Postharvest Biology and Quality Preservation of Vasconcellea pubescens: Challenges and Opportunities for Reducing Fruit Losses
Abstract
1. Introduction
1.1. Physiology of Vasconcellea pubescens: Plant and Fruit
1.1.1. Morphological and Reproductive Characteristics
1.1.2. Geographic Distribution and Agroecological Adaptation
1.1.3. Reproductive Biology and Propagation Strategies
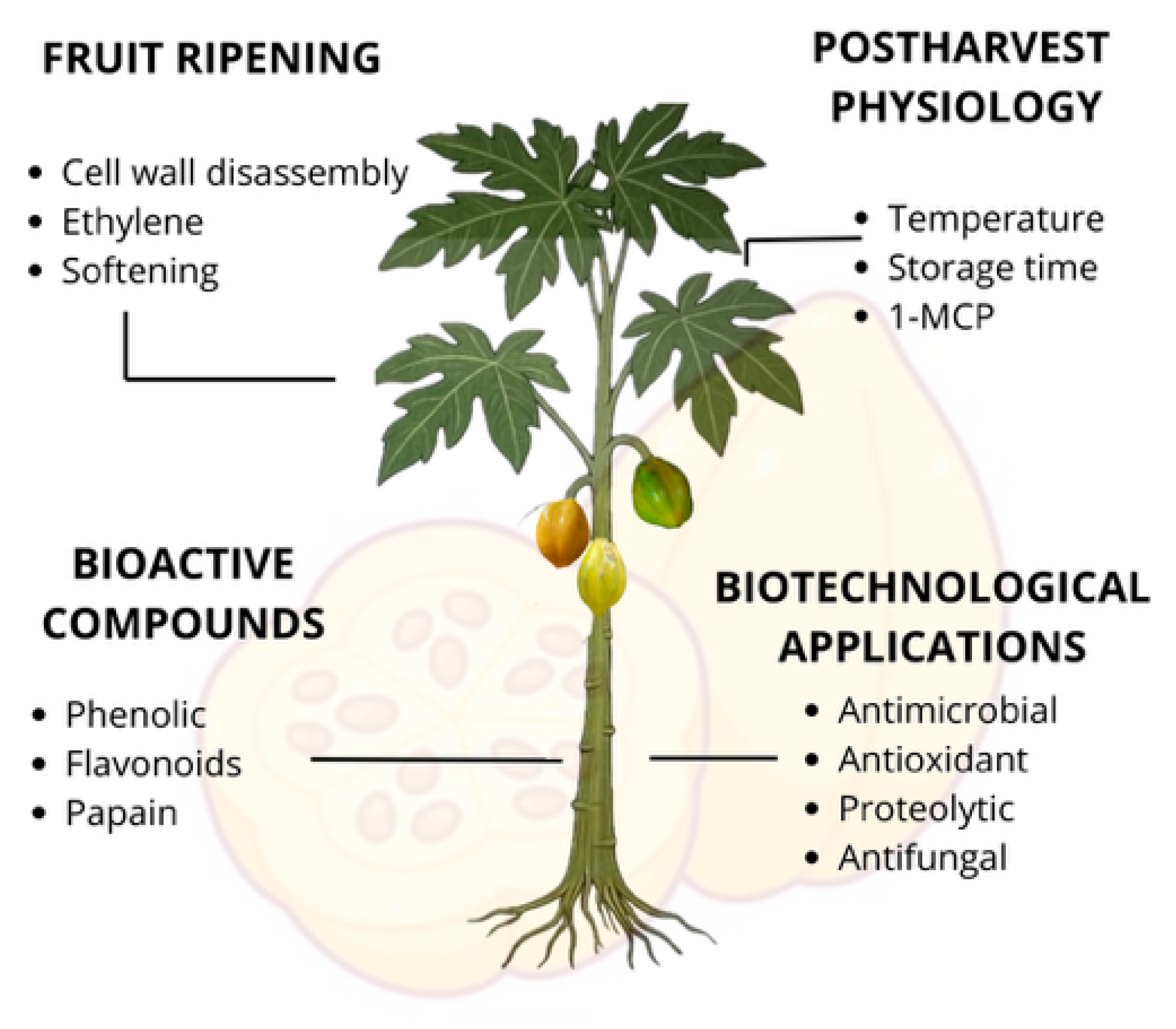
1.1.4. Postharvest Physiology and Shelf Life
1.2. Aroma, Phenolic Compounds and Antioxidants Capacity
1.3. Objective Color Assessment in Vasconcellea pubescens Fruits
1.4. Cell Wall Disassembly and Softening Mechanisms in Vaconcellea Pubescens
2. Bioinformatics and Molecular Approaches in Vasconcellea pubescens
3. Hormone Treatments and Future Perspectives
4. Biomedical and Antimicrobial Potential of Vasconcellea pubescens: From Enzymatic Activity to Functional Ingredients
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chong-Pérez, B.; Carrasco, B.; Silva, H.; Herrera, F.; Quiroz, K.; Garcia-Gonzales, R. Regeneration of Highland Papaya (Vasconcellea pubescens) from Anther Culture. Appl. Plant Sci. 2018, 6, e01182. [Google Scholar] [CrossRef] [PubMed]
- Scheldeman, X.; Willemen, L.; Coppens d’Eeckenbrugge, G.; Romeijn-Peeters, E.; Restrepo, M.T.; Romero Motoche, J.; Jiménez, D.; Lobo, M.; Medina, C.I.; Reyes, C.; et al. Distribution, Diversity and Environmental Adaptation of Highland Papayas (Vasconcellea spp.) in Tropical and Subtropical America. In Plant Conservation and Biodiversity; Hawksworth, D.L., Bull, A.T., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 293–310. [Google Scholar] [CrossRef]
- Scheldeman, X.; Kyndt, T.; d’Eeckenbrugge, G.C.; Ming, R.; Drew, R.; Van Droogenbroeck, B.; Van Damme, P.; Moore, P.H. Vasconcellea. In Wild Crop Relatives: Genomic and Breeding Resources: Tropical and Subtropical Fruits; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–249. [Google Scholar] [CrossRef]
- Tineo, D.; Bustamante, D.E.; Calderon, M.S.; Mendoza, J.E.; Huaman, E.; Oliva, M. An Integrative Approach Reveals Five New Species of Highland Papayas (Caricaceae, Vasconcellea) from Northern Peru. PLoS ONE 2020, 15, e0242469. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, B.; Avila, P.; Perez-Diaz, J.; Muñoz, P.; García, R.; Lavandero, B.; Zurita-Silva, A.; Retamales, J.B.; Caligari, P.D.S. Genetic Structure of Highland Papayas (Vasconcellea pubescens (Lenné et C. Koch) Badillo) Cultivated along a Geographic Gradient in Chile as Revealed by Inter Simple Sequence Repeats (ISSR). Genet. Resour. Crop Evol. 2009, 56, 331–337. [Google Scholar] [CrossRef]
- Carrasco, B.; García-Gonzáles, R.; Díaz, C.; Ávila, P.; Cáceres, P.; Lobos, G.A.; Silva, H.; Caligari, P.D.S. Genetic and Morphological Characterization of the Endangered Austral Papaya Vasconcellea chilensis (Planch. Ex A. DC.) Solms. Genet. Resour. Crop Evol. 2014, 61, 1423–1432. [Google Scholar] [CrossRef]
- Latcham, R.E. La Agricultura Precolombina en Chile y los Países Vecinos; Ediciones de la Universidad de Chile: Santiago, Chile, 1936. [Google Scholar]
- Salvatierra-González, M.A.; Jana-Ayala, C. Floral Expression and Pollen Germination Ability in Productive Mountain Papaya (Vasconcellea pubescens A.DC.) Orchards. Chil. J. Agric. Res. 2016, 76, 136–142. [Google Scholar] [CrossRef]
- Vegas, A.; Trujillo, G.; Sandrea, Y. Obtención, regeneración y evaluación de híbridos intergenéricos entre Carica papaya Y Vasconcellea cauliflora. Interciencia 2003, 28, 710–714. [Google Scholar]
- Balbontín, C.; Gaete-Eastman, C.; Vergara, M.; Herrera, R.; Moya-León, M.A. Treatment with 1-MCP and the Role of Ethylene in Aroma Development of Mountain Papaya Fruit. Postharvest Biol. Technol. 2007, 43, 67–77. [Google Scholar] [CrossRef]
- Cañas-Sarazúa, R.; Briones-Labarca, V.; Giovagnoli-Vicuña, C. Encapsulation of Papaya Seed Oil in Casein-Alginate-Based Shell Materials. Future Foods 2024, 9, 100301. [Google Scholar] [CrossRef]
- Fuentes, Y.; Giovagnoli-Vicuña, C.; Faúndez, M.; Giordano, A. Microencapsulation of Chilean Papaya Waste Extract and Its Impact on Physicochemical and Bioactive Properties. Antioxidants 2023, 12, 1900. [Google Scholar] [CrossRef]
- Sanhueza, D.; Sepúlveda-Orellana, P.; Salazar-Carrasco, A.; Zúñiga, S.; Herrera, R.; Moya-León, M.A.; Saez-Aguayo, S. Mucilage Extracted from Chilean Papaya Seeds Is Enriched with Homogalacturonan Domains. Front. Plant Sci. 2024, 15, 1219–1226. [Google Scholar] [CrossRef]
