Preservation of Fruit Quality at Postharvest Through Plant-Based Extracts and Elicitors
Abstract
1. Introduction
2. Main Sources of Literature and Bibliometric Analysis
2.1. Publication Trends
2.2. Most Popular Keywords
2.3. Publications by Country/Territory
3. Essential Oils (EOs), Botanical Extracts, and Volatile Constituents
3.1. Leaves Compared with Other Parts as Sources of Bioactive Compounds
3.2. Fruits, Vegetables and Flowers as Sources of Natural Preservatives
4. Isolation Methods for Plant Extracts
5. The Efficacy of Extracts from Medicinal Plants
5.1. Aloe Vera (AV)
5.2. Lemongrass
5.3. Neem
5.4. Other Extracts
6. Plant-Derived Elicitors for Enhancing Fruits Quality
6.1. Methyl Jasmonic Acid (MeJA)
| Treatment | Fruit | Effect | Reference |
|---|---|---|---|
| MeJA | Table grapes | Accelerated fruit ripening. Application of 0.1 mM and 0.01 mM MeJA significantly increased total yield while enhancing berry quality attributes and bioactive compound levels. | [141] |
| Lemons | Increased content of antioxidants such as phenolics at 0.1 mM MeJA. Antioxidant enzyme activities were significantly elevated, with no adverse effects on yield or fruit quality. | [148] | |
| Blood oranges | Decreased electrolyte leakage and MDA content and enhanced SOD, APX and CAT activities. Suppressed electrolyte leakage and MDA levels, concomitant with elevated activities of SOD, APX, and CAT. | [144] | |
| Pomegranates | Treatments with 1 mM and 5 mM MeJA accelerated on-tree fruit maturation while suppressing storage losses of firmness, weight, and organic acid content at 10 °C. And aril coloration was significantly enhanced. | [97] | |
| Pomegranates | Reduced internal/external CI symptoms and ion leakage, attributed to preserved harvest-stage unsaturated fatty acids, enhanced membrane stability, and maintained antioxidant levels during storage. | [143] | |
| Lemons | Elevated antioxidant parameters including total antioxidant activity, phenolic content, key phenolics (eriocitrin, hesperidin), and enzymatic activity. | [149] | |
| Pomegranates | Alleviated CI and maintained intact pericarp structure. | [150] | |
| Persimmons | Preserved fruit quality, content of phenolic compounds and antioxidant properties, reduced CI and membrane peroxidation, and enhanced membrane integrity during cold storage. | [147] | |
| Sweet cherries | Improvement in abiotic stress tolerance and reduction in fruit cracking and ripening delay. | [140] | |
| Plums | Elevated carotenoid and phenolic levels at harvest and antioxidant activity, with no effect on ripening of fruits on the tree. | [151] | |
| SA | Plums | Elevated bioactive and antioxidant levels upon harvesting. | [151] |
| Sweet cherries | Improved physicochemical properties (color, SSC, firmness), bioactive constituents (phenolics, anthocyanins), and antioxidant metrics (hydrophilic capacity, enzyme activities). | [152] | |
| Plums | Enhanced postharvest quality evidenced by increased weight, firmness, TA; elevated phenolics (anthocyanins), carotenoids; sustained antioxidant enzyme activity; delayed ripening/senescence; and extended shelf-life. | [153] | |
| Plums | Elevated harvest-stage phenolics and carotenoids with enhanced antioxidant capacity, without altering on-tree fruit ripening. | [151] | |
| Plums | At harvest, higher levels of firmness, weight, sugars, acids, phenolics, carotenoids, and anthocyanins were observed. SA treatment delayed softening, color shifts, and acidity loss upon storage. | [154] | |
| Pomegranates | The best improvement in quality was attained with 10 mM SA, mainly in terms of color and maintaining concentrations of anthocyanins, phenolics, and ascorbic acid during prolonged storage at 10 °C. | [96] | |
| Table grapes | 0.1 mM SA treatment elevated antioxidants and yield, promoted faster on-vine ripening, and maintained storage stability. | [155] | |
| Table grapes | Notable increases occurred in TA levels, bioactive compound concentrations, antioxidant enzyme functionality, and resistance to B. cinerea infection. | [156] | |
| Jujubes | Increased antioxidant capacity and total phenolics. | [157] | |
| Table grapes | Elevated antioxidant activity, total phenolic content, and bioactive constituent levels were observed. | [158] | |
| ASA | Sweet cherries | Enhanced firmness, color, total phenolics, SSC, total anthocyanin content, hydrophilic total antioxidant activity and antioxidant enzyme activity. | [152] |
| Plums | Enhanced weight, firmness, acid/sugar profiles, phenolic content, anthocyanins, and total carotenoids at harvest, with postponed softening, discoloration, and acidity loss during storage. | [154] | |
| Table grapes | 0.1 mM MeSA maximally promoted ripening, yield, and anthocyanin-mediated color enhancement in berries. | [155] | |
| Table grapes | Increased TA, content of bioactive compounds, activity of antioxidant enzymes, and resistance to Botrytis cinerea spoilage. | [156] | |
| Loquats | ASA was proven to be the most effective compound for both maintaining postharvest appearance and enhancing fruit quality attributes. | [159] | |
| MeSa | Plums | Harvested fruit exhibited heightened firmness, mass, organic acid diversity, phenolic compounds, soluble sugars, carotenoid content, and anthocyanin levels. Postharvest storage manifested suppressed softening, chromatic alterations, and acid depletion. | [154] |
| Pomegranates | Treatment with 10 mM SA was most effective, with improvement in red color and maintenance of anthocyanin/phenolic/ascorbic acid levels under prolonged 10 °C storage. | [96] | |
| Table grapes | Accelerated ripening, enhanced yield and berry color via elevated anthocyanin accumulation. | [156] | |
| Table grapes | Increased TA, bioactive compounds, antioxidant enzyme efficacy, and B. cinerea resistance. | [156] | |
| Apricots | Enhanced antioxidant capacity, reduced CI, maintenance of soluble solid and organic acids, and reduced decay. | [160] | |
| Oxalic Acid (OA) | Sweet cherries | Elevated fresh mass, textural rigidity, and SSC. | [152] |
| Pineapples | Diminished internal browning and increased ascorbic acid accumulation. | [161] | |
| Strawberries | Higher fruit yield and ascorbic acid levels and improved sensory attributes. | [162] | |
| Plums | Enhanced crop yield, fruit weight, and antioxidant level. Delayed maturation on-tree and during cold storage. | [163] | |
| Kiwifruit | Lower off-flavor intensity, reduced acetaldehyde/ethanol levels, and higher ascorbate content. | [120] | |
| Pomegranates | Yield enhancement and preharvest ripening acceleration exhibited dose dependency. Optimal firmness, peel chromaticity, respiratory activity, and organoleptic properties occurred with application of 10 mM OA. | [164] | |
| Apricots | Diminished moisture loss, ethylene emission, and respiration intensity. | [165] | |
| Apricots | Augmented firmness, ascorbic acid, mass and juice yield. Diminished moisture loss and antioxidative efficacy. | [166] | |
| Lemons | Reduced losses in weight and firmness, SSC and TA. Antioxidant enzymatic activity and total phenolic levels showed significant augmentation. | [167] | |
| Table grapes | Delayed senescence with stimulation of antioxidant enzyme activity. | [168] | |
| Blueberries | Elevated textural integrity, anthocyanin accumulation, and free radical scavenging capacity. | [169] | |
| Polyamines (PAs) | Apricots | Increased shelf-life up to 30 d with good quality under MAP conditions. | [170] |
| Jujubes | Improved antioxidant capacity and total phenolics. | [157] | |
| Pistachios | Increased fresh pistachio storability, delayed softening and weight loss, and inhibition of fungal infection. | [171] | |
| Table grapes | Greater firmness, lower susceptibility to microbial infection, elevated phenolic/anthocyanin accumulation with enhanced antioxidant capacity. | [172] | |
| Pears | Spermidine (Spd) (0.05 mM) and putrescine (Put) (0.25 mM) elicited significantly higher June fruit set. Maturity index peaked at 0.25 mM Put, with 0.05 mM Spd ranking second. Spd (0.25 mM) markedly elevated total sugars, antioxidants (anthocyanins and phenolics), and phytochemical accumulation. | [173] |
6.2. Salicylates
6.3. Oxalic Acid (OA)
6.4. Effects of Polyamines (PAs) on Fruit Preservation
6.5. Effectiveness of Plant Essential Oils, Extracts, and Elicitors in Fruit Preservation
7. Efficacy, Mechanisms, and Commercial Challenges
7.1. Controlling Postharvest Fruit Decay with Plant Extracts
7.2. Preventive Functions of Plant Extracts
7.3. Limits and Practical Challenges to Commercialization
7.3.1. Limits of Plant Extracts and Elicitors
7.3.2. Industrial Feasibility and Practical Challenges for Commercialization
8. Current Status, Limits, and Future Prospects
8.1. Current Status
8.2. Limitations
8.3. Future Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | Acetyl salicylic acid |
| ADC | Arginine decarboxylase |
| APX | Ascorbate peroxidase |
| APK | Ascorbate |
| ACEO | Acorus calamus essential oil |
| APS | Astragalus polysaccharides |
| AV | Aloe vera |
| CI | Chilling injury |
| CMC | Carboxymethyl cellulose |
| CBF | C-repeat/dehydration response element binding factor |
| CAT | Catalase |
| DW | Distilled water |
| ECPE | Edible coatings based on plant extracts |
| EOs | Essential oils |
| FDA | The US Food and Drug Administration |
| FI | Fagonia indica |
| GRAS | Generally recognized as safe |
| GA | Gum arabic |
| HAEs | Homogenizer-assisted extractions |
| HSPs | Heat shock proteins |
| JA | Jasmonic acid |
| MeJA | Methyl jasmonic acid |
| MeSa | Methyl salicylate |
| MAEs | Modern advanced microwave-extractions |
| MLE | moringa leaf extract |
| MAPs | Medicinal and aromatic plants |
| NLE | Neem leaf extract |
| M | Moringa |
| MDA | Malondialdehyde |
| OA | Oxalic acid |
| ODC | Ornithine decarboxylase |
| PAs | Polyamines |
| PAL | Phenylalanine ammonia lyase |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| Put | Putrescine |
| RH | Relative humidity |
| RE | Rice bran extract |
