Impact of Nitrogen on Downy Mildew Infection and Its Effects on Growth and Physiological Traits in Early Growth Stages of Cucumber
Abstract
1. Introduction
2. Materials and Methods
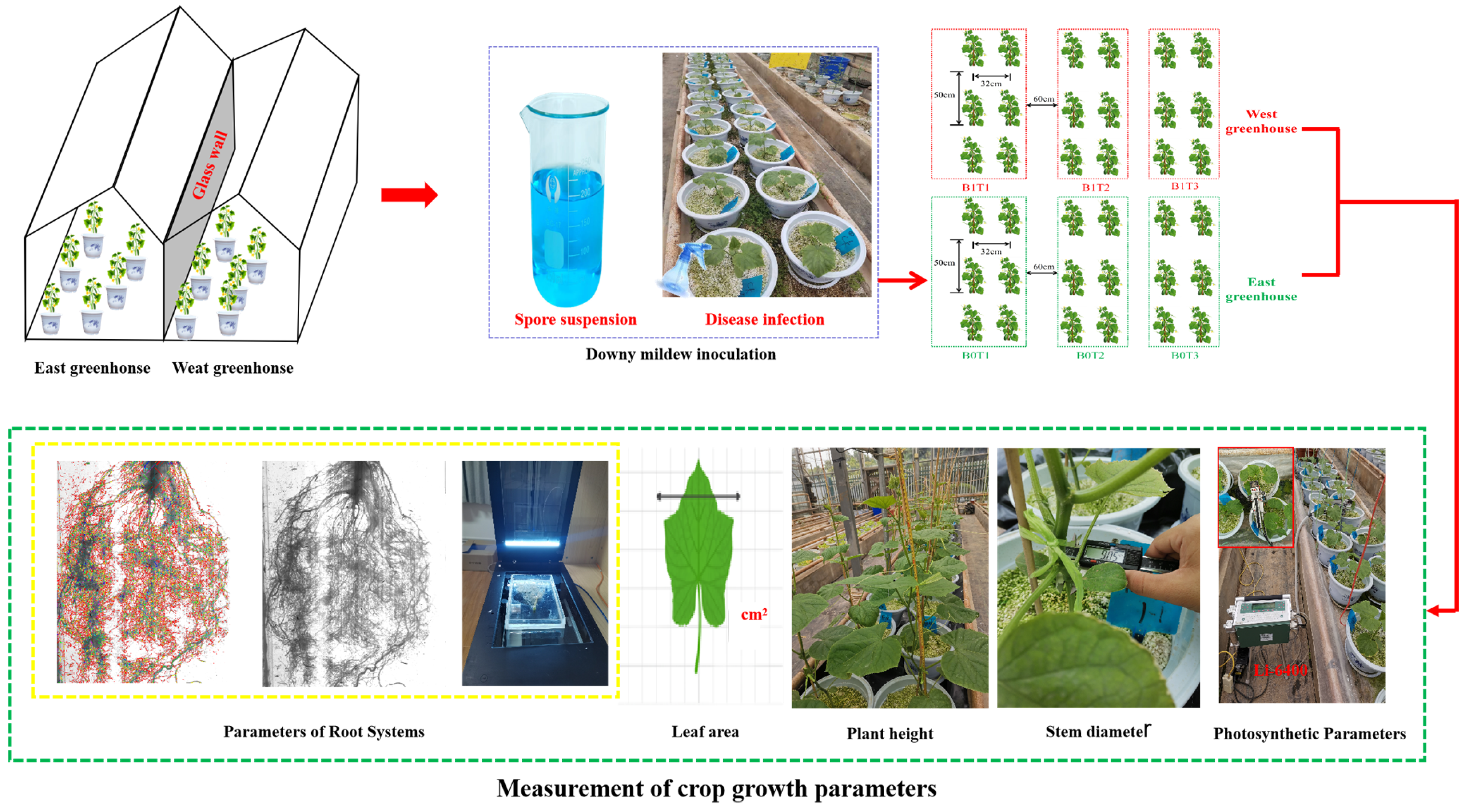
2.1. Study site and Plant Material
2.2. Downy Mildew and Nitrogen Treatments
2.2.1. Downy Mildew Inoculation
2.2.2. Nitrogen Application
2.3. Experimental Data Acquisition
2.4. Statistical Analyses
3. Results
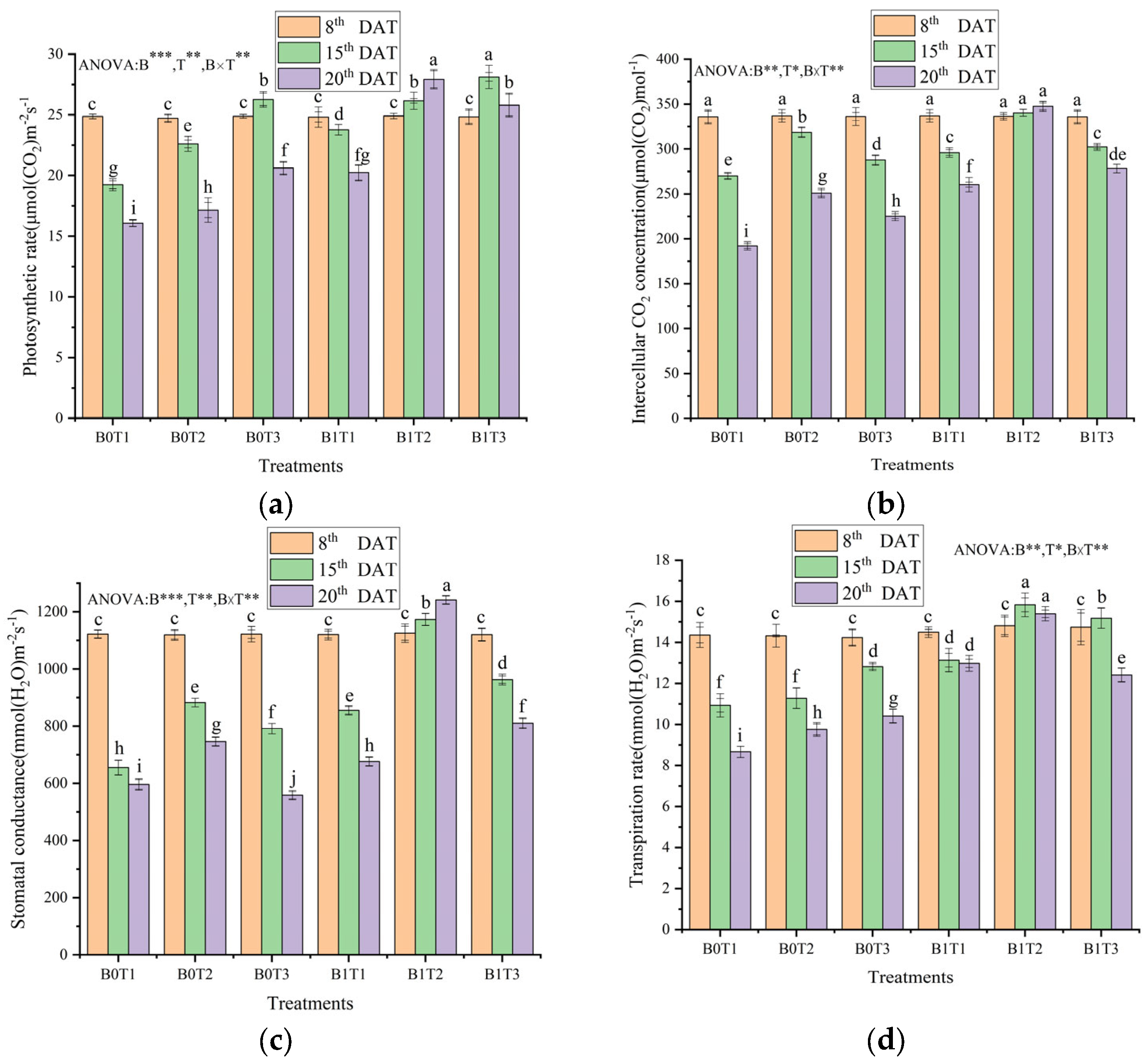
3.1. Photosynthetic Parameters
3.2. Cucumber Plants’ Growth Parameters
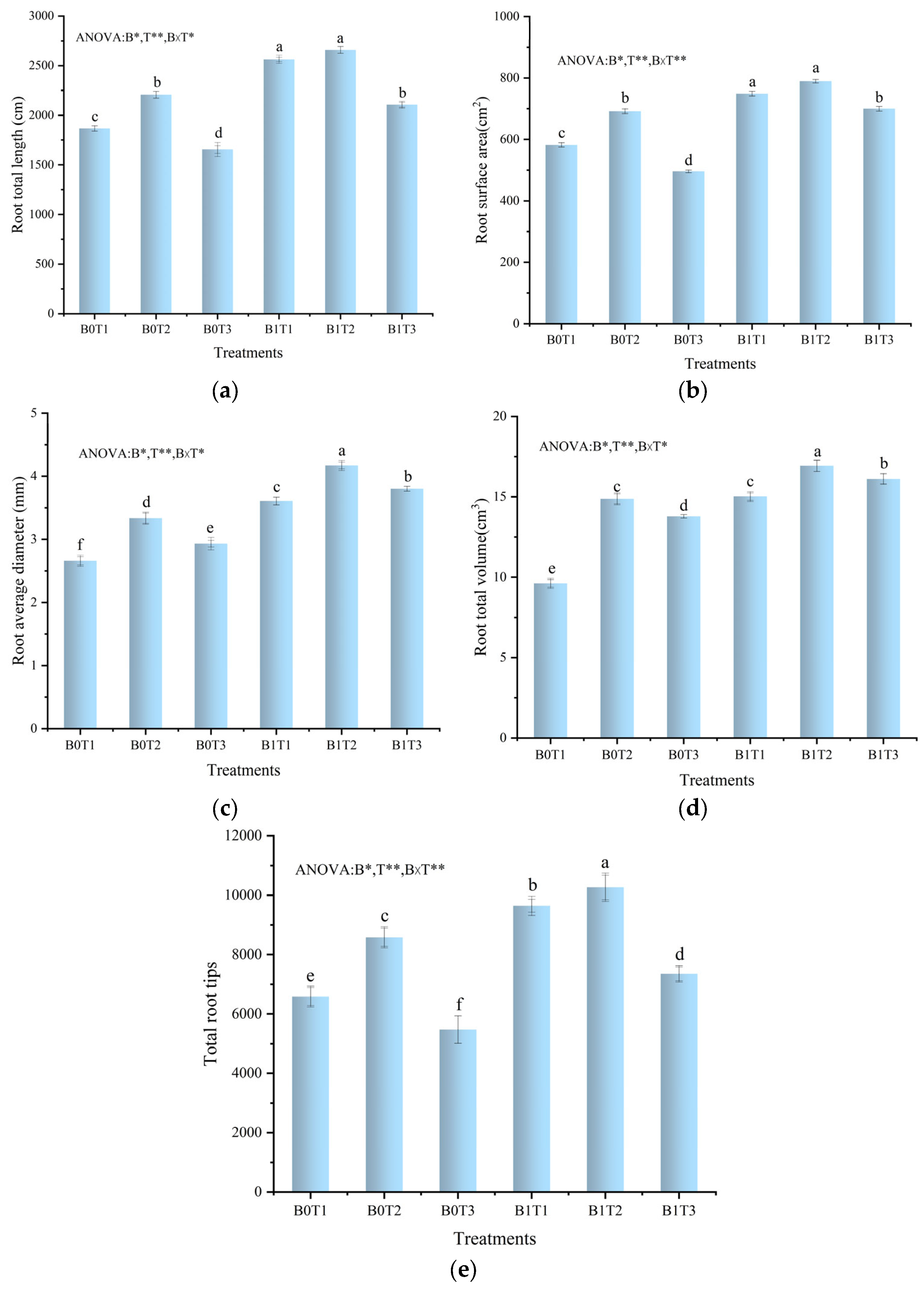
3.3. Parameters of Root Systems
4. Discussions
4.1. Effects of Downy Mildew Stress and Nitrogen Stress on Gas Exchange Parameters of Cucumber Plants
4.2. Effects of Downy Mildew Stress and Nitrogen Stress on Cucumber Plants’ Growth Parameters
4.3. Effects of Downy Mildew Stress and Nitrogen Stress on Cucumber Plants’ Root System Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.F.; Shi, Q.; Xu, G.L.; Yang, N.; Chen, T.H.; Taha, M.F.; Mao, H.P. Transmission Route of Airborne Fungal Spores for Cucumber Downy Mildew. Horticulturae 2025, 11, 336. [Google Scholar] [CrossRef]
- Acquah, S.J.; Yan, H.F.; Zhang, C.; Wang, G.Q.; Zhao, B.S.; Wu, H.M.; Zhang, H.N. Application and evaluation of Stanghellini model in the determination of crop evapotranspiration in a naturally ventilated greenhouse. Int. J. Agric. Biol. Eng. 2018, 11, 95–103. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, Z.Y.; Li, Y.F.; Lu, X.L.; Li, G.R.; Qi, S.S.; Khan, I.U.; Li, G.L.; Dai, Z.C.; Du, D.L. The Degradability of Microplastics May Not Necessarily Equate to Environmental Friendliness: A Case Study of Cucumber Seedlings with Disturbed Photosynthesis. Agriculture 2024, 14, 53. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shi, Q.; Lin, J.L.; Lu, X.T.; Ye, B.; Lv, H.X.; Du, X.X.; Chen, T.H. Hormone Metabolism and Substance Accumulation in Cucumber Plants: Downy Mildew Infection and Potassium Stress. Agriculture 2025, 15, 994. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, N.; Ma, G.X.; Taha, M.F.; Mao, H.P.; Zhang, X.D.; Shi, Q. Detection of spores using polarization image features and BP neural network. Int. J. Agric. Biol. Eng. 2024, 17, 213–221. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.S. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT Food Sci. Technol. 2021, 149, 111779. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, C.; Yan, H.F.; Li, J.; Ren, J.T.; Akhlaq, M.; Hameed, M.U.; Disasa, K.N. Physiological Response of Tomato and Cucumber Plants to Micro-Spray in High-Temperature Environment: A Scientific and Effective Means of Alleviating Crop Heat Stress. Agronomy 2023, 13, 2798. [Google Scholar] [CrossRef]
- Yan, H.F.; Deng, S.S.; Zhang, C.; Wang, G.Q.; Zhao, S.; Li, M.; Liang, S.W.; Jiang, J.H.; Zhou, Y.D. Determination of energy partition of a cucumber grown Venlo-type greenhouse in southeast China. Agric. Water Manag. 2023, 276, 108047. [Google Scholar] [CrossRef]
- Yan, H.F.; Zhang, C.; Gerrits, M.C.; Acquah, S.J.; Zhang, H.N.; Wu, H.M.; Zhao, B.S.; Huang, S.; Fu, H.W. Parametrization of aerodynamic and canopy resistances for modeling evapotranspiration of greenhouse cucumber. Agric. For. Meteorol. 2018, 262, 370–378. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.Y.; Yan, H.F.; Ullah, I.; Zuo, Z.Y.; Li, L.L.; Yu, J.J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Rehman, M.M.U.; Liu, J.Z.; Nijabat, A.; Faheem, M.; Wang, W.Y.; Zhao, S.Y. Leveraging Convolutional Neural Networks for Disease Detection in Vegetables: A Comprehensive Review. Agronomy 2024, 14, 2231. [Google Scholar] [CrossRef]