- Lemos, F.O.; Dittz, D.; Santos, V.G.; Pires, S.F.; de Andrade, H.M.; Salas, C.E.; Lopes, M.T.P. Cysteine Proteases from V. cundinamarcensis (C. candamarcensis) Inhibit Melanoma Metastasis and Modulate Expression of Proteins Related to Proliferation, Migration and Differentiation. Int. J. Mol. Sci. 2018, 19, 2846. [Google Scholar] [CrossRef]
- Vidal, L.V.; Finot, V.L.; del C. Mora, K.; Venegas, F.A. Características Físico-Químicas Del Látex de Papayuelo (Vasconcellea cundinamarcensis Badillo, Caricaceae). Inf. Tecnológica 2009, 20, 93–103. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of Phenolic Compounds from the Fruits of the Mountain Papaya Vasconcellea pubescens A. DC. Grown in Chile by Liquid Chromatography–UV Detection–Mass Spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Puente-Díaz, L.; Cifuentes, A.; Lizama, K.; González, P. Chilean Papaya (Vasconcellea pubescens): A Native Fruit with a High Health-Promoting Functional Potential. Antioxidants 2024, 13, 1521. [Google Scholar] [CrossRef]
- Pino-Ramos, L.L.; Farias, D.R.; Olivares-Caro, L.; Mitsi, C.; Mardones, C.; Echeverria, J.; Avila, F.; Gutierrez, M. Chilean Papaya (Vasconcellea pubescens A. DC.) Residues as a Source of Bioactive Compounds: Chemical Composition, Antioxidant Capacity, and Antiglycation Effects. Heliyon 2024, 10, e38837. [Google Scholar] [CrossRef]
- Caetano, C.; Lagos Burbano, T.; Sierra, C.; Tique, C.; Nunes, D. Cytogenetic of Vasconcellea Species (Caricaceae). Acta Agronómica 2008, 57, 241–245. [Google Scholar]
- Carvalho, F.A.; Renner, S.S. A Dated Phylogeny of the Papaya Family (Caricaceae) Reveals the Crop’s Closest Relatives and the Family’s Biogeographic History. Mol. Phylogenet. Evol. 2012, 65, 46–53. [Google Scholar] [CrossRef]
- Gaete-Eastman, C.; Figueroa, C.R.; Balbontín, C.; Moya, M.; Atkinson, R.G.; Herrera, R.; Moya-León, M.A. Expression of an Ethylene-Related Expansin Gene during Softening of Mountain Papaya Fruit (Vasconcellea pubescens). Postharvest Biol. Technol. 2009, 53, 58–65. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Stucken, K.; Cantuarias, C.; Lamas, F.; García, V.; Pastén, A. Antimicrobial Properties of Papaya (Vasconcellea pubescens) Subjected to Low-Temperature Vacuum Dehydration. Innov. Food Sci. Emerg. Technol. 2021, 67, 102563. [Google Scholar] [CrossRef]
- Uribe, E.; Delgadillo, A.; Giovagnoli-Vicuña, C.; Quispe-Fuentes, I.; Zura-Bravo, L. Extraction Techniques for Bioactive Compounds and Antioxidant Capacity Determination of Chilean Papaya (Vasconcellea pubescens) Fruit. J. Chem. 2015, 2015, e347532. [Google Scholar] [CrossRef]
- Meza, S.D.; Osorio Guerrero, K.V.; Lagos Burbano, T.C. Evaluación del crecimiento, la morfología floral y el fruto de chilacuán (Vasconcellea cundinamarcensis B.). Rev. Cienc. Agrícolas 2011, 28, 9–23. [Google Scholar]
- Balbontín, C.; Gaete-Eastman, C.; Fuentes, L.; Figueroa, C.R.; Herrera, R.; Manriquez, D.; Latché, A.; Pech, J.-C.; Moya-León, M.A. VpAAT1, a Gene Encoding an Alcohol Acyltransferase, Is Involved in Ester Biosynthesis during Ripening of Mountain Papaya Fruit. J. Agric. Food Chem. 2010, 58, 5114–5121. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Fuentes, L.; Gaete-Eastman, C.; Herrera, R.; Moya-León, M.A. Structural Characterization and Substrate Specificity of VpAAT1 Protein Related to Ester Biosynthesis in Mountain Papaya Fruit. J. Mol. Graph. Model. 2011, 29, 635–642. [Google Scholar] [CrossRef]
- Benítez, S.P.; Mario, L.; Delgado, O.A.; Medina, C.I. Estudios de germinación y remoción de latencia en semillas de papayuelas Vasconcellea cundinamarcensis y Vasconcellea goudotiana. Cienc. Tecnol. Agropecu. 2013, 14, 187–197. [Google Scholar] [CrossRef]
- Faúndez-Acuña, J.Y.; Verdugo, D.; Vergara, D.; Olivares, G.; Ballesteros, G.I.; Quiroz, K.; Villarroel, C.A.; González, G. The Mountain Papaya May Be a Possible Reservoir of the Kashmir Bee Virus. PeerJ 2025, 13, e18634. [Google Scholar] [CrossRef] [PubMed]
- Letelier, L.; Gaete-Eastman, C.; Peñailillo, P.; Moya-León, M.A.; Herrera, R. Southern Species From the Biodiversity Hotspot of Central Chile: A Source of Color, Aroma, and Metabolites for Global Agriculture and Food Industry in a Scenario of Climate Change. Front. Plant Sci. 2020, 11, 1002. [Google Scholar] [CrossRef]
