Manganese Deficiency Exacerbates Boron Deficiency-Induced Corky Split Vein in Citrus by Disrupting Photosynthetic Physiology and Enhancing Lignin Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Gas Exchange and Chlorophyll Fluorescence Parameters
2.3. Determination of Chlorophyll Content
2.4. Measurement of Plant Growth Parameters, Mineral Nutrient Concentrations, and Lignin Contents
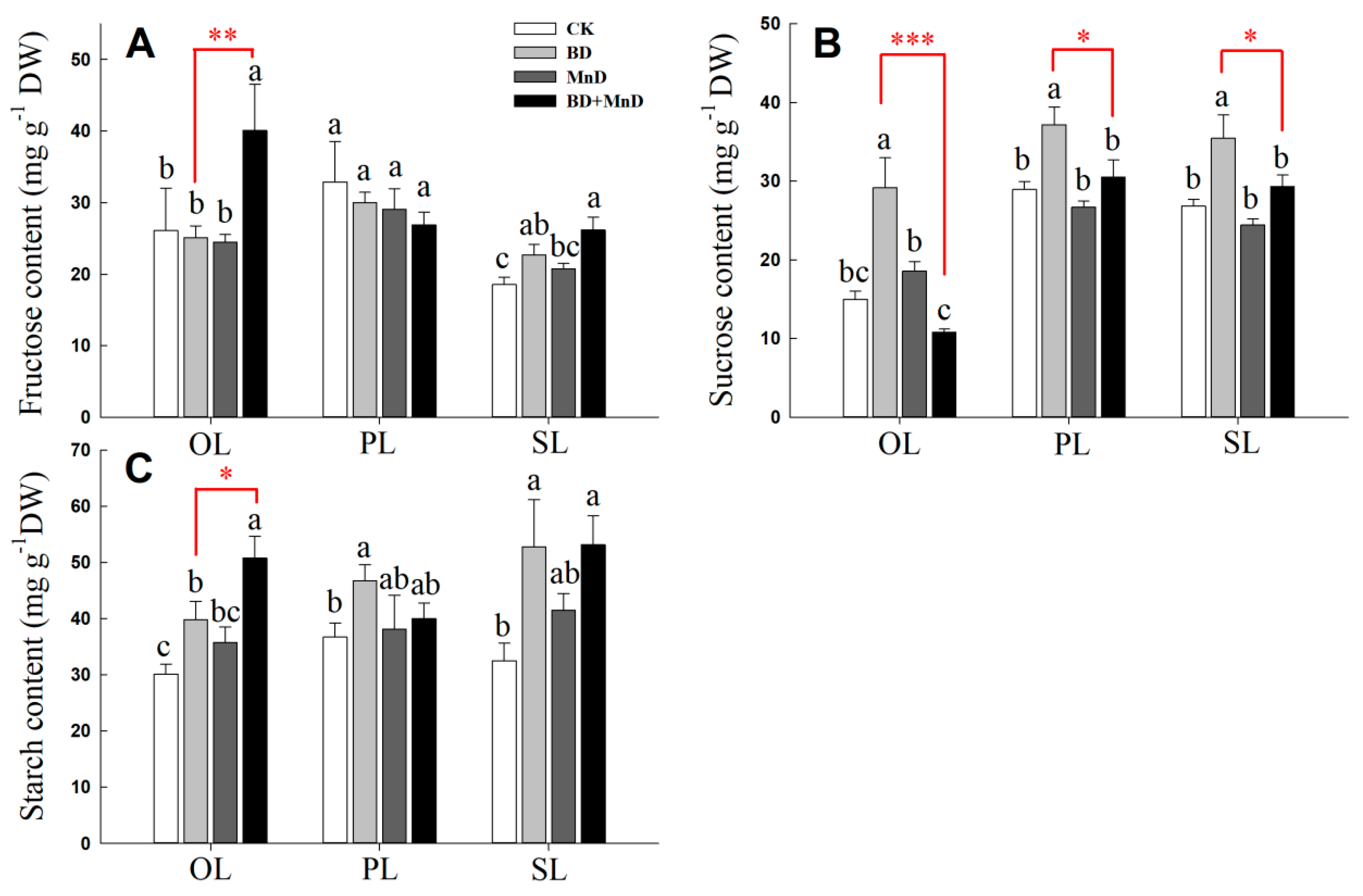
2.5. Determination of Fructose, Sucrose and Starch Contents
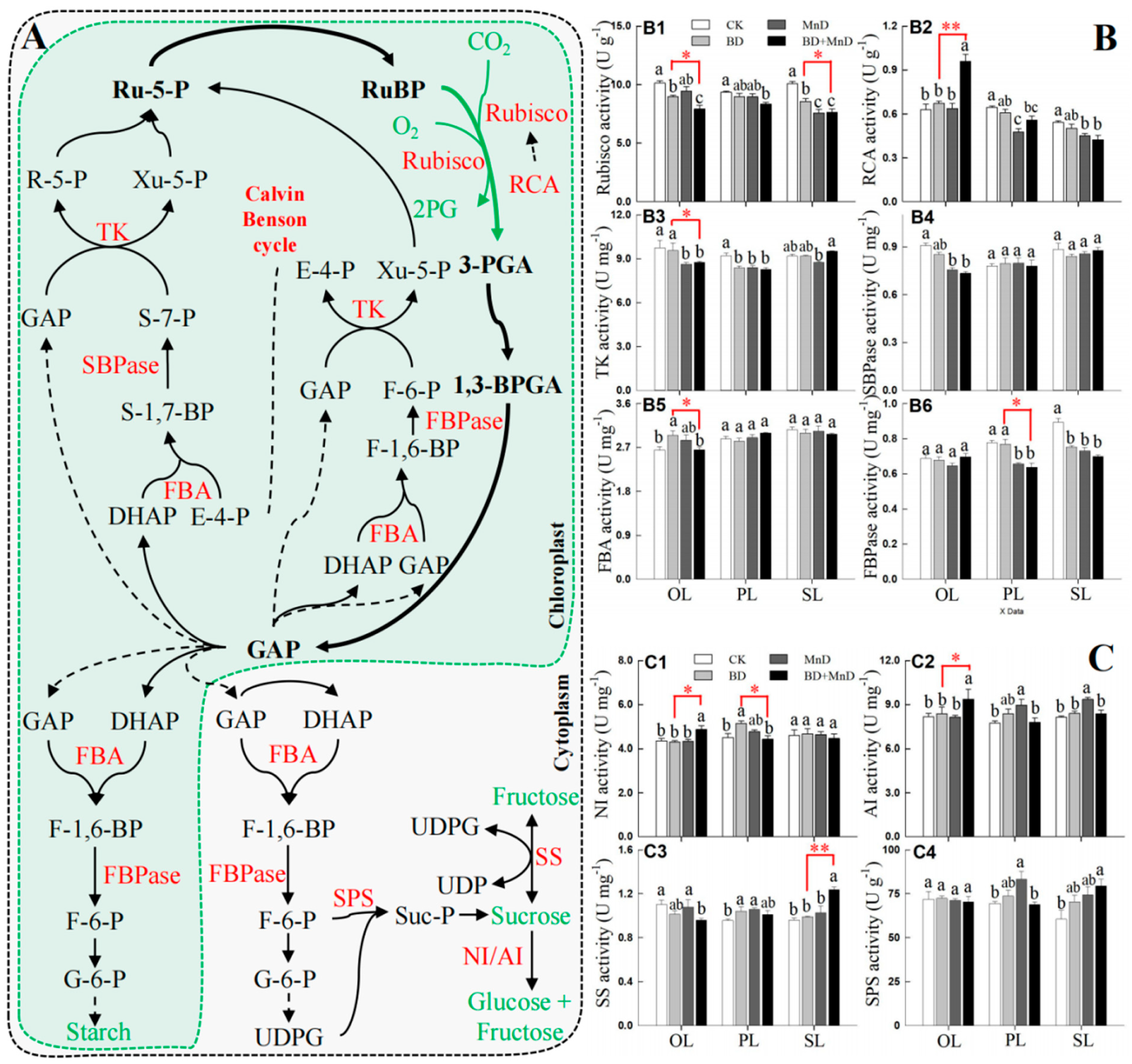
2.6. Measurement of Enzyme Activity Related to Photosynthesis
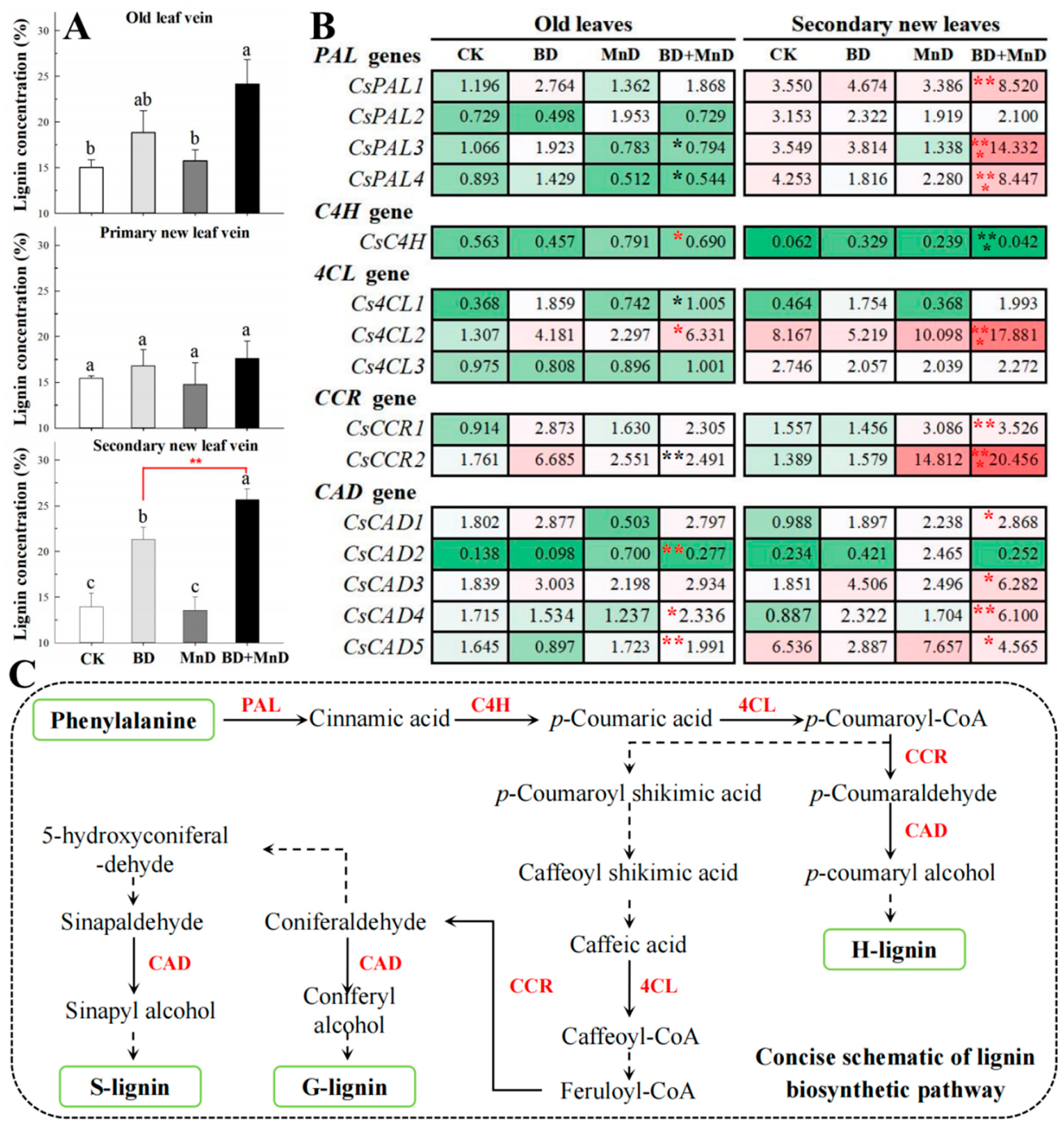
2.7. Expression Analysis of Key Enzyme Genes Involved in Lignin Biosynthesis
2.8. Experimental Design and Statistical Analysis
3. Results
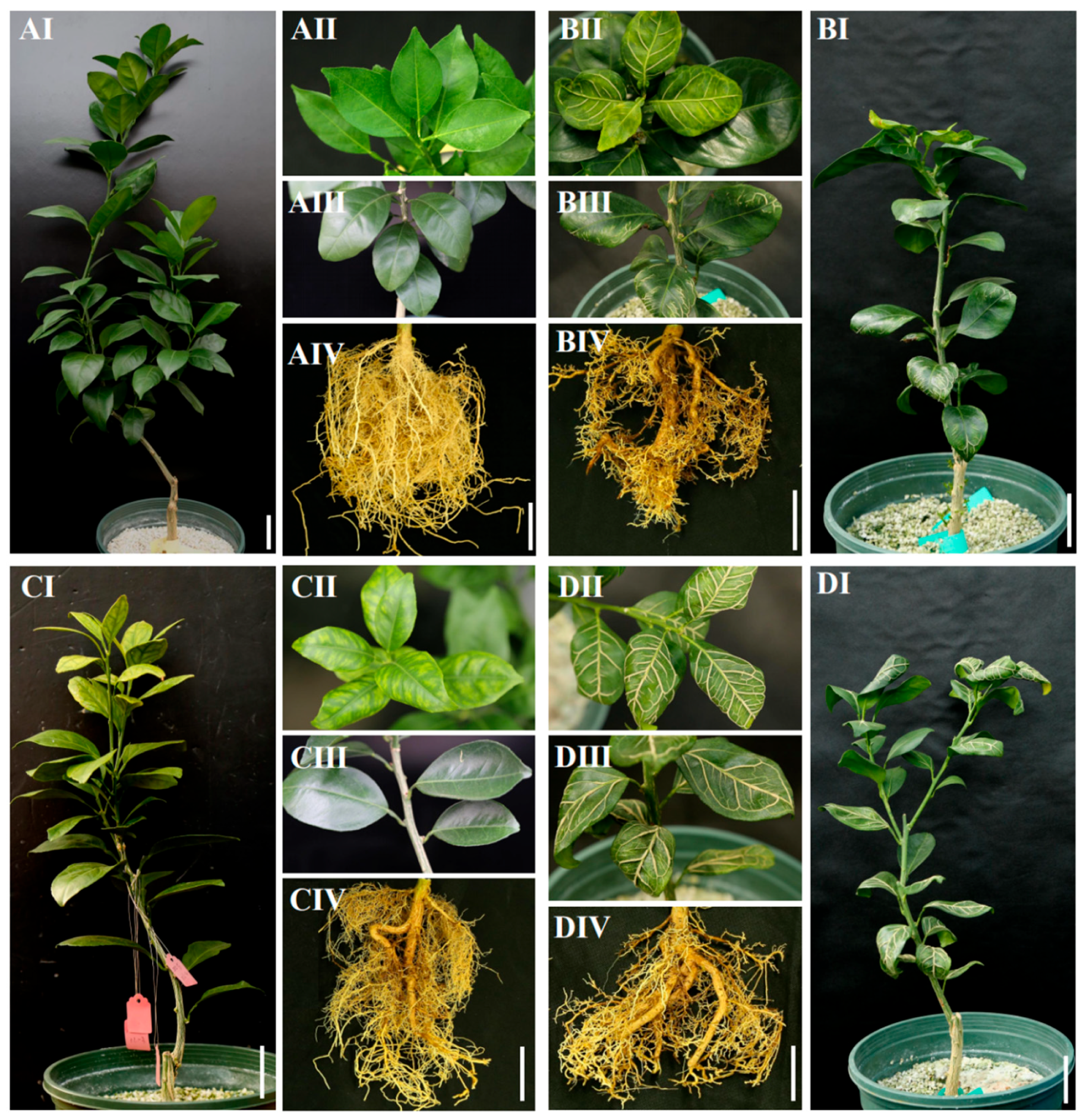
3.1. Visible Symptoms and Plant Growth
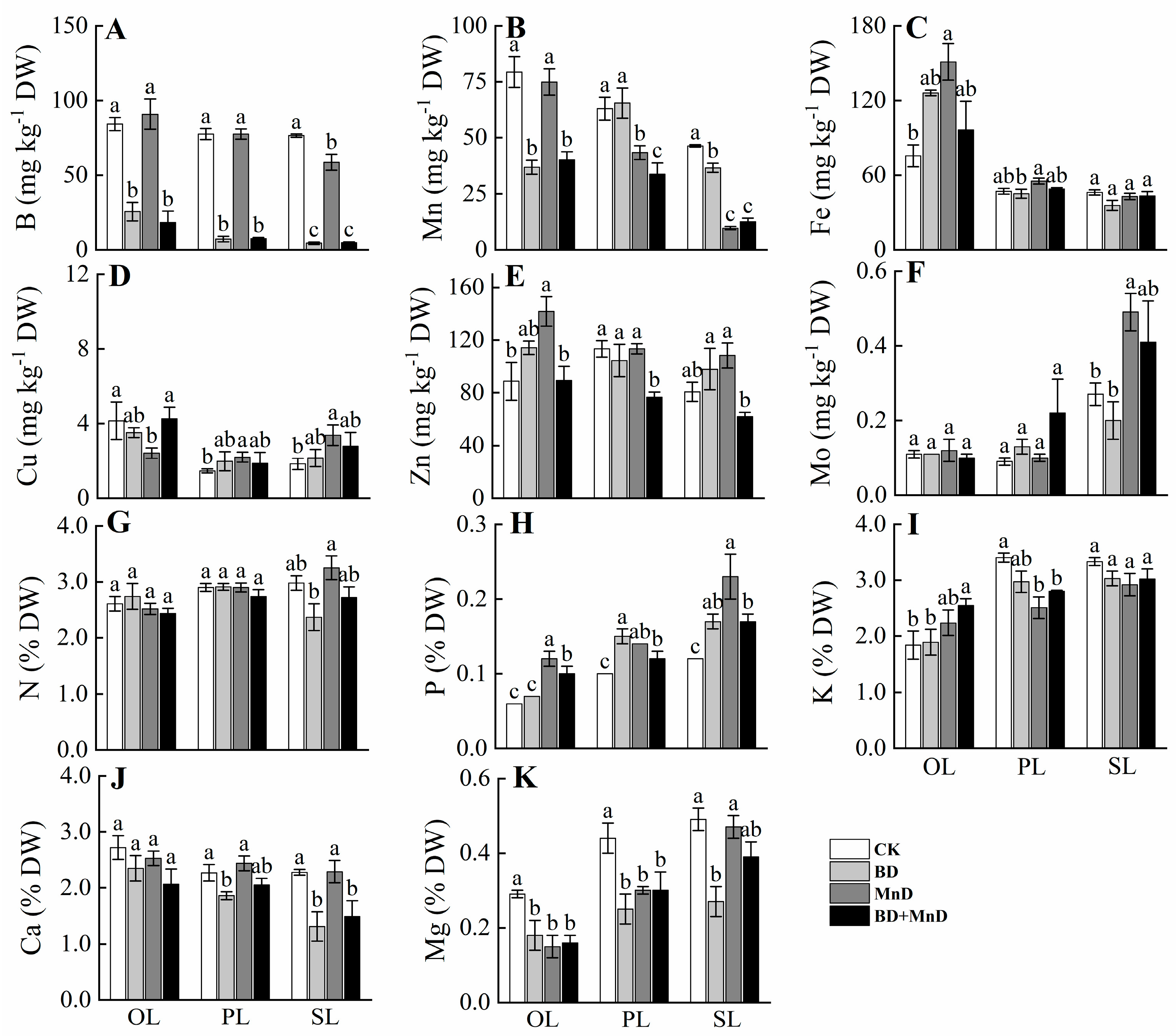
3.2. Mineral Nutrient Concentrations
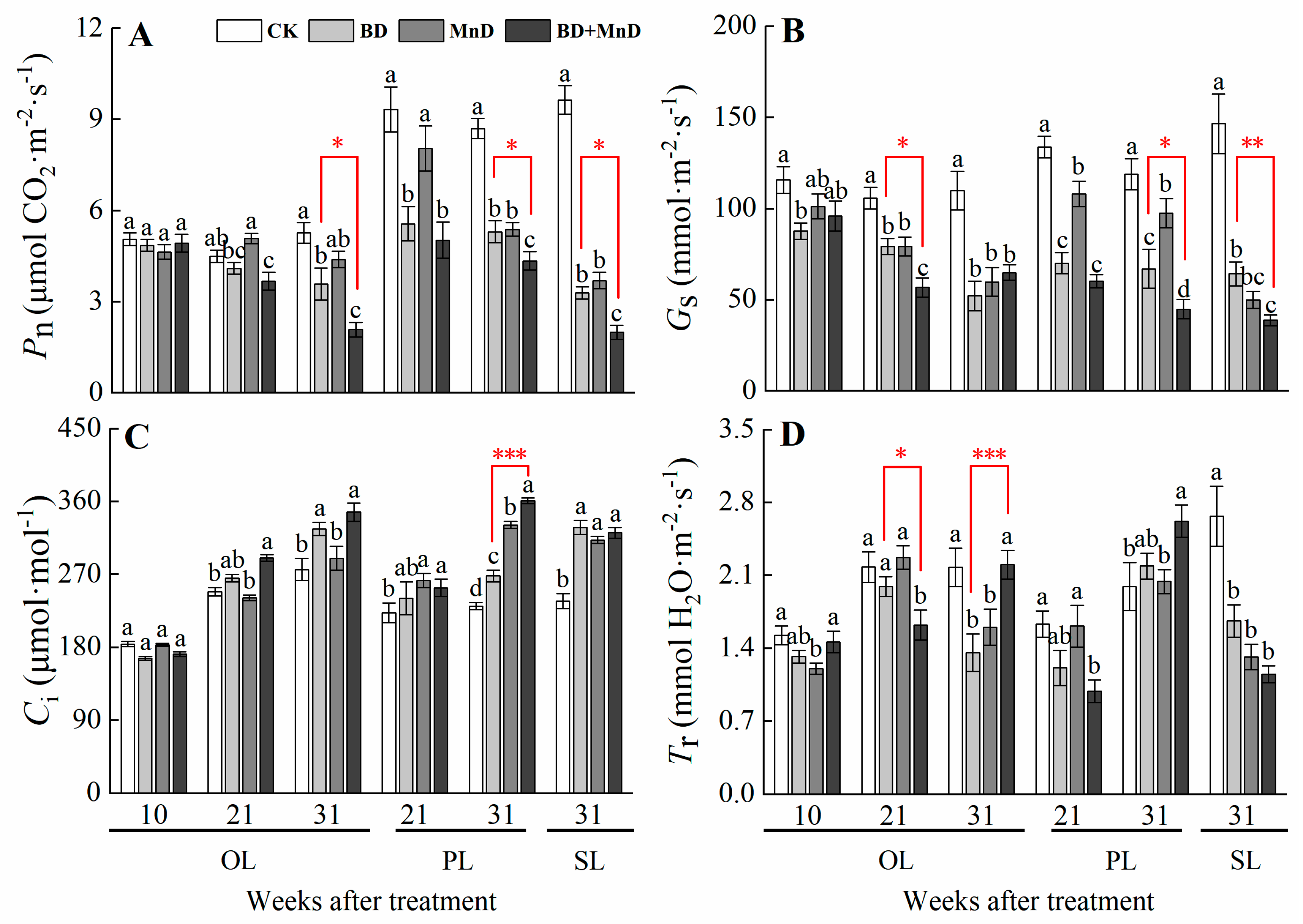
3.3. Gas Exchange Parameters and Photosynthetic Pigment Content
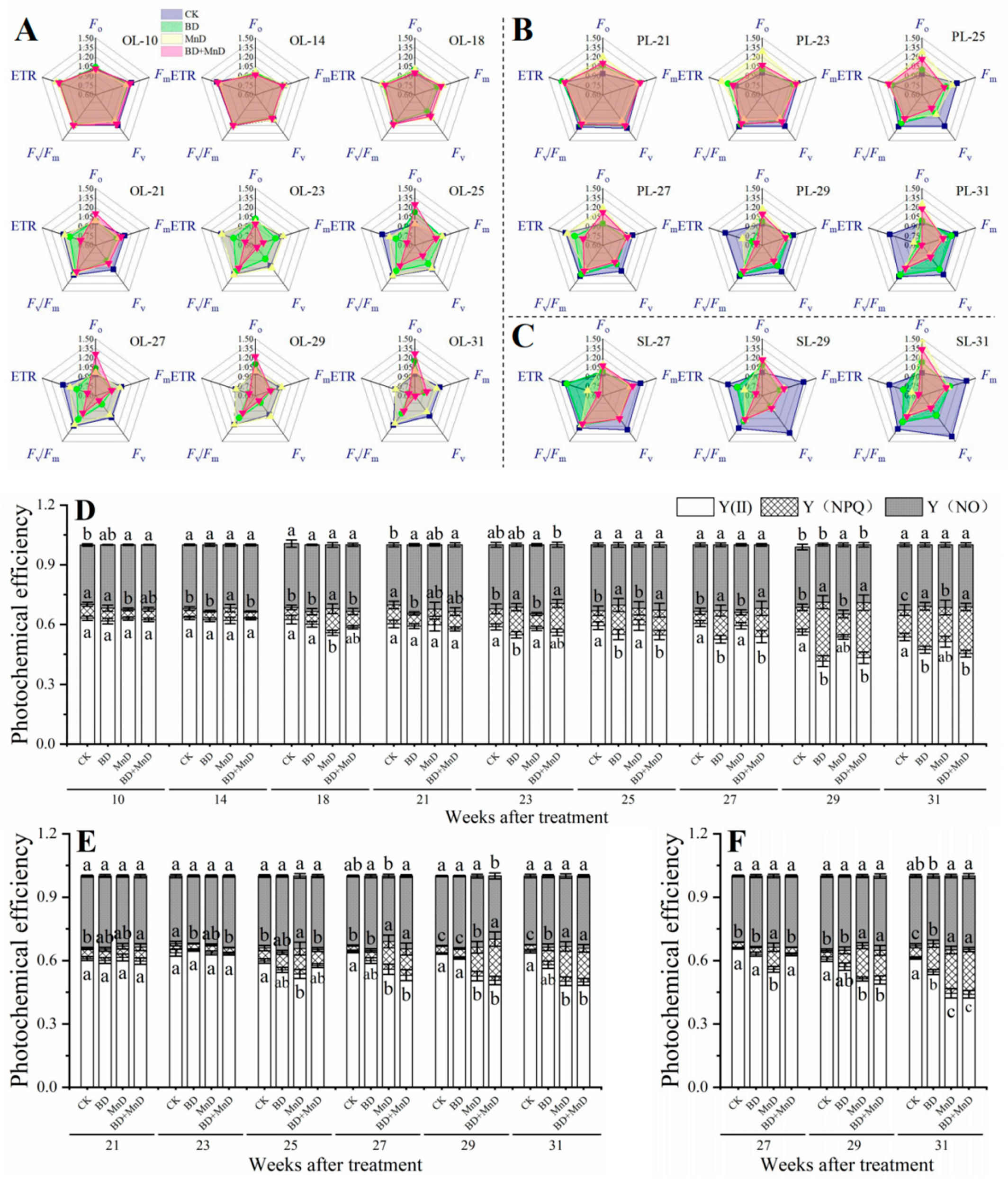
3.4. Chlorophyll Fluorescence Parameters
3.5. Leaf Photosynthetic Product Content and Related Enzyme Activity
3.6. Lignin Content and Related Key Gene Expression Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSV | Corky split vein |
| BD | Boron deficiency |
| MnD | Manganese deficiency |
| BD + MnD | Combined boron and manganese deficiency |
| CK | Control |
| OL | Old leaf |
| PL | Primary new leaf |
| SL | Secondary new leaf |
| OS | Old scion stem |
| PNS | Primarynew scion stem |
| SNS | Secondary new scion stem |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Car | Carotenoid |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Ci | Intercellular CO2 concentration |
| Gs | Stomatal conductance |
| Fo | Minimal fluorescence |
| Fm | Maximal fluorescence |
| Fv | Variable fluorescence |
| Fv/Fm | Maximal photochemical efficiency of PSII |
| ETR | Electron transport efficiency |
| Y(II) | Actual photochemical efficiency |
| Y(NPQ) | Quantum yield of regulated energy dissipation |
| Y(NO) | Quantum yield of non-regulated energy dissipation |
| Rubisco | Ribulose bisphosphate carboxylase/oxygenase |
| RCA | Rubisco activase |
| TK | Rubisco, thymidine kinase |
| SBPase | Sedoheptulose 1,7-bisphosphatase |
| FBA | Fructose 1,6-bisphosphate aldolase |
| FBPase | Fructose 1,6-bisphosphatase |
| NI | Neutral invertas |
| AI | Acid invertase |
| SS | Sucrose synthetase |
References
- Matthes, M.S.; Robil, J.M.; Mcsteen, P. From element to development: The power of the essential micronutrient boron to shape morphological processes in plants. J. Exp. Bot. 2020, 71, 1681–1693. [Google Scholar] [CrossRef]
