Development of Fruit-Specific Spectral Indices and Endmember-Based Analysis for Apple Cultivar Classification Using Hyperspectral Imaging
Abstract
1. Introduction
2. Materials and Methods
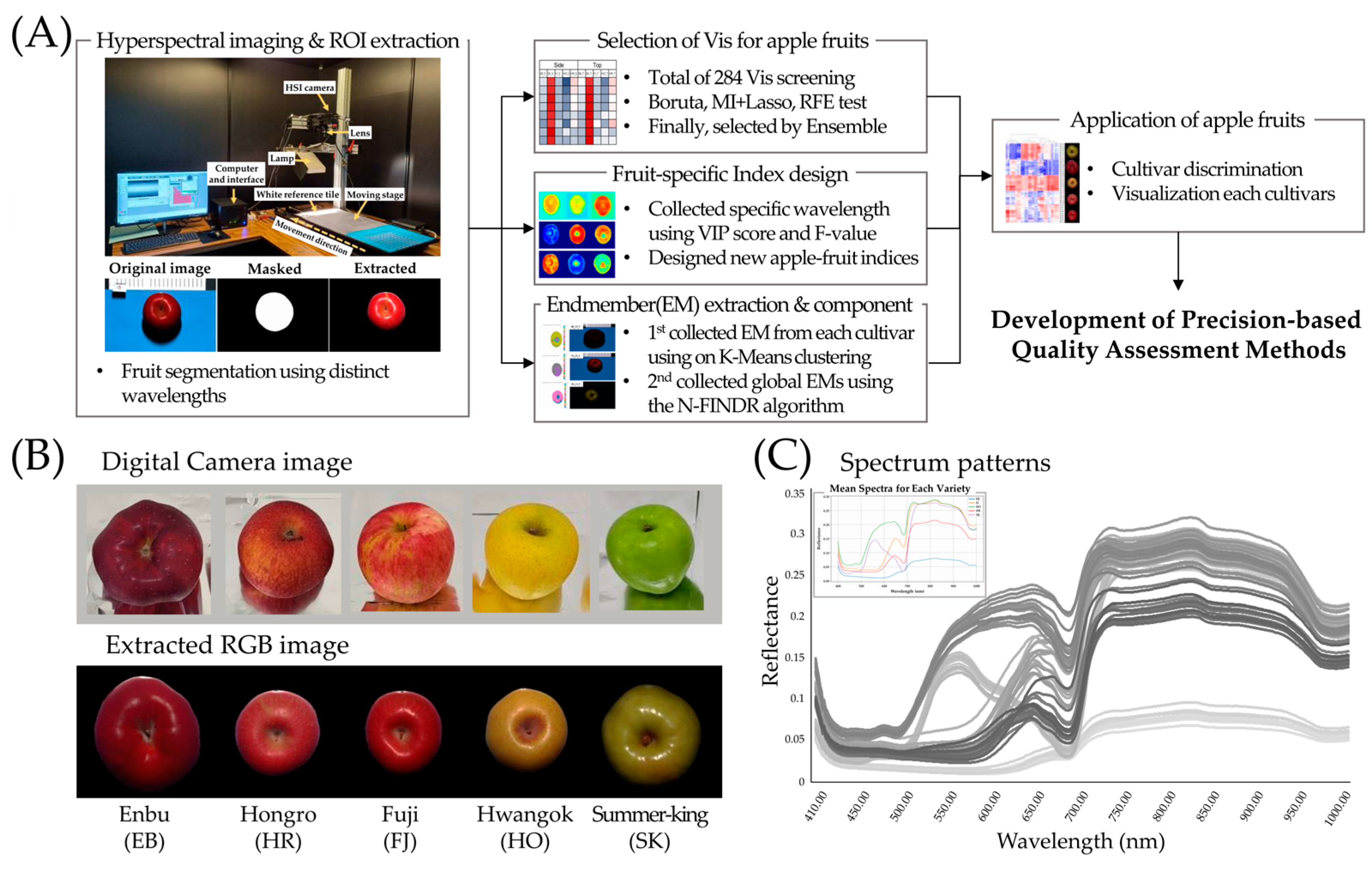
2.1. Plant Materials and Hyperspectral Imaging
2.2. Calibration and ROI Extraction
2.3. Feature Selection and Spectral Discrimination Workflow for Hyperspectral Apple Data
2.4. Spectral Differentiation of Five Apple Cultivars
2.5. Cultivar-Specific Optimal Index Selection
2.6. Spectral Unmixing for Identifying Apple Fruit Specific Endmembers
3. Results
3.1. Extraction of Spectral Reflectance Data from Apple Fruits
3.2. Identification of Robust Vegetation Indices Reflecting Pigment Diversity in Apple Cultivars
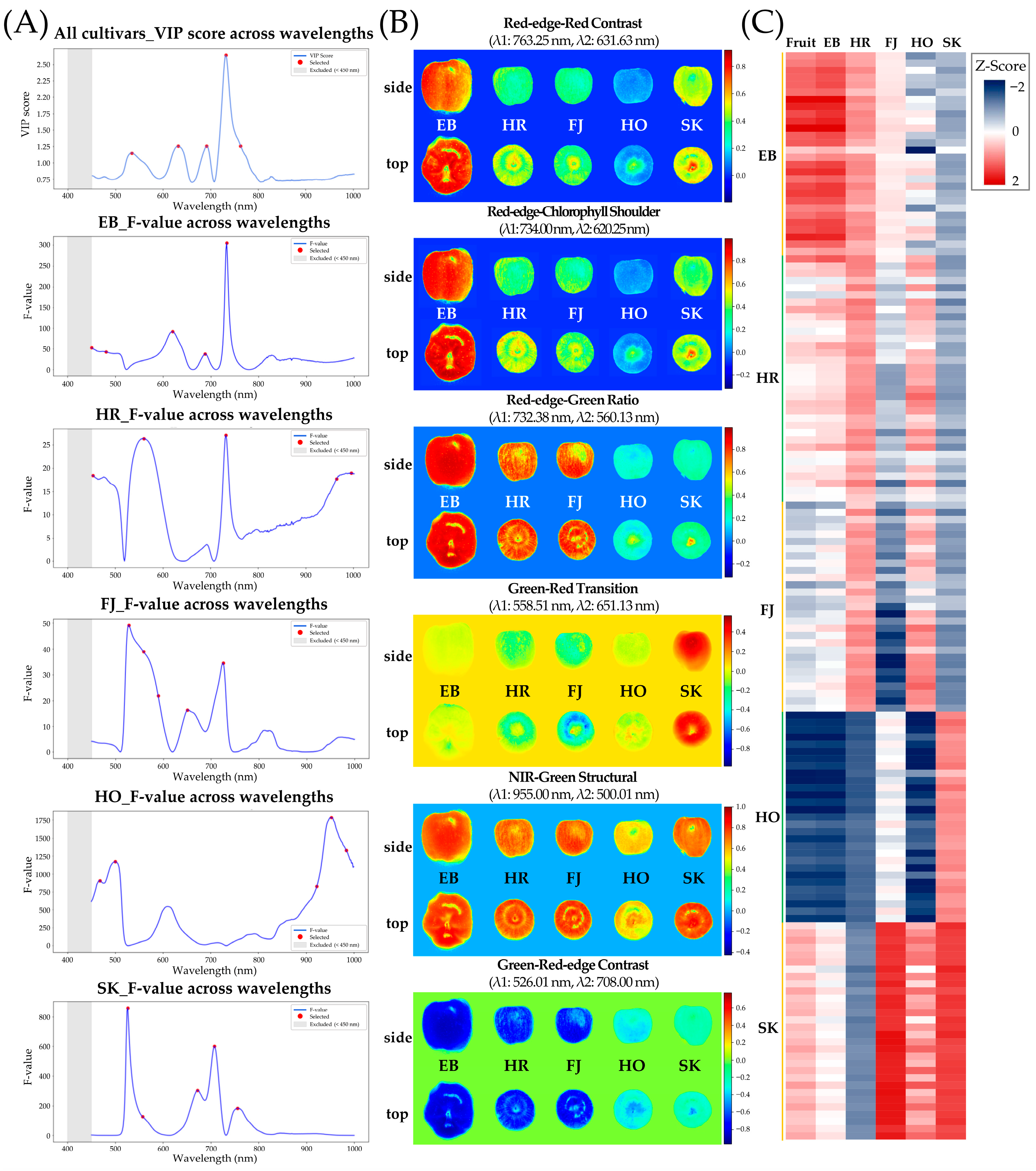
3.3. Development of Cultivar-Specific Spectral Indices Based on Pigment-Associated Bands in Apples
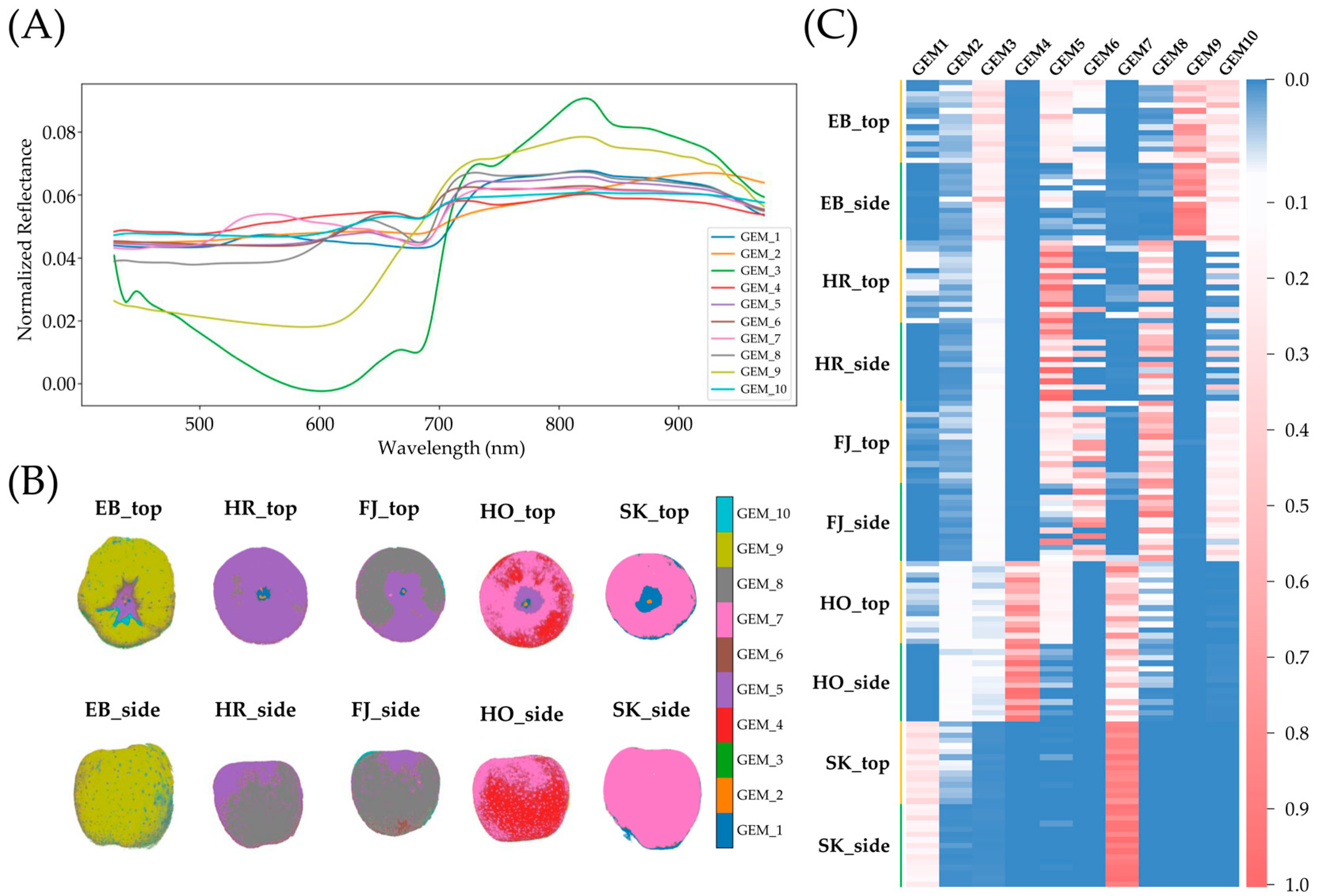
3.4. Spectral Unmixing and Endmember Mapping Across Cultivars
4. Discussion
4.1. Ensemble-Based Selection of Vegetation Indices for Apple Fruits
4.2. Development of Pigment-Sensitive Spectral Indices Through Dual Statistical Band Selection
4.3. Spectral Unmixing Optimization for Robust Endmember Extraction in Apple Fruits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Bonany, J.; Buehler, A.; Carbó, J.; Codarin, S.; Donati, F.; Echeverria, G.; Egger, S.; Guerra, W.; Hilaire, C.; Höller, I.; et al. Consumer eating quality acceptance of new apple varieties in different European countries. Food Qual. Prefer. 2013, 30, 250–259. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral imaging–an emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Baranowski, P.; Mazurek, W.; Wozniak, J.; Majewska, U. Detection of early bruises in apples using hyperspectral data and thermal imaging. J. Food Eng. 2012, 110, 345–355. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; Vigneault, C. Detecting chilling injury in Red Delicious apple using hyperspectral imaging and neural networks. Postharvest Biol. Technol. 2009, 52, 1–8. [Google Scholar] [CrossRef]
- Çetin, N.; Karaman, K.; Kavuncuoğlu, E.; Yıldırım, B.; Jahanbakhshi, A. Using hyperspectral imaging technology and machine learning algorithms for assessing internal quality parameters of apple fruits. Chemom. Intell. Lab. Syst. 2022, 230, 104650. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-Throughput Phenotyping of Maize Leaf Physiological and Biochemical Traits Using Hyperspectral Reflectance. Plant Physiol. 2016, 173, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.; Zur, Y.; Stark, R.; Gritz, U. Non-destructive and remote sensing techniques for estimation of vegetation status. In Proceedings of the 3rd European Conference on Precision Agriculture, Montpelier, France, 18–20 June 2001. [Google Scholar]
- Li, J.; Zhang, Y.; Gu, L.; Li, Z.; Li, J.; Zhang, Q.; Zhang, Z.; Song, L. Seasonal variations in the relationship between sun-induced chlorophyll fluorescence and photosynthetic capacity from the leaf to canopy level in a rice crop. J. Exp. Bot. 2020, 71, 7179–7197. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Deering, D.; Schell, J.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Legacy CDMS: Singapore, 1974. [Google Scholar]
- Gamon, J.A.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of fungal infections in strawberry fruit by VNIR/SWIR hyperspectral imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Jeong, S.W.; Lyu, J.I.; Jeong, H.; Baek, J.; Moon, J.-K.; Lee, C.; Choi, M.-G.; Kim, K.-H.; Park, Y.