Abstract
Pistachio (Pistacia vera L.) holds significant importance due to its diverse applications and nutritional benefits. The nuts are rich in essential amino acids, antioxidants, fiber, healthy fats, and minerals, making them highly valuable for human nutrition. However, pistachios are significantly challenged by salinity stress, which negatively affects their growth and metabolism. Understanding the impact of salinity stress on pistachios is crucial for developing effective strategies to enhance their tolerance, improve growth, and ensure sustainable production in saline environments. To investigate the effects of salinity on energy metabolism and amino acid composition, we monitored key metabolites and free amino acid levels in UCB-1 pistachio leaves at 7- and 21-day salt stress treatments using Liquid Chromatography–Mass Spectrometry (LC-MS) and Ultra Performance Liquid Chromatography (UPLC). Our findings revealed that salinity affected nearly all analyzed metabolites, with varied patterns observed at different time points. Notably, all free amino acids except threonine accumulated significantly in response to salt stress. Meanwhile, reductions in 3PGA, Fru1,6bP, and Glu6P+Fru6P (glycolysis and Calvin cycle intermediates) suggest a decrease in photosynthetic activity, which may ultimately impact respiration rates. These results demonstrate that salinity stress affects both amino acid metabolism and central carbon metabolism, with the magnitude and pattern of these changes depending on the duration of exposure. The observed metabolic adjustments likely represent an adaptive response, enabling the plant to partially mitigate the detrimental effects of salt stress.
1. Introduction
Salinity stress, defined as the adverse effect of excessive salts, particularly sodium chloride (NaCl), on plants, is one of the major abiotic stresses worldwide. It poses significant environmental challenges that negatively impact plant growth, development, productivity, yield, and overall survival [1,2,3,4]. Due to the high rate of agricultural land salinization worldwide, salinity is a significant agricultural issue that poses a threat to food security [5,6]. Consequently, understanding salinity-induced changes in plants and their tolerance mechanisms is crucial, particularly in relation to stress-related alterations in metabolites.
Salt damages plants in two major ways. First, it reduces the plant’s ability to absorb water, a phenomenon known as the osmotic effect. Second, salt ions accumulate inside plant cells, leading to toxic effects such as enzyme inhibition and disruption of the electrochemical gradients across cellular membranes [1,2,3,4]. Additionally, salinity stress is frequently associated with oxidative stress, resulting from excessive production of reactive oxygen species (ROS) [7]. Salinity also triggers complex regulatory mechanisms at the molecular level, including alterations in gene expression, phytohormone signaling, and osmotic adjustment through the accumulation of compatible solutes such as proline and glycine betaine [8]. Plants respond to salinity in several ways, including physiological and biochemical alterations as well as morphological changes. These complex responses to salt depend on the plant species, genotype, developmental stage, affected organ, and the severity and duration of the stress [9].
Metabolites, which vary widely in their chemical composition and structure, act as both intermediates and end products of cellular metabolic pathways, reflecting the organism’s physiological state under diverse environmental and developmental conditions [10,11]. Investigation of the metabolome through metabolomics enhances our understanding of how organisms interact with their environment. Metabolomics enables the identification and quantification of metabolites under specific environmental conditions [12] and employs several analytical techniques, among which LC–MS is one of the most common. Numerous studies on plant metabolomics under stress conditions have revealed extensive changes in metabolites. Kumari and Parida [10] reported that salt stress induces significant metabolic alterations in amino acids, fatty acids, sugars, and organic acids in Salvadora persica (commonly known as the toothbrush tree), which in turn leads to reduced activity of the TCA cycle. Research on rice varieties has also revealed differential responses to salt treatment; salt-tolerant varieties showed increased levels of serotonin and gentisic acid, both of which act as signaling molecules under NaCl stress [13]. Furthermore, Skliros et al. [14] reported that the catabolism of uric acid during salinity stress represents a novel mechanism to overcome carbon and nitrogen starvation in lentils.
Under salinity stress, one of the most prominent metabolic responses in plants is the alteration of amino acid composition. Salinity induces diverse changes in the amino acid profile, with concentrations of non-essential amino acids often increasing while those of essential amino acids may decline, reflecting metabolic adjustments to cope with ionic and osmotic stress [15]. These alterations are part of a broader physiological strategy in which the synthesis and degradation of amino acids are tightly regulated to promote the accumulation of compatible solutes and protect cellular structures from damage [16,17].
Pistachio is a commercially valuable crop from the Anacardiaceae family, thriving in arid and semi-arid regions of Iran, Turkey, Sudan, and the United States. This woody perennial nut crop is compatible with adverse environmental conditions, including salinity, which poses challenges for agriculture in regions where pistachios are cultivated [18,19,20]. Pistachio is a highly valued nut known for their nutritional benefits and health-promoting properties. It is rich in monounsaturated fatty acids, proteins, dietary fiber, vitamins, and minerals, making it a beneficial addition to the diet [21]. The UCB-1 cultivar of pistachio has shown promising characteristics in seedling growth, outperforming other varieties in terms of trunk diameter and height during early growth stages [22]. This cultivar is particularly noted for its adaptability and performance under Mediterranean conditions, which is crucial for sustainable cultivation practices [23].
The majority of studies conducted on pistachio have primarily focused on classical physiological, morphological, and biochemical traits, while metabolic and free amino acid responses under salinity stress remain largely unexplored. For instance, Jamshidi Goharrizi et al. [24] evaluated the effects of salinity and drought on the physiological and biochemical traits of several pistachio rootstocks, including UCB-1. Similarly, Jamshidi Goharrizi et al. [25] examined changes in physiological, biochemical, and ion-homeostasis traits in four pistachio rootstocks over a 60-day period under salinity and drought conditions. Other studies, including Rezayian et al. [26], Akbari et al. [27], Hakimnejad et al. [28], Rahneshan et al. [29], and Mirabi et al. [30], have also primarily addressed physiological, biochemical, antioxidant, and morphological responses of pistachio rootstocks, without evaluating comprehensive profiles of metabolites and free amino acids.
Salt tolerance is a complex trait involving multiple interacting mechanisms. Despite considerable advances in plant metabolomics under abiotic stresses, research at the horticultural level remains limited, particularly in perennial nut crops such as pistachio. Previous studies on pistachio salinity tolerance have primarily focused on physiological and morphological traits, including ion homeostasis, antioxidant enzyme activities, growth, and yield. In contrast, metabolic and free amino acid responses under salinity stress have remained largely unexplored. The simultaneous analysis of selected metabolites and free amino acids provides critical insight into the biochemical strategies employed by pistachio to cope with salinity stress, a perspective not addressed in prior research.
In this study, we aim to address the following core scientific questions: (i) how the duration of salinity stress regulates the coordinated response of carbon metabolism and amino acid metabolism in UCB-1 pistachio rootstock, (ii) which key metabolites and amino acids act as markers of salt stress adaptation, and (iii) how dynamic metabolic adjustments contribute to the plant’s physiological resilience under prolonged salt exposure. To achieve these objectives, we conducted the first integrated analysis of metabolic adjustments and amino acid changes using advanced LC-MS and UPLC techniques, focusing on dynamic biochemical responses rather than conventional physiological traits. Given the scarcity of similar investigations worldwide, these findings provide novel insights and a valuable reference for future research on stress adaptation in perennial crops.
