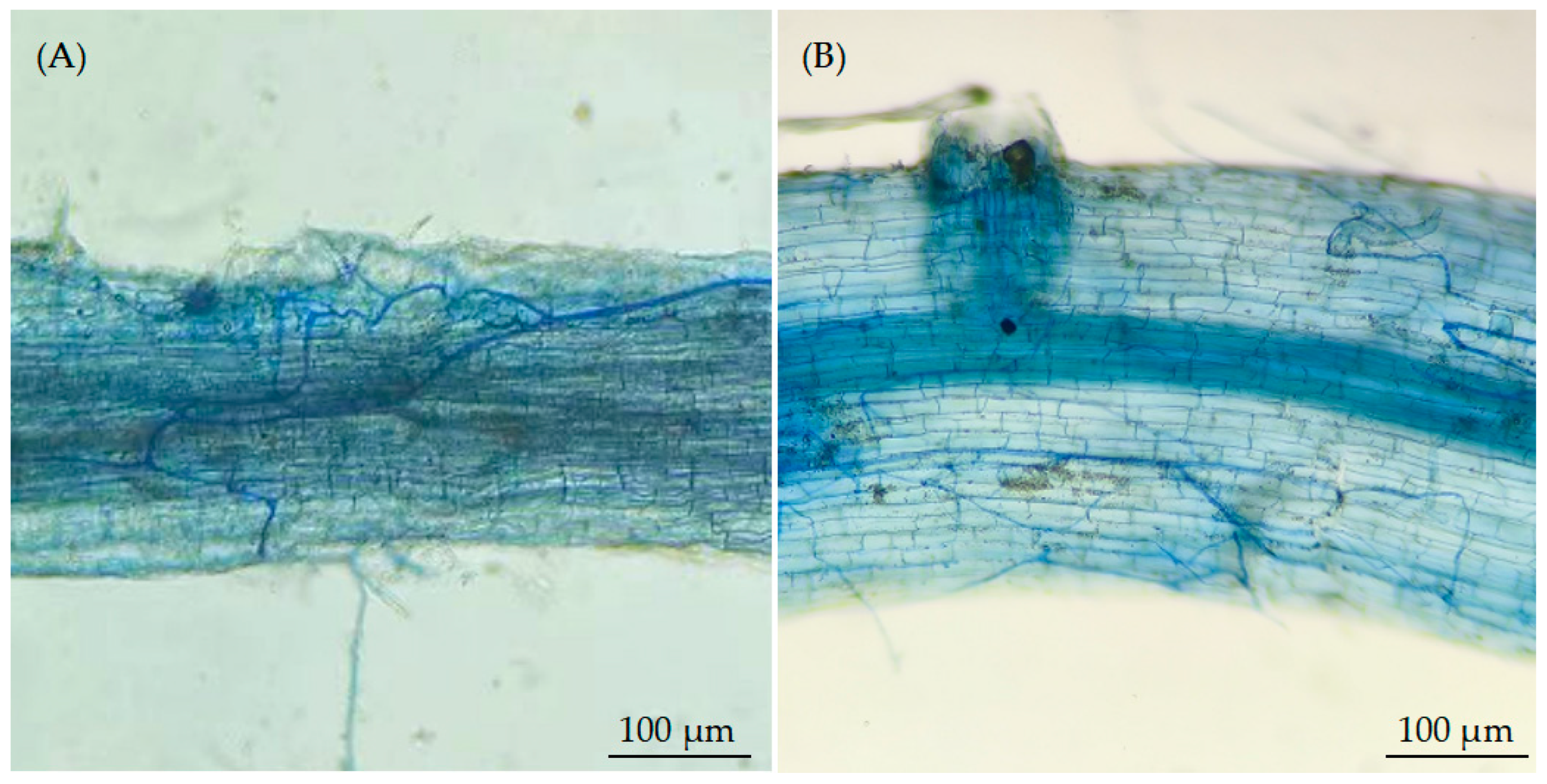
As symbiotic fungi, arbuscular mycorrhizal fungi (AMF) are widely present in soils and can form symbiotic relationships with most terrestrial plants [
16]. Under heavy metal stress, AMF infect plant roots to form mycorrhizal symbioses. In particular, the AMF exogenous hyphal network enhances the plant’s absorption of mineral nutrients and alleviates the negative effects of heavy metals on plant growth [
17,
18]. Shaher et al. [
19] demonstrated that AMF alleviated the toxic effects of heavy metals (Cd and Pb) by promoting nutrient absorption and secondary metabolite accumulation in
Calendula officinalis. AMF (
Rhizophagus irregularis) inoculation lowered Cd influx in Perennial Ryegrass (
Lolium perenne L.), enhanced the availability of nutrients in the rhizosphere, and mitigated Cd phytotoxicity [
20]. AMF also cause changes in the root exudates of plants. For instance, low-molecular-weight organic acids can form “heavy metal–low-molecular-weight organic acid” complexes with soil heavy metals, which reduces the mobility and bioavailability of heavy metals and alleviates heavy metals’ phytotoxicity [
21]. The influence process of low-molecular-weight organic acids on the availability of heavy metals is relatively complex. It is not only related to the types and properties of organic acids themselves, but also to factors such as soil conditions and planting patterns [
22]. Under Cd stress, AMF affect the secretion amounts of different types of low-molecular-weight organic acids, causing changes to the forms of heavy metals [
23]. The root systems of plants can alter the soil redox potential and pH by secreting low-molecular-weight organic acids (such as malic acid and citric acid) to reduce the solubility and mobility of heavy metal elements, thereby alleviating the growth conditions of plants under heavy metal stress [