- Bolaños, L.; Abreu, I.; Bonilla, I.; Camacho-Cristóbal, J.J.; Reguera, M. What can boron deficiency symptoms tell us about its function and regulation. Plants 2023, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.B.; Yang, L.T.; Li, Y.; Xu, J.; Liao, T.T.; Chen, Y.B.; Chen, L.S. Effects of boron deficiency on major metabolites, key enzymes and gas exchange in leaves and roots of Citrus sinensis seedlings. Tree Physiol. 2014, 34, 608–618. [Google Scholar] [CrossRef]
- Chatter, M.; Tabi, Z.; Galli, M.; Malcomber, S.; Buck, A.; Muszynski, M.; Gallavotti, A. The boron efflux transporter rotten ear is required for maize inflorescence development and fertility. Plant Cell 2014, 26, 2962–2977. [Google Scholar] [CrossRef]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef]
- Zhou, G.F.; Sun, X.N.; Zhang, L.P.; Zeng, X.L.; Liu, G.D.; Sheng, O. Lignin metabolism plays an essential role in the formation of corky split vein caused by boron deficiency in ‘Newhall’ navel orange (Citrus sinensis Osb.). Sci. Hortic. 2022, 294, 110763. [Google Scholar] [CrossRef]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Wang, N.N.; Yang, C.Q.; Pan, Z.Y.; Liu, Y.Z.; Peng, S.A. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef]
- Cakmak, I.; Kurz, H.; Marschner, H. Short-term effects of boron, germanium and high light intensity on membrane permeability in boron deficient leaves of sunflower. Physiol. Plant. 1995, 95, 11–18. [Google Scholar] [CrossRef]
- Dell, B.; Huang, L. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Xiao, J.X.; Yan, X.; Peng, S.A.; Deng, X.X.; Fang, Y.W. Relationship between boron deficiency occurrence and annual changes in contents of boron and sugar of ‘Newhall’ navel orange. Acta Hortic. Sin. 2006, 33, 356–359. [Google Scholar]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yang, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Li, S.; Yang, C.Q.; Peng, S.A. Effects of boron-deficiency on anatomical structures in the leaf main vein and fruit mesocarp of pummelo [Citrus grandis (L.) Osbeck]. Korean J. Hortic. Sci. 2013, 88, 693–700. [Google Scholar] [CrossRef]
- Ling, L.L.; Peng, L.Z.; Chun, C.P.; Cao, L.; Jiang, C.L. Characteristics analysis of microelement contents in ‘Newhall’ navel orange leaves in Southern Jiangxi Province. Acta Hortic. Sin. 2010, 37, 1388–1394. [Google Scholar]
- Wang, R.D.; Jiang, C.C.; Liu, G.D.; Wang, Y.H.; Peng, S.A.; Zeng, Q.L. Status and analysis on available boron content in soil of Gannan navel orange orchards. S. China Fruits 2011, 40, 1–3+7. [Google Scholar]