- Peña T., D.F.; Villena O., P.G.; Aguirre, Á.J.; Jiménez M., C. Diversidad genética de accesiones de la familia Caricaceae en el sur de Ecuador. Maskana 2017, 8, 85–102. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Na, J.-K.; Yu, Q.; Moore, R.C.; Zee, F.; Huber, S.C.; Ming, R. The Origin of the Non-Recombining Region of Sex Chromosomes in Carica and Vasconcellea. Plant J. 2010, 63, 801–810. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Drew, R.A. Potential for Using Vasconcellea parviflora as a Bridging Species in Intergeneric Hybridisation between V. pubescens and Carica papaya. Aust. J. Bot. 2009, 57, 592–601. [Google Scholar] [CrossRef]
- Drew, R.A.; Siar, S.V.; O’Brien, C.M.; Sajise, A.G.C. Progress in Backcrossing between Carica papaya × Vasconcellea quercifolia Intergeneric Hybrids and C. papaya. Aust. J. Exp. Agric. 2006, 46, 419–424. [Google Scholar] [CrossRef]
- Drew, R.A.; Magdalita, P.M.; O’Brien, C.M. Development of Carica Interspecific Hybrids. Acta Hortic. 1998, 461, 285–292. [Google Scholar] [CrossRef]
- Van Droogenbroeck, B.; Kyndt, T.; Romeijn-Peeters, E.; Van Thuyne, W.; Goetghebeur, P.; Romero-Motochi, J.P.; Gheysen, G. Evidence of Natural Hybridization and Introgression between Vasconcellea Species (Caricaceae) from Southern Ecuador Revealed by Chloroplast, Mitochondrial and Nuclear DNA Markers. Ann. Bot. 2006, 97, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Ramage, C.; Ashmore, S.; Drew, R.A. Development of a Codominant CAPS Marker Linked to PRSV-P Resistance in Highland Papaya. Theor. Appl. Genet. 2006, 113, 1159–1169. [Google Scholar] [CrossRef]
- Kanchana-udomkan, C.; Nantawan, U.; Drew, R.; Ford, R. Progress in Introgression of Papaya Ringspot Virus Resistance from Vasconcellea pubescens to Carica papaya. Acta Hortic. 2018, 1203, 59–66. [Google Scholar] [CrossRef]
- Copeland, R.G.R.; Bar, I.; Prasad, S.H.; Mir, R.R.; Garg, V.; Henry, R.; Ming, R.; Varshney, R.K. Harnessing Novel Genomic Resources and Emerging Breeding Tools to Fast-Track Genetic Improvement in Papaya. Hortic. Plant J. 2025, in press. [Google Scholar] [CrossRef]
- Culqui, Y.K.L.; Alvarado, J.J.T.; Mori, J.B.M.; Valqui, N.C.V.; Huaman, E.H.; Cruz, S.M.O. Establecimiento y multiplicación in vitro de papayas de montaña: Vasconcellea chachapoyensis Y Vasconcellea x Heilbornii. Bioagro 2021, 33, 135–142. [Google Scholar] [CrossRef]
- Nelson, R.; Chaudhary, R.; Sharma, P.; Thakur, V.; Rachna, R.; Thakur, N. Analysis of the Constraints Faced by the Farmers in Adoption of Electronic National Agriculture Platform (E NAM) in Himachal Pradesh. J. Sci. Res. Rep. 2025, 31, 273–277. [Google Scholar] [CrossRef]
- Rachna; Thakur, V.; Sharma, P.; Nelson, R.; Thakur, N. Strengthening Food Safety and Consumer Trust: Unlocking Blockchain’s Potential in the Indian Agri Market. Int. J. Agric. Food Sci. 2025, 7, 247–255. [Google Scholar] [CrossRef]
- Prasad, K.; Paul, J. Postharvest Losses of Papaya and Practice for Its Management. Food Sci. Rep. 2021, 2, 49–53. [Google Scholar]
- Vinod, B.R.; Asrey, R.; Sethi, S.; Prakash, J.; Meena, N.K.; Menaka, M.; Mishra, S.; Shivaswamy, G. Recent Advances in Physical Treatments of Papaya Fruit for Postharvest Quality Retention: A Review. eFood 2023, 4, e79. [Google Scholar] [CrossRef]
- Islam, M.S.; Kao, N.; Bhattacharya, S.N.; Gupta, R.; Bhattacharjee, P.K. Effect of Low Pressure Alkaline Delignification Process on the Production of Nanocrystalline Cellulose from Rice Husk. J. Taiwan Inst. Chem. Eng. 2017, 80, 820–834. [Google Scholar] [CrossRef]
- Ogalde, F.; Nazer, A. Evaluación técnica de un panel aislante aglomerado basado en semillas de papaya. Obras. Proy. 2025, 37, 15–24. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Robles Ardila, D.P.; Rodríguez Pardo, N.; Pataquiva-Mateus, A.; Robles Ardila, D.P.; Rodríguez Pardo, N.; Pataquiva-Mateus, A. Synthesis of Magnetite Nanoparticles Using Papaya Peel Extract for the Azo Dyes Degradation in Aqueous Solutions. Ingeniare. Rev. Chil. Ing. 2019, 27, 431–442. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High Hydrostatic Pressure and Ultrasound Extractions of Antioxidant Compounds, Sulforaphane and Fatty Acids from Chilean Papaya (Vasconcellea pubescens) Seeds: Effects of Extraction Conditions and Methods. LWT—Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Chen, X.; Quek, S.Y. Free and Glycosidically Bound Aroma Compounds in Fruit: Biosynthesis, Transformation, and Practical Control. Crit. Rev. Food Sci. Nutr. 2022, 63, 9052–9073. [Google Scholar] [CrossRef]