| RS | Reducing sugars |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SAR | Systemic acquired resistance |
| SSC | Soluble solid content |
| Spd | Spermidine |
| Spm | Spermine |
| SA | Salicylic acid |
| TA | Total acidity |
| TS | Total sugars |
| UAE | Ultrasonic-extraction |
| VC | Vitamin C |
References
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the balkan region (Southeast europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, J.; Hossain, M.I.; Saha, S.R.; Rob, M.; Afroz, T.; Pramanik, S.; Islam, M.R.; Nath, D.D. Postharvest physiology of fruits and vegetables and their management technology: A review. J. Anim. Plant Sci. 2024, 34, 291–303. [Google Scholar] [CrossRef]
- Zhang, W.L.; Pan, Y.G.; Jiang, Y.M.; Zhang, Z.K. Advances in control technologies and mechanisms to treat peel browning in postharvest fruit. Sci. Hortic. 2023, 311, 111798. [Google Scholar] [CrossRef]
- Li, C.H.; Cao, S.F.; Yang, Z.F.; Watkins, C.B.; Wang, K.T. The physiology, molecular biology and biochemistry in ripening and stored fruit. Front. Plant Sci. 2023, 14, 1296816. [Google Scholar] [CrossRef]
- Abdullahi, A.; Tijjani, A.; Abubakar, A.; Khairulmazmi, A.; Ismail, M. Plant biomolecule antimicrobials: An alternative control measures for food security and safety of the chapter. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–406. [Google Scholar]
- Roy, S.; Priyadarshi, R.; Lopusiewicz, L.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha Prasad, J.; Yadav, A.; Upadhyay, A.; Neeraj Shukla, S.; Petkoska, A.T.; Heena Suri, S.; Gniewosz, M.; Kieliszek, M. Recent trends in edible packaging for food applications—Perspective for the future. Food Eng. Rev. 2023, 15, 718–747. [Google Scholar] [CrossRef]
- Tian, B.R.; Liu, J.Y.; Yang, W.Z.X.; Wan, J.B. Biopolymer food packaging films incorporated with essential oils. J. Agric. Food Chem. 2023, 71, 1325–1347. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, L.; Mao, C.Y.; Jin, L.G.; Wu, S.L.; Zheng, Y.F.; Cui, Z.D.; Li, Z.Y.; Zhang, Y.; Zhu, S.L.; et al. Natural extracts for antibacterial applications. Small 2024, 20, e2306553. [Google Scholar] [CrossRef]
- Li, C.C.; Chen, L.; Mcclements, D.J.; Liu, W.M.; Long, J.; Qiu, C.; Wang, Y.; Yang, Z.Y.; Xu, Z.L.; Meng, M.; et al. Utilization of plant extracts to control the safety and quality of fried foods-a review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2310–2345. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Sabir, F.K.; Sabir, A. Aloe vera treatments extend the postharvest life of table grapes by delaying weight loss, berry softening, rachis browning, and biochemical changes. Erwerbs-Obstbau 2022, 64, 767–775. [Google Scholar] [CrossRef]
- Sowmyashree, A.; Sharma, R.R.; Rudra, S.G.; Verma, M.K.; Grover, M. Influence of plant extract and edible coatings on quality of nectarine (Prunus persica) fruits. Indian J. Agric. Sci. 2021, 91, 113–116. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; De Oliveira, J.G.; Lamonica, G.C.; Bezerra, C.; Martins, V.D.A.; Ferreira, M.D.; Plepis, A.M.D.; Junior, S.B. Improvement of the physical-chemical, microbiological, volatiles and sensory quality of strawberries covered with chitosan/gelatin/pomegranate peel extract-based coatings. Food Chem. 2025, 471, 142755. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; Abou El Ghit, H.M.; Fikry, M.; Bahloul, A.M.E.; Morsy, A.R.; Lo’ay, A.A.; Abdelaziz, A.; Alhaithloul, H.A.S.; et al. The effects of a gum arabic-based edible coating on guava fruit characteristics during storage. Coatings 2022, 12, 90. [Google Scholar] [CrossRef]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Innovative edible coatings for postharvest storage of sweet cherries. Sci. Hortic. 2023, 310, 111738. [Google Scholar] [CrossRef]
- Durovic, S.D.; Smyatskaya, Y.A.; Tosti, T. Editorial: Extracts from plants and other natural sources: Application, characterization, optimization, and their use. Front. Nutr. 2024, 11, 1506537. [Google Scholar] [CrossRef]
- Oesper, L.; Merico, D.; Isserlin, R.; Bader, G.D. Wordcloud: A cytoscape plugin to create a visual semantic summary of networks. Source Code Biol. Med. 2011, 6, 7. [Google Scholar] [CrossRef]
- Zhang, L.L.; Ling, J.; Lin, M.W. Risk management research in east asia: A bibliometric analysis. Int. J. Intell. Comput. Cybern. 2023, 16, 574–594. [Google Scholar] [CrossRef]
- Ingulsrud, J.E.; Kai, K.; Kadowaki, S.; Kurobane, S.; Shiobara, M. The assessment of cross-cultural experience: Measuring awareness through critical text analysis. Int. J. Intercult. Relat. 2002, 26, 473–491. [Google Scholar] [CrossRef]
- Pham-Duc, B.; Nguyen, H.; Le Minh, C.; Khanh, L.H.; Trung, T. A bibliometric and content analysis of articles in remote sensing from vietnam indexed in scopus for the 2000–2019 period. Ser. Rev. 2020, 46, 275–285. [Google Scholar] [CrossRef]
- Nasir, A.; Shaukat, K.; Hameed, I.A.; Luo, S.H.; Mahboob, T.; Iqbal, F. A bibliometric analysis of corona pandemic in social sciences: A review of influential aspects and conceptual structure. IEEE Access 2020, 8, 133377–133402. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Sandal, S. Neem essential oil: Extraction, characterization, and encapsulation. Food Chem. Adv. 2024, 4, 100702. [Google Scholar] [CrossRef]
- Li, J.Y.; Dadmohammadi, Y.; Abbaspourrad, A. Flavor components, precursors, formation mechanisms, production and characterization methods: Garlic, onion, and chili pepper flavors. Crit. Rev. Food Sci. Nutr. 2022, 62, 8265–8287. [Google Scholar] [CrossRef]
- Baicus, A.; Mattuzzi, F.C.; Paraschiv, A.M.; Dinu, R.S.; Dumitrescu, M.C.; Marinescu, A.A.; Ionescu, D.; Dragos, D. Antibacterial activity of clove, oregano, thyme, eucalyptus, and tea tree essential oils against Escherichia coli and Klebsiella pneumoniae strains. Rev. Romana De Med. De Lab. 2022, 30, 327–338. [Google Scholar] [CrossRef]
- Faheem, F.; Liu, Z.W.; Rabail, R.; Haq, I.U.; Gul, M.; Bryla, M.; Roszko, M.; Kieliszek, M.; Din, A.; Aadil, R.M. Uncovering the industrial potentials of lemongrass essential oil as a food preservative: A review. Antioxidants 2022, 11, 720. [Google Scholar] [CrossRef]
- Szewczyk, A.; Marino, A.; Molinari, J.; Ekiert, H.; Miceli, N. Phytochemical characterization, and antioxidant and antimicrobial properties of agitated cultures of three rue species: Ruta chalepensis, Ruta corsica and Ruta graveolens. Antioxidants 2022, 11, 592. [Google Scholar] [CrossRef]
- Abou-Zaid, E.S.A.; Hussein, A.S.; Sultan, R.; Abo-Elyousr, K.A.M.; Sallam, N.M.A.; Bagy, H. Improvement of post-harvest quality of balady lime fruit with aloe vera gel and tea tree oil against green mold disease caused by Penicillium digitatum. J. Plant Pathol. 2024, 106, 1715–1729. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liao, P. Current insights into plant volatile organic compound biosynthesis. Curr. Opin. Plant Biol. 2025, 85, 102708. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, J.; Xu, Y.; Li, Z.; Li, L.; Li, D.; Belwal, T.; Jeandet, P.; Luo, Z.; Xu, Y. Deterioration of plant volatile organic compounds in food: Consequence, mechanism, detection, and control. Trends Food Sci. Technol. 2023, 131, 61–76. [Google Scholar] [CrossRef]
- Pheko-Ofitlhile, T.; Makhzoum, A. Impact of hydrodistillation and steam distillation on the yield and chemical composition of essential oils and their comparison with modern isolation techniques. J. Essent. Oil Res. 2024, 36, 105–115. [Google Scholar] [CrossRef]
- Qian, L.; Jia, R.; Zhao, Q.; Sun, N.; Yang, J.; Wen, J.; Li, H.; Yang, J.; Mo, L.; Gao, W. Tough, antibacterial, and antioxidant chitosan-based composite films enhanced with proanthocyanidin and carvacrol essential oil for fruit preservation. Food Res. Int. 2025, 208, 116269. [Google Scholar] [CrossRef]
- Ashraf, J.; Ismail, N.; Tufail, T.; Zhang, J.; Awais, M.; Zhang, Q.; Ahmed, Z.; Qi, Y.; Liu, S.; Xu, B. Fabrication of novel pullulan/carboxymethyl chitosan-based edible film incorporated with ultrasonically equipped aqueous zein/turmeric essential oil nanoemulsion for effective preservation of mango fruits. Int. J. Biol. Macromol. 2025, 294, 139330. [Google Scholar] [CrossRef]
- Wang, L.; Fu, H.; Tan, Q.; Wu, S.; Wang, Y.; Peng, X. Preservation effects of chitosan/polyvinyl alcohol/clove essential oil antifungal film on yellow peaches: Physicochemical properties and fruit quality assessment. Food Control 2024, 164, 110582. [Google Scholar] [CrossRef]
- Felicia, W.X.L.; Kobun, R.; Aqilah, N.M.N.; Mantihal, S.; Huda, N. Chitosan/aloe vera gel coatings infused with orange peel essential oils for fruits preservation. Curr. Res. Food Sci. 2024, 8, 100680. [Google Scholar] [CrossRef]
- Das, S.; Chaudhari, A.K.; Singh, V.K.; Dwivedy, A.K.; Dubey, N.K. Angelica archangelica essential oil loaded chitosan nanoemulsion as edible coating for preservation of table grape fruit against botrytis cinerea contamination and storage quality deterioration. Postharvest Biol. Technol. 2023, 205, 112482. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ahsan, H.; Shahid, M.; Aslam, B.; Nawaz, Z.; Hussain, R.; Ahamd, M.Z.; Ataya, F.S.; Li, K. Preparation and characterization of essential oil from lavandula spica plant and its antimicrobial activity against pseudomonas aeruginosa and staphylococcus aureus. Microb. Pathog. 2025, 198, 107157. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; Aguilar-Méndez, M.A.; Ronquillo-De Jesús, E.; Jaime-Fonseca, M.R.; Von Borries-Medrano, E. Preparation and characterization of gelatin-carboxymethylcellulose active film incorporated with pomegranate (Punica granatum L.) peel extract for the preservation of raspberry fruit. Food Hydrocoll. 2024, 150, 109677. [Google Scholar] [CrossRef]
- Ma, J.; Liu, S.; Zeng, J.; Zhang, Y.; Chang, W.; Meng, Z.; Zhou, Y.; Zhang, W.; Ding, X.; Pan, X. Comparative metabolome and transcriptome analyses reveal the role of MeJA in improving postharvest disease resistance and maintaining the quality of rosa roxburghii fruit. Postharvest Biol. Technol. 2025, 220, 113314. [Google Scholar] [CrossRef]