- Liu, X.S.; Zhang, Z.Y.; Wei, J.P. Influencing factors and intensity change of soil pH value in South Jiangxi Province. Bull. Soil Water Conserv. 2021, 41, 100–105. [Google Scholar]
- Yang, C.Q.; Liu, Y.Z.; An, J.C.; Li, S.; Jin, L.F.; Zhou, G.F.; Wei, Q.J.; Yan, H.Q.; Wang, N.N.; Fu, L.N.; et al. Digital gene expression analysis of corky split vein caused by boron deficiency in ‘Newhall’ navel orange (Citrus sinensis Osbeck) for selecting differentially expressed genes related to vascular hypertrophy. PLoS ONE 2013, 8, e65737. [Google Scholar] [CrossRef]
- Liu, G.D.; Dong, X.C.; Liu, L.C.; Wu, L.S.; Peng, S.A.; Jiang, C.C. Metabolic profiling reveals altered pattern of central metabolism in navel orange plants as a result of boron deficiency. Physiol. Plant. 2015, 153, 513–524. [Google Scholar] [CrossRef]
- Zhou, G.F.; Li, B.X.; Zhang, L.P.; Sheng, O.; Wei, Q.J.; Yao, F.X.; Guan, G.; Liu, G.D. Physiological and nutritional responses of ‘HB’ Pummelo [Citrus grandis (L.) Osbeck ‘Hirado Buntan’] to the combined effects of low pH levels and boron deficiency. HortScience 2020, 55, 449–456. [Google Scholar] [CrossRef]
- Zhou, G.F.; Peng, S.A.; Liu, Y.Z.; Wei, Q.J.; Han, J.; Islam, M.Z. The physiological and nutritional responses of seven different citrus rootstock seedlings to boron deficiency. Trees-Struct. Funct. 2014, 28, 295–307. [Google Scholar] [CrossRef]
- Zhou, G.F.; Zhang, L.P.; Li, B.X.; Sheng, O.; Wei, Q.J.; Yao, F.X.; Guan, G.; Liu, G.D. Genome-wide identification of long non-coding RNA in trifoliate orange (Poncirus trifoliata L. Raf) leaves in response to boron deficiency. Int. J. Mol. Sci. 2019, 20, 5419. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y.; Achor, D.S. Early events of citrus greening (Huanglongbing) disease development at the ultrastructural level. Phytopathology 2010, 100, 949–958. [Google Scholar] [CrossRef]
- Huang, J.H.; Xu, J.; Ye, X.; Luo, T.Y.; Ren, L.H.; Fan, G.C.; Qi, Y.P.; Li, Q.; Ferrarezi, R.S.; Chen, L.S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees-Struct. Funct. 2019, 33, 171–182. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.F.; Cai, L.Y.; Lai, N.W.; Deng, C.L.; Guo, J.X.; Yang, L.T.; Chen, L.S. Molecular and physiological mechanisms underlying magnesium-deficiency-induced enlargement, cracking and lignification of Citrus sinensis leaf veins. Tree Physiol. 2020, 40, 1277–1291. [Google Scholar] [CrossRef]
- Liang, D.; Ma, F.W. Photosynthesis and assimilate transport in fruit trees. In Modern Fruit Tree Biology; Li, T.Z., Zhang, Z.H., Eds.; Science Press: Beijing, China, 2008; pp. 141–152. ISBN 978-7-030-20116-4. [Google Scholar]