- Pan, L.X.; Zhou, C.G.; Jing, J.; Zhuang, M.; Zhang, J.C.; Wang, K.; Zhang, H.Y. Metabolomics analysis of cucumber fruit in response to foliar fertilizer and pesticides using UHPLC-Q-Orbitrap-HRMS. Food Chemistry. 2022, 369, 130960. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Acquah, S.J.; Zhang, J.Y.; Wang, G.Q.; Zhang, C.; Darko, R.O. Overview of modelling techniques for greenhouse microclimate environment and evapotranspiration. Int. J. Agric. Biol. Eng. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ma, G.X.; Du, X.X.; Liu, Y.; Wang, B.; Xu, G.L.; Mao, H.P. Effects of Nutrient Solution Irrigation Quantity and Downy Mildew Infection on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2020, 10, 1921. [Google Scholar] [CrossRef]
- Ali, A.B.; Elshaikh, N.A.; Hussien, G.; Abdallah, F.E.; Hassan, S. Biochar Addition for Enhanced Cucumber Fruit Quality Under Deficit Irrigation. Biosci. J. 2020, 36, 1930–1937. [Google Scholar] [CrossRef]
- Abdelfatah, A.; Mazrou, Y.S.A.; Arafa, R.A.; Makhlouf, A.H.; El-Nagar, A. Control of cucumber downy mildew disease under greenhouse conditions using biocide and organic compounds via induction of the antioxidant defense machinery. Sci. Rep. 2025, 15, 11705. [Google Scholar] [CrossRef]
- Xu, Y.H.; Ma, Y.; Luz, C.M.; Angel, S.M.M.; Wang, Q.J. Compost biochemical quality mediates nitrogen leaching loss in a greenhouse soil under vegetable cultivation. Geoderma 2020, 358, 113984. [Google Scholar] [CrossRef]
- Atri, A.; .Bhardwaj, N.R.; Roy, A.K.; Kaur, A. Efficacy of biocontrol agents against Sclerospora graminicola causing downy mildew in fodder pearl millet. Range Manag. Agrofor. 2023, 44, 76–83. [Google Scholar] [CrossRef]
- Huang, S.; Yan, H.F.; Zhang, C.; Wang, G.Q.; Acquah, S.J.; Yu, J.J.; Li, L.L.; Mam, J.M.; Darko, R.O. Modeling evapotranspiration for cucumber plants based on the Shuttleworth-Wallace model in a Venlo-type greenhouse. Agric. Water Manag. 2020, 228, 105861. [Google Scholar] [CrossRef]