23]. Therefore, after AMF have established a symbiotic relationship with plants, they can improve the morphology of the roots. At the same time, they affect the roots’ secretion of low-molecular-weight organic acids, thereby facilitating the host plants’ absorption of nutrient elements, inhibiting heavy metals’ mobility, and regulating the transport process of heavy metals in the host plants through the mycorrhizal structure and the complex mycelial network. As a result, heavy metals’ phytotoxicity on the host plants is reduced and the growth of the host plants is promoted, as AFM help the plants resist non-biological adverse conditions such as heavy metal stress [
17,
18,
24].
As an annual or perennial herbaceous plant of the Solanaceae family, tomato (
Solanum lycopersicum) is rich in lycopene, vitamins, and polyphenolic compounds, and is one of the most widely cultivated fruits and vegetables worldwide. Non-biological stress mainly refers to adverse conditions caused by environmental factors, including not only temperature stress, light stress, drought stress, and salt stress, but also various heavy metal stresses, such as Pb and Cd stress [
25,
26,
27,
28]. To date, most studies on non-biological stress in tomatoes have focused on the effects of salt stress, drought stress, and high-temperature stress. However, some existing studies have shown that tomatoes are sensitive to Cd. Moreover, Cd pollution is becoming increasingly severe, which not only reduces the yield and quality of vegetables such as tomatoes, but also poses a threat to human health [
4]. A significant body of research has focused on the response mechanisms of vegetables such as cucumber, lettuce, and pepper under Cd stress. However, few studies have focused on the mechanism of tomato’s tolerance to Cd stress in relation to microorganisms such as AMF. Therefore, it is of significance to study the growth and physiological and biochemical changes in tomato under Cd stress conditions in relation to AMF. This study used tomato (
Ailsa Craig) as the study material and applied AMF (
Diversispora versiformis) and CdSO
4 to interactively treat tomato seedlings. The study explored the growth and physiological–biochemical responses of tomato plants to AMF and CdSO
4, and further analyzed the mechanism by which AMF enhances tomato’s tolerance to Cd stress. This study provides a theoretical basis for using microorganisms to remediate heavy metal-contaminated soil and improve plant stress resistance.