- Mishra, S.; Heckathorn, S.A.; Frantz, J.M.; Krause, C. The effect of boron availability, CO2, and irradiance on relative accumulation of the major boron transport proteins, bor1 and nip5;1. Biol. Plant. 2018, 62, 121–128. [Google Scholar] [CrossRef]
- Sharma, P.N.; Ramchandra, T. Water relations and photosynthesis in mustard plants subjected to boron deficiency. Indian J. Plant Physiol. 1990, 85, 150–154. [Google Scholar]
- Lu, X.P.; Jiang, C.C.; Dong, X.C.; Wu, X.W.; Yan, L. Leaf photosynthetic characteristics of citrus rootstocks with different boron efficiency. Plant Nutr. Fert. Sci. 2017, 23, 476–483. [Google Scholar]
- García-sánchez, F.; Simón-grao, S.; Martínez-nicolás, J.J.; Alfosea-simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-pérez, J.G.; Cámara-zapata, J.M. Multiple stresses occurring with boron toxicity and deficiency in plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef]
- Song, X.; Song, B.Q.; Huo, J.L.; Liu, H.J.; Adil, M.F.; Jia, Q.; Wu, W.Y.; Kuerban, A.; Wang, Y.; Huang, W.G. Effect of boron deficiency on the photosynthetic performance of sugar beet cultivars with contrasting boron efficiencies. Front. Plant Sci. 2023, 13, 1101171. [Google Scholar] [CrossRef]
- Fujiyama, B.S.; Silva, A.R.B.E.; Júnior, M.L.D.S.; Cardoso, N.R.P.; Fonseca, A.B.D.; Viana, R.G.; Sampaio, L.S. Boron fertilization enhances photosynthesis and water use efficiency in soybean at vegetative growth stage. J. Plant Nutr. 2019, 42, 2498–2506. [Google Scholar] [CrossRef]
- Gimeno, V.; Simón, I.; Nieves, M.; Martínez, V.; Cámara-zapata, J.M.; García, A.L.; García-Sánchez, F. The physiological and nutritional responses to an excess of boron by Verna lemon trees that were grafted on four contrasting rootstocks. Trees-Struct. Funct. 2012, 26, 1513–1526. [Google Scholar] [CrossRef]
- Han, S.; Tang, N.; Jiang, H.X.; Yang, L.T.; Li, Y.; Chen, L.S. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 2009, 176, 143–153. [Google Scholar] [CrossRef]
- Sheng, O.; Song, S.W.; Peng, S.A.; Deng, X.X. The effects of low boron on growth, gas exchange, boron concentration and distribution of ‘Newhall’ navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks. Sci. Hortic. 2009, 121, 278–283. [Google Scholar] [CrossRef]
- Zhou, G.F.; Li, B.X.; Lin, H.Z.; Guan, G.; Liu, G.D.; Yao, F.X.; Zhong, B.L. Early and late symptoms of micronutrient deficiency and diurnal changes of photosynthesis of ‘Newhall’ navel orange. Plant Nutr. Fert. Sci. 2019, 25, 1957–1966. [Google Scholar]