- Zhu, W.D.; Sun, J.; Wang, S.M.; Shen, J.F.; Yang, K.F.; Zhou, X. Identifying Field Crop Diseases Using Transformer-Embedded Convolutional Neural Network. Agriculture 2022, 12, 1083. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wang, Y.Y.; Li, Z.H.; Huang, X.W.; Shen, T.T.; Zou, X.B. Simultaneous and nondestructive diagnostics of nitrogen/magnesium/potassium-deficient cucumber leaf based on chlorophyll density distribution features. Biosyst. Eng. 2021, 212, 458–467. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Li, J.; AL-Huqail, A.A.; Hamed, M.A.; Du, D.L.; EL-Khamisy, R.R. Slow-released fertilizers optimization and experimental impacts on soil fertility and wheat- maize cropping system. Sci. Agric. 2024, 81, e20230234. [Google Scholar] [CrossRef]
- Yan, H.F.; Ma, J.M.; Zhang, J.Y.; Wang, G.Q.; Zhang, C.; Akhlaq, M.; Huang, S.; Yu, J.J. Effects of film mulching on the physiological and morphological parameters and yield of cucumber under insufficient drip irrigation. Irrig. Drain. 2022, 71, 897–911. [Google Scholar] [CrossRef]
- Yan, H.F.; Acquah, S.J.; Zhang, C.; Wang, G.Q.; Huang, S.; Zhang, H.N.; Zhao, B.S.; Wu, H.M. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manag. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.P.; Wang, Z.C.; Chen, S.; Ullah, I.; Ali, M.; Saifullah, M. Effect of Fertigation Levels on Water Consumption, Soil Total Nitrogen, and Growth Parameters of Brassica Chinensis under Straw Burial. Commun. Soil Sci. Plant Anal. 2021, 52, 32–34. [Google Scholar] [CrossRef]