-I. SUnSeT: Spectral unmixing of hyperspectral images for phenotyping soybean seed traits. Plant Cell Rep. 2024, 43, 164. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Saeys, Y.; Abeel, T.; Van de Peer, Y. Robust feature selection using ensemble feature selection techniques. In Proceedings of the Joint European Conference on Machine Learning and Knowledge Discovery in Databases, Antwerp, Belgium, 14–18 September 2008; pp. 313–325. [Google Scholar]
- Barnes, R.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. J. Chemom. Soc. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.; Noland, T.L.; Sampson, P. Vegetation stress detection through chlorophyll a + b estimation and fluorescence effects on hyperspectral imagery. J. Environ. Qual. 2002, 31, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Chang, C.-I. Hyperspectral Data Exploitation: Theory and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lightenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Nagy, A.; Szabó, A.; Elbeltagi, A.; Nxumalo, G.S.; Bódi, E.B.; Tamás, J. Hyperspectral indices data fusion-based machine learning enhanced by MRMR algorithm for estimating maize chlorophyll content. Front. Plant Sci. 2024, 15, 1419316. [Google Scholar] [CrossRef]
- Zhou, J.; Li, F.; Wang, X.; Yin, H.; Zhang, W.; Du, J.; Pu, H. Hyperspectral and fluorescence imaging approaches for nondestructive detection of rice chlorophyll. Plants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.; He, X.; Zhang, C.; Li, J.; Huang, W. Detection of early bruises on apples using hyperspectral reflectance imaging coupled with optimal wavelengths selection and improved watershed segmentation algorithm. J. Sci. Food Agric. 2023, 103, 6689–6705. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, B.; Li, J.; Liu, C.; Huang, W.; Tian, X. Prediction of soluble solids content of apple using the combination of spectra and textural features of hyperspectral reflectance imaging data. Postharvest Biol. Technol. 2016, 121, 51–61. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar]
- Zhang, B.; Zhou, J. Hyperspectral imaging and their applications in the nondestructive quality assessment of fruits and vegetables. In Hyperspectral Imaging in Agriculture, Food and Environment; IntechOpen: London, UK, 2018; p. 27. [Google Scholar]
- Qian, Y.; Ye, M.; Zhou, J. Hyperspectral image classification based on structured sparse logistic regression and three-dimensional wavelet texture features. IEEE Trans. Geosci. Remote Sens. 2012, 51, 2276–2291. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Chen, Y.; Tan, K.; Wang, L.; Yan, X. Stability analysis of hyperspectral band selection algorithms based on neighborhood rough set theory for classification. Chemom. Intell. Lab. Syst. 2017, 169, 35–44. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Li, X.; Yu, K.-Q.; Cheng, F.; He, Y. Hyperspectral imaging for determining pigment contents in cucumber leaves in response to angular leaf spot disease. Sci. Rep. 2016, 6, 27790. [Google Scholar] [CrossRef] [PubMed]
- Van Beers, R.; Aernouts, B.; Watte, R.; Schenk, A.; Nicolai, B.; Saeys, W. Effect of maturation on the bulk optical properties of apple skin and cortex in the 500–1850 nm wavelength range. J. Food Eng. 2017, 214, 79–89. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; Vigneault, C.; Qiao, J.; ElSayed, A. Early detection of apple bruises on different background colors using hyperspectral imaging. LWT-Food Sci. Technol. 2008, 41, 337–345. [Google Scholar] [CrossRef]