- Zhang, G.J.; Jiang, H.; Zheng, L.Q.; Chen, J.; Qiu, D.L.; Liu, X.H. Effect of copper stress on photosynthesis of navel orange seedlings. Chin. J. Eco-Agric. 2009, 17, 130–134. [Google Scholar] [CrossRef]
- Zhou, G.F.; Li, B.X.; Fu, Y.L.; Guan, G.; Yao, F.X.; Liu, G.D. Effects of iron, manganese and zinc deficiency on the symptom, photosynthetic characteristics and nutrient status of ‘Nanfeng’ tangerine. Acta Hortic. Sin. 2019, 46, 691–700. [Google Scholar]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Xie, D.X.; Ma, X.N.; Zhao, Y.Q.; Li, J.X.; Fu, D.L.; Zhang, Z.Q.; Ju, C.F.; Wang, C. Absorption, transport and regulation of manganese in plants. Sci. Sin. 2023, 53, 1199–1212. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil. Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Liu, C.Z.; Piao, L.; Yang, F.Q.; Liu, J.Q.; Faheem, J.M.; Li, M. Effect of Mn deficiency on carbon and nitrogen metabolism of different genotypes seedlings in maize (Zea mays L.). Plants 2023, 12, 1407. [Google Scholar] [CrossRef]
- Pittman, J.K. Managing the manganese: Molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interf. Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Setter, T.L.; Waters, I. Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J. Plant Nutr. 2007, 29, 899–911. [Google Scholar] [CrossRef]
- Hansel, C.M.; Zeiner, C.A.; Santelli, C.M.; Webb, S.M. Mn(II) oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 12621–12625. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil. Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Huang, X.; Shabala, S.; Shabala, L.; Rengel, Z.; Wu, X.; Zhang, G.; Zhou, M. Linking waterlogging tolerance with Mn2+ toxicity: A case study for barley. Plant Biol. 2015, 17, 26–33. [Google Scholar] [CrossRef]
- Ying, J.G.; Liu, X.H.; Li, J.B.; Wu, Q.; Peng, S.A.; Jiang, C.C. Analysis of Cu and Mn contents in the soil and leaves of citrus orchards in Nanfeng and Quzhou. S. China Fruits 2016, 45, 15–18. [Google Scholar]
- Cheng, J.J.; Ding, C.F.; Li, X.G.; Zhang, T.L.; Wang, X.X. Soil quality evaluation for navel orange production systems in central subtropical China. Soil Till. Res. 2016, 155, 225–232. [Google Scholar] [CrossRef]
- Chun, C.P.; Peng, L.Z.; Ling, L.L.; Lai, J.J.; Cao, L.; Jiang, C.L. Study on the contents of macronutrients and medium nutrients in ‘Newhall’ navel orange leaves in Southern Jiangxi province of China. J. Fruit Sci. 2010, 27, 678–682. [Google Scholar]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, Y.; Han, L.H.; Xu, J.Z. The interactive effects of nitrogen and calcium on photosynthetic characteristics and chlorophyll fluorescence parameters of nectarine under protected culture. Plant Nutr. Fert. Sci. 2013, 19, 893–900. [Google Scholar]