4. Discussion
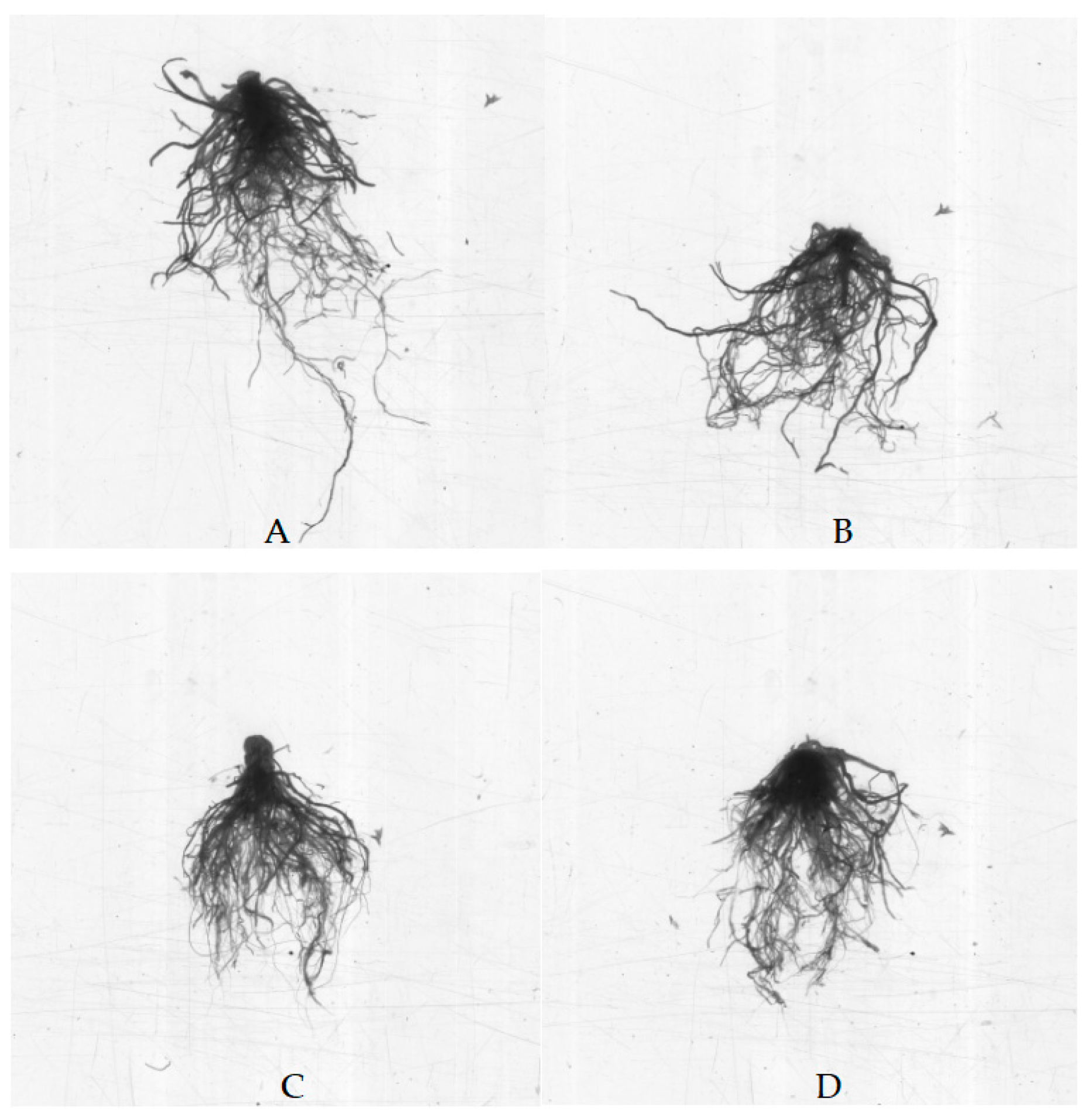
The phytotoxicity of Cd refers to the damaging effects it has on photosynthesis, growth, secondary metabolism, oxidative stress responses, and other plant processes [
18]. The morphological indicators of plants include total plant weight, leaf number, plant height, fresh weight of root system, fresh weight of aboveground part, total root length, and total root surface area, which can be used to reflect the response of plants to Cd toxicity [
30,
31,
32,
33]. Yang et al. [
20] used
Lolium perenne L. as the material to study the impact of 100 mg/kg Cd stress on its growth and found that Ca significantly inhibited various growth indicators such as leaf fresh weight and root fresh weight. In another study, 400 mg·kg
−1 Cd severely reduced the growth indicators of
Rosa rugosa and also caused chlorosis and leaf desiccation [
34]. In this study, the growth of the aboveground parts and roots of tomato plants treated with 50 μmol/L Cd was significantly inhibited. At the same time, phenomena such as leaf wilting, edge curling, darkening of color, chlorosis, slow addition of new leaves, and shedding of old leaves occurred. These findings suggest that Cd competes with mineral nutrients for the same transport pathways, thereby altering the absorption and distribution of mineral nutrients in plants, resulting in nutrient deficiency in the plants and inhibition of plant growth [
35].