2. Materials and Methods
The experiment was conducted in a completely randomized design (CRD) with three biological replicates. UCB-1 pistachio seedlings were divided into three treatment groups: (i) control, (ii) salinity stress for 7 days (200 mM Sodium chloride (NaCl), Sigma-Aldrich, St. Louis, MO, USA), and (iii) salinity stress for 21 days (200 mM NaCl). Each replicate consisted of three pots, and metabolic characteristics and amino acid profiles were analyzed in leaves collected from these seedlings.
The selection of 200 mM NaCl was based on both previous studies and field relevance. Earlier experiments on pistachio and related reports have demonstrated that this concentration induces significant physiological and biochemical responses while maintaining plant survival. Moreover, 200 mM NaCl corresponds to an electrical conductivity (EC) of approximately 20 dS/m, which closely reflects the severe salinity conditions reported in pistachio growing regions of Iran, where soil EC can exceed 16 dS/m [31]. Thus, this concentration provides a realistic simulation of natural saline environments in arid and semi-arid areas. To better capture the dynamics of salinity tolerance, plants were sampled at 7 days (medium-term) and 21 days (long-term) after treatment, since previous studies have indicated that plants exhibit distinct early stress responses and later adaptive mechanisms. This approach enabled a comprehensive evaluation of both immediate and long-term metabolic adjustments in UCB-1 pistachio rootstock under salinity stress.
2.1. Plant Material, Growth Conditions, and Salinity Treatment
The UCB-1 pistachio rootstocks used in this study were obtained as six-month-old seedlings from Tooba Tissue Culture Company, a knowledge based company located in Kerman, Iran. All seedlings were grown under uniform conditions before the experiment, ensuring consistency in growth parameters such as size and developmental stage across all treatment groups. The UCB-1 variety is a hybrid pistachio rootstock, developed at the University of California, USA, through the controlled cross-pollination of Atlantic and Integrima varieties. This hybrid rootstock was specifically bred to enhance resistance to pests and diseases, including Verticillium wilt (commonly known as the pistachio wilt disease), and has been increasingly used for pistachio cultivation due to its high resistance to various pests and diseases. It is a non-native species that has recently been introduced into Iran for commercial cultivation.
Six-month-old UCB-1 pistachio rootstocks were planted in pots with a height and diameter of 50 cm, containing a 1:1 mixture of sand and clay, in a greenhouse maintained at a 16/8 h light/dark cycle, 28/18 °C day/night temperature, and 70% relative humidity. Before the salinity treatment, pots were irrigated weekly with ½ strength Hoagland’s nutrient solution according to soil test results. Salinity treatment was applied by irrigating each pot every four days with 200 mM NaCl, using a fixed volume of 2 L per pot. Soil salinity was monitored with a conductivity meter (EC meter (model 5TE), Decagon Devices, Pullman, WA, USA) before each irrigation, and the outlet water from pots was used to adjust subsequent irrigation salinity to maintain the target 200 mM NaCl. Leaching was performed as needed to prevent salt accumulation and ensure uniform experimental conditions, using 1 L of deionized water per pot for 10 min. After 7 and 21 days of salinity treatment, leaves from three biological replicates were harvested, immediately frozen in liquid nitrogen, and stored at −80 °C.
2.2. Extraction and Quantification of Metabolites
For metabolite profiling, approximately 20 mg of pistachio leaf material was freeze-dried at −50 °C under a vacuum of 0.05 mbar for 48 h. Metabolites were then extracted using a chloroform–methanol solution (2:1, v/v) (Sigma-Aldrich, St. Louis, MO, USA) following established plant metabolomics protocols. This biphasic extraction efficiently separates polar and non-polar metabolites. After phase separation, samples were dried under a gentle nitrogen stream and re-dissolved in 300 µL of ultrapure water (Milli-Q system, Merck, Darmstadt, Germany). Prior to large-scale measurements, preliminary analyses were conducted using representative samples from each tissue type to optimize extraction efficiency and metabolite detection. Quantification was performed using an external standard mixture containing 30 reference metabolites at six different concentrations. Standard mixtures were injected at three time points during each analytical run (beginning, middle, and end) to monitor instrument stability. Calibration curves were constructed for each metabolite, showing high linearity (R2 > 0.99), and quantification was performed individually using the corresponding calibration curve.
LC–MS analyses were performed at the Bioanalytics Unit, Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany, under controlled laboratory conditions. Analyses were carried out using a high-performance ion chromatography system (Dionex ICS series, Thermo Fisher Scientific, Bremen, Germany) coupled to a quadrupole time-of-flight mass spectrometer (Q-TOF LC–MS, Agilent Technologies, Waldbronn, Germany). Chromatographic separation was achieved on an IonPac AS11-HC column (2 × 250 mm, 4 µm particle size; Thermo Fisher Scientific), which is optimized for the separation of highly polar and ionic metabolites. The mobile phase consisted of (A) ultrapure water and (B) 100 mM KOH (Sigma-Aldrich, St. Louis, MO, USA) solution, with a gradient elution program optimized for metabolite separation: 0–2 min, 1% B; 2–10 min, linear increase to 50% B; 10–20 min, increase to 100% B; 20–25 min, hold at 100% B; followed by re-equilibration at 1% B. The flow rate was maintained at 0.25 mL min−1, the column temperature at 30 °C, and the injection volume at 5 µL.
Mass spectrometry was performed in negative electrospray ionization (ESI−) mode, as this provided better ionization efficiency for acidic and polar metabolites. The MS parameters were as follows: capillary voltage, 3.0 kV; source temperature, 120 °C; desolvation temperature, 350 °C; and full-scan acquisition range, m/z 50–1000. Raw data were processed and analyzed using MassHunter Qualitative and Quantitative Analysis software (Agilent Technologies).
2.3. Extraction and Quantification of Amino Acids
For amino acid profiling, approximately 10 mg of freeze-dried pistachio leaf material was weighed for each extraction. Prior to large-scale analysis, preliminary tests with representative samples from each tissue type were conducted to optimize chromatographic separation and achieve maximum resolution of amino acids.
Derivatization was performed using the fluorescing reagent 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (ACQ), synthesized in-house at the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany. For each reaction, 10 µL of sample extract was mixed with 90 µL of ultrapure water and treated with ACQ (final concentration: 10 mM) in a borate buffer at pH 8.5, following established protocols. The reaction was carried out at 55 °C for 10 min, after which the final sample volume was adjusted to 400 µL. ACQ derivatization was selected because it enhances fluorescence signals and improves chromatographic separation of amino acids, which are otherwise poorly retained on reversed-phase columns.