- Luo, D.; Huang, T.; Kou, X.; Zhang, Y.; Ba, L.; Wang, X.; Cao, S. MeJA enhances antioxidant activity and reduces membrane lipid degradation by maintaining energy charge levels in crystal grapes. Postharvest Biol. Technol. 2024, 216, 113078. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Abdallah, E.M.; Alanazi, N.A.; Ed-Dra, A.; Jamal, A.; Idriss, H.; Alshammari, A.S.; Shommo, S.A. Spices as sustainable food preservatives: A comprehensive review of their antimicrobial potential. Pharmaceuticals 2023, 16, 1451. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Spices, condiments, extra virgin olive oil and aromas as not only flavorings, but precious allies for our wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef]
- Camele, I.; De Feo, V.; Altieri, L.; Mancini, E.; De Martino, L.; Rana, G.L. An attempt of postharvest orange fruit rot control using essential oils from mediterranean plants. J. Med. Food 2010, 13, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.L.; Laudy, A.E.; Majewska, E.; Tarnowska, K.; Małajowicz, J.; Ziarno, M. Antioxidant and antibacterial activity of extracts from selected plant material. Appl. Sci. 2022, 12, 9871. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martin-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Jin, L.; Abdollahi, M.; Zhao, G.; Venkatachalam, K.; Ban, Z. Controlled release and stability enhancement of cinnamon essential oil in glutathione-modified soy protein particles: Its antimicrobial application for fresh-cut cantaloupe. Food Res. Int. 2025, 211, 116523. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.; Ortega-Ramirez, L.; Cruz-Valenzuela, M.; Silva-Espinoza, B.; Gonzalez-Aguilar, G.; Gutiérrez-Pacheco, S.; Ayala-Zavala, J. Combinational approaches for antimicrobial packaging: Pectin and cinnamon leaf oil of the chapter. In Antimicrobial Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 879–886. [Google Scholar]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.; Cruz-Valenzuela, M.; Leyva, J.; Ortega-Ramírez, L.; Carrazco-Lugo, D.; Pérez-Carlón, J.; Melgarejo-Flores, B.; González-Aguilar, G.; Miranda, M. Pectin–cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Tripathi, P. Evaluation of Some Plant Products Against Fungi Causing Post Harvest Diseases of Some Fruits. Ph.D. Thesis, Department of Botany, Banaras Hindu University, Varanasi, India, 2001. [Google Scholar]
- Al-Mijalli, S.H.; Mrabti, H.N.; Abdallah, E.M.; Assaggaf, H.; Qasem, A.; Alenazy, R.; Bouyahya, A.; Alshabrmi, F.M.; El Hachlafi, N. Acorus calamus as a promising source of new antibacterial agent against pseudomonas aeruginosa and staphylococcus aureus: Deciphering volatile compounds and mode of action. Microb. Pathog. 2025, 200, 107357. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Ma, D.; Liu, Z.; Qi, P.; Wang, Z.; Di, S.; Wang, X. Review of fruits flavor deterioration in postharvest storage: Odorants, formation mechanism and quality control. Food Res. Int. 2024, 182, 114077. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Baljeet, S.Y.; Simmy, G.; Ritika, Y.; Roshanlal, Y. Antimicrobial activity of individual and combined extracts of selected spices against some pathogenic and food spoilage microorganisms. Int. Food Res. J. 2015, 22, 2594–2600. [Google Scholar]
- Vajpayee, M.; Singh, M.; Dave, H.; Ledwani, L. Application of acid protease for eco-friendly pre-treatment of goat skin to improve antimicrobial finish using herbal natural extracts. J. Am. Leather Chem. Assoc. 2023, 118, 219–234. [Google Scholar] [CrossRef]
- Li, J.H.; Azam, M.; Noreen, A.; Umer, M.A.; Ilahy, R.; Akram, M.T.; Qadri, R.; Khan, M.A.; Rehman, S.U.; Hussain, I.; et al. Application of methyl jasmonate to papaya fruit stored at lower temperature attenuates chilling injury and enhances the antioxidant system to maintain quality. Foods 2023, 12, 2743. [Google Scholar] [CrossRef]
- Wijewardane, R.N.A.; Guleria, S. Effect of pre-cooling, fruit coating and packaging on postharvest quality of apple. J. Food Sci. Technol. 2013, 50, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Q.; Yin, Z.F.; Zhang, T.H.; Yang, W.C.; Fang, T.Q.; Wang, Y.; Guo, N. Preparation and characterization of carvacrol/e-polylysine loaded antimicrobial nanobilayer emulsion and its application in mango preservation. Food Chem. 2024, 446, 138831. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Azam, M.; Shen, J.; Ghani, M.A.; Khan, A.S.; Ahmad, S.; Iqbal, M.A.; Anjum, N.; Zhang, J.; Anjum, M.A. Overall quality maintenance of grapefruit during cold storage using pre-storage neem leaf extract dipping. J. Food Meas. Charact. 2021, 15, 1727–1736. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Sangprayoon, P.; Supapvanich, S.; Youryon, P.; Wongs-Aree, C.; Boonyaritthongchai, P. Efficiency of salicylic acid or methyl jasmonate immersions on internal browning alleviation and physicochemical quality of queen pineapple cv. “Sawi” fruit during cold storage. J. Food Biochem. 2019, 43, e13059. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, S.M.; Jeong, I.; Heo, W.; Hwang, K.A.; Han, B.K.; Kim, Y.J. Anti-browning and oxidative enzyme activity of rice bran extract treatment on freshly cut ‘fuji’ apple. Agronomy 2022, 12, 86. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Meng, W.B.; Chen, Y.L.; Peng, Y. Browning inhibition of plant extracts on fresh-cut fruits and vegetables-a review. J. Food Process. Preserv. 2022, 46, 16532. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.K.; Wang, C.J.; Zhang, R.X.; Jin, L.S.; He, Z.T.; Tian, S.P.; Wu, K.H.; Wang, F.M. Prospect-pmp plus: Simultaneous retrievals of chlorophyll a and b, carotenoids and anthocyanins in the leaf optical properties model. Sensors 2022, 22, 25. [Google Scholar] [CrossRef]
- Hamann, D.; Puton, B.M.S.; Comin, T.; Colet, R.; Valduga, E.; Zeni, J.; Steffens, J.; Junges, A.; Backes, G.T.; Cansian, R.L. Active edible films based on green tea extract and gelatin for coating of fresh sausage. Meat Sci. 2022, 194, 108966. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.; FHetta, H.; Jeandet, P.; Yahia, R.; El-Saber Batiha, G.; Ali, E. Effects of olive leaf extracts as natural preservative on retailed poultry meat quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- El Dessouky Abdel-Aziz, M.; Samir Darwish, M.; Mohamed, A.H.; El-Khateeb, A.Y.; Hamed, S.E. Potential activity of aqueous fig leaves extract, olive leaves extract and their mixture as natural preservatives to extend the shelf life of pasteurized buffalo milk. Foods 2020, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Naher, J.; Nilsuwan, K.; Palamae, S.; Hong, H.; Zhang, B.; Osako, K.; Benjakul, S. Ethanolic extracts from mint (Mentha arvensis) and basil (Ocimum basilicum) leaves: Antioxidant, antimicrobial capacities and shelf-life extension of refrigerated squid mantle cut. Int. Aquat. Res. 2023, 15, 313–332. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, J.; Song, Z.; Wang, W.; Cao, Y.; Yu, Q. Preparation and characterization of chitosan/polyvinyl alcohol/ginkgo biloba leaf extract composite film and its effect on chilled beef preservation. Int. J. Biol. Macromol. 2025, 305, 141124. [Google Scholar] [CrossRef]
- Lestari, E.N.E.; Nisa, K.; Suryani, A.E.; Kusumaningsih, T. Encapsulation of Peperomia pellucida (l.) kunth leaf extract for postharvest preservation of malang apple (Malus sylvestris) at ambient storage. Food Biosci. 2024, 61, 104808. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; De Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Donat, A.; Sucu, S. The effect of pre-harvest and post-harvest aloe vera gel treatments on fruit quality and storage performance of table grapes. Sci. Hortic. 2024, 331, 113117. [Google Scholar] [CrossRef]
- Siddiqua, M.; Khan Sa, K.U.; Tabassum, P.; Sultana, S. Effects of neem leaf extract and hot water treatments on shelf life and quality of banana: Effect of plant extract and hot water on banana. J. Bangladesh Agric. Univ. 2018, 16, 351–356. [Google Scholar] [CrossRef]
- Joshi, R.L.; Sharma, H.; Mehta, V.N.; Patel, S.K.; Bambharoliya, K. Azadirachta indica derived copper oxide nanoparticles: A sustainable approach for reducing post-harvest losses and enhancing mango quality. Food Chem. 2025, 480, 143625. [Google Scholar] [CrossRef]
- Turan, E.; Şimşek, A. Effects of lyophilized black mulberry water extract on lipid oxidation, metmyoglobin formation, color stability, microbial quality and sensory properties of beef patties stored under aerobic and vacuum packaging conditions. Meat Sci. 2021, 178, 108522. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) peel as a sustainable source of anthocyanins and ellagitannins delivered by phospholipid vesicles for alleviating oxidative stress in human keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef]
- Al-Juhaimi, F.; Babtain, I.A.; Ahmed Ia, M.; Alsawmahi, O.N.; Ghafoor, K.; Adiamo, O.Q.; Babiker, E.E. Assessment of oxidative stability and physicochemical, microbiological, and sensory properties of beef patties formulated with baobab seed (Adansonia digitata) extract. Meat Sci. 2020, 162, 108044. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.; Monir, M.; Ahmed, K. Comprehensive effects of black cumin (Nigella sativa) and synthetic antioxidant on sensory and physicochemical quality of beef patties during refrigerant storage. J. Agric. Food Res. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Jeżo, A. Valorization of tree bark-derived suberin in applications for the bio-based composites industry--a recent review. J. Renew. Mater. 2024, 12, 16585–16593. [Google Scholar] [CrossRef]
- Yitbarek, R.M.; Admassu, H.; Idris, F.M.; Fentie, E.G. Optimizing the extraction of essential oil from cinnamon leaf (Cinnamomum verum) for use as a potential preservative for minced beef. Appl. Biol. Chem. 2023, 66, 47. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Yang, Y.; Zhang, Y.; Zuo, P.; Guo, Y.; Shen, Z. Preservative effects of osmanthus fragrans flower flavonoids on fresh-cut yuluxiang pear. Heliyon 2024, 10, e29748. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Elhadef, K.; Akermi, S.; Ben Akacha, B.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; Rebezov, M. Novel active food packaging films based on gelatin-sodium alginate containing beetroot peel extract. Antioxidants 2022, 11, 2095. [Google Scholar] [CrossRef]