AMF are important functional microorganisms widely present in soils. After AMF infect plants and form a mycorrhizal symbiotic structure, they can improve the host plants’ nutrient and water absorption, thereby promoting the growth of the host plants and enhancing their stress resistance [
26,
36]. In this study, the AMF treatment significantly enhanced tomato growth. Especially under Cd stress conditions, the AMF treatment had a better recovery effect on the growth potential of tomato seedlings. This is similar to what was reported by Yang et al. [
20] in their study on the effects of AMF on Perennial Ryegrass (
Lolium perenne L.) under Cd stress, wherein inoculation with AMF reduced Cd influx in plants, enhanced nutrient availability, and thus mitigated Cd phytotoxicity. The findings of this study are also consistent with the research results reported by Zhuang et al. [
12], who found that inoculation with AMF (
Rhizophagus intraradices) can effectively alleviate the negative effects of Cd stress (300 μM) on the growth characteristics and nutrient element content of
Malus hupehensis Rehd. Furthermore, the results of this study also indicated that after the AMF inoculation, the Cd content in the root systems of tomato seedlings under Cd stress conditions decreased significantly from 19.32 mg/kg to 11.54 mg/kg. This is consistent with the results of previous studies. For example, AMF dramatically reduced the Cd level in the roots and shoots of maize (
Zea mays L.), which weakened the phytotoxicity of excessive Cd [
37]. In another study, AMF significantly increased maize height and biomass and decreased the available Cd content in both the soil and maize [
38]. Mycorrhization (
Rhizophagus intraradices) could prevent Cd-induced growth inhibition and reduce Cd accumulation in the roots of
Glycine max (L.) Merr [
39]. AMF (
D. eburnea) markedly altered soil Cd speciation by increasing the proportion of exchangeable Cd and decreasing residual Cd, resulting in changes to the Cd content in the roots of
L. perenne and
A. fruticosa [
40]. In addition, AMF inoculation reduced the Cd level in
P. yunnanensis [
41].
The toxic effects of heavy metals also manifest in the destruction of the chloroplast structure in plant leaves. Chloroplasts are crucial sites for photosynthesis in plants, and chlorophyll, as an important photosynthetic pigment, plays a decisive role in the accumulation of plant biomass [
42,
43,
44,
45]. Li et al. [
17] subjected
Medicago truncatula to Cd stress (20 mg/g) and found that the chlorophyll content in the leaves significantly decreased. The chlorophyll a, chlorophyll b, and total chlorophyll levels in ‘Baizizhi’ and ‘Zizhi’ decreased with increasing Cd contents [
34]. The chlorophyll pigments were significantly reduced in 100 mg/kg Cd-contaminated
Brassica chinensis L. seedlings when compared to seedlings not subjected to Cd treatment [
42]. The damage caused by Cd to chloroplasts and thylakoid membranes occurs concurrently with the activities of enzymes involved in chlorophyll synthesis, which activates enzymes related to chlorophyll degradation and ROS production, leading to a decrease in chlorophyll synthesis and content [
46]. In this study, Cd dramatically lowered the chlorophyll b, chlorophyll a, and total chlorophyll concentrations in tomato seedling leaves, indicating that Cd stress could inhibit the synthesis of chlorophyll in tomato seedlings. It is notable that the AMF treatment significantly increased the chlorophyll a, chlorophyll b, and total chlorophyll levels in tomato seedlings, especially under Cd stress conditions where the AMF treatment had a better recovery effect. These results are similar to the results reported by Wang et al. [
47], who found that after AMF inoculation, the agronomic traits of tomato significantly improved in moderately Cd-contaminated soil, specifically manifested as increased plant height, stem diameter, and chlorophyll content. Therefore, Cd stress significantly inhibited the synthesis of chlorophyll in tomato, but AMF could effectively alleviate this Cd phytotoxic effect.