Subsequently, 1 µL of the derivatized solution was injected for UPLC separation. Detection of ACQ-derivatized amino acids was performed using a UV/fluorescence detector at 254 nm, where the derivatives exhibit strong absorbance. Quantification was achieved using external amino acid standard mixtures at six concentrations, analyzed at three points throughout the analytical sequence (beginning, middle, and end) to ensure instrument stability and reproducibility. Calibration curves generated from these standards showed excellent linearity (R2 > 0.99) across the tested concentration range. In addition, single amino acid standards were measured to verify retention times and analytical accuracy.
All analyses were conducted at the Bioanalytics Unit, IPK Gatersleben (Am Schwabenplan 1b, 06466 Seeland, Germany) under controlled laboratory conditions. UPLC–MS was performed on an ACQUITY UPLC system (Waters Corp., Milford, MA, USA) coupled to a quadrupole time-of-flight mass spectrometer (Q-TOF LC–MS Agilent Technologies, Waldbronn, Germany), equipped with an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm particle size; Waters Corp.). The use of UPLC, compared to conventional HPLC, allowed for higher resolution, faster analysis, and improved sensitivity due to the sub-2 µm particle size of the column.
The mobile phase consisted of water with 0.1% formic acid (Sigma-Aldrich, St. Louis, MO, USA) (solvent A) and acetonitrile (Sigma-Aldrich, St. Louis, MO, USA) with 0.1% formic acid (solvent B). The gradient program was as follows: 0–2 min, 2% B; 2–10 min, linear increase to 30% B; 10–15 min, linear increase to 90% B; 15–20 min, hold at 90% B; 20–25 min, re-equilibration to 2% B. The flow rate was maintained at 0.3 mL min−1, the column temperature at 40 °C, and the injection volume at 1 µL.
2.4. Statistical Analysis
Statistical analyses were performed using SAS software (version 9.1). Data are presented as means ± standard deviation (SD). Each dataset was analyzed using one-way ANOVA for the different time points (0, 7, and 21 days) under salt treatment (200 mM NaCl), at a significance level of p < 0.01, followed by the LSD test to compare means of different time points. Principal component analysis (PCA) was conducted using XLSTAT software (version 2016) (Addinsoft, Paris, France), with all data standardized using Z-score normalization prior to analysis. Hierarchical cluster analysis was performed using JMP software (version 16.2.0) with Euclidean distance as the similarity measure and UPGMA linkage method to group samples based on their metabolic and amino acid profiles.
3. Results
In this experiment, the medium-term (7-day) and long-term (21-day) effects of salinity on UCB-1 pistachio rootstocks were investigated. Free amino acids and key metabolites, including components of the sucrose synthesis and degradation pathways, glycolysis, and the TCA cycle, were quantified using LC-MS to evaluate the impact of salinity on energy production and primary metabolism. Plants exhibited distinct metabolic responses between the 7-day and 21-day exposures. In total, 20 key metabolites and 19 free amino acids were detected, all of which showed significant changes under salinity stress (p < 0.01).
3.1. Metabolites Associated
ANOVA results revealed that salinity treatments exerted highly significant effects (p < 0.01) on all measured metabolites and key intermediates in pistachio leaves (Table 1). Central glycolytic intermediates and energy nucleotides including 3-phosphoglycerate (3PGA), phosphoenolpyruvate (PEP), ADP-glucose (ADPGlc), UDP-glucose (UDPGlc), ATP, ADP, and AMP were strongly affected, indicating substantial perturbations in carbohydrate metabolism and cellular energy balance under salinity stress. Similarly, TCA cycle intermediates such as malate, succinate, citrate, fumarate, cis-aconitate, and trans-aconitate displayed highly significant treatment-dependent changes, reflecting dynamic adjustments in carbon flux and energy production. Sugar phosphates and soluble sugars, including glucose 1-phosphate, glucose 6-phosphate + fructose 6-phosphate, fructose-1,6-bisphosphate, and sucrose, also varied significantly among treatments, highlighting modifications in carbohydrate partitioning and osmolyte accumulation. Nucleotide sugars (UTP, UDP, UMP) were similarly influenced, suggesting impacts on polysaccharide synthesis and metabolic signaling. Collectively, these statistically robust alterations across multiple metabolic pathways demonstrate that salinity stress induces a coordinated reprogramming of pistachio leaf metabolism.

Table 1.
Analysis of variance (ANOVA) for key metabolites and energy nucleotides in pistachio leaves under salinity stress.
In our study, almost all key metabolites were significantly altered in response to salinity stress. For clarity, metabolites were classified into functional modules, including photosynthesis-related metabolites, energy metabolism intermediates, TCA cycle components, and nucleotide metabolism.
Photosynthesis and sugar metabolism: Sucrose levels increased by ~42% at 7 days (15.7 vs. 11.1 in control), suggesting an initial osmoprotective response, but declined to slightly below control at 21 days (9.6). Glucose, fructose, and sucrose 6-phosphate were below detection limits, and trehalose 6-phosphate remained undetectable (Figure 1).
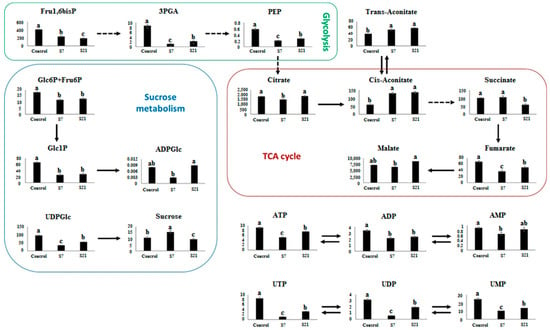
Figure 1.
Alterations in key metabolite contents (nmol g−1 DW) from UCB-1 pistachio leaves after 7 (S7) and 21 days (S21) of salt stress. Error bars indicate standard deviation (SD) of three biological replicates. Different letters above bars indicate statistically significant differences at p < 0.01 according to the LSD test; bars sharing the same letter are not significantly different.
Energy metabolism: Several glycolytic intermediates were strongly reduced under salinity. 3-phosphoglycerate (3PGA) declined from 8.68 in the control to 1.25 (−86%) at day 7 and remained low at day 21 (2.29). PEP dropped by ~66% at day 7 (0.20 vs. 0.58) and stayed suppressed (0.27) at 21 days. Glc1P and Glc6P+Fru6P were reduced by 62% and 35%, respectively, at day 7 and remained low thereafter. ATP decreased by 44% at day 7 (5.18 vs. 9.18) and partially recovered at day 21 (7.63). Similar patterns were observed for UTP, UDP, and UMP, which declined sharply during the first week (e.g., UTP from 8.67 to 1.15) but partially recovered by day 21 (3.31). ADP-glucose and AMP remained relatively unchanged, while ADP showed a moderate decrease at day 7 (−37%).
TCA 7. (6478 vs. 7299; −11%) but exceeded control values at day 21 (8756; +20%), indicating dynamic regulation of the TCA cycle. Citrate declined initially (1521 vs. 1836; −17%) but returned close to baseline by 21 days (1889). Succinate showed little change at day 7 but was reduced by 40% at day 21 (58.9 vs. 99.4). Both cis-aconitate and trans-aconitate nearly doubled at day 7 (130 vs. 60 and 53 vs. 40, respectively) and remained elevated at day 21. Fumarate decreased markedly at day 7 (−46%) and remained suppressed over time.