- Chang, C.-I.; Plaza, A. A fast iterative algorithm for implementation of pixel purity index. IEEE Geosci. Remote Sens. Lett. 2006, 3, 63–67. [Google Scholar] [CrossRef]
- Bioucas-Dias, J.M.; Plaza, A.; Dobigeon, N.; Parente, M.; Du, Q.; Gader, P.; Chanussot, J. Hyperspectral unmixing overview: Geometrical, statistical, and sparse regression-based approaches. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 354–379. [Google Scholar] [CrossRef]
- Borsoi, R.A.; Imbiriba, T.; Bermudez, J.C.M.; Richard, C.; Chanussot, J.; Drumetz, L.; Tourneret, J.-Y.; Zare, A.; Jutten, C. Spectral variability in hyperspectral data unmixing: A comprehensive review. IEEE Geosci. Remote Sens. Mag. 2021, 9, 223–270. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Dias, J.M. Vertex component analysis: A fast algorithm to unmix hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2005, 43, 898–910. [Google Scholar] [CrossRef]
- Winter, M.E. N-FINDR: An algorithm for fast autonomous spectral end-member determination in hyperspectral data. In Imaging Spectrometry V; SPIE: Bellingham, WA, USA, 1999; pp. 266–275. [Google Scholar]
- Xu, L.; Li, J.; Wong, A.; Peng, J. Kp-means: A clustering algorithm of k “purified” means for hyperspectral endmember estimation. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1787–1791. [Google Scholar]
- Bilius, L.B.; Pentiuc, S.G. Unsupervised clustering for hyperspectral images. Symmetry 2020, 12, 277. [Google Scholar] [CrossRef]
- Qin, J.; Burks, T.F.; Ritenour, M.A.; Bonn, W.G. Detection of citrus canker using hyperspectral reflectance imaging with spectral information divergence. J. Food Eng. 2009, 93, 183–191. [Google Scholar] [CrossRef]
- Haut, J.M.; Paoletti, M.; Plaza, J.; Plaza, A. Cloud implementation of the K-means algorithm for hyperspectral image analysis. J. Supercomput. 2017, 73, 514–529. [Google Scholar] [CrossRef]