- Giampieri, F.; Battino, M. Bioactive phytochemicals and functional food ingredients in fruits and vegetables. Int. J. Mol. Sci. 2020, 21, 3278. [Google Scholar] [CrossRef]
- Derbassi, N.; Pedrosa, M.C.; Heleno, S.; Fernandes, F.; Dias, M.I.; Calhelha, R.C.; Rodrigues, P.; Carocho, M.; Ferreira, I.; Barros, L. Arbutus unedo leaf extracts as potential dairy preservatives: Case study on quark cheese. Food Funct. 2022, 13, 5442–5454. [Google Scholar] [CrossRef]
- Parafati, L.; Siracusa, L.; Pesce, F.; Restuccia, C.; Fallico, B.; Palmeri, R. Mango (Mangifera indica L.) young leaf extract as brine additive to improve the functional properties of mozzarella cheese. Food Chem. 2023, 425, 136474. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Uribe-Velázquez, T.; Rosales-De La Cruz, M.F.; Raygoza-Murguía, L.V.; Garcia-Amezquita, L.; García-Cayuela, T. Utilization of blueberry-based ingredients for formulating a synbiotic petit suisse cheese: Physicochemical, microbiological, sensory, and functional characterization during cold storage. Lwt-Food Sci. Technol. 2023, 183, 114955. [Google Scholar] [CrossRef]
- Sekhavatizadeh, S.S.; Abadariyan, N.; Ebrahimi, L.; Hasanzadeh, M. Effects of free and encapsulated siah-e-samarghandi grape seed extract on the physicochemical, textural, microbial, and sensorial properties of uf-feta cheese. Food Sci. Nutr. 2023, 11, 3923–3938. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martinez-Romero, D.; Guillen, F.; Valero, D.; Serrano, M. The effects of salicylic acid and its derivatives on increasing pomegranate fruit quality and bioactive compounds at harvest and during storage. Front. Plant Sci. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘mollar de elche’ at harvest and during postharvest storage. J. Sci. Food Agric. 2020, 100, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Babaoğlu, A.S.; Unal, K.; Dilek, N.M.; Poçan, H.B.; Karakaya, M. Antioxidant and antimicrobial effects of blackberry, black chokeberry, blueberry, and red currant pomace extracts on beef patties subject to refrigerated storage. Meat Sci. 2022, 187, 108765. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Matos, L.C.; Santos, L. Harnessing the potential of chestnut shell extract to enhance fresh cheese: A sustainable approach for nutritional enrichment and shelf-life extension. J. Food Meas. Charact. 2024, 18, 1559–1573. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Nesic, A.; Bravo-Arrepol, G.; Rodríguez-Llamazares, S.; Valdés, O.; Banerjee, A.; Castaño, J.; Delattre, C. Bioactive pectin-murta (Ugni molinae t.) seed extract films reinforced with chitin fibers. Molecules 2021, 26, 7477. [Google Scholar] [CrossRef]
- Atwaa, E.H.; Shahein, M.R.; Radwan, H.A.; Mohammed, N.S.; Aloraini, M.A.; Albezrah, N.K.A.; Alharbi, M.A.; Sayed, H.H.; Daoud, M.A.; Elmahallawy, E.K. Antimicrobial activity of some plant extracts and their applications in homemade tomato paste and pasteurized cow milk as natural preservatives. Fermentation 2022, 8, 428. [Google Scholar] [CrossRef]
- Gumus, D.; Kizil, M. Comparison of the reducing effects of blueberry and propolis extracts on heterocyclic aromatic amines formation in pan fried beef. Meat Sci. 2022, 186, 108746. [Google Scholar] [CrossRef]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of identification and quantification of polyphenolic compounds in skins and seeds of four grape varieties. Molecules 2023, 28, 61. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.; Bendary, E.S.A.; Danial, A.W. Physical characteristics, mineral content, and antioxidant and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.; Godjevargova, T. Antimicrobial polymer films with grape seed and skin extracts for food packaging. Microorganisms 2024, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Zhao, C.H.; Fu, X. Physicochemical characterization, antioxidative and immunoregulatory activity of polysaccharides from the flower of Hylocereus undatus (haw.) britton et rose. Int. J. Biol. Macromol. 2023, 251, 126408. [Google Scholar] [CrossRef] [PubMed]
- Ergin, K.N.; Karakaya, S.; Göger, G.; Sytar, O.; Demirci, B.; Duman, H. Anatomical and phytochemical characteristics of different parts of Hypericum scabrum L. Extracts, essential oils, and their antimicrobial potential. Molecules 2022, 27, 1228. [Google Scholar] [CrossRef]
- Belfekih, F.; Moussaif, A.; El-Mzibri, M.; Moutaouakkil, A.; Benbacer, L.; Bengueddour, R.; Iddar, A. Potential effect of fruit and flower extracts of Arbutus unedo L. On tetrahymena pyriformis exposed to a cobalt-60 source. J. Exp. Biol. Agric. Sci. 2024, 12, 237–247. [Google Scholar] [CrossRef]
- Nkogo, L.F.E.; Mouendou, M.S.M.; Dumarçay, S.; Engonga, P.E.; Gérardin, P. Phytochemical study, ftir and gc-ms characterization and evaluation of the antioxidant activity of Letestua durissima extracts. Forests 2024, 15, 429. [Google Scholar] [CrossRef]
- Chen, C.; Cai, J.; Ren, Y.H.; Xu, Y.; Liu, H.L.; Zhao, Y.Y.; Chen, X.F.; Liu, Z.B. Antimicrobial activity, chemical composition and mechanism of action of Chinese chive (Allium tuberosum rottler) extracts. Front. Microbiol. 2022, 13, 1028627. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Srinivasa, C.; Mellappa, G.; Patil, S.M.; Ramu, R.; Shreevatsa, B.; Dharmashekar, C.; Kollur, S.P.; Syed, A.; Shivamallu, C. Plants and endophytes–a partnership for the coumarin production through the microbial systems. Mycology 2022, 13, 243–256. [Google Scholar] [CrossRef]
- Ali, S.; Mir, S.A.; Dar, B.; Ejaz, S. Sustainable Postharvest Technologies for Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Farooq, S.; Mir, S.A.; Shah, M.A.; Manickavasagan, A. Extraction techniques of the chapter. In Plant Extracts: Applications in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–37. [Google Scholar]
- El Maaiden, E.; Bouzroud, S.; Nasser, B.; Moustaid, K.; El Mouttaqi, A.; Ibourki, M.; Boukcim, H.; Hirich, A.; Kouisni, L.; El Kharrassi, Y. A comparative study between conventional and advanced extraction techniques: Pharmaceutical and cosmetic properties of plant extracts. Molecules 2022, 27, 2074. [Google Scholar] [CrossRef]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A glimpse into the extraction methods of active compounds from plants. Crit. Rev. Anal. Chem. 2022, 52, 667–696. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction methods for the isolation of isoflavonoids from plant material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Green, R.J. Antioxidant Activity of Peanut Plant Tissues. Master’s degree Thesis, North Carolina State University, Raleigh, NC, USA, 2004. [Google Scholar]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of aloe vera gel coating enriched with fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Liu, M.M.; Wang, Z.E.; Li, S.E.; Jiang, T.J.; Zheng, X.L. Pre-harvest spraying of oxalic acid improves postharvest quality associated with increase in ascorbic acid and regulation of ethanol fermentation in kiwifruit cv. Bruno during storage. J. Integr. Agric. 2019, 18, 2514–2520. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan-aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Mendy, T.K.; Misran, A.; Mahmud, T.M.M.; Ismail, S.I. Application of Aloe vera coating delays ripening and extend the shelf life of papaya fruit. Sci. Hortic. 2019, 246, 769–776. [Google Scholar] [CrossRef]
- Sempere-Ferre, F.; Giménez-Santamarina, S.; Roselló, J.; Santamarina, M.P. Antifungal in vitro potential of aloe vera gel as postharvest treatment to maintain blueberry quality during storage. LWT 2022, 163, 113512. [Google Scholar] [CrossRef]
- Delta, B.A. Antifungal effect of aloe vera extract on otomycosis: A review. Eureka Herba Indones. 2022, 3, 159–163. [Google Scholar] [CrossRef]
- Mazón-Suástegui, J.M.; Salas-Leiva, J.; Teles, A.; Tovar-Ramírez, D. Immune and antioxidant enzyme response of longfin yellowtail (Seriola rivoliana) juveniles to ultra-diluted substances derived from phosphorus, silica and pathogenic vibrio. Homeopathy 2019, 108, 43–53. [Google Scholar] [CrossRef]
- Jagana, D.; Hegde, Y.R.; Lella, R. Bioefficacy of essential oils and plant oils for the management of banana anthracnose-a major post-harvest disease. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2359–2365. [Google Scholar] [CrossRef]
- Pérez-Cordero, A.; Chamorro-Anaya, L.; Vitola-Romero, D.; Hernández-Gómez, J. Antifungal activity of Cymbopogon citratus against Colletotrichum gloesporioides. Agron. Mesoam. 2017, 28, 465–475. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, E.; Contreras-Oliva, A.; Salinas-Ruiz, J.; Hernández-Ramírez, G.; Spinoso-Castillo, J.L.; Cuevas, S.I.C. Plant extracts control in vitro growth of disease-causing fungi in chayote. Plants 2023, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Protection, Citrus and lemongrass essential oils inhibit botrytis cinerea on ‘golden delicious’,‘pink lady’and ‘granny smith’apples. J. Plant Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mditshwa, A.; Mbili, N. Carboxyl methylcellulose (cmc) incorporated with moringa leaf and seed extracts as new postharvest organic edible coating for avocado (Persea americana mill.) fruit. In Proceedings of the 7th International Conference on Managing Quality in Chains (MQUIC)/2nd International Symposium on Ornamentals/13th International Protea Research Symposium, Stellenbosch, South Africa, 4–7 September 2017; pp. 161–168. [Google Scholar]