- Sasongko, H.; Lestari, R.; Yugatama, A.; Farida, Y.; Farida, Y.; Farida, Y. Antidiabetic and Antioxidant Effect Combination Vasconcellea pubescens A.DC. and Momordica Charantia L. Extract in Alloxan- Induced Diabetic Rats. Pharmacogn. J. 2020, 12, 311–315. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Poblete, J.; Quispe-Fuentes, I.; Uribe, E.; Bilbao-Sainz, C.; Pastén, A. Chemical and Bioactive Characterization of Papaya (Vasconcellea pubescens) under Different Drying Technologies: Evaluation of Antioxidant and Antidiabetic Potential. Food Meas. 2019, 13, 1980–1990. [Google Scholar] [CrossRef]
- Sasongko, H.; Efendi, N.R.; Sugiyarto. The Ethanolic Extract of Mountain Papaya (Vasconcellea pubescens A.DC.) Fruit against Lipid Peroxidation of Rat Liver Tissues. AIP Conf. Proc. 2018, 2019, 050001. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Smith, D.E. Color Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 573–586. [Google Scholar] [CrossRef]
- Ruslan, R.; Roslan, N. Assessment on the Skin Color Changes of Carica papaya L. Cv. Sekaki Based on CIE L*a*b* and CIE L*C*h Color Space. Int. Food Res. J. 2016, 23, S173–S178. [Google Scholar]
- Sañudo-Barajas, J.A.; Labavitch, J.; Greve, C.; Osuna-Enciso, T.; Muy-Rangel, D.; Siller-Cepeda, J. Cell Wall Disassembly during Papaya Softening: Role of Ethylene in Changes in Composition, Pectin-Derived Oligomers (PDOs) Production and Wall Hydrolases. Postharvest Biol. Technol. 2009, 51, 158–167. [Google Scholar] [CrossRef]
- Cáez Ramírez, G.; Téllez-Medina, D.I.; García-Armenta, E.; -López, G.F.G. Digital Image Analysis and Fractal Metrics as Potential Tools to Monitor Colour Changes in Fresh-Cut Papaya (Carica papaya L.). Int. J. Food Prop. 2017, 20 (Suppl. S1), S177–S189. [Google Scholar] [CrossRef]
- Gil i Cortiella, M.; Vasquez-Rojas, C.; Castro, R.I.; Muñoz-Vera, M.; Parra-Palma, C.; Méndez-Yáñez, Á.; Sáez, D.; Ramos, P.; Morales-Quintana, L. Evolution of the Fruit Ripening and Development of the Strawberry ‘Aroma’; through Transcriptional, Physiological and Chemicals Analysis. Food Meas. 2024, 18, 3160–3175. [Google Scholar] [CrossRef]
- Bartels, D.; Baumann, A.; Maeder, M.; Geske, T.; Heise, E.M.; von Schwartzenberg, K.; Classen, B. Evolution of Plant Cell Wall: Arabinogalactan-Proteins from Three Moss Genera Show Structural Differences Compared to Seed Plants. Carbohydr. Polym. 2017, 163, 227–235. [Google Scholar] [CrossRef]
- Gray, J.; Picton, S.; Shabbeer, J.; Schuch, W.; Grierson, D. Molecular Biology of Fruit Ripening and Its Manipulation with Antisense Genes. Plant Mol. Biol. 1992, 19, 69–87. [Google Scholar] [CrossRef]
- Jia, K.; Wang, W.; Zhang, Q.; Jia, W. Cell Wall Integrity Signaling in Fruit Ripening. Int. J. Mol. Sci. 2023, 24, 4054. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant Cell Walls: Wall-Associated Kinases and Cell Expansion. Curr. Biol. 2001, 11, R558–R559. [Google Scholar] [CrossRef]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.N.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S.; et al. Mutations in the Pectin Methyltransferase QUASIMODO2 Influence Cellulose Biosynthesis and Wall Integrity in Arabidopsis [OPEN]. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Moya, M.; Herrera, R. Ripening of Mountain Papaya (Vasconcellea pubescens) and Ethylene Dependence of Some Ripening Events. Postharvest Biol. Technol. 2004, 34, 211–218. [Google Scholar] [CrossRef]
- Mattus-Araya, E.; Stappung, Y.; Herrera, R.; Moya-León, M.A. Molecular Actors Involved in the Softening of Fragaria chiloensis Fruit Accelerated by ABA Treatment. J. Plant Growth Regul. 2023, 42, 433–448. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular Events Occurring During Softening of Strawberry Fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH Family of Enzymes Involved in Xyloglucan Endotransglucosylation and Endohydrolysis: Current Perspectives and a New Unifying Nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell Wall Modifications during Fruit Ripening: When a Fruit Is Not the Fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Goulao, L.F.; Cosgrove, D.J.; Oliveira, C.M. Cloning, Characterisation and Expression Analyses of cDNA Clones Encoding Cell Wall-Modifying Enzymes Isolated from Ripe Apples. Postharvest Biol. Technol. 2008, 48, 37–51. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Changes in Cell Wall Polysaccharides and Cell Wall Degrading Enzymes during Ripening of Fragaria chiloensis and Fragaria ×ananassa Fruits. Sci. Hortic. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Merino, D. El Cultivo Del Babaco; Paraninfo.es: Madrid, Spain, 1989; ISBN 9788471142627. Available online: https://www.paraninfo.es/catalogo/9788471142627/el-cultivo-del-babaco (accessed on 11 September 2025).