Overall interpretation: These quantitative changes reveal distinct temporal patterns of metabolic adjustment. Early responses were characterized by osmoprotection (sucrose accumulation) and suppression of energy-demanding pathways (ATP and glycolytic intermediates). Longer-term responses included partial recovery of nucleotides and accumulation of certain TCA intermediates (malate, citrate), highlighting a metabolic rebalancing that supports cellular adaptation under prolonged salinity stress.
3.2. Amino Acids Assay
The analysis of variance (ANOVA) revealed significant differences among treatments for all measured free amino acids in pistachio leaves under salinity stress. The salinity stress mean squares were substantially higher than the error mean squares for each amino acid, indicating that the applied salinity treatments (0, 7, and 21 days) had a pronounced effect on amino acid accumulation (Table 2). Specifically, amino acids such as Asparagine (Asn), Arginine (Arg), Glutamine (Gln), Glutamate (Glu), Proline (Pro), and Histidine (His) exhibited highly significant changes (p < 0.01) across the treatments, reflecting strong metabolic adjustments to salinity stress. Other essential amino acids, including Lysine (Lys), Threonine (Thr), Leucine (Leu), and Phenylalanine (Phe), also showed significant variation, though their absolute values differed in magnitude. The low error mean squares indicate low variability among biological replicates, confirming the reliability and reproducibility of the observed responses. Overall, the results demonstrate that salinity stress substantially modulates the free amino acid profile in pistachio leaves, highlighting the importance of amino acid metabolism in osmotic adjustment and stress tolerance. The significant alterations observed in both non-essential (e.g., Glu, Gln, Pro) and essential amino acids (e.g., Lys, Leu, Phe) underscore the comprehensive metabolic reprogramming that pistachio undergoes under prolonged salinity conditions.

Table 2.
Analysis of variance (ANOVA) of free amino acid concentrations in pistachio leaves under different salinity stress.
Under salinity stress, the levels of almost all free amino acids increased significantly, with the exception of threonine (Thr). Thr content consistently decreased throughout the stress period, from 44.64 µmol g−1 FW in the control to 11.81 at day 7 (−74%) and further to 3.19 at day 21 (−93%) (Figure 2).
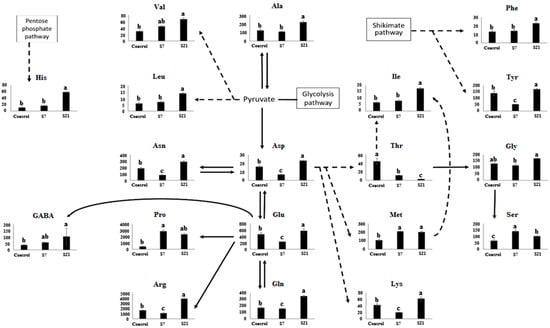
Figure 2.
Alterations in amino acid pools (nmol g−1 DW) from UCB-1 pistachio leaves after 7 (S7) and 21 days (S21) of salinity stress. Error bars indicate standard deviation (SD) of three biological replicates. Different letters above bars indicate statistically significant differences at p < 0.01 according to the LSD test; bars sharing the same letter are not significantly different.
Amino acids displayed distinct temporal patterns depending on the duration of stress. Histidine (His) increased moderately at day 7 (16.96 vs. 11.06; +53%) but accumulated strongly under long-term stress (58.73; +430%). Similar long-term accumulation was observed for γ-aminobutyric acid (GABA), which rose from 37.4 in the control to 58.0 at day 7 (+55%) and 153.5 at day 21 (+310%), and for valine (Val), which increased from 30.5 to 45.3 at day 7 (+48%) and 68.2 at day 21 (+123%).
Proline (Pro) exhibited a sharp increase at day 7 (2830 vs. 418; +577%), followed by a decline at day 21 (2341), though levels remained ~5.5-fold above control. Serine (Ser) nearly doubled at day 7 (140 vs. 67; +110%) but decreased to 100 by day 21, still above control (+50%).
Asparagine (Asn), arginine (Arg), aspartate (Asp), lysine (Lys), glutamate (Glu), and tyrosine (Tyr) showed a biphasic response: all decreased during the first week (e.g., Arg: 1623 → 1123, −31%; Lys: 42.5 → 20.0, −53%; Tyr: 138.6 → 53.1, −62%) but then recovered strongly by day 21 (e.g., Arg: 3859, +138%; Lys: 62.5, +47%; Tyr: 170.5, +23%).
Alanine (Ala), glutamine (Gln), glycine (Gly), isoleucine (Ile), leucine (Leu), methionine (Met), and phenylalanine (Phe) exhibited delayed accumulation, showing little change at day 7 but significantly higher levels at day 21 (e.g., Ala: 122 → 220, +80%; Gln: 171 → 351, +105%; Met: 107 → 201, +88%; Phe: 13.2 → 22.9, +73%).
Overall interpretation: These quantitative changes indicate that amino acid metabolism in UCB-1 pistachio rootstock is highly dynamic and temporally regulated. Early responses included rapid accumulation of osmoprotective amino acids such as proline and serine, while later stages were characterized by recovery of initially suppressed amino acids (e.g., Arg, Lys, Tyr) and de novo accumulation of branched-chain and aromatic amino acids. This reflects a coordinated adjustment of nitrogen allocation and stress adaptation strategies over time under salinity stress.
3.3. Evaluating the Effect of Salinity Stress on Pistachio Using Cluster Analysis
Metabolites and amino acids were visualized as a heatmap (Figure 3), and cluster analysis revealed distinct patterns among the three treatments. For amino acids, the control grouped closely with the 7-day salinity treatment (distance coefficient 0.40), while the 21-day treatment clustered separately, merging with the control–7-day group at 0.59. For central metabolites including sugars, glycolytic intermediates, TCA cycle compounds, and nucleotide derivatives such as sucrose, citrate, malate, succinate, ADP, PEP, Glc1P, Glc6P+Fru6P, Fru1,6bP, fumarate, cis-aconitate, trans-aconitate, UDPGlc, ATP, UTP, UDP, and UMP the 7- and 21-day salinity treatments clustered together at 0.35, joining the control at 0.49. These central metabolites represent key nodes of primary metabolism governing energy provision, carbon flux regulation, and cellular adaptation. The clustering patterns indicate that prolonged salinity induces coordinated and stabilized reprogramming of central metabolic pathways, whereas amino acid profiles reflect both short and long-term biochemical responses to salt stress.
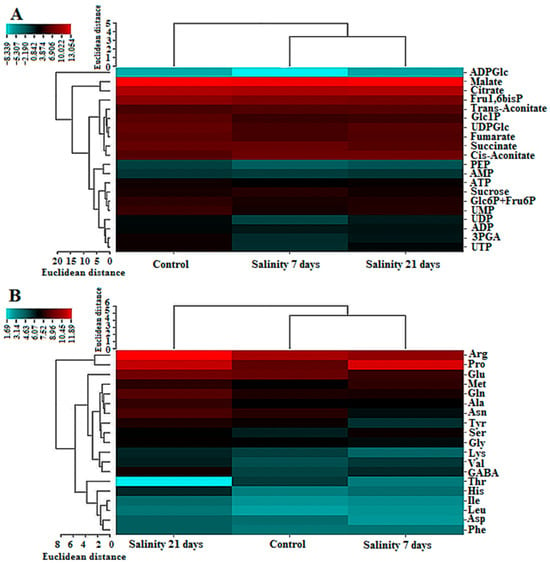
Figure 3.