- Bordoh, P.K.; Ali, A.; Dickinson, M.; Siddiqui, Y. Antimicrobial effect of rhizome and medicinal herb extract in controlling postharvest anthracnose of dragon fruit and their possible phytotoxicity. Sci. Hortic. 2020, 265, 109249. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘gola’ guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Zaidi, M.; Akbar, A.; Ali, S.; Akram, H.; Ercisli, S.; Ilhan, G.; Sakar, E.; Marc, R.A.; Sonmez, D.A.; Ullah, R.; et al. Application of plant-based edible coatings and extracts influences the postharvest quality and shelf life potential of “surahi” guava fruits. Acs Omega 2023, 8, 19523–19531. [Google Scholar] [CrossRef]
- Kilic, M.; Gollan, P.J.; Aro, E.-M.; Rintamaki, E. Jasmonic acid signaling and glutathione coordinate plant recovery from high light stress. Plant Physiol. 2025, 197, 143. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Maintaining postharvest quality of fresh produce with volatile compounds. Innov. Food Sci. Emerg. Technol. 2007, 8, 111–116. [Google Scholar] [CrossRef]
- Jia, H.F.; Zhang, C.; Pervaiz, T.; Zhao, P.C.; Liu, Z.J.; Wang, B.J.; Wang, C.; Zhang, L.; Fang, J.G.; Qian, J.P. Jasmonic acid involves in grape fruit ripening and resistant against Botrytis cinerea. Funct. Integr. Genom. 2016, 16, 79–94. [Google Scholar] [CrossRef]
- Glowacz, M.; Roets, N.; Sivakumar, D. Control of anthracnose disease via increased activity of defence related enzymes in ‘hass’ avocado fruit treated with methyl jasmonate and methyl salicylate. Food Chem. 2017, 234, 163–167. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Guillen, F.; Diaz-Mula, H.M.; Martinez-Romero, D.; Serrano, M.; Valero, D. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 2. Improvement of fruit quality and antioxidant systems during postharvest storage. Postharvest Biol. Technol. 2014, 98, 115–122. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Sweet cherry (Prunus avium L.) cracking during development on the tree and at harvest: The impact of methyl jasmonate on four different growing seasons. Agriculture 2023, 13, 1244. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Liao, L.; Li, S.C.; Li, Y.J.; Huang, Z.H.; Li, J.H.; Xiong, B.; Zhang, M.F.; Sun, G.C.; Wang, Z.H. Pre- or post-harvest treatment with MeJA improves post-harvest storage of lemon fruit by stimulating the antioxidant system and alleviating chilling injury. Plants 2022, 11, 2840. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trends Food Sci. Technol. 2021, 113, 355–365. [Google Scholar] [CrossRef]
- Min, D.D.; Li, F.J.; Ali, M.; Zhang, X.H.; Liu, Y.G. Application of methyl jasmonate to control chilling tolerance of postharvest fruit and vegetables: A meta-analysis and eliciting metabolism review. Crit. Rev. Food Sci. Nutr. 2024, 64, 12878–12891. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Esna-Ashari, M. Effects of postharvest methyl jasmonate treatment on persimmon quality during cold storage. Sci. Hortic. 2022, 294, 110756. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.; Li, H.; Jia, X.; Guo, Y.; Luo, J.; Li, X. Methyl jasmonate alleviates chilling injury and keeps intact pericarp structure of pomegranate during low temperature storage. Food Sci. Technol. Int. 2021, 27, 22–31. [Google Scholar] [CrossRef]
- Barman, K.; Sharma, S.; Siddiqui, M.W. Effects of methyl jasmonate treatment on fruit quality properties. In Emerging Postharvest Treatment of Fruits and Vegetables; Apple Academic Press: New York, NK, USA, 2018; pp. 111–132. [Google Scholar]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martinez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest salicylate treatments enhance antioxidant compounds, color and crop yield in low pigmented-table grape cultivars and preserve quality traits during storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Zapata, P.J.; Guillén, F.; Valverde, J.M.; Serrano, M.; Valero, D. Preharvest application of methyl salicylate, acetyl salicylic acid and salicylic acid alleviated disease caused by Botrytis cinerea through stimulation of antioxidant system in table grapes. Int. J. Food Microbiol. 2020, 334, 108807. [Google Scholar] [CrossRef]
- Shanbehpour, F.; Rastegar, S.; Ghasemi, M. Effect of preharvest application of calcium chloride, putrescine, and salicylic acid on antioxidant system and biochemical changes of two indian jujube genotypes. J. Food Biochem. 2020, 44, 13474. [Google Scholar] [CrossRef]
- Gomes, E.P.; Borges, C.V.; Monteiro, G.C.; Belin Ma, F.; Minatel, I.O.; Junior, A.P.; Tecchio, M.A.; Lima, G.P.P. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘niagara rosada’ table grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Georgiadou, E.C.; Drogoudi, P.; Fotopoulos, V.; Manganaris, G.A. The efficacy of acetylsalicylic acid, spermidine and calcium preharvest foliar spray applications on yield efficiency, incidence of physiological disorders and shelf-life performance of loquat fruit. Sci. Hortic. 2021, 289, 110439. [Google Scholar] [CrossRef]
- Fan, X.; Du, Z.; Cui, X.; Ji, W.; Ma, J.; Li, X.; Wang, X.; Zhao, H.; Liu, B.; Guo, F.; et al. Preharvest methyl salicylate treatment enhance the chilling tolerance and improve the postharvest quality of apricot during low temperature storage. Postharvest Biol. Technol. 2021, 177, 111535. [Google Scholar] [CrossRef]
- Youryon, P.; Chimphakdee, N.; Supapvanich, S. Effects of preharvest salicylic acid or oxalic acid on quality of’queen’pineapple fruit harvested at different month stored at room temperature. Acta Hortic. 2017, 1256, 489–494. [Google Scholar] [CrossRef]
- Anwar, R.; Gull, S.; Nafees, M.; Amin, M.; Hussain, Z.; Khan, A.; Malik, A. Pre-harvest foliar application of oxalic acid improves strawberry plant growth and fruit quality. J. Hortic. Sci. Technol. 2018, 1, 35–41. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Martinez-Romero, D.; Valero, D.; Zapata, P.J. Oxalic acid preharvest treatment increases antioxidant systems and improves plum quality at harvest and during postharvest storage. J. Sci. Food Agric. 2019, 99, 235–243. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Valverde, J.M.; Guillen, F.; Castillo, S.; Martinez-Romero, D.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest application of oxalic acid improved pomegranate fruit yield, quality, and bioactive compounds at harvest in a concentration-dependent manner. Agronomy 2020, 10, 1522. [Google Scholar] [CrossRef]
- Üzümcü, S.S.; Koyuncu, M.A.; Onursal, C.E.; Güneyli, A.; Erbaş, D. Effect of pre-harvest oxalic acid treatment on shelf-life of apricot cv.‘Roxana’. Nevşehir Bilim Ve Teknol. Derg. 2020, 9, 73–80. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, S.; Razzaq, K.; Rajwana, I.; Akhtar, G.; Naz, A.; Amin, M.; Khalid, M.; Khalid, S. Pre-harvest oxalic acid application improves fruit size at harvest, physico chemical and sensory attributes of ‘red flesh’ apricot during fruit ripening. J. Hortic. Sci. Technol. 2021, 4, 48–55. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest treatment with oxalic acid improves postharvest storage of lemon fruit by stimulation of the antioxidant system and phenolic content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Serna-Escolano, V.; Guillén, F.; Valero, D.; Serrano, M.; García-Martínez, S.; Terry, L.A.; Alamar, M.C.; Zapata, P.J. Oxalic acid preharvest treatment improves colour and quality of seedless table grape ‘magenta’ upregulating on-vine abscisic acid metabolism, relative vvnced1 gene expression, and the antioxidant system in berries. Front. Plant Sci. 2021, 12, 740240. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Adaos, G.; Cedeño-García, G.; Ospino-Olivella, S.; Vergara-Retamales, R.; Lopéz, M.; Olivares, R.; Hirzel, J.; Olivares-Soto, H.; Betancur, M. Preharvest applications of oxalic acid and salicylic acid increase fruit firmness and polyphenolic content in blueberry (Vaccinium corymbosum L.). Horticulturae 2023, 9, 639. [Google Scholar] [CrossRef]
- Erbaș, D.; Koyuncu, M.; Onursal, C. Effects of pre-and postharvest spermidine treatments on storage life and quality of ‘aprikoz’ apricot. Acta Hortic. 2018, 785–792. [Google Scholar] [CrossRef]
- Mirdehghan, S.; Khanamani, Z.; Shamshiri, M.; Hokmabadi, H. Preharvest foliar application of putrescine and spermine on postharvest quality of fresh pistachio. In Proceedings of the VII International Postharvest Symposium, Kuala Lumpur, Malaysia, 25–29 June 2012; pp. 299–303. [Google Scholar]
- Mirdehghan, S.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Sayyad-Amin, P.; Davarynejadl, G.; Abedi, B. Effects of preharvest application of chemical compounds on pear (Pyrus communis cv. ‘Shekari’) fruit traits. Erwerbs-Obstbau 2022, 64, 657–662. [Google Scholar] [CrossRef]
- Jalili, I.; Ebadi, A.; Askari, M.A.; Kalatehjari, S.; Aazami, M.A. Foliar application of putrescine, salicylic acid, and ascorbic acid mitigates frost stress damage in Vitis vinifera cv. ‘Giziluzum’. BMC Plant Biol. 2023, 23, 1–15. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.H.; Ortega, M.A.; Tsai, C.J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349. [Google Scholar] [CrossRef]
- Li, A.X.; Sun, X.; Liu, L.J. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Serrano, M.; Moral, J.; Castillo, S. Methyl salicylate treatments of sweet cherry trees improve fruit quality at harvest and during storage. Sci. Hortic. 2015, 197, 665–673. [Google Scholar] [CrossRef]
- Valverde, J.M.; Giménez, M.J.; Guillén, F.; Valero, D.; Martínez-Romero, D.; Serrano, M. Methyl salicylate treatments of sweet cherry trees increase antioxidant systems in fruit at harvest and during storage. Postharvest Biol. Technol. 2015, 109, 106–113. [Google Scholar] [CrossRef]
- Yuan, R.M.; Mao, L.L.; Min, T.; Zhao, Y.Y.; Duan, Y.Q.; Wang, H.X.; Lin, Q. Salicylic acid treatment inhibits ethylene synthesis and starch-sugar conversion to maintain apple fruit quality during shelf life. Sci. Hortic. 2023, 308, 111586. [Google Scholar] [CrossRef]