- Maselli, A.; Rosales, L.C.; Guevara, Y.; Suárez, H.Z. Comportamiento De Materiales De Los Géneros Carica Y Vasconcellea Frente A Erwinia papayae, Meloidogyne incognita Y Rotylenchulus reniformis. Rev. Protección Veg. 2010, 25, 157–165. [Google Scholar]
- Kabir, M.Y.; Hossain, S.K. Botanical Extracts Improve Postharvest Quality and Extend the Shelf Life of Papaya (Carica papaya L. Cv. Shahi). N. Z. J. Crop Hortic. Sci. 2025, 53, 605–621. [Google Scholar] [CrossRef]
- Sivakumar, D.; Wall, M.M. Papaya Fruit Quality Management during the Postharvest Supply Chain. Food Rev. Int. 2013, 29, 24–48. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Jaskani, M.J.; Ahmad, R. Papaya Treatment with Putrescine Maintained the Overall Quality and Promoted the Antioxidative Enzyme Activities of the Stored Fruit. Sci. Hortic. 2020, 268, 109367. [Google Scholar] [CrossRef]
- Getnet, M.; Alemu, K.; Tsedaley, B. Status of Postharvest Papaya Anthracnose (Colletotrichum gloeosporioides) in Assosa Zone, Western Ethiopia. Discov. Food 2024, 4, 25. [Google Scholar] [CrossRef]
- Vilaplana, R.; Chicaiza, G.; Vaca, C.; Valencia-Chamorro, S. Combination of Hot Water Treatment and Chitosan Coating to Control Anthracnose in Papaya (Carica papaya L.) during the Postharvest Period. Crop Prot. 2020, 128, 105007. [Google Scholar] [CrossRef]
- Landi, L.; Peralta-Ruiz, Y.; Chaves-López, C.; Romanazzi, G. Chitosan Coating Enriched with Ruta graveolens L. Essential Oil Reduces Postharvest Anthracnose of Papaya (Carica papaya L.) and Modulates Defense-Related Gene Expression. Front. Plant Sci. 2021, 12, 765806. [Google Scholar] [CrossRef]
- Ayón-Reyna, L.E.; González-Robles, A.; Rendón-Maldonado, J.G.; Báez-Flores, M.E.; López-López, M.E.; Vega-García, M.O. Application of a Hydrothermal-Calcium Chloride Treatment to Inhibit Postharvest Anthracnose Development in Papaya. Postharvest Biol. Technol. 2017, 124, 85–90. [Google Scholar] [CrossRef]
- Shu, C.; Wall, M.M.; Follett, P.A.; Sugimoto, N.; Bai, J.; Sun, X. Effect of Humidity-Triggered Controlled-Release 1-Methylcyclopropene (1-MCP) on Postharvest Quality of Papaya Fruit. Horticulturae 2023, 9, 1062. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, L.; Ding, X.; Gao, Q.; Xiao, S.; Tan, Q.; Huang, J.; Chen, W.; Li, X. Transcriptomic Analysis Reveals Key Factors in Fruit Ripening and Rubbery Texture Caused by 1-MCP in Papaya. BMC Plant Biol. 2019, 19, 309. [Google Scholar] [CrossRef]
- Fabi, J.P.; Cordenunsi, B.R.; de Mattos Barreto, G.P.; Mercadante, A.Z.; Lajolo, F.M.; Oliveira do Nascimento, J.R. Papaya Fruit Ripening: Response to Ethylene and 1-Methylcyclopropene (1-MCP). J. Agric. Food Chem. 2007, 55, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Lu, B.G.; Feng, L.; Yang, F.Y.; Geng, J.J.; Ming, R.; Chen, X.J. Isolation of Ripening-Related Genes from Ethylene/1-MCP Treated Papaya through RNA-Seq. BMC Genom. 2017, 18, 671. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.E.; Chambers, A.H. CRISPR/Cas9-Mediated Genome Editing of Phytoene Desaturase in Carica papaya L. J. Hortic. Sci. Biotechnol. 2022, 97, 580–592. [Google Scholar] [CrossRef]
- Silva, M.L.d. Silenciamento do gene da β-1,3-Glucanase de Carica papaya por CRISPR-Cas9. 2021. Available online: http://repositorio.ufes.br/handle/10/15917 (accessed on 11 September 2025).