Cluster map of some key metabolites (A) and amino acids (B) in UCB-1 pistachio leaves after 7 and 21 days of salt stress. Blue and red reveal relatively low and high expressions, respectively.
3.4. Evaluating the Effect of Salinity Stress on Pistachio Using PCA
The PCA result indicated the alteration due to salinity. There is an obvious separation between control and salinity treatments in the first principal component (F1), which accounts for 52.55% of the total variance. Thus, two distinct groups were obtained: one group included control plants and the second group included plants exposed to 7 and 21-day salinity. Two salinity treatments were separated by the second principal component (F2) representing 47.45% of the total variance (Figure 4). 20 metabolites including Ser, Thr, Pro, Met, Val, 3PGA, PEP, UDPGlc, Glc1P, Glc6P+Fru6P, Fru1,6bisP, ATP, ADP, AMP, UTP, UDP, UMP, cis-aconitate, trans-aconitate, and fumarate were correlated with PCA1 (F1). Also, 19 metabolites including His, Asn, Arg, Gln, Asp, Gly, Glu, Ala, Lys, Tyr, Ile, Leu, Phe, GABA, ADPGlc, malate, succinate, citrate, and sucrose were correlated with PCA2 (F2).
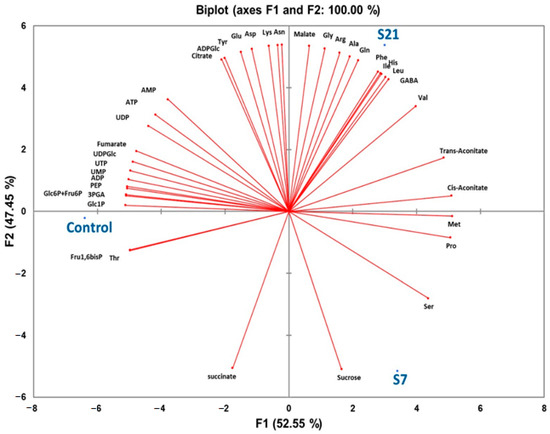
Figure 4.
Principal component analysis (PCA) of leaf metabolites from UCB-1 pistachio under control or salinity conditions with varied lengths of time (7 and 21-day exposure). Principal components 1 and 2 account for 100% of the variance in the data.
4. Discussion
Since plants are sessile organisms, their survival under abiotic stress depends on rapid and complex adaptive responses that involve morphological, physiological, and biochemical adjustments. Among these stresses, salinity represents a major global challenge, adversely affecting nearly 20% of arable land and severely restricting plant growth and productivity [12,32,33]. While previous studies have largely characterized salinity responses in pistachio through classical physiological and biochemical traits, the present work provides novel insights at the metabolic and amino acid levels by applying LC-MS-based metabolomics. This approach enables the detection of subtle but critical alterations in central metabolites, offering a more precise understanding of salinity-induced reprogramming at the molecular level. Such knowledge not only complements earlier physiological studies but also advances the identification of potential metabolic markers associated with salt tolerance, which is a crucial step toward the development of resilient pistachio rootstocks for sustainable cultivation in saline environments [27,28]. The ANOVA results provide strong statistical evidence that salinity stress profoundly alters both metabolite and free amino acid profiles in pistachio leaves. Highly significant differences (p < 0.01) were observed among treatments for central glycolytic intermediates, TCA cycle metabolites, sugar phosphates, soluble sugars, and energy nucleotides, indicating extensive reprogramming of carbohydrate metabolism, carbon flux, and cellular energy balance under stress. Similarly, all measured amino acids exhibited significant treatment-dependent changes. The low error variability confirms the reliability and reproducibility of these responses across biological replicates.
4.1. Photosynthesis and Carbon Assimilation
Salt stress markedly impairs photosynthetic efficiency [34]. This reduction arises from multiple interrelated factors, including stomatal closure that limits CO2 availability, dehydration of cell membranes that lowers their permeability to CO2, and a decline in chlorophyll content resulting from Cl− ion accumulation. Moreover, Na+ ions disrupt the proton-motive force and interfere with CO2-fixing enzymes. Structural disorganization of the thylakoid membranes within chloroplasts further contributes to the overall reduction in photosynthetic capacity under saline conditions [1,4,34].
Our metabolite data provide direct evidence for these impairments. For instance, 3-phosphoglycerate (3PGA) decreased by ~40% after 7 days of salt stress and remained 30% lower than control even after 21 days, indicating a sustained reduction in Calvin cycle flux. Similarly, fructose-1,6-bisphosphate (Fru1,6bP) declined by ~35% at day 7, but partially recovered to ~80% of control levels at day 21, suggesting short-term inhibition followed by partial compensation. Glucose-6-phosphate plus fructose-6-phosphate (Glc6P+Fru6P) exhibited a 25% reduction at day 7 and did not fully recover at day 21, further reflecting limited carbon assimilation. In contrast, cis-aconitate and trans-aconitate accumulated up to 1.5–2-fold during the early stress phase, reflecting rerouting of carbon toward the TCA cycle for stress adaptation.
Interestingly, some metabolites displayed recovery trends. For example, malate and citrate, which decreased by ~20% and ~30% at day 7, returned close to control levels by day 21, consistent with metabolic adjustments to sustain mitochondrial energy supply. However, succinate and several nucleotide sugars (ATP, UDP, UTP, UMP) remained persistently depleted (30–50% lower than controls), highlighting ongoing impairment of photosynthesis-derived carbon flux.
Together, these dynamic changes illustrate that salt stress initially triggers sharp declines in carbon assimilation intermediates, followed by partial metabolic reprogramming. Nevertheless, persistent reductions in 3PGA and nucleotide pools confirm that the Calvin cycle and energy metabolism remain constrained, ultimately limiting photosynthetic performance under prolonged salinity, a pattern consistent with earlier reports [35].
