Optimizing Target Metabolites Production in Coleus blumei Indoor Cultivation: Combined Effects of LED Light and Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Material, Light Treatment, and Indoor Setting
2.2. Irrigation
2.3. Sampling Scheme
2.4. Biomass and Bioactive Compounds Quantification
2.5. Experimental Design and Statistical Analysis
- Leaf and root DW;
- Leaf and root concentrations of RA and QU;
- Leaf, root, and total yields of RA and QU.
3. Results
3.1. ANOVA Results
3.2. Dry Weight
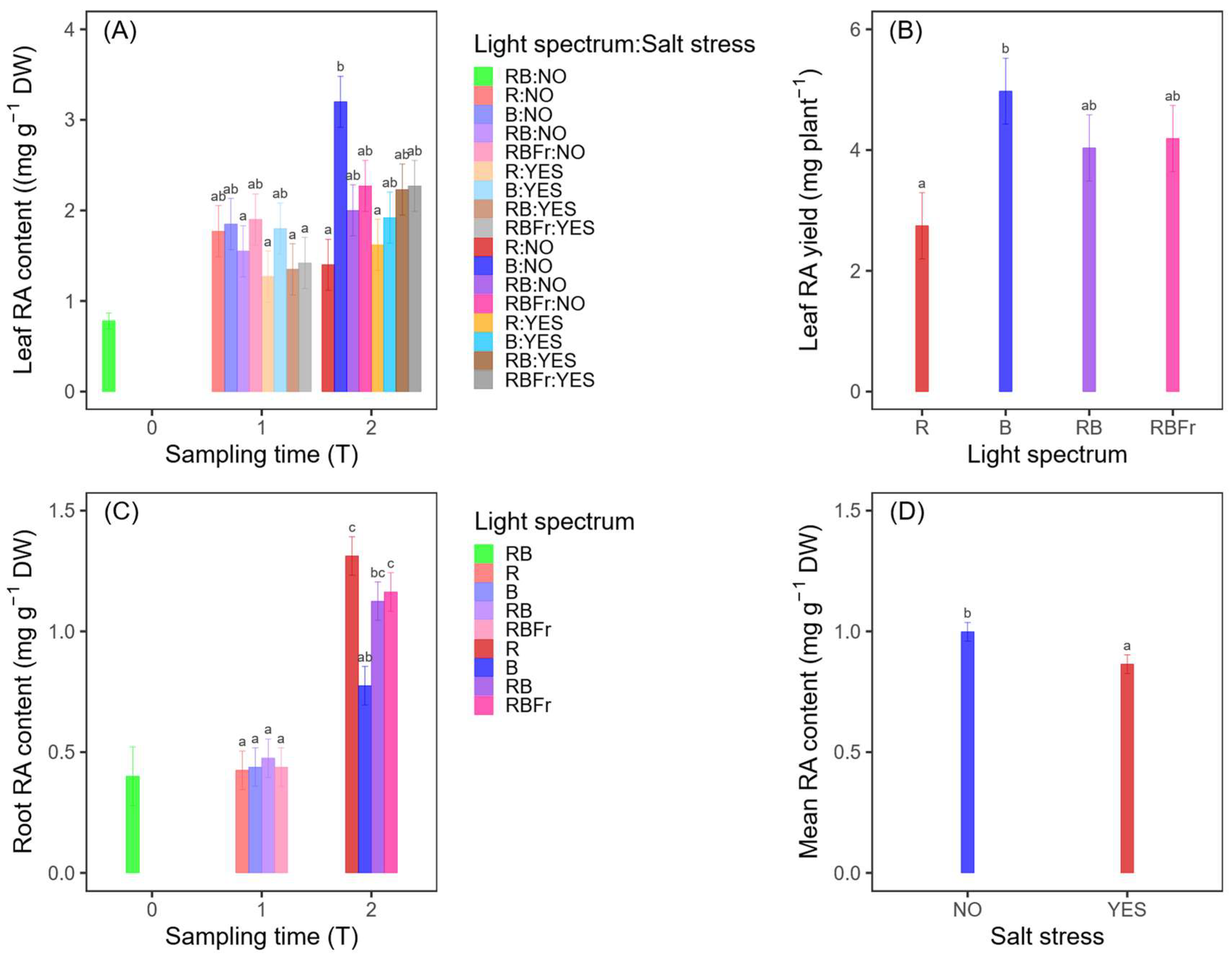
3.3. Rosmarinic Acid
3.3.1. Leaves
3.3.2. Roots
3.3.3. Total Plant
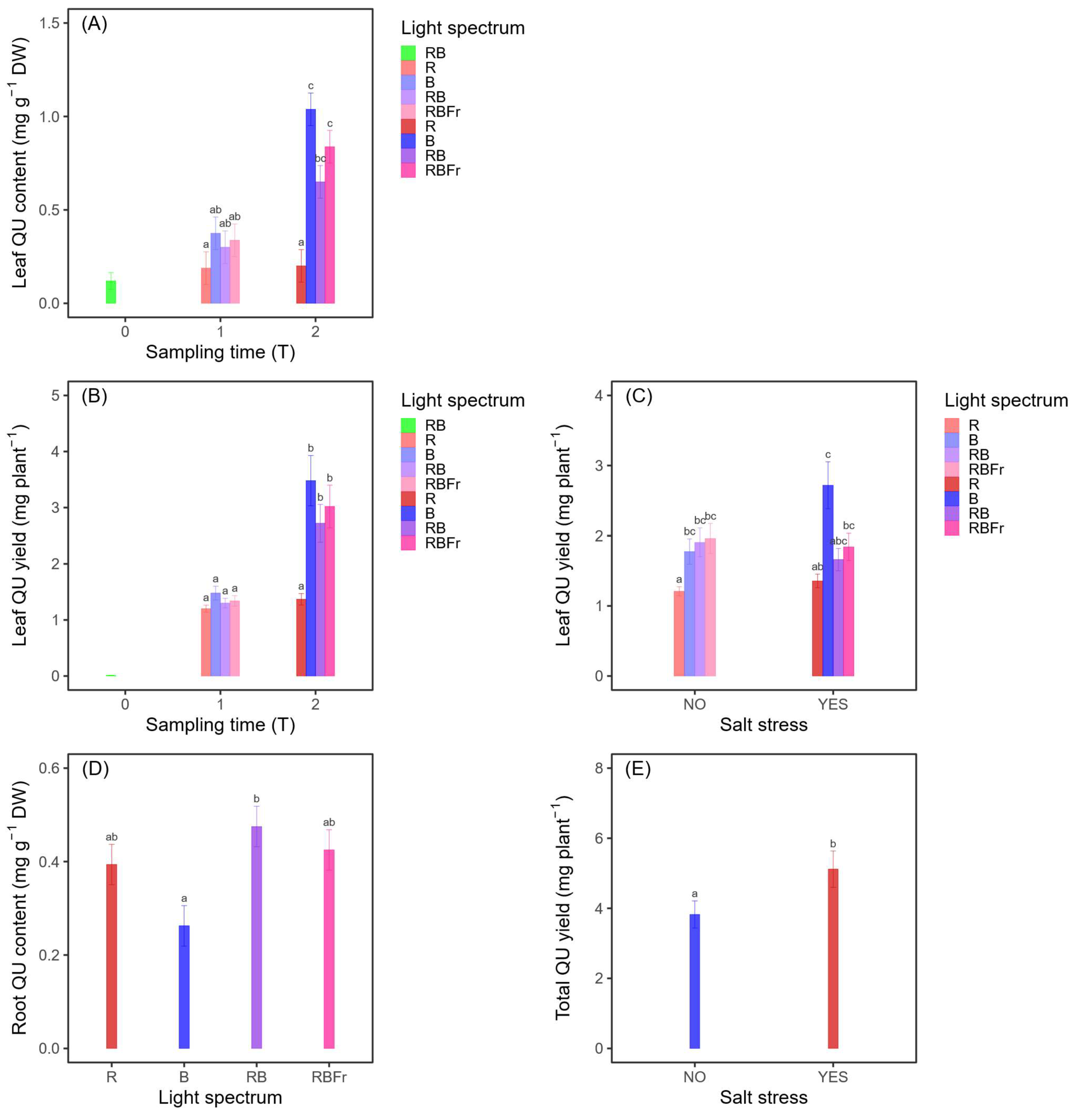
3.4. Quercetin
3.4.1. Leaves
3.4.2. Roots
3.4.3. Total Plant
4. Discussion
4.1. Effects of Salt Stress and Light on Biomass Accumulation
4.2. Effect of Salt Stress and Light on Secondary Metabolites
4.3. Strategic Optimization of Rosmarinic Acid and Quercetin Yields: Implications and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade-Cetto, A. Ethnobotanical Study of the Medicinal Plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- Jakobina, M.; Łyczko, J.; Szumny, A.; Galek, R. The Influence of Cultivation Conditions on the Formation of Psychoactive Salvinorin A, Salvinorin B, Rosmarinic Acid and Caffeic Acid in Coleus Scutellarioides. Sci. Rep. 2024, 14, 6693. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, A.; Ellis, B.E. Rosmarinic Acid Production in Coleus Cell Cultures. Planta 1997, 137, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Vuković, R.; Likić, S.; Jelaska, S. Potential of Different Coleus Blumei Tissues for Rosmarinic Acid Production. Food Technol. Biotechnol. 2015, 53, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bismelah, N.A.; Ahmad, R.; Mohamed Kassim, Z.H.; Ismail, N.H.; Rasol, N.E. The Antibacterial Effect of Plectranthus scutellarioides (L.) R.Br. Leaves Extract against Bacteria Associated with Peri-Implantitis. J. Tradit. Complement. Med. 2022, 12, 556–566. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Hazrati, S.; Pignata, G.; Casale, M.; Hosseini, S.J.; Nicola, S. Influence of Nutrient Solutions in an NGS® Soilless System on the Yield, Quality and Shelf Life of Fresh-Cut Commercial Mint at Different Harvest Times. Agronomy 2024, 14, 610. [Google Scholar] [CrossRef]
- Quadri, A.; Barbaresi, A.; Tassinari, P.; Bertaccini, A.; Contaldo, N.; Mercolini, L.; Protti, M.; Montalbetti, R.; Laurita, R.; Torreggiani, D. Use of LED Light and Plasma Activated Water (PAW) to Stimulate Pharmaceutical Compound Production in Catharanthus roseus Plants. In Proceedings of the X International Symposium on Light in Horticulture, Seoul, Republic of Korea, 19–22 May 2024; pp. 255–268. [Google Scholar] [CrossRef]