- Estrella-Maldonado, H.; Ramírez, A.G.; Ortiz, G.F.; Peraza-Echeverría, S.; la Vega, O.M.-D.; Góngora-Castillo, E.; Santamaría, J.M. Transcriptomic Analysis Reveals Key Transcription Factors Associated to Drought Tolerance in a Wild Papaya (Carica papaya) Genotype. PLoS ONE 2021, 16, e0245855. [Google Scholar] [CrossRef]
- Pan, L.; Jiang, L. Identification and Expression of the WRKY Transcription Factors of Carica papaya in Response to Abiotic and Biotic Stresses. Mol. Biol. Rep. 2014, 41, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, R.; Ali, A.; Gururani, M. Abscisic Acid and Ethylene Coordinating Fruit Ripening under Abiotic Stress. Plant Sci. 2024, 349, 112243. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Rojas, C.; Muñoz-Vera, M.; Flores, S.; Betancourt, M.; Castro, R.I.; Ramos, P.; Laporte, D.; Parra-Palma, C.; Morales-Quintana, L. Impact of Methyl Jasmonate on Blueberry Ripening Fruits: Assessment of Cell Wall Thermal Stability, Nutritional Parameters and Antioxidant Enzymatic Activity. Front. Plant Sci. 2025, 16, 1550131. [Google Scholar] [CrossRef]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different Regulatory Mechanisms of Plant Hormones in the Ripening of Climacteric and Non-Climacteric Fruits: A Review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Q.; Zhu, H. Editorial: Towards a Better Understanding of Fruit Ripening: Crosstalk of Hormones in the Regulation of Fruit Ripening. Front. Plant Sci. 2023, 14, 1173877. [Google Scholar] [CrossRef]
- Mello, V.J.; Gomes, M.T.R.; Lemos, F.O.; Delfino, J.L.; Andrade, S.P.; Lopes, M.T.P.; Salas, C.E. The Gastric Ulcer Protective and Healing Role of Cysteine Proteinases from Carica candamarcensis. Phytomedicine 2008, 15, 237–244. [Google Scholar] [CrossRef]
- Freitas, K.M.; e Silva, A.C.A.; Veloso, E.S.; Ferreira, Ê.; Barcelos, L.S.; Caliari, M.V.; Salas, C.E.; Lopes, M.T.P. P1G10, the Proteolytic Fraction from Vasconcellea cundinamarcensis, Stimulates Tissue Repair after Acute Exposure to Ultraviolet B Radiation. Int. J. Mol. Sci. 2019, 20, 4373. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.M.; Pizzitola, M.P.; Araújo e Silva, A.C.; Dittz, D.; Freitas, K.M.; Ferreira, Ê.; Salas, C.E.; Lopes, M.T.P. The Proteolytic Fraction From Vasconcellea cundinamarcensis Latex Displays Anti-Inflammatory Effect in A Mouse Model of Acute TNBS-Induced Colitis. Sci. Rep. 2020, 10, 3074. [Google Scholar] [CrossRef]
- Torres-Ossandón, M.J.; Vega-Gálvez, A.; Salas, C.E.; Rubio, J.; Silva-Moreno, E.; Castillo, L. Antifungal Activity of Proteolytic Fraction (P1G10) from (Vasconcellea cundinamarcensis) Latex Inhibit Cell Growth and Cell Wall Integrity in Botrytis cinerea. Int. J. Food Microbiol. 2019, 289, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Rodríguez, A.; Duarte-Casar, R.; Rojas-Le-Fort, M.; Romero-Benavides, J.C. Food Uses, Functional Activities, and Bioactive Compounds of Three Ecuadorian Vasconcellea Fruits: Bibliometric Analysis and Review. J. Agric. Food Res. 2024, 17, 101244. [Google Scholar] [CrossRef]
- Souza, D.P.; Freitas, C.D.T.; Pereira, D.A.; Nogueira, F.C.; Silva, F.D.A.; Salas, C.E.; Ramos, M.V. Laticifer Proteins Play a Defensive Role against Hemibiotrophic and Necrotrophic Phytopathogens. Planta 2011, 234, 183–193. [Google Scholar] [CrossRef]
- Valdes, O.; Bustos, D.; Guzmán, L.; Muñoz-Vera, M.; Urra, G.; Castro, R.I.; Morales-Quintana, L. The Controlled Release of Abscisic Acid (ABA) Utilizing Alginate–Chitosan Gel Blends: A Synergistic Approach for an Enhanced Small-Molecule Delivery Controller. Gels 2024, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Pudhuvai, B.; Sharma, C.; Kumar, A.; Sharma, V.; Yadav, D.; Jin, J.-O. Carica papaya L.: A Tropical Fruit with Benefits beyond the Tropics. Diversity 2022, 14, 683. [Google Scholar] [CrossRef]
- Matsuane, C.; Kavoo, A.M.; Kiage, B.N.; Karanja, J.; Rimberia, F.K. Nutrient content and biochemical analysis of papaya (Carica papaya L.) hybrids grown in central Kenya. Plant Sci. Today 2023, 10, 263–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, Y.; Li, J.; Agar, O.T.; Barrow, C.; Dunshea, F.; Suleria, H.A. Screening and characterization of phenolic compounds by LC-ESI-QTOF-MS/MS and their antioxidant potentials in papaya fruit and their by-products activities. Food Biosci. 2023, 52, 102480. [Google Scholar] [CrossRef]
- Babalola, B.A.; Akinwande, A.I.; Otunba, A.A.; Adebami, G.E.; Babalola, O.; Nwufo, C. Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain. Arab. J. Chem. 2024, 17, 105369. [Google Scholar] [CrossRef]