- Liang, C.C.; Cui, X.Z.; Sun, C.C.; Ye, S.X.; Huang, N.X.; Chen, R.; Zhang, A.D.; Yang, Y.Q.; Gong, H.S.; Sun, S.Y.; et al. Synergistic and antagonistic effects of preharvest salicylic acid and postharvest 1-methylcyclopropene treatments on the storage quality of apricot. Food Chem. 2023, 405, 134764. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Li, S.L.; Deng, M.Y.; Gui, R.; Liu, Y.Q.; Chen, X.P.; Lin, Y.X.; Li, M.Y.; Wang, Y.; He, W.; et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem.-X 2022, 15, 100384. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Zamani, Z.; Moghadam, M.R.F.; Saba, M.K. Combination effects of preharvest tree net-shading and postharvest fruit treatments with salicylic acid or hot water on attributes of pomegranate fruit. Sci. Hortic. 2022, 304, 111257. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Song, C.C.; Brummell, D.A.; Qi, S.N.; Lin, Q.; Bi, J.F.; Duan, Y.Q. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; Pinchu, C.; Li, X.; Yang, J.; Fan, W. Oxalate in plants: Metabolism, function, regulation, and application. J. Agric. Food Chem. 2022, 70, 16037–16049. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Ayere, J.E.; Amune, U.O.; Otuya, I.C.; Abuga, E.C.; Anyalewechi, C.L.; Okoro, O.V.; Okolie, J.A.; Oyefolu, P.K.; Eshiemogie, S.O. A comprehensive review of recent advances in the applications and biosynthesis of oxalic acid from bio-derived substrates. Environ. Res. 2024, 251, 118703. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A.; Afrifa-Yamoah, E.; Malik, A.U. Oxalic acid: A blooming organic acid for postharvest quality preservation of fresh fruit and vegetables. Postharvest Biol. Technol. 2023, 206, 112574. [Google Scholar] [CrossRef]
- Cai, X.F.; Ge, C.H.; Xu, C.X.; Wang, X.L.; Wang, S.; Wang, Q.H. Expression analysis of oxalate metabolic pathway genes reveals oxalate regulation patterns in spinach. Molecules 2018, 23, 1286. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, N.; Liu, R.C.; Wang, R.; Chen, C.K.; Ma, C.; Nie, H.L.; Lei, J.Q.; Tao, Q.Y. Effects of pre-harvest spraying with Salicylic acid (sa) and Sodium nitroprusside (snp) on storage quality and pathogenic fungal species in ‘manaohong’ cherries. Agronomy 2023, 13, 2853. [Google Scholar] [CrossRef]
- Salyari, R.; Seifi, E.; Varasteh, F.; Alizadeh, M. Effects of pre-harvest salicylic acid treatment on the post-harvest quality of peach cultivar robin. J. Chem. Health Risks 2022, 12, 1041. [Google Scholar] [CrossRef]
- Erogul, D.; Kibar, H.; Sen, F.; Gundogdu, M. Role of postharvest oxalic acid treatment on quality properties, phenolic compounds, and organic acid contents of nectarine fruits during cold storage. Horticulturae 2023, 9, 1021. [Google Scholar] [CrossRef]
- Li, P.Y.; Zheng, X.L.; Chowdhury, M.G.F.; Cordasco, K.; Brecht, J.K.; Fla State Hort, S. Prestorage application of oxalic acid to alleviate chilling injury in mango fruit, In Proceedings of the 128th Annual Meeting of the Florida-State-Horticultural-Society (FSHS), St Augustine, FL, USA, 31 May–2 June 2015. pp. 190–195.
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.M.; Zheng, Y.H. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.K.; Jiang, W.B. Changes in sugar metabolism caused by exogenous oxalic acid related to chilling tolerance of apricot fruit. Postharvest Biol. Technol. 2016, 114, 10–16. [Google Scholar] [CrossRef]
- Chen, J.X.; Tang, L.; Guo, W.L.; Wang, D.; Sun, Y.G.; Guo, C.H. Oxalic acid secretion alleviates saline-alkali stress in alfalfa by improving photosynthetic characteristics and antioxidant activity. Plant Physiol. Biochem. 2024, 208, 108475. [Google Scholar] [CrossRef]
- Shi, J.Y.; Gong, Z.Q.; Cao, Z.M.; Hou, F.R.; Cui, W.J.; Jia, F.J.; Jiao, J.; Zhou, Q.X.; Wang, W.L.; Wang, Y.S. Mechanism of oxalic acid delaying browning of fresh-cut apples mediated by synergistic regulation of phenol metabolism and oxidative stress. Postharvest Biol. Technol. 2025, 227, 113609. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Yu, J.; Brecht, J.K.; Jiang, T.J.; Zheng, X.L. Pre-harvest application of oxalic acid increases quality and resistance to penicillium expansum in kiwifruit during postharvest storage. Food Chem. 2016, 190, 537–543. [Google Scholar] [CrossRef]
- Lehner, A.; Meimoun, P.; Errakhi, R.; Madiona, K.; Barakate, M.; Bouteau, F. Toxic and signalling effects of oxalic acid: Oxalic acid-natural born killer or natural born protector? Plant Signal. Behav. 2008, 3, 746–748. [Google Scholar] [CrossRef]
- Batool, M.; Bashir, O.; Amin, T.; Wani, S.M.; Masoodi, F.A.; Jan, N.U.; Bhat, S.A.; Gul, A. Effect of oxalic acid and salicylic acid treatments on the post-harvest life of temperate grown apricot varieties (Prunus armeniaca) during controlled atmosphere storage. Food Sci. Technol. Int. 2022, 28, 557–569. [Google Scholar] [CrossRef]
- Qiao, J.T.; Cai, W.W.; Wang, K.; Haubruge, E.; Dong, J.; El-Seedi, H.R.; Xu, X.; Zhang, H.C. New insights into identification, distribution, and health benefits of polyamines and their derivatives. J. Agric. Food Chem. 2024, 72, 5089–5106. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent modulators of plant responses to stress. J. Plant Interact. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Cruz-Pulido, Y.E.; Lomascolo, N.J.; May, D.; Hatahet, J.; Thomas, C.E.; Chu, A.K.W.; Stacey, S.P.; Guzman, M.D.V.; Aubert, G.; Mounce, B.C. Polyamines mediate cellular energetics and lipid metabolism through mitochondrial respiration to facilitate virus replication. PLoS Path. 2024, 20, e1012711. [Google Scholar] [CrossRef]
- Liu, T.A.; Stewart, T.M.; Casero, R.A., Jr. The synergistic benefit of combination strategies targeting tumor cell polyamine homeostasis. Int. J. Mol. Sci. 2024, 25, 8173. [Google Scholar] [CrossRef]
- Gao, F.; Mei, X.; Li, Y.; Guo, J.; Shen, Y. Update on the roles of polyamines in fleshy fruit ripening, senescence, and quality. Front. Plant Sci. 2021, 12, 610313. [Google Scholar] [CrossRef]
- Kakkar, R.K.; Sawhney, V.K. Polyamine research in plants–a changing perspective. Physiol. Plant. 2002, 116, 281–292. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-based active coatings incorporated with bioactive compounds for reducing postharvest losses of fresh fruits. Coatings 2021, 12, 8. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-based coating enriched with hairy fig (Ficus hirta vahl.) fruit extract for “newhall” navel orange preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible coatings incorporating pomegranate peel extract and biocontrol yeast to reduce penicillium digitatum postharvest decay of oranges. Food Microbiol. 2018, 74, 107–112. [Google Scholar] [CrossRef]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Biotechnology, Induction of defense responses against alternaria rot by different elicitors in harvested pear fruit. Appl. Microbiol. 2006, 70, 729–734. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.Y.; Zhang, X.Y.; Zhao, L.A.; Wang, K.L.; Dhanasekaran, S.; Mehari, T.G.; Zhang, H.Y. Biotechnological and biocontrol approaches for mitigating postharvest diseases caused by fungal pathogens and their mycotoxins in fruits: A review. J. Agric. Food Chem. 2023, 71, 17584–17596. [Google Scholar] [CrossRef]
- Chen, J.Y.; Shen, Y.T.; Chen, C.Y.; Wan, C.P. Inhibition of key citrus postharvest fungal strains by plant extracts in vitro and in vivo: A review. Plants 2019, 8, 26. [Google Scholar] [CrossRef]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as alternative fungicides for controlling plant diseases: A comprehensive review of their efficacy, commercial representatives, advantages, challenges for adoption, and possible solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Aremu, A.O.; Fawole, O.A. Potentials of medicinal plant extracts as an alternative to synthetic chemicals in postharvest protection and preservation of horticultural crops: A review. Sustainability 2021, 13, 5897. [Google Scholar] [CrossRef]
- Petcu, C.D.; Tapaloaga, D.; Mihai, O.D.; Gheorghe-Irimia, R.A.; Negoita, C.; Georgescu, I.M.; Tapaloaga, P.R.; Borda, C.; Ghimpeteanu, O.M. Harnessing natural antioxidants for enhancing food shelf life: Exploring sources and applications in the food industry. Foods 2023, 12, 3176. [Google Scholar] [CrossRef]
- Wang, X.K. Managing land carrying capacity: Key to achieving sustainable production systems for food security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Hanif, K.; Zubair, M.; Hussain, D.; Ali, S.; Saleem, M.; Khan, H.A.; Nazir, T.; Ul Hassan, M.W. Biopesticides and insect pest management. Int. J. Trop. Insect Sci. 2022, 42, 3631–3637. [Google Scholar] [CrossRef]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising natural products in crop protection and food preservation: Basis, advances, and future prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Sharif, Z.M.; Mustapha, F.; Jai, J.; Yusof, N.M.; Zaki, N.M. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bull. 2017, 19, 145–153. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Valadez-Blanco, R.; Hernández-Carlos, B.; Guadarrama-Mendoza, P.C. Natural antioxidant extracts as food preservatives. Acta Sci. Pol. Technol. Aliment. 2017, 16, 361–370. [Google Scholar] [CrossRef]
- Hosseini, A.; Saba, M.K.; Watkins, C.B. Microbial antagonists to biologically control postharvest decay and preserve fruit quality. Crit. Rev. Food Sci. Nutr. 2024, 64, 7330–7342. [Google Scholar] [CrossRef]
- Yan, F.J.; Tangpong, J.; Chen, W. Antioxidant properties of phytochemicals. Front. Nutr. 2024, 11, 1354987. [Google Scholar] [CrossRef]
- Shan, Z.J.; Ye, J.F.; Hao, D.C.; Xiao, P.G.; Chen, Z.D.; Lu, A.M. Distribution patterns and industry planning of commonly used traditional Chinese medicinal plants in China. Plant Divers. 2022, 44, 255–261. [Google Scholar] [CrossRef]
- Xue, D.; Wu, J.; Liu, X.; Lu, B.; Pei, S. Biodiversity inventory and researches of the chapter. In Contemporary Ecology Research in China; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–28. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 1778. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Heng, J.; Bechard, S.; Lach, D.; Rothstein, J.; Wang, M.H.; Ubal, S.; Mcclements, D.J.; Corvalan, C.M.; Lu, J.K. Evaluating essential oils as biocidal anti-drift adjuvants for safe and sustainable agricultural spray enhancement. J. Aerosol Sci. 2024, 181, 106421. [Google Scholar] [CrossRef]