- Mohammadi, H.; Khoshi, N.; Hazrati, S.; Aghaee, A.; Falakian, M.; Ghorbanpour, M. Interaction of NaCl Salinity and Light Intensity Affect Growth, Physiological Traits and Essential Oil Constituents in Artemisia dracunculus L. (Tarragon). Biochem. Syst. Ecol. 2023, 107, 104626. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Kotagiri, D.; Kolluru, V.C. Effect of Salinity Stress on the Morphology & Physiology of Five Different Coleus Species. Biomed. Pharmacol. J. 2017, 10, 1639–1649. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Raghavendra, A.S.; Reddy, K.J. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Giménez, A.; Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and Led Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Lu, Z.; Pang, S.; Wang, L.; Wang, L.; Li, W. Gene Expression Profiles and Flavonoid Accumulation during Salt Stress in Ginkgo biloba Seedlings. Plants 2020, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Sambuco, B.; Quadri, A.; Trenta, M.; Tassinari, P.; Torreggiani, D.; Barbaresi, A.; Mercolini, L.; Protti, M. Enhancing Secondary Metabolites Accumulation in Coleus blumei through LED Light Application. In Proceedings of the X International Symposium on Light in Horticulture, Seoul, Republic of Korea, 19–22 May 2024; pp. 243–254. [Google Scholar] [CrossRef]
- Posit team. RStudio: Integrated Development Environment for R. Posit Software, Version 2025.05.01; PBC: Boston, MA, USA, 2025. Available online: http://www.posit.co/ (accessed on 15 June 2025).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package, Version 1.8.4-1. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 June 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wang, J.; Han, Z.; He, J.; Kang, H.; Li, Q.; Chen, H.; Zhang, X.; Miao, W.; Shang, X.; Chen, W.; et al. Exploring the Effects of Light–Water Interaction in Plant Factory to Improve the Yield and Quality of Panax notoginseng (Burkill) F. H. Chen. Agronomy 2025, 15, 368. [Google Scholar] [CrossRef]