4.2. Respiration and Energy Production
Salinity stress induced substantial alterations in glycolytic intermediates and energy nucleotides in pistachio leaves, as evidenced by decreases in 3-phosphoglycerate (3PGA), fructose-1,6-bisphosphate (Fru1,6bP), phosphoenolpyruvate (PEP), and ATP, indicating a potential decline in glycolytic flux. The imbalance in ion uptake under salinity can be partially mitigated by adjustments in organic acid concentrations, highlighting the link between salinity and respiration modulation [36]. Consequently, variations in TCA cycle intermediates are expected, though the magnitude of these changes depends on species, tissue type, salinity level, duration of exposure, and any pre-treatments [36,37,38]. In the present work, accumulations of the TCA cycle components were changed as reported by previous studies [36,37,38,39,40]. TCA cycle components including citrate, succinate, fumarate, and malate—exhibited significant shifts, reflecting a redistribution of carbon towards pathways essential for osmotic adjustment [9]. Reactive oxygen species (ROS) generated under salinity may inhibit key TCA cycle enzymes, such as succinate dehydrogenase, leading to decreased conversion of succinate to fumarate [41]. The observed reduction in glycolysis and TCA cycle activity likely contributes to a lowered respiration rate, consistent with reports that salinity-tolerant plants often reduce respiration to conserve energy and allocate carbon for growth and stress adaptation, For example, accumulation of Clˉ and H2O2 during salinity has been shown to inhibit the activity of succinate dehydrogenase, which converts succinate to fumarate [36]. Literature reports that respiration rates decline in approximately 42% of salinity-tolerant plant species, a response that may conserve energy and redirect carbon toward new tissue synthesis and growth [10,41].
Additionally, the decrease in ATP, ADP, UTP, UDP, and UMP pools may result from limited phosphate availability in saline soils, exacerbating the reduction in cellular energy currency [4]. Moreover, the expression and activity of H+-ATPase, especially V-ATPase, are stimulated by salinity for removing Na+ from the cytosol [5,34] which could contribute to ATP consumption and reduction in its pool. V-ATPase is essential for establishing the proton gradient necessary for Na+/H+ antiporter activity, which transports excess Na+ to the vacuole [42]. A decrease in ATP content might also correlate with reductions in respiration and photosynthesis. Notably, the shifts in UTP and UDP levels were particularly significant.
4.3. Osmotic Adjustment
The osmotic effect resulting from salt accumulation in the soil decreases osmotic potential, thereby limiting water uptake by plants. This condition induces water deficit, reduced turgor pressure, and a decline in cell volume, ultimately constraining plant growth. To mitigate these effects, plant cells synthesize and accumulate compatible osmolytes or osmoprotectants, low-molecular-weight compounds that not only contribute to osmotic adjustment but also stabilize subcellular structures by scavenging reactive oxygen species (ROS) and preventing protein denaturation [1,9,10]. In the present study, pistachio leaves showed significant modulation of key metabolites involved in osmotic adjustment under both 7-day and 21-day salinity treatments, indicating an active cellular response to maintain water balance. Antunes et al. [43] examined the metabolome of strawberry fruit grown under osmotic stress. LC-MS/MS and GC-MS data showed accumulation of phenolic compounds, glycerophospholipids, phytosterols, carbohydrates, and an aromatic amino acid under drought and salt stress conditions. Untargeted metabolomics confirmed the presence of stress-induced metabolites, which are related to ROS inhibition and biosynthesis of cell wall lipids, potentially serving as biomarkers for osmotic stress.
Compatible osmolytes can include various compounds like sugars, amino acids, and their derivatives, such as glycine betaine, β-alanine betaine, and proline betaine [9]. The specific osmolyte utilized can vary among plant species; for instance, some may accumulate proline while others produce sucrose. For instance, some species accumulate proline and others produce sucrose [1]. In pistachio, sucrose levels increased after 7 days of salinity exposure and returned to near-control levels after 21 days, whereas malate content initially decreased and later exceeded pre-stress levels, indicating a dynamic adjustment of organic acids for osmotic balance. This suggests that the type of osmoprotectant is influenced not only by the species but also by the duration of the salinity stress. Additionally, malate, recognized as the most abundant organic anion in plants, plays a significant role in osmotic adjustment [44]. Yang et al. [11] indicated that in soybean, carbohydrate contents decreased while amino acid pools increased, while in tomatoes, proline levels were found to decrease [45].
During saline stress, carbon partitioning favors the production of osmoprotectants such as sucrose over starch [1,9]. In addition to osmotic adjustment, sucrose is involved in radical scavenging and carbon storage [9,34,37]. Even though sucrose levels rose after 7 days of salinity, the pools of key components involved in sucrose biosynthesis like Glc6+Fru6P, Glc1P, and UDPGlc decreased, likely due to reduced photosynthesis rates. It can be inferred that these compounds were redirected towards sucrose production to mitigate any declines in sucrose levels. Wang et al. [46] studied the effects of saline-alkaline stress on the sugar metabolome of jujube fruit. They found glucose and fructose accumulated mainly during early growth stages, whereas sucrose was the predominant sugar in later stages. Metabolomic network maps revealed close correlations between six accumulated metabolites and specific genes, with sucrose identified as a key metabolite in metabolic pathways.
4.4. Amino Acids
Nitrogen-containing compounds, such as amino acids and amides, play a crucial role in plant responses to salinity by contributing to osmotic adjustment, serving as nitrogen reservoirs, scavenging reactive radicals, and protecting macromolecules from stress-induced damage [4,47]. In pistachio leaves, our study confirmed that the levels of free amino acids were substantially modified under 7-day and 21-day salinity stress, consistent with their roles in osmotic adjustment and stress protection. The type of nitrogen-containing compound and its accumulation vary among different species [4,48].
A notable trend was the transient decrease of asparagine (Asn), arginine (Arg), aspartate (Asp), glutamate (Glu), tyrosine (Tyr), and lysine (Lys) at day 7, followed by an increase at day 21. This pattern reflects the dynamic regulation of nitrogen metabolism. Asn is a well-known nitrogen storage and transport molecule in perennial woody species, including pistachio. Its reduction at day 7 may indicate rapid utilization of stored nitrogen for the biosynthesis of osmoprotectants and stress-responsive proteins during the initial stress response. By day 21, the subsequent increase in Asn and related amino acids suggests a reallocation and storage of nitrogen, possibly linked to recovery processes and the long-term adjustment of carbon–nitrogen metabolism under salinity stress. Thus, pistachio appears to regulate amino acid pools not only for osmotic adjustment but also for nitrogen recycling and storage, a mechanism that enhances survival under prolonged stress.
Our study aligns with previous reports demonstrating that amino acid metabolism is strongly influenced by environmental stress [11,37,39]. Proline, recognized as the most prevalent amino acid in many plant species, notably increased during salinity stress [10,42,49]. In our experiment, proline levels were elevated after 7-day stress and partially maintained after 21-day stress, highlighting its central role in osmotic adjustment and ROS mitigation in pistachio. It functions as a chaperone, osmotic regulator, and a scavenger of ROS, as well as playing a role in the regulation of genes responsive to salinity [6,10,11].
Furthermore, the serine pool, a precursor to choline, was found to be enhanced, which can lead to increased glycine betaine production [42]. Glycine betaine plays a vital role in protection of thylakoid membranes and major enzyme structures, thus maintaining the photosynthetic efficiency, while mitigating the formation of ROS [6,9]. Additionally, alanine, which serves as a precursor to β-alanine betaine, was also elevated, although proline and serine levels were more prominent during a 7-day stress period compared to a 21-day stress period, with alanine only showing significant increases after 21 days.