- Li, J.; Hanif, M.; Azam, M.; Khan, M.A.; Umer, M.A.; Chaudhary, B.; Hussain, I.; Khan, T.A.; Rehman, S.U.; Awais, M.; et al. Effects of neem leaf extract on physiochemical traits and antioxidant activity of peach fruit during storage. J. Stored Prod. Res. 2025, 111, 102555. [Google Scholar] [CrossRef]
- Prasad, K.; Asrey, R.; Sethi, S.; Srivastav, M.; Singh, D.; Arora, A.; Joshi, A.; Reddy, V.R.; Meena, N.K.; Thakur, A.; et al. Preservation potential of essential oils on the postharvest quality and shelf-life attributes of mango fruit. S. Afr. J. Bot. 2024, 172, 8–18. [Google Scholar] [CrossRef]
- Tian, J.; Xie, S.; Zhang, P.; Wang, Q.; Li, J.; Xu, X. Attenuation of postharvest peel browning and chilling injury of banana fruit by astragalus polysaccharides. Postharvest Biol. Technol. 2022, 184, 111783. [Google Scholar] [CrossRef]
- Passafiume, R.; Gugliuzza, G.; Gaglio, R.; Busetta, G.; Tinebra, I.; Sortino, G.; Farina, V.J.H. Aloe-based edible coating to maintain quality of fresh-cut italian pears (Pyrus communis L.) during cold storage. Horticulturae 2021, 7, 581. [Google Scholar] [CrossRef]
- Kubheka, S.F.; Tesfay, S.Z.; Mditshwa, A.; Magwaza, L.S. Evaluating the efficacy of edible coatings incorporated with moringa leaf extract on postharvest of ‘maluma’avocado fruit quality and its biofungicidal effect. HortScience 2020, 55, 410–415. [Google Scholar] [CrossRef]
- Philemon, Y.K.; Matasyoh, J.C.; Wagara, I.N. Chemical composition and antifungal activity of the essential oil from Lippia javanica (verbenaceae). Int. J. Biotechnol. Food Sci. 2016, 4, 1–6. [Google Scholar]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana mill.) against anthracnose during post-harvest storage. Crop Protect. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Ahmad, I.; Tanveer, M.U.; Liaqat, M.; Dole, J.M. Comparison of corm soaks with preharvest foliar application of moringa leaf extract for improving growth and yield of cut freesia hybrida. Sci. Hortic. 2019, 254, 21–25. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Ugboko, H.U.; Nwinyi, O.C.; Oranusi, S.U.; Fatoki, T.H.; Omonhinmin, C.A. Antimicrobial importance of medicinal plants in nigeria. Sci. World J. 2020, 2020, 7059323. [Google Scholar] [CrossRef]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ben Aoumar, A.A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Chen, H.Z.; Liu, Y.Q.; Liu, Y.S. Proteomic analysis of kiwifruit in response to the postharvest pathogen, botrytis cinerea. Front. Plant Sci. 2018, 9, 158. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Dumitrascu, L.; Aprodu, I. Aromatic herbs as a source of bioactive compounds: An overview of their antioxidant capacity, antimicrobial activity, and major applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef]
- Eckardt, J.; Tondi, G.; Fanchin, G.; Lach, A.; Junker, R.R. Effect of tannin furanic polymer in comparison to its mimosa tannin extract on the growth of bacteria and white-rot fungi. Polymers 2023, 15, 175. [Google Scholar] [CrossRef]
- Kauffmann, A.C.; Castro, V.S. Phenolic compounds in bacterial inactivation: A perspective from brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B.J. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.V.; Palou, L.; Taberner, V.; Fernández-Catalán, A.; Martínez-Blay, V.; Argente-Sanchis, M.; Pérez-Gago, M.B. Essential oils and natural plant extracts as antifungal ingredients of pectin -based edible composite coatings to control green mold and maintain postharvest quality of ‘valencia’ oranges. In Proceedings of the 6th International Symposium on Postharvest Pathology—Innovation and Advanced Technologies for Managing Postharvest Pathogens, Limassol, Cyprus, 29 May–2 June 2022; pp. 229–236. [Google Scholar]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, D.; Fu, X.; Xiong, C.M.; Nie, Q.Y. Peel essential oil composition and antibacterial activities of citrus x sinensis L. Osbeck ‘tarocco’ and Citrus reticulata blanco. Horticulturae 2022, 8, 793. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Emanuel, I.B.; Laird, A.E.; Hand, F.P. Understanding environmental and physiological factors affecting the biology of diaporthe ilicicola, the fungus causing latent fruit rot in winterberry. Plant Dis. 2023, 107, 2986–2996. [Google Scholar] [CrossRef]
- Sar, T.; Marchlewicz, A.; Harirchi, S.; Mantzouridou, F.T.; Hosoglu, M.I.; Akbas, M.Y.; Hellwig, C.; Taherzadeh, M.J. Resource recovery and treatment of wastewaters using filamentous fungi. Sci. Total Environ. 2024, 951, 175752. [Google Scholar] [CrossRef]
- Yao, Y.J.; Li, Y.R.; Zhao, L.T.; Li, S.J.; Zhou, Z.Q. Citrus lemon (Citrus limon (L.) burm. F. Cv. Eureka) essential oil controls blue mold in citrus by damaging the cell membrane of penicillium italicum. Lwt-Food Sci. Technol. 2023, 188, 115456. [Google Scholar] [CrossRef]
- Yusoff, S.F.; Haron, F.F.; Asib, N.; Mohamed, M.T.M.; Ismail, S.I. Development of vernonia amygdalina leaf extract emulsion formulations in controlling gray mold disease on tomato (Lycopersicon esculentum mill.). Agronomy 2021, 11, 373. [Google Scholar] [CrossRef]
- Plesken, C.; Pattar, P.; Reiss, B.; Noor, Z.N.; Zhang, L.S.; Klug, K.; Huettel, B.; Hahn, M. Genetic diversity of botrytis cinerea revealed by multilocus sequencing, and identification of B. Cinerea populations showing genetic isolation and distinct host adaptation. Front. Plant Sci. 2021, 12, 663027. [Google Scholar] [CrossRef]
- Yang, X.N.; Khan, I.; Kang, S.C. Chemical composition, mechanism of antibacterial action and antioxidant activity of leaf essential oil of Forsythia koreana deciduous shrub. Asian Pac. J. Trop. Med. 2015, 8, 682–688. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F. Antioxidant and anticholinesterase effects of the ethanol extract, ethanol extract fractions and total alkaloids from the cultivated Ruta chalepensis. S. Afr. J. Bot. 2019, 120, 163–169. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; El-Mokhtar, M.A.; Abdel-Rahman, E.A.; Ahmed, I.A.; Nicola, M.A. Terpenoid-rich elettaria cardamomum extract prevents alzheimer-like alterations induced in diabetic rats via inhibition of gsk3β activity, oxidative stress and pro-inflammatory cytokines. Cytokine 2019, 113, 405–416. [Google Scholar] [CrossRef]
- Yamaguchi, T. Antibacterial effect of the combination of terpenoids. Arch. Microbiol. 2022, 204, 1–7. [Google Scholar] [CrossRef]
- Smulek, W.; Makiej, A.; Jarzebski, M.; Zdarta, A.; Jeszka-Skowron, M.; Ciesielczyk, F.; Jesionowski, T.; Zdarta, J.; Kaczorek, E. Nanoemulsions of essential oils stabilized with saponins exhibiting antibacterial and antioxidative properties. Rev. Adv. Mater. Sci. 2023, 62, 337. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, M.R.; Haque, A.R.; Kabir, M.R.; Khushe, K.J.; Hasan, S.K. Trends and challenges of fruit by-products utilization: Insights into safety, sensory, and benefits of the use for the development of innovative healthy food: A review. Bioresour. Bioprocess. 2024, 11, 10. [Google Scholar] [CrossRef]
- Chinnasamy, N.; Harishankar, N.; Kumar, P.U.; Rukmini, C. Toxicological studies on debitterized neem oil (Azadirachta indica). Food Chem. Toxicol. 1993, 31, 297–301. [Google Scholar] [CrossRef]
- Gibriel, A.Y.; Al-Sayed, H.M.A.; Rady, A.H.; Abdelaleem, M.A. Synergistic antibacterial activity of irradiated and nonirradiated cumin, thyme and rosemary essential oils. J. Food Saf. 2013, 33, 222–228. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Protect. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Schnarr, L.; Olsson, O.; Kümmerer, K.J.C. Biodegradation of flavonoids–influences of structural features. Chemosphere 2024, 359, 142234. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yu, Y.; Jo, Y.; Han, J.H.; Xue, Y.; Cho, M.; Bae, S.-J.; Ryu, D.; Park, W.; Ha, K.-T. Impact of extraction techniques on phytochemical composition and bioactivity of natural product mixtures. Front. Pharmacol. 2025, 16, 1615338. [Google Scholar] [CrossRef] [PubMed]
- Le Bloch, J.; Rouault, M.; Langhi, C.; Hignard, M.; Iriantsoa, V.; Michelet, O. The novel food evaluation process delays access to food innovation in the european union. Sci. Food 2025, 9, 117. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and modern techniques for bioactive compounds recovery from plants. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Erazo-Lara, A.E.; Garcia-Pastor, M.E.; Padilla-Gonzalez, P.A.; Serrano, M.; Valero, D. Yellow pitahaya (Selenicereus megalanthus haw.) growth and ripening as affected by preharvest elicitors (salicylic acid, methyl salicylate, methyl jasmonate, and oxalic acid): Enhancement of yield, and quality at harvest. Horticulturae 2024, 10, 493. [Google Scholar] [CrossRef]
- Antón-Domínguez, B.I.; Díaz-Díaz, M.; Acedo-Antequera, F.A.; Trapero, C.; Agustí-Brisach, C. Use of natural-based commercial products as an alternative for providing bioprotection against verticillium wilt of olive. J. Sci. Food Agric. 2024, 104, 6311–6321. [Google Scholar] [CrossRef]
- Travičić, V.; Cvanić, T.; Ćetković, G. Plant-based nano-emulsions as edible coatings in the extension of fruits and vegetables shelf life: A patent review. Foods 2023, 12, 2535. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant antimicrobials for food quality and safety: Recent views and future challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
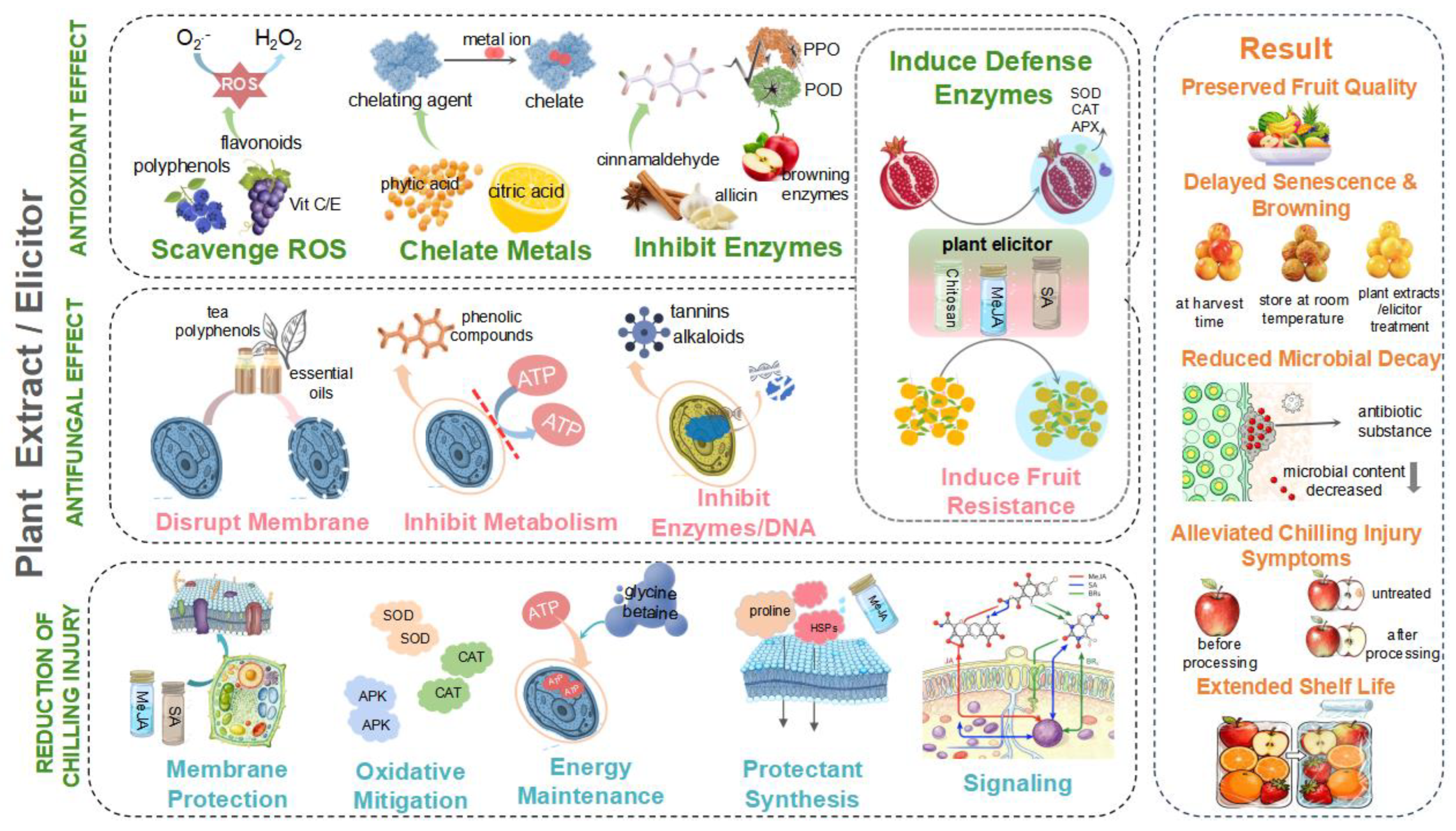
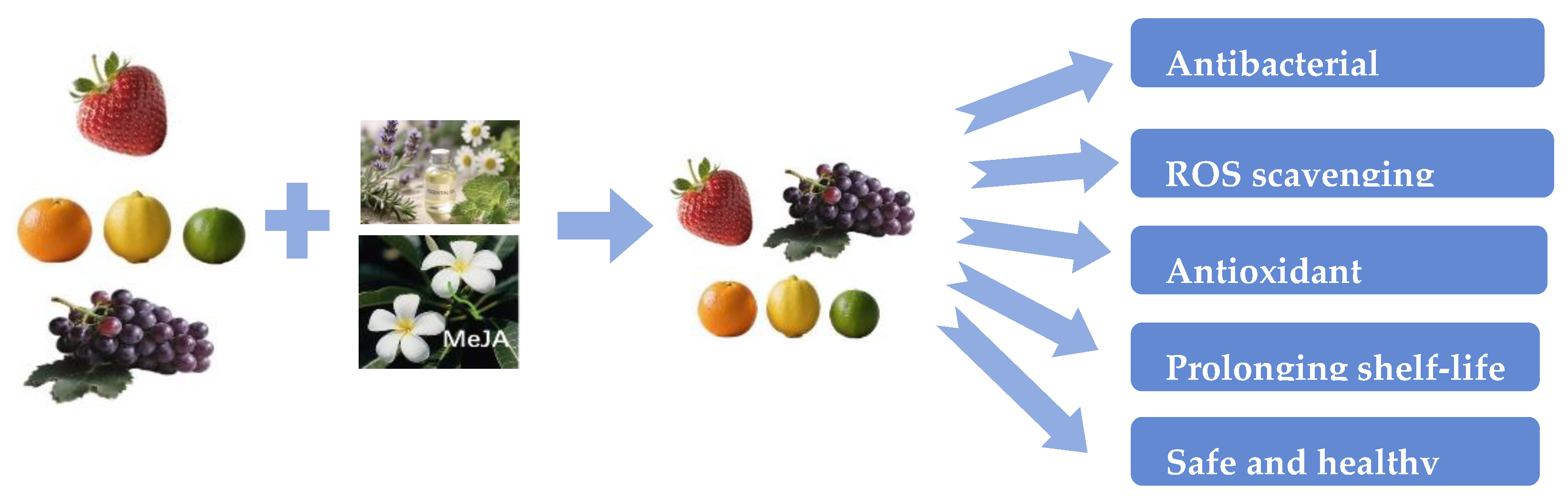
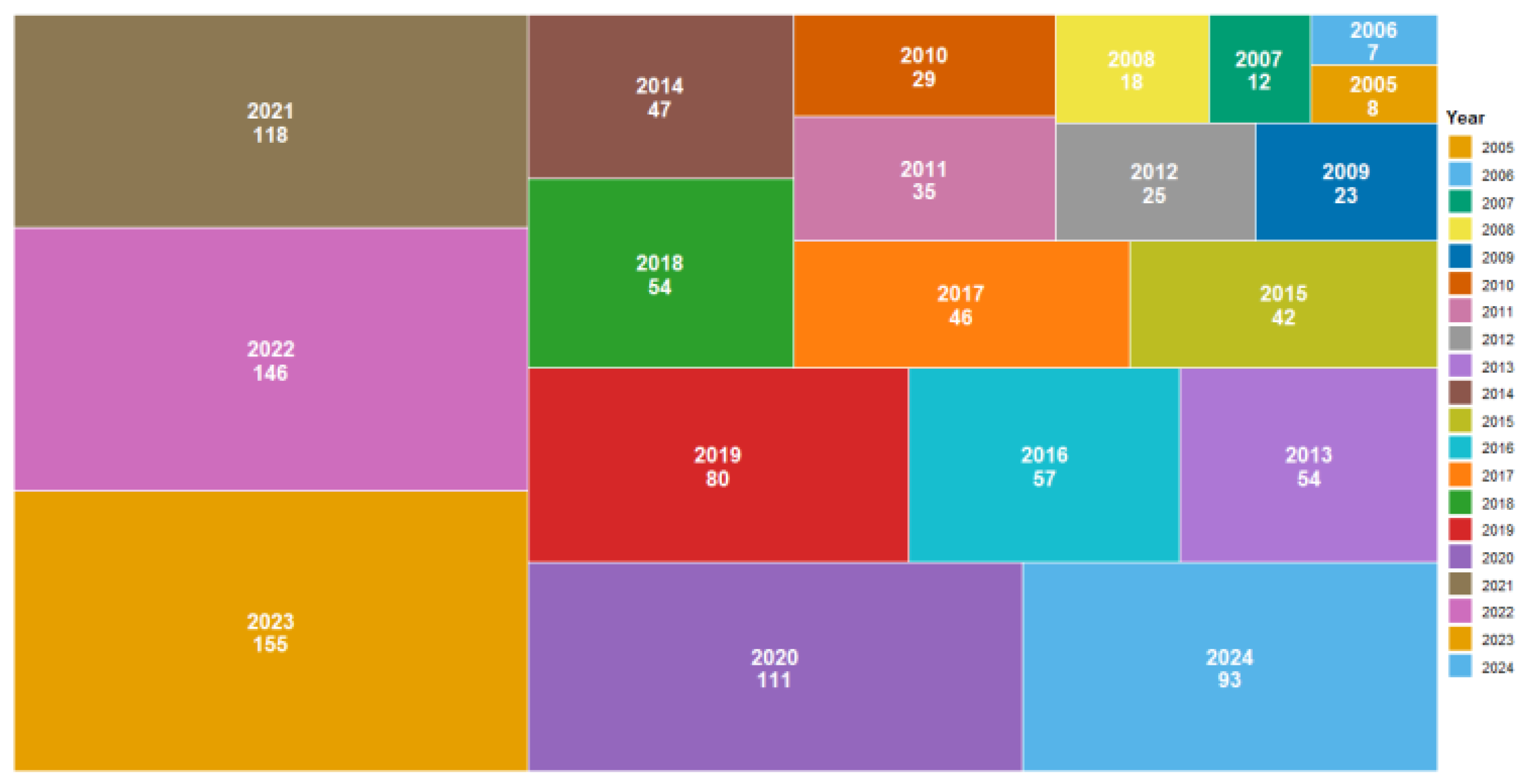
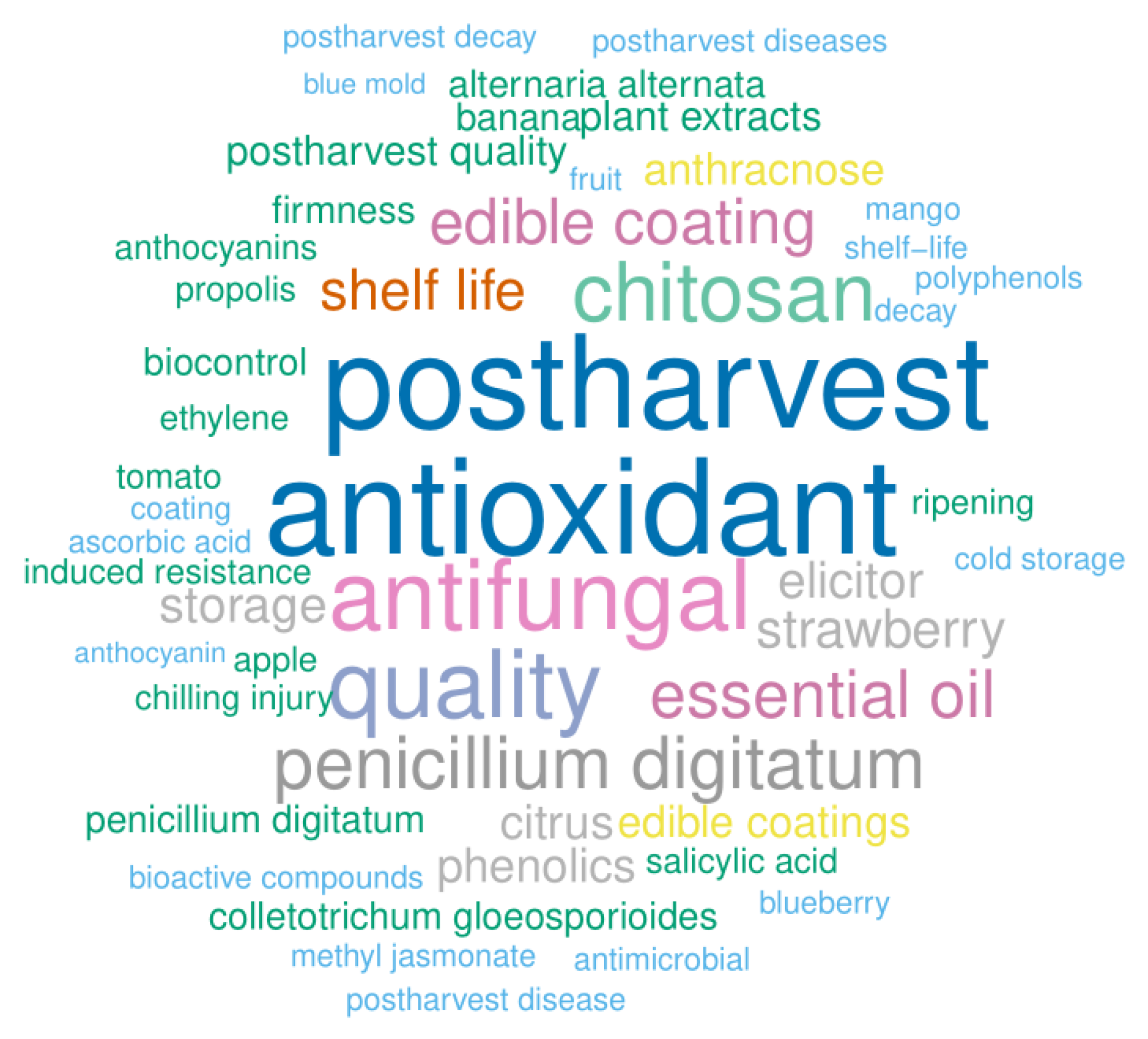

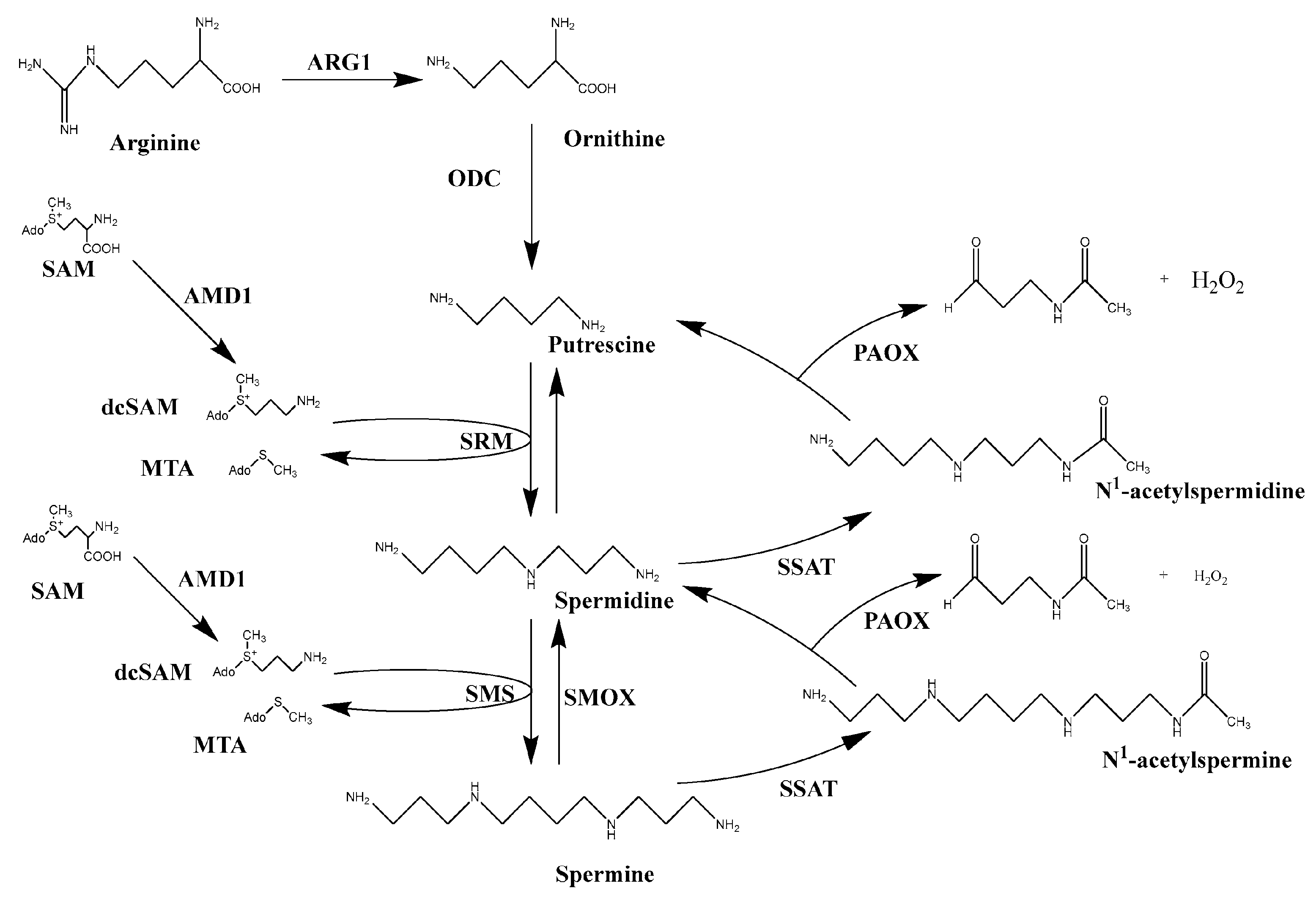
| Plant Species | Extracts/Key Compounds | Biological Activities | References |
|---|---|---|---|
| Oregano and thyme | Proanthocyanidins and carvacrol EOs | Proanthocyanidins possess extensive bioactive profiles, primarily scavenging free radicals in the human body, and reducing inflammation. Carvacrol EOs inhibit a wide range of microorganisms. | [38] |