- Quadri, A.; Sambuco, B.; Trenta, M.; Tassinari, P.; Torreggiani, D.; Mercolini, L.; Protti, M.; Zambonelli, A.; Barbaresi, A. Bioengineered Indoor Farming Approaches: LED Light Spectra and Bi-2 Ostimulants for Enhancing Vindoline and Catharanthine Production in 3 Catharanthus roseus. Horticulturae 2025, 11, 828. [Google Scholar] [CrossRef]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. The Effects of Moderate and Severe Salinity on Composition and Physiology in the Biomass Crop Miscanthus × Giganteus. Plants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of Salinity Stress on Crop Plants: Improving Salt Tolerance through Genetic and Molecular Dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Luo, S.; Zou, J.; Shi, M.; Lin, S.; Wang, D.; Liu, W.; Shen, Y.; Ding, X.; Jiang, Y. Effects of Red-Blue Light Spectrum on Growth, Yield, and Photosynthetic Efficiency of Lettuce in a Uniformly Illumination Environment. Plant Soil Environ. 2024, 70, 305–316. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, H.; Chu, Y.; Sun, C.; Wei, N.; Yang, M.; Zheng, C. Effects of Salt Stress on Chlorophyll Fluorescence and the Antioxidant System in Ginkgo biloba L. Seedlings. HortScience 2019, 54, 2125–2133. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Wilson, C.; Madore, M.A. Root-Zone Salinity Alters Raffinose Oligosaccharide Metabolism and Transport in Coleus. Plant Physiol. 1997, 115, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Trócsányi, E.; György, Z.; Zámboriné-Németh, É. New Insights into Rosmarinic Acid Biosynthesis Based on Molecular Studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.J. Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Ma, D.; Guo, Y.; Ali, I.; Lin, J.; Xu, Y.; Yang, M. Accumulation Characteristics of Plant Flavonoids and Effects of Cultivation Measures on Their Biosynthesis: A Review. Plant Physiol. Biochem. 2024, 215, 108960. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The Ascorbate-Glutathione Cycle Coming of Age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef]
- Chibani, K.; Gherli, H.; Fan, M. The Role of Blue Light in Plant Stress Responses: Modulation through Photoreceptors and Antioxidant Mechanisms. Front. Plant Sci. 2025, 16, 1554281. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential Regulation of Closely Related R2R3-MYB Transcription Factors Controls Flavonol Accumulation in Different Parts of the Arabidopsis Thaliana Seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols Modulate Plant Development, Signaling, and Stress Responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Nile, S.H.; Sharma, K.; Li, G.H.; Park, S.W. Effect of Different Exposed Lights on Quercetin and Quercetin Glucoside Content in Onion (Allium cepa L.). Saudi J. Biol. Sci. 2015, 22, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Ru, M.; Wang, K.; Bai, Z.; Peng, L.; He, S.; Wang, Y.; Liang, Z. A Tyrosine Aminotransferase Involved in Rosmarinic Acid Biosynthesis in Prunella vulgaris L. Sci. Rep. 2017, 7, 4892. [Google Scholar] [CrossRef]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production and Applications of Rosmarinic Acid and Structurally Related Compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef]
- Chen, I.G.J.; Lee, M.S.; Lin, M.K.; Ko, C.Y.; Chang, W. The Blue Light Decreases Tanshinone IIA Content in Salvia Miltiorrhiza Hairy Roots via Genes Regulation. J. Photochem. Photobiol. B 2018, 183, 164–171. [Google Scholar] [CrossRef]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl Changes the Nutrient and Polyphenolic Composition of Basil Leaves. Ind. Crops Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Cioć, M.; Szopa, A.; Prokopiuk, B.; Pawłowska, B.; Łopusiewicz, Ł. Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro. Agronomy 2025, 15, 2197. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The Combination of Blue and Red LED Light Improves Growth and Phenolic Acid Contents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of Light on Secondary Metabolites in Selected Leafy Greens: A Review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and Calcium Modulate the Production of Rosmarinic Acid, Luteolin, and Apigenin in Dracocephalum kotschyi under Salinity Stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. Plant Factories in the Water-Food-Energy Nexus Era: A Systematic Bibliographical Review. Food Secur. 2020, 12, 253–268. [Google Scholar] [CrossRef]
- Shorrocks, A.F. Decomposition Procedures for Distributional Analysis: A Unified Framework Based on the Shapley Value. Econ. Inequal. 2013, 11, 99–126. [Google Scholar] [CrossRef]

| Main Factor | Factors Combination | ||||||
|---|---|---|---|---|---|---|---|
| Leaves | Light | Salt Stress | Time | L:S | L:T | S:T | L:S:T |
| Dry weight | ns | ns | *** | · | ns | ns | ns |
| RA concentration | * | · | *** | ns | ns | ns | · |
| RA yield | * | ns | *** | ns | ns | ns | ns |
| QU concentration | *** | · | *** | ns | ** | ns | ns |
| QU yield | *** | ns | *** | · | * | ns | ns |
| Roots | Light | Salt stress | Time | L:S | L:T | S:T | L:S:T |
| Dry weight | * | ns | *** | ns | ns | ns | ns |
| RA concentration | * | ns | *** | ns | * | ns | ns |
| RA yield | ns | ns | *** | ns | ns | ns | ns |
| QU concentration | ** | ns | *** | ns | ns | ns | ns |
| QU yield | ns | ns | *** | ns | ns | ns | ns |