- Jeon, Y.A.; Chung, S.W.; Kim, S.C.; Lee, Y.J. Comprehensive assessment of antioxidant and anti-inflammatory properties of papaya extracts. Foods 2022, 11, 3211. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Wu, Y.T.; Chang, H.J.; Huang, C.C.; Cheng, K.C.; Hsu, H.Y.; Hsieh, C.W. Anti-inflammatory and anti-oxidative effects of polysaccharides extracted from unripe Carica papaya L. fruit. Antioxidants 2023, 12, 1506. [Google Scholar] [CrossRef]
- Shoyshob, T.Z.; Heya, I.A.; Afrin, N.; Enni, M.A.; Asha, I.J.; Moni, A.; Hannan, A.; Uddin, J. Protective Mechanisms of Carica papaya Leaf Extract and Its Bioactive Compounds Against Dengue: Insights and Prospects. Immuno 2024, 4, 629–645. [Google Scholar] [CrossRef]
- Bustos, D.; Guzmán, L.; Valdés, O.; Muñoz-Vera, M.; Morales-Quintana, L.; Castro, R.I. Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel. Polymers 2023, 15, 3217. [Google Scholar] [CrossRef]
- Limongi, T.; Susa, F.; Marini, M.; Allione, M.; Torre, B.; Pisano, R.; di Fabrizio, E. Lipid-Based Nanovesicular Drug Delivery Systems. Nanomaterials 2021, 11, 3391. [Google Scholar] [CrossRef]
- Rusli, W.; Jackson, A.W.; Van Herk, A. A Roadmap towards Successful Nanocapsule Synthesis via Vesicle Templated RAFT-Based Emulsion Polymerization. Polymers 2018, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, E.F.; Ferreira, L.M.; Gehrcke, M.; Cruz, L.; Pedra, N.S.; Ramos, P.T.; Bona, N.P.; Soares, M.S.P.; Rodrigues, R.; Spanevello, R.M.; et al. 2-(2-Methoxyphenyl)-3-((Piperidin-1-yl)ethyl)thiazolidin-4-One-Loaded Polymeric Nanocapsules: In Vitro Antiglioma Activity and In Vivo Toxicity Evaluation. Cell Mol. Neurobiol. 2019, 39, 783–797. [Google Scholar] [CrossRef] [PubMed]
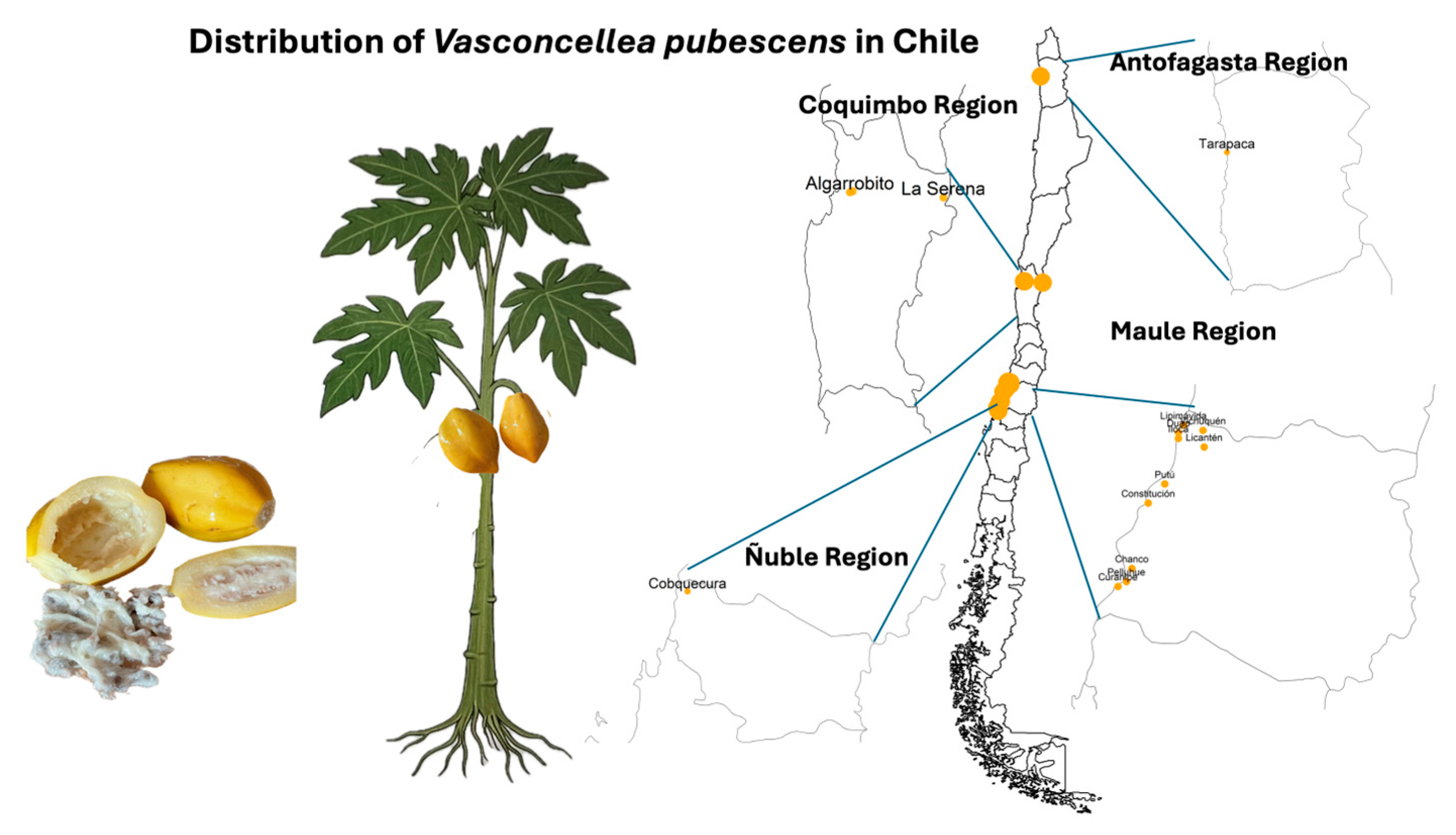
| Scientific Name | Other Names | Synonimus | Extension |
|---|---|---|---|
| Vasconcellea pubescens [10,11,12,13] | Chamburo, chilacuán, mountain pawpaw, papayer de la montagne, chamburo, papaya de tierra fría, chihualcán, siglalón, chichuacacón, titi-ish, bonete, papaya de altura, papayuela, tapaculo, ababai, bonete. | Vasconcellea cundinamarcensis [14,15], Carica candamarcensis Hook. F [16], Carica cestriflora (A. DC.) Solms, Carica chiriquensis Woodson [16], Carica pubescens (A. DC.) [17], Carica pubescens Lenné & C. Koch, Carica cundinamarcensis Linden, Papaya cundinamarcensis (Linden) Kuntze, Papaya pubescens (A. DC.) Kuntze, Vasconcellea cestriflora A. DC. | Colombia, Ecuador, Venezuela, Peru, Bolivia, Panama, Chile, Costa Rica |
| Female Parent | Male Parent | Mortality Rate in Glasshouse [39] | Color Flower | Color Fruit | Article |
|---|---|---|---|---|---|
| Carica papaya | Vasconcellea cauliflora | high | - | - | [9,34] |
| Carica papaya | Vasconcellea parviflora | low | pink | - | [32,34] |
| Vasconcellea pubescens | yellow or cream | [34] | |||
| Vasconcellea quercifolia | yellow | [34] | |||
| Vasconcellea spitulata | [34] | ||||
| Carica papaya | Vasconcellea goudotiana | high | yellow-purple | [34] | |
| Vasconcellea monoica | [34] | ||||
| Vasconcellea monoica | [34] | ||||
| Vasconcellea spitulata | Vasconcellea pubescens | - | orange, green or greenish-yellow | [2,35] | |
| V. heilbornii var. chrysopetala | Vasconcellea pubescens | high | - | [2,35,39] | |
| Specie | Tissue | Application |
|---|---|---|
| Prunus domestica | Carozo | Chipboard [44,45] |
| Oryza sativa | Rice husk | |
| Rice straw | ||
| Juglans regia | Nut shell | |
| Oryza sativa | Rice straw | NCC [44,46] |
| Cocos nucifera | Coconut shell | |
| Carica papaya | Papaya peel extract | Magnetite nanoparticles [47] |
| Peels and seeds | Oil from Unripe and ripe papaya seeds [11,48] | |
| Vasconcellea pubescens | Peel and seeds | Microencapsulation of bioactive extracts [12] |
| Mucilage and seeds Seeds | Antiglycans, [45] Chipboard [45] |
| Main Causes of Post-Harvest Loss | Mitigation Strategies | |
|---|---|---|
| Pathogenic fungi and others | Colletotrichum spp. Fusarium spp. Phytium spp. Oidium spp. Alternaria spp. Mycosphaerella spp. Meloidogyne incognita Tetranychus urticae [70] Aphis spp. [70] Erwinia papaya [71] Meloidogyne incognita [71] Rotylenchulus reniformis [71] | Hot water immersion ozonation, radiation, cold storage, chitosan, essential oils and silicones, Application of a hydrothermal-calcium chloride [43,72,73,74,75,76,77,78] |
| Mechanical damage | Bumps and bruises during harvesting, transport, and handling increase susceptibility to infection and accelerate deterioration. | Careful harvesting, sorting, ergonomic packaging and suitable transport [42,72,73] |
| Environmental factors | Improper temperatures, low humidity and light exposure can increase susceptibility to damage. | |