| Turmeric | Curcumin and turmerone | Turmeric roots have remarkable antimicrobial, antioxidant, antiviral, anti-inflammatory, and anticancer activities, and its antioxidant activity prevents enzymatic browning of fruits and prolongs shelf life. | [39] |
| Cloves | β-caryophyllene, eugenol, and other phenolic compounds | Antioxidant, anti-inflammatory, antibacterial, and anticancer properties. | [40] |
| Orange | Limonene and β-myrcene | Remarkable antimicrobial and antioxidant properties. | [41] |
| Angelica sinensis | δ-3-carene and limonene | δ-3-carene and limonene were predominantly effective against migraine, anorexia, bronchitis, menstrual complaints, and gastrointestinal disorders. As a chitosan nanoemulsion, its EO inhibited the biosynthesis of ergosterol and enhanced the release of Ca2+, Mg2+, and K+ ions, suggesting the plasma membrane as possible antifungal site of action, against the growth and development of Botrytis cinerea mycelia. | [42] |
| Curcumin and orange | Oxygenated turmerone and limonene | Excellent bacteriostatic properties against Escherichia coli and Staphylococcus aureus. Weight loss and deterioration of strawberries were effectively reduced, and shelf life was prolonged. | [43] |
| Lavender | Linalool, 1,8-cineole, camphor, and borneol | L. spica EO showed antimicrobial activity against Gram-positive bacteria. | [44] |
| Pomegranate | Polyphenols | Exhibited antioxidant, antidiabetic, anticancer, antiviral, anti-inflammatory, and antimicrobial properties. | [45] |
| Prickly pear | Methyl jasmonate | MeJA treatment effectively reduced decay, maintained fruit firmness and brightness, suppressed respiration, and decreased malondialdehyde concentration. | [46] |
| Crystal grapes | Methyl jasmonate | MeJA treatment inhibited accumulation of soluble solids, reduced decay, weight loss, and browning, and improved fruit appearance. | [47] |
| Plant Species | Source | Industrial Applications | Functions | Reference |
|---|---|---|---|---|
| Tea | Leaves | Packaging films | Provide mechanical strength, act as a water barrier, inhibit spoilage by microbes and prevent rancidity. | [73] |
| Olive | Leaves | Natural preservative | Reduce microbial growth and maintain the quality and sensory attributes of poultry. | [74] |
| Fig | Leaves | Natural preservative | Prolong shelf life of pasteurized buffalo milk, without altering its properties. | [75] |
| Mentha arvensis | Leaves | Prolong shelf life | Increase the shelf life of squid mantle cuts during refrigerated storage. | [76] |
| Ginkgo | Leaves | Bioactive composite films | Inhibit microbial growth and loss of color and delay fat and protein oxidation in chilled beef during storage. | [77] |
| Peperomia pellucida | Leaves | Edible coatings | Preserve the quality and prolong the shelf life of apples by combating oxidation and inhibiting microbes. | [78] |
| Aloe | Leaves | Edible coatings | Inhibit the growth of microorganisms in blueberries, reduce water loss, and extend shelf life. | [79] |
| Aloe | Leaves | Edible coatings | Increase phenolic compounds and total antioxidant capacity of fresh grapes during storage. | [80] |
| Neem | Leaves | Preservatives impact | Extend the shelf life of bananas and reduce the incidence of spoilage. | [81] |
| Neem | Leaves | Preservative impact | Inhibit fungi, maintain firmness and delay fruit ripening. | [82] |
| Black mulberry | Fruit | Preservative impact | Reduce lipid oxidation and microbial invasion during storage. | [83] |
| Jaboticaba | Peel | Cosmeceutical | Counteract the toxic effects of peroxide, accelerate wound healing, and treat skin diseases and wounds related to oxidative stress. | [84] |
| Baobab tree | Seeds | Preservative impact | Improve storage stability, antioxidant content and cooking properties of beef patties. | [85] |
| Black cumin | Seeds | Edible coatings | Retard oxidative rancidity. | [86] |
| Quercus suber | Bark | Packaging films | Enhance film pliability and photostability while strengthening antimicrobial efficacy and antioxidant performance. | [87] |
| Cinnamon | Bark | Edible coatings | Inhibit invasion by pathogenic microbes in minced beef. | [88] |
| Osmanthus | Flower | Preservatives impact | Prevents pear tissue oxidation, effectively reduces microbial growth, and extends shelf life. | [89] |
| Beet | Root peel | Packaging films | Delay protein and lipid oxidation, stabilize meat color, inhibit microbial growth, and prolong shelf life. | [90] |
| Plant Name | Part Used | Research Focus | Treatment | Reference |
|---|---|---|---|---|
| Azadirachta indica | NLE | Effects of NLE application on the antioxidant activity and physicochemical characteristics of peaches. | Peaches treated with different NLE concentrations were stored at room temperature for 9 days, then tested for antioxidant activity and physicochemical properties. The 30% NLE treatment reduced weight loss and SSC, maintained higher acidity, and extended shelf life. | [231] |
| Thyme | EO | Preservation ability of different plant EOs on post-harvest quality and shelf life of mangoes. | Mangoes were treated with thyme oil and postharvest properties were recorded every 3 d. Thyme oil was most effective at preservation of mangoes compared with other EOs. | [232] |
| Astragalus | Astragalus polysaccharides (APS) | The effect of APS on peel browning and CI of banana fruit. | Bananas were immersed in a solution of 0.1 g/L APS, 0.5 g/L APS, or sterile water at 25 °C for 5 min. The treated fruit were placed into perforated plastic boxes and stored at 7 ± 1 °C. | [233] |
| Aloe | Leaf extract | Effects of AV coating on grapes, before and after harvest, and the combined application before and after harvest, on storage characteristics and grape quality. | For both preharvest and postharvest periods, AV coatings were applied at a concentration of 33%. For the preharvest treatment, the entire foliage was sprayed with the solution 10 d before harvesting. On the day of harvest, clusters were picked, immersed in 33% AV solution for 5 min and stored. The control group was treated with distilled water (DW). | [80] |
| Aloe | Leaf extract | Aloe-based edible coating to maintain quality of fresh-cut Italian pears (Pyrus communis L.) during cold storage. | Two edible coatings were tested: (1) 120 mL AV gel + 6 g hydroxypropyl-methylcellulose (HPMC) + 3 g pomegranate seeds oil (PSO) dissolved in 300 mL of DW (2) 120 mL AV gel + 3 g HPMC dissolved in 300 mL DW. | [234] |
| Moringa | Leaf extract | Preservative effect of coatings made of gum arabic (GA) and carboxymethyl cellulose (CMC), containing moringa (M) leaf extract effective against Colletotrichum gloeosporioides on Maluma avocados. | Avocados were coated with 10% and 15% GA, 10% and 15% GA + M, or 1% CMC + M. Uncoated fruit served as control. The fruits were kept at 5.5 °C and 95% relative humidity for 21 days before being transferred to 21.1 °C and 60% RH for 7 days to imitate the natural environment. | [235] |
| Lippia javanica | EO from leaves | The minimum inhibitory concentration of EO for visible growth of Fusarium graminearum. | Experimental concentrations of 0.87, 0.65, 0.43, 0.22, 0.11, 0.054 and 0.027 mg/mL of EO were tested in bioassays. | [236] |
| Aloe, Indian fagonbush | Plant powder | Effect of AV gel alone or enriched with Fagonia indica (FI) extract on storage of sapodilla fruit. | Treatments: DW control, 50% AV, 100% AV, 50% AV + 1% FI, and 100% AV + 1% FI. Fruit were dipped in the corresponding solutions for 5 min and then air dried at ambient temperature and stored. | [119] |
| Thyme, aloe | Thyme oil + extract | Effect of thyme oil and edible coatings on the growth of anthracnose on artificially infected avocados. | The ‘poisoned food technique’ was used to compare the effect of various plant extract treatments. Infected avocados were coated with chitosan, aloe, thyme oil, chitosan + thyme oil (3:1), or aloe + thyme oil (3:1) before storage at room temperature for 5 d. | [237] |
| Aloe, ginger, garlic | AV gel | Effect of GA, AV gel, ginger and garlic extracts on preservation of guava. | Treatments: DW control, 20% ginger extract + 10% GA, 20% garlic extract + 10% GA, 100% AV gel + 10% GA. | [133] |
| Moringa | Moringa leaf extract (MLE) | Effect of preharvest foliar applications of MLE on morphological and physiological attributes and yield of freesia corms, Freesia hybrida L. | Experiment I: corms were soaked in 1%, 2%, 5% or 10% MLE for 24 h and air dried before planting. Experiment II: untreated corms were planted and 1%, 2%, 3% or 5% MLE was applied to the planted area until runoff at 30 and 60 d after planting. | [238] |
| Aloe | Leaf extract | Effect of edible coating of salicylic acid (SA) and AV on microbial load and CI of oranges. | Thomson navel oranges (Citrus sinensis L. Osbeck) were coated with SA and AV, stored at 4 ± 1 °C and 80 ± 5% RH, and microbial load, CI and quality were evaluated. | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Liu, L.; Gao, Z.; Zhao, J.; Yang, Y.; Shen, Z. Preservation of Fruit Quality at Postharvest Through Plant-Based Extracts and Elicitors. Horticulturae 2025, 11, 1186. https://doi.org/10.3390/horticulturae11101186
Chen D, Liu L, Gao Z, Zhao J, Yang Y, Shen Z. Preservation of Fruit Quality at Postharvest Through Plant-Based Extracts and Elicitors. Horticulturae. 2025; 11(10):1186. https://doi.org/10.3390/horticulturae11101186
Chicago/Turabian StyleChen, Dixin, Li Liu, Zhongkai Gao, Jianshe Zhao, Yingjun Yang, and Zhiguo Shen. 2025. "Preservation of Fruit Quality at Postharvest Through Plant-Based Extracts and Elicitors" Horticulturae 11, no. 10: 1186. https://doi.org/10.3390/horticulturae11101186
APA StyleChen, D., Liu, L., Gao, Z., Zhao, J., Yang, Y., & Shen, Z. (2025). Preservation of Fruit Quality at Postharvest Through Plant-Based Extracts and Elicitors. Horticulturae, 11(10), 1186. https://doi.org/10.3390/horticulturae11101186