When plants are subjected to heavy metal stress during their growth process, the inner membrane of the chloroplasts will be damaged, which affects photosynthesis and the production of assimilates in the plants [
12,
48]. In PSII, when plants are subjected to abiotic stress, the
Fv′/
Fm′ ratio decreases, indicating that the photosystem II has been damaged [
49]. Under Cd stress (20 mg/Kg), the chlorophyll fluorescence parameters (φPSII and qP) of
Medicago truncatula Gaertn decreased significantly, indicating that the Cd
2+ stress damaged the photosynthetic organ [
17]. Shaari et al. [
42] found that
Brassica chinensis L. seedlings subjected to 100 mg/kg Cd presented the lowest
Fv′/
Fm′ ratio (0.73), indicating that these seedlings were stressed as compared to the control. This study found that Cd stress significantly reduced φPSII,
Fv′/
Fm′, and qP in the leaves of tomato seedlings. However, after inoculation with the AMF, these indicators returned to normal levels. This finding is consistent with the results of other studies. In response to Cd stress (300 μM),
Fv′/
Fm′ was significantly increased in mycorrhizal (
R. intraradices) compared to non-mycorrhizal
M. hupehensis Rehd seedlings [
12]. Under Cd stress conditions (20 mg/kg), after inoculation with arbuscular mycorrhizal fungi, the chlorophyll fluorescence parameters (φPSII and NPQ) of alfalfa (
Medicago truncatula) were significantly improved, effectively alleviating the damage to the PSII reaction center caused by Cd stress [
17]. This indicates that AMF can alleviate and even restore the damage to PSII caused by Cd stress. Under Cd stress conditions, AMF (
Funneliformis mosseae) significantly increased the
Fv′/
Fm′, φPSII, and qP in
Oryza sativa L. [
48]. The above results indicate that Cd stress weakens the efficiency of light energy utilization by reducing chlorophyll fluorescence parameters. However, inoculation with AMF can effectively alleviate the reduction effect of Cd stress on these parameters, thereby mitigating the adverse effects of Cd on the PSII reaction center and enhancing the light energy utilization efficiency.
Photosynthesis is the process by which plants synthesize compounds rich in energy. It is the ultimate carbon synthesis pathway in various biochemical and physiological processes of plants, and it forms the basis of plant life activities [
50]. Photosynthesis is highly sensitive to many adverse environmental conditions, including water stress, high temperature, salt damage, and heavy metal stress [
18,
51]. All these stresses reduce the photosynthetic efficiency of plants and thereby affect plant growth and development. Photosynthetic intensity parameters can precisely reflect the photosynthetic intensity in plants. As the degree of Cd stress increases, the Pn, Tr, and Gs of
Rosa rugosa leaves showed gradually decreasing trends [
34]. This is in agreement with this study, where Cd acts as an effective inhibitor of photosynthesis, suppressing plant photosynthesis through stomatal closure, and leading to damage to the photosynthetic apparatus and the destruction of the light-harvesting complexes and photosystems I and II [
52]. There have been some reports on the effects of AMF inoculation in alleviating the negative impact of Cd stress on plant photosynthesis. AMF could mitigate Cd-induced photosynthesis and growth phytotoxicity and nutrient ion disorders in
Malus hupehensis Rehd [
12]. AMF mitigated the Cd phytotoxicity on photosynthesis efficiency in
Cicer arietinum [
53]. These results are consistent with those of this study, which found that under the condition of no AMF inoculation, Cd dramatically lowered the values of Pn, Gs, Ci, and Tr. However, after the AMF inoculation, these indicators returned to levels close to those without Cd stress. Therefore, Cd has an inhibitory effect on the photosynthetic intensity and efficiency of plants. However, AMF can effectively alleviate the negative effects of Cd on photosynthesis, thereby increasing photosynthetic products and alleviating the damage caused by Cd to plants.