- Zhu, J.J.; Sun, L.Y.; Ju, F.Y.; Wang, Z.; Xiong, C.; Yu, H.L.; Yu, K.; Huo, Y.Y.; Khattak, W.A.; Hu, W.; et al. Potassium Application Increases Cotton (Gossypium hirsutum L.) Fiber Length by Improving K+/Na+ Homeostasis and Potassium Transport Capacity in the Boll-Leaf System under Moderate Salinity. Agronomy 2020, 12, 2962. [Google Scholar] [CrossRef]
- Hou, P.F.; Yuan, W.S.; Li, G.H.; Petropoulos, E.; Xue, L.X.; Feng, Y.F.; Xue, L.H.; Yang, L.Z.; Ding, Y.F. Deep fertilization with controlled-release fertilizer for higher cereal yield and N utilization in paddies: The optimal fertilization depth. Agron. J. 2021, 113, 5027–5039. [Google Scholar] [CrossRef]
- Li, M.Q.; Li, J.Y.; Wei, X.H.; Zhu, W.J. Early diagnosis and monitoring of nitrogen nutrition stress in tomato leaves using electrical impedance spectroscopy. Int. J. Agric. Biol. Eng. 2017, 10, 194–205. [Google Scholar]
- Chen, W.; Gao, Y.; Yang, J.; Fan, F.J.; Zhang, W.G.; Li, J.Y.; Zhou, C.; Shi, G.L.; Tong, F.; Fan, G.P. Taxonomical and functional bacterial community selection in the rhizosphere of the rice genotypes with different nitrogen use efficiencies. Plant Soil 2022, 470, 111–125. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Li, Y.; Li, M. Effect of high-voltage electrostatic field on inorganic nitrogen uptake by cucumber plants. Trans. ASABE 2016, 59, 25–29. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, J.; Wang, J.D.; Zhang, Y.C. Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl. Soil Ecol. 2022, 170, 104266. [Google Scholar] [CrossRef]
- Abubaker, B.A.; Yan, H.F.; Hong, L.; You, W.Y.; Elshaikh, N.A.; Hussei, G.; Pandab, S.; Hassan, S. Enhancement of Depleted Loam Soil as Well as Cucumber Productivity Utilizing Biochar Under Water Stress. Commun. Soil Sci. Plant Anal. 2019, 50, 49–64. [Google Scholar] [CrossRef]
- Yan, Z.N.; Cao, X.X.; Bing, L.X.; Song, J.X.; Qi, Y.; Han, Q.Y.; Yang, Y.J.; Lin, D. Responses of Growth, Enzyme Activity, and Flower Bud Differentiation of Pepper Seedlings to Nitrogen Concentration at Different Growth Stages. Agronomy 2024, 14, 2270. [Google Scholar] [CrossRef]