The presence of GABA was also notable in this study, where it acts as a signaling molecule to trigger salinity responses and provides energy and carbon skeletons during such stress [6]. Its catabolism further supports energy production and nitrogen–carbon balance during salinity [41]. Moreover, the depletion of glutamate aligns with findings from other studies, likely due to its conversion into proline and chlorophyll [10,50]. Tyrosine content decreased slightly in pistachio leaves, consistent with its conversion into polyphenols for ROS scavenging.
Additionally, levels of malic and citric acids were elevated, indicating their contribution to osmotic adjustment and carbon flux redistribution under salinity stress. Several studies have explored the accumulation of metabolites in plants under environmental stress conditions [10]. Renault et al. [51] reported an increase in gamma-aminobutyric acid (GABA) levels in response to salt stress in tobacco (Nicotiana tabacum) and Arabidopsis thaliana. Similarly, Similarly, Zhang et al. [52] observed elevated GABA concentrations in tobacco plants under salt stress, highlighting its role in stress tolerance. Additionally Usadel et al. and Kaya et al. [53,54] reported elevated levels of malic acid and citric acid in plants exposed to environmental stresses, reinforcing the role of these organic acids in stress adaptation.
4.5. Identification of Metabolic Markers Under Salinity Stress
Integration of PCA and hierarchical cluster analysis allowed the identification of key metabolites that are most responsive to salinity stress in UCB-1 pistachio rootstocks. PCA revealed clear separation of the treatments, with the first principal component (F1, 52.55% variance) distinguishing control plants from salinity-exposed plants (7- and 21-day), and the second component (F2, 47.45% variance) separating the 7- and 21-day treatments. Metabolites strongly correlated with F1 included Ser, Thr, Pro, Met, Val, 3PGA, PEP, UDPGlc, Glc1P, Glc6P+Fru6P, Fru1,6bisP, ATP, ADP, AMP, UTP, UDP, UMP, cis-aconitate, trans-aconitate, and fumarate, indicating that these compounds largely drive the distinction between control and salt-treated plants. Metabolites correlated with F2, including His, Asn, Arg, Gln, Asp, Gly, Glu, Ala, Lys, Tyr, Ile, Leu, Phe, GABA, ADPGlc, malate, succinate, citrate, and sucrose, contributed to the differentiation between medium- and long-term salinity treatments.
Hierarchical clustering further supported these results. Amino acids showed that the control and 7-day treatments clustered together at a distance coefficient of 0.40, merging with the 21-day treatment at 0.59. Central metabolites including sugars, glycolytic intermediates, TCA cycle compounds, and nucleotide derivatives such as sucrose, citrate, malate, succinate, ADP, PEP, Glc1P, Glc6P+Fru6P, Fru1,6bP, fumarate, cis-aconitate, trans-aconitate, UDPGlc, ATP, UTP, UDP, and UMP clustered the 7- and 21-day salinity treatments together at 0.35, joining the control at 0.49.
Taken together, cis-aconitate, trans-aconitate, and GABA consistently showed strong responses in both PCA and clustering analyses, highlighting their potential as core biochemical indicators for salt tolerance in pistachio. Monitoring these metabolites could provide a practical approach for evaluating and selecting salt-tolerant pistachio rootstocks.
5. Conclusions
We conducted a metabolomics study to understand how pistachio plants respond to salt stress. Our findings indicate that salinity triggers nearly all the metabolites measured. By comparing the metabolic responses, we determined that the response to salinity stress varies depending on the duration of exposure. Short-term (7-day) and long-term (21-day) salinity stress led to distinct changes in key metabolites involved in photosynthesis, glycolysis, and the TCA cycle, reflecting alterations in energy production and carbon assimilation. The differential accumulation of amino acids and metabolites like proline, sucrose, and various TCA cycle intermediates suggests that pistachio plants employ multiple strategies to mitigate the detrimental effects of salt stress. These include osmotic adjustment, energy conservation, and the activation of protective biochemical pathways. Our findings highlight the significance of understanding the temporal dynamics of metabolic responses to salinity. These insights could aid in developing more resilient pistachio cultivars and enhancing management practices in saline environments. Ultimately, the ability of pistachios to adapt metabolically to prolonged salt exposure highlights the potential for optimizing their growth and productivity in challenging conditions. This study focuses exclusively on leaf metabolism. Future investigations could expand to include root systems and fruits to provide a more comprehensive understanding of the metabolic responses of pistachio to salinity. Additionally, integrating multi-omics approaches and exploring different cultivars under varied environmental conditions may further elucidate mechanisms of salt tolerance.
Author Contributions
H.S.: Investigation, Data curation, Formal analysis, Visualization, Validation, Software, Writing—original draft. F.F.: Conceptualization, Investigation, Methodology, Data curation, Validation, Writing—review & editing. S.H.: Formal analysis, Visualization, and Writing—original draft. R.K.: Formal analysis, Visualization, and Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted with the financial support of the National Researchers and Technologists Support Fund (INSF) (grant number 4027652), for which we are grateful.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by the Iran National Science Foundation (INSF), and the authors would like to express gratitude to the INSF for their support (grant number 4027652). The authors acknowledge the National Science Foundation Grant Number 2150087 for financial support. Additionally, we thank the technical assistance of Payam Noor University Faculty of Graduate Studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, F.; Hosseinzadeh, A.; Alizadeh, H.; Brimavandi, T. The proteome response of Hordeum spontaneum to salinity stress. Cereal Res. Commun. 2013, 41, 78–87. [Google Scholar] [CrossRef]
- Boustani, A.; Fatehi, F.; Azizinezhad, R. The proteome response of “Hordeum marinum” to long-term salinity stress. Cereal Res. Commun. 2017, 45, 401–410. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Li, J.; Liu, M. Biological features and regulatory mechanisms of salt tolerance in plants. J. Cell. Biochem. 2019, 120, 10914–10920. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Jia, Q.; Ibrahim, A.K.; Niyitanga, S.; Zhang, L. Mechanisms and signaling pathways of salt tolerance in crops: Understanding from the transgenic plants. Trop. Plant Biol. 2020, 13, 297–320. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Zhang, S.; Tao, X.; Mesbah, N.M.; Mao, X.; Wang, H.; Wiegel, J.; Zhao, B. The polyextremophile Natranaerobius thermophilus adopts a dual adaptive strategy to long-term salinity stress, simultaneously accumulating compatible solutes and K+. Appl. Environ. Microbiol. 2024, 90, e00145-24. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008, 54, 89. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Yang, D.-S.; Zhang, J.; Li, M.-X.; Shi, L.-X. Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J. Plant Growth Regul. 2017, 36, 460–471. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef]