- Huang, C.; Barker, S.J.; Langridge, P.; Smith, F.W.; Graham, R.D. Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and -deficient barley roots. Plant Physiol. 2000, 124, 415–422. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Matas, M.A.; González-Fontes, A.; Camacho-Cristóbal, J.J. Effect of boron supply on nitrate concentration and its reduction in roots and leaves of tobacco plants. Biol. Plant. 2009, 53, 120–124. [Google Scholar] [CrossRef]
- Koshiba, T.; Kobayashi, M.; Ishihara, A.; Matoh, T. Boron nutrition of cultured tobacco BY-2 cells. VI. Calcium is involved in early responses to boron deprivation. Plant Cell Physiol. 2010, 51, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Pando, C.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; González-Fontes, A. Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+-related genes in Arabidopsis thaliana roots. Plant Physiol. Biochem. 2013, 65, 55–60. [Google Scholar] [CrossRef]
- Zhou, G.F.; Wei, Q.J.; Li, B.X.; Zeng, X.L.; Liu, G.D. Establishment and optimization of hydroponic culture system for root morphologic and nutritional analysis of trifoliate orange [Poncirus trifoliata (L.) Raf]. Sci. Agr. 2020, 77, e20180261. [Google Scholar] [CrossRef]
- Ling, L.L.; Huang, Y.; Peng, L.Z.; Wu, Y.T.; Jiang, C.L.; Cao, L.; Chun, C.P. Influence of magnesium deficiency and excess on chlorophyll fluorescence characteristics of ‘Newhall’ navel orange leaves. Acta Ecol. Sin. 2014, 34, 1672–1680. [Google Scholar] [CrossRef][Green Version]
- Xiong, S.M.; Zuo, X.F.; Zhu, Y.Y. Determination of cellulose, hemi-cellulose and lignin in rice hull. Cereal Feed Ind. 2005, 8, 40–41. [Google Scholar][Green Version]
- Chowdhury, S.; Dutt, S. Vein-splitting, a boron deficiency disease of citrus in Assam. Sci. Cult. 1950, 15, 358–359. [Google Scholar][Green Version]
- Li, J.; Xie, Z.C.; Xie, W.L.; Wu, X.M.; Shi, Q. Relationship between leaf vein splitting and mineral nutrition of citrus. Acta Hortic. Sin. 2011, 38, 425–433. [Google Scholar][Green Version]
- Xi, F.S.; Wu, Y.J.; Xiang, D.Y. The causes of severe physiological shoot blight of fast-growing eucalyptus and emergency control measures. Guangxi For. Sci. 2005, 34, 73–75. [Google Scholar][Green Version]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Morphology and oxidative physiology of boron-deficient mulberry plants. Tree Physiol. 2010, 30, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.D.; Jiang, C.C.; Wang, Y.H. Distribution of boron and its forms in young ‘Newhall’ navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks in response to deficient and excessive boron. Soil Sci. Plant Nutr. 2011, 57, 93–104. [Google Scholar] [CrossRef][Green Version]