- Shi, Q.; You, L.; Wang, Y.; Du, X.; Chen, T. Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2025, 15, 1017. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Leather, S.R. Effect of nitrogen fertilizer on the growth and survival of Rhopalosiphum padi (L.) and Sitobion avenae (F.) (Homoptera: Aphididae) on different wheat cultivars. Crop Prot. 2011, 30, 216–221. [Google Scholar] [CrossRef]
- Mandal, K.; Saravanan, R.; Maiti, S. Effect of different levels of N, P and K on downy mildew (Peronospora plantaginis) and seed yield of isabgol (Plantago ovata). Crop Prot. 2008, 27, 988–995. [Google Scholar] [CrossRef]
- Leser, C.; Treutter, D. Effects of nitrogen supply on growth, contents of phenolic compounds and pathogen (scab) resistance of apple trees. Physiol. Plant. 2005, 123, 49–56. [Google Scholar] [CrossRef]
- Hoffland, E.; Jeger, M.J.; van Beusichem, M.L. Effect of nitrogen supply rate on disease resistance in tomato depends on the pathogen. Plant Soil 2000, 218, 239–247. [Google Scholar] [CrossRef]
- Sivaprakasam, K.; Pillayarsamy, K.; Rajagopalan, S.C.K. Influence of nitrogen on the incidence of downy mildew disease of pearl millet (Pennisetum typhoides Stapf and Hubb.). Plant Soil 1974, 41, 677–679. [Google Scholar] [CrossRef]
- Mao, H.P.; Wang, Y.F.; Yang, N.; Liu, Y.; Zhang, X.D. Effects of nutrient solution irrigation quantity and powdery mildew infection on the growth and physiological parameters of greenhouse cucumbers. Int. J. Agric. Biol. Eng. 2022, 15, 68–74. [Google Scholar] [CrossRef]
- GB/T 17980.26-2000; Pesticide—Guidelines for the Field Efficacy Trials(I)—Fungicides Against Downy Mildew of Cucumber. Standardization Administration of China: Beijing, China, 5 January 2000.