- Skliros, D.; Kalloniati, C.; Karalias, G.; Skaracis, G.N.; Rennenberg, H.; Flemetakis, E. Global metabolomics analysis reveals distinctive tolerance mechanisms in different plant organs of lentil (Lens culinaris) upon salinity stress. Plant Soil 2018, 429, 451–468. [Google Scholar] [CrossRef]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.; Mousa, N.H.; Hanafy, R.S.; Latef, A.A.H.A. Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative stress in wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Torabi, M. Physiological and biochemical responses of plants to salt stress. In Proceedings of the 1st International Conference on New Ideas in Agriculture, Isfahan, Iran, 26 January 2014; pp. 26–27. [Google Scholar]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Pakzad, R.; Fatehi, F.; Kalantar, M.; Maleki, M. Evaluating the antioxidant enzymes activities, lipid peroxidation and proteomic profile changing in UCB-1 pistachio rootstock leaf under drought stress. Sci. Hortic. 2019, 256, 108617. [Google Scholar] [CrossRef]
- Khalilpour, M.; Mozafari, V.; Abbaszadeh-Dahaji, P. Tolerance to salinity and drought stresses in pistachio (Pistacia vera L.) seedlings inoculated with indigenous stress-tolerant PGPR isolates. Sci. Hortic. 2021, 289, 110440. [Google Scholar] [CrossRef]
- Zhang, S.; Quartararo, A.; Betz, O.K.; Madahhosseini, S.; Heringer, A.S.; Le, T.; Shao, Y.; Caruso, T.; Ferguson, L.; Jernstedt, J. Root vacuolar sequestration and suberization are prominent responses of Pistacia spp. rootstocks during salinity stress. Plant Direct 2021, 5, e00315. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio nuts (Pistacia vera L.): Production, nutrients, bioactives and novel health effects. Plants 2021, 11, 18. [Google Scholar] [CrossRef]
- Atli, H.; Arpaci, S.; Ayanoglu, H. Comparison of seedling characteristics of some Pistacia species. Options Méditerr. 2001, 56, 215–218. [Google Scholar]
- Gadže, J.; Lovrić, J.; Kramarić, J.M.; Radunić, M.; Blašković, L.; Batelja Lodeta, K. Propagation, varieties and rootstocks of pistachios. Pomol. Croat. Glas. Hrvat. Agron. Druš. 2023, 27, 71–88. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Baghizadeh, A.; Kalantar, M.; Fatehi, F. Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci. Hortic. 2020, 261, 108970. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Amirmahani, F.; Salehi, F. Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought+ salinity stresses. Physiol. Plant. 2020, 168, 973–989. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Akbari, M.; Katam, R.; Husain, R.; Farajpour, M.; Mazzuca, S.; Mahna, N. Sodium chloride induced stress responses of antioxidative activities in leaves and roots of pistachio rootstock. Biomolecules 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Hakimnejad, S.; Karimi, H.R.; Sahhafi, S.R.; Esmaeilizadeh, M. Evaluation of the tolerance of four pistachio rootstocks to salinity stress based on morphological, physiological and biochemical parameters. J. Nuts 2022, 13, 87–103. [Google Scholar]
- Rahneshan, Z.; Nasibi, F.; Lakehal, A.; Bellini, C. Unravelling salt stress responses in two pistachio (Pistacia vera L.) genotypes. Acta Physiol. Plant. 2018, 40, 172. [Google Scholar] [CrossRef]
- Mirabi, E.; Seifi, E.; Hokmabadi, H. Effects of Salinity on Antioxidant Enzymes and some Morphophysiological Traits of Two Interspecies Hybrid Pistachio Rootstocks. J. Plant Prod. Res. 2024, 31, 213–229. [Google Scholar]
- Shahrayini, E.; Noroozi, A.A. Modeling and mapping of soil salinity and alkalinity using remote sensing data and topographic factors: A case study in Iran. Environ. Model. Assess. 2022, 27, 901–913. [Google Scholar] [CrossRef]
- Fatehi, F.; Hosseinzadeh, A.; Alizadeh, H.; Brimavandi, T.; Struik, P.C. The proteome response of salt-resistant and salt-sensitive barley genotypes to long-term salinity stress. Mol. Biol. Rep. 2012, 39, 6387–6397. [Google Scholar] [CrossRef] [PubMed]
- Bowne, J.; Bacic, A.; Tester, M.; Roessner, U. Abiotic stress and metabolomics. In Annual Plant Reviews, Volume 43: Biology of Plant Metabolomics; Hall, R.D., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 61–85. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Vijayalakshmi, C.; Thiyagarajan, K.; Kannanbapu, J.; Suneetha Kota, S.K.; Padmavathi, G.; Geetha, S.; Sritharan, N.; Viraktamath, B. Changes in photosynthesis, chlorophyll fluorescence, gas exchange parameters and osmotic potential to salt stress during early seedling stage in rice (Oryza sativa L.). SABRAO J. Breed. Genet. 2014, 46, 120–135. [Google Scholar]
- Saha, P.; Kunda, P.; Biswas, A.K. Influence of sodium chloride on the regulation of Krebs cycle intermediates and enzymes of respiratory chain in mungbean (Vigna radiata L. Wilczek) seedlings. Plant Physiol. Biochem. 2012, 60, 214–222. [Google Scholar] [CrossRef]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stevanato, P.; Yu, L.; Zhao, H.; Sun, X.; Sun, F.; Li, J.; Geng, G. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J. Plant Res. 2017, 130, 1079–1093. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, Y.-H. Sodium chloride causes variation in organic acids and proteins in tomato root. Afr. J. Biotechnol. 2010, 9, 8161. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.C.; Acunha, T.d.S.; Perin, E.C.; Rombaldi, C.V.; Galli, V.; Chaves, F.C. Untargeted metabolomics of strawberry (Fragaria x ananassa ‘Camarosa’) fruit from plants grown under osmotic stress conditions. J. Sci. Food Agric. 2019, 99, 6973–6980. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsuchiya, Y.; Ariyoshi, M.; Nakano, R.; Ushijima, K.; Kubo, Y.; Mori, I.C.; Higashiizumi, E.; Galis, I.; Yamamoto, Y. Two members of the aluminum-activated malate transporter family, SlALMT4 and SlALMT5, are expressed during fruit development, and the overexpression of SlALMT5 alters organic acid contents in seeds in tomato (Solanum lycopersicum). Plant Cell Physiol. 2016, 57, 2367–2379. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Yan, M.; Zhou, X.; Yuan, Z.; Zhang, Q.; Yan, H.; Wu, C. Effects of saline-alkali stress on sugar metabolism of jujube fruit: A metabolomic analysis. Agronomy 2023, 13, 2239. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, V.; Madaan, N.; Mudgal, A. Biochemical mechanisms of salt tolerance in plants: A review. Int. J. Bot. 2010, 6, 136–143. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Roessner, U.; Jayasinghe, N.S.; Lutz, A.; Rupasinghe, T.; Bazihizina, N.; Bohm, J.; Alharbi, S.; Hedrich, R.; Shabala, S. Epidermal bladder cells confer salinity stress tolerance in the halophyte quinoa and Atriplex species. Plant Cell Environ. 2017, 40, 1900–1915. [Google Scholar] [CrossRef]
- Renault, H.; Roussel, V.; El Amrani, A.; Arzel, M.; Renault, D.; Bouchereau, A.; Deleu, C. The Arabidopsis pop2–1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Du, Y.; Chen, S.; Tang, H. Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Kuschinsky, A.; Steinhauser, D.; Pauly, M. Transcriptional co-response analysis as a tool to identify new components of the wall biosynthetic machinery. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2005, 139, 69–73. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).