- Ye, X.; Huang, H.Y.; Wu, F.L.; Cai, L.Y.; Chen, L.S. Molecular mechanisms for magnesium-deficiency-induced leaf vein lignification, enlargement and cracking in Citrus sinensis revealed by RNA-Seq. Tree Physiol. 2020, 41, 280–301. [Google Scholar] [CrossRef]
- Bagherian, S.A.A.; Izadpanah, K. Two novel variants of hop stunt viroid associated with yellow corky vein disease of sweet orange and split bark disorder of sweet lime. J. Kühn Arch. 2010, 427, 105–113. [Google Scholar]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.M.S.; Bonine, C.A.V.; Viana, J.D.O.F.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2020, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Barreiro, M.; Ramalho, J. Manganese accumulation in rice: Implications for photosynthetic functioning. J. Plant Physiol. 2004, 161, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Linker, R.; Gepstein, S.; Tanimoto, E.; Yamamoto, R.; Neumann, P.M. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiol. 2006, 140, 603–612. [Google Scholar] [CrossRef]
- Wei, H.; Dhanaraj, A.L.; Arora, R.; Rowland, L.J.; Fu, Y.; Sun, L. Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: Importance of moderately abundant ESTs in genomic studies. Plant Cell Environ. 2006, 29, 558–570. [Google Scholar] [CrossRef]
- Moura-Sobczak, J.; Souza, U.; Mazzafera, P. Drought stress and changes in the lignin content and composition in Eucalyptus. BMC Proc. 2011, 5, 103. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sheng, L.P.; Zhang, H.R.; Du, X.P.; An, C.; Xia, X.L.; Chen, F.D.; Jiang, J.F.; Chen, S.M. CmMYB19 over-expression improves aphid tolerance in chrysanthemum by promoting lignin synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef]
- Liu, W.G.; Ren, M.L.; Liu, T.; Du, Y.L.; Zhou, T.; Liu, X.M.; Liu, J.; Hussain, S.; Yang, W.Y. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agr. 2018, 17, 1594–1604. [Google Scholar] [CrossRef]
- Wen, B.X.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.W.; Yang, J.Y.; Xu, M.; Qin, S.S.; Yang, W.Y.; Liu, W.G. Slight shading stress at seedling stage does not reduce lignin biosynthesis or affect lodging resistance of soybean stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef]
- Li, C.H.; Luo, Y.L.; Jin, M.; Sun, S.F.; Wang, Z.L.; Li, Y. Response of lignin metabolism to light quality in wheat population. Front. Plant Sci. 2021, 12, 729647. [Google Scholar] [CrossRef]
| Treatments | Plant Height (cm) | Dry Weight (g plant−1) | Root-Shoot Ratio | |||