- Zhu, Z.X.; Li, D.; Wang, P.; Li, J.H.; Lu, X.C. Transcriptome and ionome analysis of nitrogen, phosphorus and potassium interactions in sorghum seedlings. Theor. Exp. Plant Physiol. 2020, 32, 271–285. [Google Scholar] [CrossRef]
- Kumar, J.S.; Saravanan, R.; Ravi, V.; Sreekumar, J.; Sunitha, S.; More, S.J. Interactive Effects of CO2 Enrichment and Nitrogen Levels on Leaf Gas Exchange Capacities of Sweet Potato. J. Agron. Crop Sci. 2025, 11, e70029. [Google Scholar] [CrossRef]
- Zekker, I.; Kännaste, A.; Eremeev, V.; Kask, K.; Meinson, P.; Nassar, H.; Mäeorg, E.; Runno-Paurson, E.; Niinemets, Ü. Impacts of nitrogen fertilization and planting date on the physiology and yield of purple sweet potato at the extreme Northern edge of cultivation. PLoS ONE 2025, 20, e0318531. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, O.; Steppe, K.; Zgallai, H.; El Hadj, S.B.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Sun, T.T.; Zhang, J.K.; Zhang, Q.; Li, X.L.; Li, M.J.; Yang, Y.Z.; Zhou, J.; Wei, Q.P.; Zhou, B.B. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Qiang, B.B.; Chen, S.Y.; Fan, Z.; Cao, L.; Li, X.; Fu, C.Y.; Zhang, Y.X.; Jin, X.J. Effects of nitrogen application levels on soybean photosynthetic performance and yield: Insights from canopy nitrogen allocation studies. Field Crops Res. 2025, 326, 109871. [Google Scholar] [CrossRef]
- Si, T.; Lu, J.H.; Liang, Z.J.; Kong, J.; Yu, X.N.; Zhang, X.J.; Zou, X.X. Stratified Fertilization Increases Peanut Yield and Quality by Enhancing Photosynthesis and Modulating Antioxidative, Carbon and Nitrogen Metabolic Enzymes. J. Soil Sci. Plant Nutr. 2025, 25, 4925–4942. [Google Scholar] [CrossRef]
- Shao, C.H.; Qiu, C.F.; Qian, Y.F.; Liu, G.R. Nitrate deficiency decreased photosynthesis and oxidation-reduction processes, but increased cellular transport, lignin biosynthesis and flavonoid metabolism revealed by RNA-Seq in Oryza sativa leaves. PLoS ONE 2020, 15, e0235975. [Google Scholar] [CrossRef]
- Mu, X.H.; Chen, Y.L. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Luo, T.; Zhang, K.K.; Zhu, K.M.; Rana, M.S.; Hu, L.Y.; Jiang, Y. Compensation of high nitrogen toxicity and nitrogen deficiency with biochar amendment through enhancement of soil fertility and nitrogen use efficiency promoted rice growth and yield. GCB Bioenergy 2021, 10, 1765–1784. [Google Scholar] [CrossRef]
- Dos Santos, L.C.N.; Gaion, L.A.; Prado, R.M.; Barreto, R.F.; Carvalho, R.F. Low auxin sensitivity of diageotropica tomato mutant alters nitrogen deficiency response. An. Acad. Bras. Cienc. 2020, 92, e20190254. [Google Scholar] [CrossRef]
- Razgallah, N.; Chikh-Rouhou, H.; Abid, G.; M’hamdi, M. Identification of Differentially Expressed Putative Nitrate Transporter Genes in Lettuce. Int. J. Veg. Sci. 2017, 23, 390–399. [Google Scholar] [CrossRef]
- M’hamdi, M.; Abid, G.; Chikh-Rouhou, H.; Razgallah, N.; Hassen, A. Effect of genotype and growing season on nitrate accumulation and expression patterns of nitrate transporter genes in potato (Solanum tuberosum L.). Arch. Agron. Soil Sci. 2016, 62, 1508–1520. [Google Scholar] [CrossRef]
- Razgallah, N.; Abid, G.; Chikh-Rouhou, H.; Hassen, A.; M’hamdi, M. Nitrate Content and Expression of Putative Nitrate Transporter Genes in Lettuce Fertilized with Nitrogen Fertilizers. Int. J. Veg. Sci. 2016, 23, 173–184. [Google Scholar] [CrossRef]
- Huang, W.T.; Xie, Y.Z.; Chen, X.F.; Zhang, J.; Chen, H.H.; Ye, X.; Guo, J.X.; Yang, L.T.; Chen, L.S. Growth, Mineral Nutrients, Photosynthesis and Related Physiological Parameters of Citrus in Response to Nitrogen Deficiency. Agronomy 2021, 11, 1859. [Google Scholar] [CrossRef]