- Cecilia, A. Evaluation of Hyperspectral Unmixing Methods: A Comparative Study for Very-High Spatial Resolution Hyperspectral Images. In Proceedings of the 2024 IEEE Southwest Symposium on Image Analysis and Interpretation (SSIAI), Santa Fe, NM, USA, 17–19 March 2024; pp. 53–56. [Google Scholar]
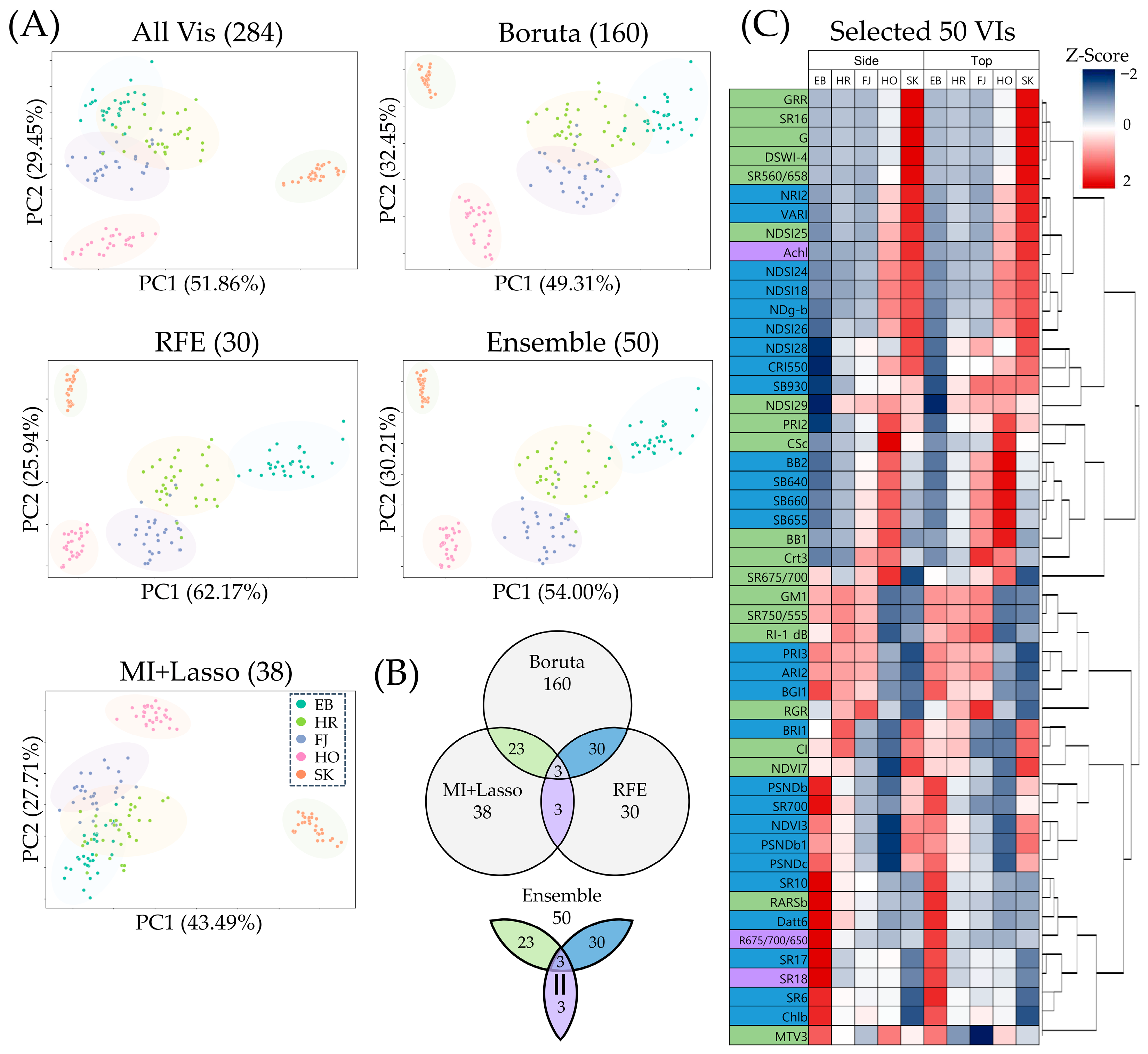
| Method | Selected VIs | Accuracy (%) | Macro F1 Score |
|---|---|---|---|
| All VIs | 284 | 97.78 | 0.977 |
| Boruta | 160 | 95.56 | 0.955 |
| MI+Lasso | 38 | 97.78 | 0.977 |
| RFE | 30 | 95.56 | 0.955 |
| Ensemble | 50 | 95.56 | 0.955 |
| Index | Selected Wavelengths (nm) | λ1:λ2 (nm) | Index Type | Acc. (%) | Derivation & Intent | Linked Pigment/ Optical Property | Refs |
|---|---|---|---|---|---|---|---|
| Red-edge–Red Contrast Index | 534.13, 631.63, 691.75, 732.38, 763.25 | 763.25:631.63 | NDSI | 89 | PLS-DA VIP (global discriminant wavelengths) | 630–640 nm: chlorophyll absorption shoulder; 760–763 nm: red-edge/structural scattering | [28,29] |
| Red-edge–Chlorophyll Shoulder Index | 451.26, 482.13, 620.25, 688.50, 734.00 | 734.00:620.25 | NDSI | 99 | ANOVA (EB vs. others; spectral difference) | 620 nm: chlorophyll a red absorption; 734 nm: red-edge transition | [29,30] |
| Red-edge–Green Ratio Index | 452.88, 560.13, 732.38, 964.75, 995.62 | 732.38:560.13 | NDSI | 87 | ANOVA (HR vs. others) | 560 nm: green/putative anthocyanin absorption; 732 nm: red-edge/NIR | [30] |
| Green–Red Transition DSI | 527.63, 558.51, 589.38, 651.13, 725.88 | 558.51:651.13 | DSI | 91 | ANOVA (FJ vs. others) | 558 nm: green (anthocyanin-sensitive region); 651 nm: chlorophyll a absorption | [28,31] |