|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Total | |||
| CK | 74.8 ± 5.6 a | 14.5 ± 1.9 a | 20.3 ± 1.3 a | 25.9 ± 1.6 a | 60.7 ± 2.3 a | 0.77 ± 0.11 b |
| BD | 56.6 ± 1.3 c | 8.6 ± 1.7 b | 13.8 ± 1.0 c | 20.4 ± 0.9 b | 42.8 ± 2.3 c | 0.92 ± 0.06 a |
| MnD | 65.4 ± 2.3 b | 12.6 ± 0.7 a | 17.7 ± 1.4 b | 22.6 ± 1.1 ab | 52.9 ± 2.7 b | 0.75 ± 0.06 b |
| BD + MnD | 54.3 ± 2.8 c | 6.6 ± 2.1 b | 14.5 ± 2.3 c | 18.0 ± 1.3 b | 39.1 ± 3.6 c | 0.90 ± 0.13 a |
| Photosynthetic Pigment | CK | BD | MnD | BD + MnD | |
|---|---|---|---|---|---|
| Chlorophyll a (mg g−1 FW) | OL | 1.67 ± 0.08 a | 1.32 ± 0.06 b | 1.79 ± 0.07 a | 1.10 ± 0.01 c |
| PL | 2.02 ± 0.11 a | 2.07 ± 0.12 a | 1.64 ± 0.06 b | 1.78 ± 0.11 ab | |
| SL | 1.98 ± 0.15 a | 1.46 ± 0.21 b | 1.16 ± 0.13 c | 1.42 ± 0.13 b | |
| Chlorophyll b (mg g−1 FW) | OL | 1.13 ± 0.07 a | 0.83 ± 0.06 b | 1.02 ± 0.11 ab | 0.68 ± 0.07 c |
| PL | 1.08 ± 0.05 a | 1.11 ± 0.10 a | 0.65 ± 0.03 b | 0.70 ± 0.04 b | |
| SL | 1.19 ± 0.05 a | 0.85 ± 0.09 b | 0.74 ± 0.01 bc | 0.62 ± 0.05 c | |
| Total chlorophyll (mg g−1 FW) | OL | 2.76 ± 0.02 a | 2.15 ± 0.09 b | 2.81 ± 0.18 a | 1.78 ± 0.07 c |
| PL | 3.10 ± 0.06 a | 3.18 ± 0.18 a | 2.30 ± 0.08 b | 2.48 ± 0.15 b | |
| SL | 3.17 ± 0.12 a | 2.31 ± 0.12 b | 1.89 ± 0.12 c | 2.04 ± 0.18 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fu, Y.; Gan, Z.; Wei, Q.; Yang, M.; Yao, F.; Zhou, G. Manganese Deficiency Exacerbates Boron Deficiency-Induced Corky Split Vein in Citrus by Disrupting Photosynthetic Physiology and Enhancing Lignin Metabolism. Horticulturae 2025, 11, 1172. https://doi.org/10.3390/horticulturae11101172
Li Y, Fu Y, Gan Z, Wei Q, Yang M, Yao F, Zhou G. Manganese Deficiency Exacerbates Boron Deficiency-Induced Corky Split Vein in Citrus by Disrupting Photosynthetic Physiology and Enhancing Lignin Metabolism. Horticulturae. 2025; 11(10):1172. https://doi.org/10.3390/horticulturae11101172
Chicago/Turabian StyleLi, Yanhong, Yiping Fu, Zhili Gan, Qingjing Wei, Mei Yang, Fengxian Yao, and Gaofeng Zhou. 2025. "Manganese Deficiency Exacerbates Boron Deficiency-Induced Corky Split Vein in Citrus by Disrupting Photosynthetic Physiology and Enhancing Lignin Metabolism" Horticulturae 11, no. 10: 1172. https://doi.org/10.3390/horticulturae11101172
APA StyleLi, Y., Fu, Y., Gan, Z., Wei, Q., Yang, M., Yao, F., & Zhou, G. (2025). Manganese Deficiency Exacerbates Boron Deficiency-Induced Corky Split Vein in Citrus by Disrupting Photosynthetic Physiology and Enhancing Lignin Metabolism. Horticulturae, 11(10), 1172. https://doi.org/10.3390/horticulturae11101172