- Gao, K.; Chen, F.J.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. A comprehensive analysis of root morphological changes and nitrogen allocation in maize in response to low nitrogen stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef]
- Chen, G.D.; Wang, L.; Fabrice, M.R.; Tian, Y.A.; Qi, K.J.; Chen, Q.; Cao, P.; Wang, P.; Zhang, S.L.; Wu, J.Y.; et al. Physiological and Nutritional Responses of Pear Seedlings to Nitrate Concentrations. Front. Plant Sci. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Song, H.F.; Cai, Z.; Liao, J.; Zhang, S. Phosphoproteomic and Metabolomic Analyses Reveal Sexually Differential Regulatory Mechanisms in Poplar to Nitrogen Deficiency. J. Proteome Res. 2020, 19, 1073–1084. [Google Scholar] [CrossRef]
- Chen, Z.P.; Li, H.P.; Yang, T.Y.; Chen, T.T.; Dong, C.X.; Gu, Q.; Cheng, X.M. Transcriptome analysis provides insights into the molecular bases in response to different nitrogen forms-induced oxidative stress in tea plant roots (Camellia sinensis). Funct. Plant Biol. 2020, 47, 1073–1082. [Google Scholar] [CrossRef]
- Matic, M.; Vukovic, R.; Vrandecic, K.; Camagajevac, I.S.; Cosic, J.; Vukovic, A.; Sabljic, K.; Sabo, N.; Dvojkovic, K.; Novoselovic, D. Oxidative Status and Antioxidative Response to Fusarium Attack and Different Nitrogen Levels in Winter Wheat Varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, Q.W.; Zou, Y.G.; Ma, S.M.; Zhang, X.D.; Xie, X.Y.; Wang, L.C. Effect of potassium deficiency on growth, antioxidants, ionome and metabolism in rapeseed under drought stress. Plant Growth Regul. 2020, 90, 455–466. [Google Scholar] [CrossRef]

| Disease Level | Symptoms Described |
|---|---|
| Level 0 | Asymptomatic |
| Level 1 | Diseased spot area occupies less than 5% of the leaf area |
| Level 3 | Diseased area accounts for 6% to 10% of the leaf area |
| Level 5 | Diseased area accounts for 11% to 25% of the leaf area |
| Level 7 | Diseased area accounts for 26% to 50% of the leaf area |
| Level 9 | Diseased spot area accounts for more than 50% of the leaf area |
| Nutrient Solution Group | Chemical Reagent | N-50% | N-100% | N-150% |
|---|---|---|---|---|
| A | Ca(NO3)2·4H2O | 413 | 826 | 826 |
| KNO3 | 303 | 606 | 606 | |
| B | NH4H2PO4 | 57 | 114 | 144 |
| MgSO4·7H2O | 492 | 492 | 492 | |
| NaNO3 | 0 | 0 | 552 | |
| KCl | 223 | 0 | 0 | |
| CaCl2 | 194 | 0 | 0 | |
| C | NaFe-EDTA | 7 | 7 | 7 |
| MnSO4 | 1.7 | 1.7 | 1.7 | |
| ZnSO4 | 1.45 | 1.45 | 1.45 | |
| CuSO4 | 0.19 | 0.19 | 0.19 | |
| Na2MoO4 | 0.12 | 0.12 | 0.12 | |
| Na2B4O7 | 2.45 | 2.45 | 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, Q.; Du, X.; Chen, T.; Taha, M.F. Impact of Nitrogen on Downy Mildew Infection and Its Effects on Growth and Physiological Traits in Early Growth Stages of Cucumber. Horticulturae 2025, 11, 1182. https://doi.org/10.3390/horticulturae11101182
Wang Y, Shi Q, Du X, Chen T, Taha MF. Impact of Nitrogen on Downy Mildew Infection and Its Effects on Growth and Physiological Traits in Early Growth Stages of Cucumber. Horticulturae. 2025; 11(10):1182. https://doi.org/10.3390/horticulturae11101182
Chicago/Turabian StyleWang, Yafei, Qiang Shi, Xiaoxue Du, Tianhua Chen, and Mohamed Farag Taha. 2025. "Impact of Nitrogen on Downy Mildew Infection and Its Effects on Growth and Physiological Traits in Early Growth Stages of Cucumber" Horticulturae 11, no. 10: 1182. https://doi.org/10.3390/horticulturae11101182
APA StyleWang, Y., Shi, Q., Du, X., Chen, T., & Taha, M. F. (2025). Impact of Nitrogen on Downy Mildew Infection and Its Effects on Growth and Physiological Traits in Early Growth Stages of Cucumber. Horticulturae, 11(10), 1182. https://doi.org/10.3390/horticulturae11101182