| Physiological disorders | Accelerated ripening, excessive softening and storage problems. | Use of 1-methylcyclopropene (1-MCP) to delay ripening and modified atmosphere technologies [10,43,72,79,80,81,82] |
| Compound Category | Specific Compounds | Concentration (Per 100 g) | Main Reported Biological Roles | Reference |
|---|---|---|---|---|
| Vitamins | Vitamin C (Ascorbic acid) | 448.30 mg (JKUAT 8 variety) | Antioxidant, supports immune function and prevents oxidative damage. | [97,98] |
| β-Carotene (Provitamin A) | 68.75 mg (Solo variety) | Antioxidant, retina and epithelial health via vitamin A conversion. | [99] | |
| Vitamin E | Variable concentrations | Antioxidant, membrane protection and lipid peroxidation prevention. | [98] | |
| B-Complex vitamins | Present in moderate amounts | Metabolic support, energy production. Cofactor in enzymatic reactions. | [98] | |
| Folate | Present | Metabolic support, DNA synthesis, cell division. | [98] | |
| Minerals | Potassium | 1145.10 mg (JKUAT 8) | Electrolyte balance, cardiovascular benefits. Regulates blood pressure, nerve function. | [98,99] |
| Calcium | Variable | Structural support, signaling. Bone health, muscle function. | [98,99] | |
| Magnesium | Variable | Enzyme cofactor, muscle function, activates over 300 enzymes. | [98,99] | |
| Carotenoids | Lycopene | 25.47 mg (Solo variety) | Antioxidant, associated with cardioprotective and anticancer properties. | [98,99] |
| Lutein/Zeaxanthin | Present | Macular protection, ocular health (blue light filtering). | [98] | |
| α-Carotene | Present | Antioxidant, free radical scavenging | [98] | |
| Enzymes | Papain | Variable (enzyme activity units) | Protein hydrolysis, tissue debridement, digestive aid, anti-inflammatory, wound healing. | [100] |
| Chymopapain | Variable | Peptide bond cleavage, proteolytic activity, inflammation reduction | [100] | |
| Phenolic Compounds | Chlorogenic acid | Identified via LC-ESI-QTOF-MS/MS | Antioxidant, anti-inflammatory, inhibits ROS production, NF-κB suppression | [101,102] |
| Neochlorogenic acid | Present | Antioxidant, free radical neutralization | [102] | |
| Cynarin | Present | Hepatoprotective, antioxidant, liver enzyme protection | [102] | |
| Eupatorine | Present | Anti-inflammatory, cytokine modulation | [102] | |
| Vicenin II | Present | Antioxidant, cardioprotective, oxidative stress reduction | [102] | |
| Flavonoids | Quercetin derivatives | Present | Anti-inflammatory, antioxidant, NF-κB pathway inhibition | [101,102] |
| Kaempferol compounds | Present | Antioxidant, anticancer potential, cell cycle regulation. | [101] | |
| Rutin | Present | Vascular protection, anti-inflammatory, Capillary strengthening. | [101] | |
| Polysaccharides | Pectin | Significant amounts | Digestive health, cholesterol reduction, gel formation, bile acid binding. | [98,103] |
| Other polysaccharides | Present | Antioxidant, anti-inflammatory, NF-κB modulation, immune support | [103] | |
| Alkaloids | Carpaine | Present (mainly in leaves/seeds) | Platelet modulation, antimicrobial, membrane interaction, enzyme inhibition | [100] |
| Other Compounds | Benzyl isothiocyanate (BITC) | Present in seeds | Antimicrobial, anticancer potential, protein modification, apoptosis induction | [98] |
| Glucosinolates | Present in seeds | Detoxification support, phase II enzyme induction | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, T.; Jara-Villacura, V.; Parra-Palma, C.; Morales-Quintana, L. Postharvest Biology and Quality Preservation of Vasconcellea pubescens: Challenges and Opportunities for Reducing Fruit Losses. Horticulturae 2025, 11, 1165. https://doi.org/10.3390/horticulturae11101165
Méndez T, Jara-Villacura V, Parra-Palma C, Morales-Quintana L. Postharvest Biology and Quality Preservation of Vasconcellea pubescens: Challenges and Opportunities for Reducing Fruit Losses. Horticulturae. 2025; 11(10):1165. https://doi.org/10.3390/horticulturae11101165
Chicago/Turabian StyleMéndez, Tamara, Valentina Jara-Villacura, Carolina Parra-Palma, and Luis Morales-Quintana. 2025. "Postharvest Biology and Quality Preservation of Vasconcellea pubescens: Challenges and Opportunities for Reducing Fruit Losses" Horticulturae 11, no. 10: 1165. https://doi.org/10.3390/horticulturae11101165
APA StyleMéndez, T., Jara-Villacura, V., Parra-Palma, C., & Morales-Quintana, L. (2025). Postharvest Biology and Quality Preservation of Vasconcellea pubescens: Challenges and Opportunities for Reducing Fruit Losses. Horticulturae, 11(10), 1165. https://doi.org/10.3390/horticulturae11101165