During normal physical metabolism, plants produce reactive oxygen species (ROS). However, the generation and clearance of ROS are in a dynamic equilibrium. Once plants are subjected to adverse stress, this dynamic equilibrium is disrupted, leading to the accumulation of ROS, which causes membrane lipid peroxidation, leading to damage to the cell membrane structure as well as to lipids, proteins, and DNA [
54,
55]. During the development of plants, there is a set of protective enzyme systems within plant cells that prevent ROS from causing damage, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) [
54]. The research by Zhang et al. [
56] indicates that Cd stress increases the activity of lipoxygenase and NADPH oxidase, causing the significant accumulation of ROS (such as hydrogen peroxide and superoxide anion radicals), which subsequently leads to membrane lipid peroxidation in plants, resulting in the disruption of the cell membrane system, cell damage, and electrolyte leakage. Treatment with Cd caused significant increase in H
2O
2 content and triggered membrane lipid peroxidation in Perennial Ryegrass (
Lolium perenne L.) [
20]. The results of this study also indicated that Cd stress triggered an outbreak of ROS in tomato roots, causing damage to the cell membrane and the outflow of cytoplasm, and subsequently leading to membrane lipid peroxidation. This study also found that the AMF treatment significantly increased antioxidant enzyme activities (POD, SOD, CAT, etc.) to reduce ROS levels (hydrogen peroxide, superoxide anion radicals, etc.), thereby alleviating the damage caused by Cd stress to tomato seedlings. In another study, AMF alleviated Cd phytotoxicity mainly by promoting the immobilization and sequestration of Cd, reducing ROS production, and accelerating their scavenging in wheat (
Triticum aestivum L.) [
57]. AMF improved ROS scavenging efficiency (by enhancing the activity of POD and CAT) and alleviated oxidative stress in Perennial Ryegrass (
Lolium perenne L.), thereby mitigating Cd poisoning [
20]. These studies are in agreement with our study, showing that Cd induces the production of excessive ROS in plants. However, inoculation with AMF could enhance the activity of antioxidant enzymes in plants under Cd stress conditions, improve the plants’ antioxidant defense ability to reduce the content of ROS, maintain the redox balance within plant cells, protect the cell membrane’s function and structure, strengthen the antioxidant capacity, and mitigate Cd phytotoxicity to the cell membrane. AMF penetration and colonization involve a series of cytological and biochemical sequence of events and intracellular changes, including anti-oxidative damaging effect and ROS promotion [
58]. In the early stages of the AMF–plant interaction, the mechanism of suppression or induction associated with plant defense holds the key to the plant–fungus compatibility in the context of this mutually beneficial symbiotic relationship [
39]. The physiological processes include changes in the activation of plasma membrane-bound enzymes, kinases, phosphatases, and phospholipases; the permeability of the plasma membrane; and the production of signal molecules, including ROS. Regarding Cd stress, this study, in conjunction with previous studies, demonstrated that AMF are also involved in defense processes and mechanisms, potentially with effects on the induction of abiotic stress tolerance.
Under heavy metal stress conditions, plants will produce a large amount of osmotic regulatory substances. These substances not only maintain the cell turgor pressure and prevent excessive water loss from the protoplasm, but also stabilize the structure of organelles, in order to regulate various physiological functions and alleviate the damage caused by heavy metal stress to plants [
59]. Proline (Pro), malondialdehyde (MDA), soluble proteins, and soluble sugars, among others, are all osmotic regulatory substances in plants [
59]. The content of MDA significantly increased by 2.5-fold under Cd
2+ stress in
M. truncatula, indicating that Cd
2+ caused oxidative damage to the cell membrane [
17]. It is consistent with the conclusion of this study, which found that Cd stress increased the contents of osmotic regulatory substances (Pro, MDA, soluble protein, and soluble sugar) in the root systems of tomato seedlings. Such an increase is an adaptive response of tomato under heavy metal Cd stress, as it reduces lipid peroxidation in the cell membrane, alleviates membrane damage, and provides protection for the plants. This study also found that AMF could significantly reduce the accumulation of osmotic regulatory substances in the root systems of tomato, thereby alleviating the damage caused by Cd stress to tomato seedlings. This is in agreement with previous research results and further confirms the beneficial role of inoculating AMF in plants subjected to Cd stress to reduce lipid peroxidation [
17,
39]. Therefore, AMF can effectively alleviate the abnormal accumulation of osmotic regulatory substances induced by Cd, reduce the damage caused by membrane lipid peroxidation, and enhance the Cd tolerance of plants.
AMF also change the levels of phytohormones such as strigolactone (SL), IAA, tZR, GA3, and ABA, which confer resistance to abiotic stresses, including drought, salt, and heavy metal stresses, in host plants by coordinating multiple signal transduction pathways [
57]. Strigolactone (SL) induces spore germination and promotes hyphal growth in AMF [
60]. Application of the strigolactone GR24 improved Cd tolerance by regulating Cd uptake and antioxidant metabolism in
Hordeum vulgare L. [
61]. The high SL level in AMF-treated seedlings could lower Cd toxic action by regulating Cd accumulation and scavenging ROS in
M. hupehensis [
12]. AMF could also increase the seedling biomass of
M. hupehensis under Cd stress conditions, possibly by increasing the IAA level in both the leaves and roots [
12]. The increased IAA level in mycorrhizal tomato under Cd stress conditions strengthened the mutualism between AMF and the host plants. AMF could inhibit the expression of Cd transport and absorption genes, increase Cd content in cell walls, promote antioxidant enzyme biosynthesis, and alleviate Cd-mediated growth inhibition [
18]. Relatively high root IAA levels were associated with higher plant Cd tolerance in mycorrhizal tomato under Cd stress conditions.