| Total plant | Light | Salt stress | Time | L:S | L:T | S:T | L:S:T |
| Dry weight | * | ns | *** | ns | ns | ns | ns |
| RA yield | ns | ns | *** | ns | ns | ns | ns |
| QU yield | ns | * | *** | ns | ns | ns | ns |
| Time Levels | |||
|---|---|---|---|
| Leaves | T0 | T1 | T2 |
| Dry weight | 0.04 ± 0.01 | 1.42 ± 0.14 a | 2.65 ± 0.14 b |
| RA concentration | 0.78 ± 0.08 | 1.62 ± 0.09 a | 2.12 ± 0.09 b |
| RA yield | 0.03 ± 0.003 | 2.35 ± 0.38 a | 5.62 ± 0.38 b |
| QU concentration | 0.12 ± 0.044 | 0.30 ± 0.04 a | 0.68 ± 0.04 b |
| QU yield | 0.002 ± 0.00 | 1.32 ± 0.04 a | 2.42 ± 0.14 b |
| Roots | T0 | T1 | T2 |
| Dry weight | 0.19 ± 0.04 | 8.29 ± 0.75 a | 17.88 ± 0.75 b |
| RA concentration | 0.26 ± 0.02 | 0.44 ± 0.03 a | 1.09 ± 0.03 b |
| RA yield | 0.05 ± 0.018 | 4.34 ± 0.41 a | 19.36 ± 1.20 b |
| QU concentration | 0.24 ± 0.04 | 0.22 ± 0.03 a | 0.55 ± 0.03 b |
| QU yield | 0.15 ± 0.06 | 1.50 ± 0.17 a | 8.22 ± 0.96 b |
| Total plant | T0 | T1 | T2 |
| Dry weight | 0.22 ± 0.05 | 9.71 ± 0.79 a | 20.53 ± 0.79 b |
| RA yield | 0.09 ± 0.01 | 6.61 ± 0.48 a | 24.98 ± 1.34 b |
| QU yield | 0.05 ± 0.01 | 1.94 ± 0.19 a | 10.09 ± 1.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambuco, B.; Barbaresi, A.; Quadri, A.; Trenta, M.; Tassinari, P.; Mercolini, L.; Protti, M.; Torreggiani, D. Optimizing Target Metabolites Production in Coleus blumei Indoor Cultivation: Combined Effects of LED Light and Salinity Stress. Horticulturae 2025, 11, 1205. https://doi.org/10.3390/horticulturae11101205
Sambuco B, Barbaresi A, Quadri A, Trenta M, Tassinari P, Mercolini L, Protti M, Torreggiani D. Optimizing Target Metabolites Production in Coleus blumei Indoor Cultivation: Combined Effects of LED Light and Salinity Stress. Horticulturae. 2025; 11(10):1205. https://doi.org/10.3390/horticulturae11101205
Chicago/Turabian StyleSambuco, Bianca, Alberto Barbaresi, Alessandro Quadri, Mattia Trenta, Patrizia Tassinari, Laura Mercolini, Michele Protti, and Daniele Torreggiani. 2025. "Optimizing Target Metabolites Production in Coleus blumei Indoor Cultivation: Combined Effects of LED Light and Salinity Stress" Horticulturae 11, no. 10: 1205. https://doi.org/10.3390/horticulturae11101205
APA StyleSambuco, B., Barbaresi, A., Quadri, A., Trenta, M., Tassinari, P., Mercolini, L., Protti, M., & Torreggiani, D. (2025). Optimizing Target Metabolites Production in Coleus blumei Indoor Cultivation: Combined Effects of LED Light and Salinity Stress. Horticulturae, 11(10), 1205. https://doi.org/10.3390/horticulturae11101205