| NIR–Green Structural Index | 467.51, 500.01, 924.12, 955.00, 985.87 | 955.00:500.01 | NDSI | 100 | ANOVA (HO vs. others) | 500 nm: carotenoid/anthocyanin-sensitive region; 955 nm: NIR structural scattering and water absorption | [32] |
| Green–Red-edge Contrast Index | 526.01, 556.88, 672.25, 708.00, 758.38 | 526.01:708.00 | NDSI | 100 | ANOVA (SK vs. others) | 525–530 nm: green (chlorophyll b/anthocyanin-related); 708 nm: red-edge onset | [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Jeong, H.; Lee, S.; Ga, E.; Baek, J.; Kim, S.L.; Kang, S.-H.; Park, Y.-I.; Kim, K.-H.; Lyu, J.I. Development of Fruit-Specific Spectral Indices and Endmember-Based Analysis for Apple Cultivar Classification Using Hyperspectral Imaging. Horticulturae 2025, 11, 1177. https://doi.org/10.3390/horticulturae11101177
Lee Y-J, Jeong H, Lee S, Ga E, Baek J, Kim SL, Kang S-H, Park Y-I, Kim K-H, Lyu JI. Development of Fruit-Specific Spectral Indices and Endmember-Based Analysis for Apple Cultivar Classification Using Hyperspectral Imaging. Horticulturae. 2025; 11(10):1177. https://doi.org/10.3390/horticulturae11101177
Chicago/Turabian StyleLee, Ye-Jin, HwangWeon Jeong, Seoyeon Lee, Eunji Ga, JeongHo Baek, Song Lim Kim, Sang-Ho Kang, Youn-Il Park, Kyung-Hwan Kim, and Jae Il Lyu. 2025. "Development of Fruit-Specific Spectral Indices and Endmember-Based Analysis for Apple Cultivar Classification Using Hyperspectral Imaging" Horticulturae 11, no. 10: 1177. https://doi.org/10.3390/horticulturae11101177
APA StyleLee, Y.-J., Jeong, H., Lee, S., Ga, E., Baek, J., Kim, S. L., Kang, S.-H., Park, Y.-I., Kim, K.-H., & Lyu, J. I. (2025). Development of Fruit-Specific Spectral Indices and Endmember-Based Analysis for Apple Cultivar Classification Using Hyperspectral Imaging. Horticulturae, 11(10), 1177. https://doi.org/10.3390/horticulturae11101177