The normal functioning of respiratory metabolism plays a crucial role in the growth and development of plants. When plants are exposed to heavy metal stress, an appropriate amount of intermediate metabolic products serves as the foundation for their adaptation to heavy metal-contaminated soil [
62]. As intermediate products of plant respiratory metabolism, succinic acid and malic acid are closely related to plant metabolic process. In this study, Cd stress significantly reduced the contents of malic acid and succinic acid in the roots, which is consistent with the research results reported in sunflower [
63]. The concentration of respiratory metabolites in the root system is remarkably correlated with the root activity in the rhizosphere soil. Malic acid and succinic acid, as respiratory metabolites of the root system, could strengthen root activity and accelerate plant growth [
62]. In this study, Cd stress might have inhibited tomato growth by reducing the levels of malic acid and succinic acid in the roots, thereby weakening the root respiration metabolism. AMF not only significantly promote plant growth and nutrient absorption, but also promote the secretion of low-molecular-weight organic acids (such as malic acid and succinic acid) by the roots. Low-molecular-weight organic acids have significant impacts on the physical and chemical properties of the soil and the toxicity of heavy metals to plants. They play a positive role in the activation and absorption of insoluble nutrients in the rhizosphere, converting insoluble substances into usable active components through acidification and other pathways, thereby promoting plant growth [
64]. In this study, the AMF inoculation treatment significantly promoted the secretion of succinic acid and malic acid in the roots of tomato seedlings. Under Cd stress, low-molecular-weight organic acids can alter the speciation and bioavailability of heavy metals, thereby affecting the absorption and accumulation of Cd in plants [
65]. The biological toxicity of Cd in soil mainly depends on its form. Cd exists in various forms in the soil, including in exchangeable form, iron–manganese oxide form, and organic-bound form. Some studies have shown that the inoculation of AMF reduces the content of exchangeable Cd, possibly because the change in the number of soil microorganisms improves the growth of plant roots and their absorption of nutrients, thereby altering the form of Cd [
66]. Low-molecular-weight organic acids secreted by the root system are also one of the factors that affect the form of Cd. Among them, citric acid and malic acid can increase the content of exchangeable heavy metals in soil, thereby achieving the purpose of activating heavy metals [
66]. Lactic acid and malic acid can also cause changes to the form of Cd by altering the pH value. The content of iron–manganese complexed Cd in soil treated with AMF increased significantly after inoculation [
67]. A possible reason is that under the space limitation of the root bags, organic acids are concentrated, making it easier to alter the pH value and redox potential of the soil, thereby promoting the formation of iron–manganese complexed Cd [
67]. The content of organic Cd is significantly reduced. A possible reason for this is that AMF, in order to provide more nutrients to the host plant, promote the decomposition of organic matter into small molecules that are easily absorbed by the host plant, thereby reducing the combination of organic matter and Cd, and resulting in a decrease in the content of organic-bound Cd [
67]. This study found that AMF could, to some extent, alleviate the negative effects of Cd on the secretion of citric acid and malic acid by tomato roots and enhance the plant’s tolerance to heavy metals, thereby alleviating the inhibitory effect of Cd stress on plant growth. In this study, the AMF treatment significantly reduced the Cd content in tomato plants, indicating that the AMF treatment enhanced the tolerance of tomato plants to Cd by increasing the contents of malic acid and citric acid in the roots, thereby promoting root growth. The main reasons are as follows: First, the mycelia of AMF contain binding sites for heavy metals, allowing heavy metals to be adsorbed, bound, and fixed, thereby reducing the stress induced by heavy metals on the host plants. Second, the AMF inoculation significantly increased the biomass of tomato plants to be much higher than that of the control, which indirectly led to a decrease in the Cd content in the plants as the larger biomass had a dilution effect. Yu et al. [
68] also indicated that the mycelium has a strong ability to adsorb Cd, which supports the significance of the AMF ecological function in this study. This research fully utilized the role of the AMF root exudate mycelium and examined the concentrations of low-molecular-weight organic acids secreted by the root system. The AMF inoculation promoted the complexation and chelation reactions between organic acids and Cd and reduced the toxicity of Cd to tomato plants, thereby enhancing the tolerance of tomato plants to Cd and promoting their growth.