Regulation of Cell Metabolism and Changes in Berry Shape of Shine Muscat Grapevines Under the Influence of Different Treatments with the Plant Growth Regulators Gibberellin A3 and N-(2-Chloro-4-Pyridyl)-N′-Phenylurea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Treatment
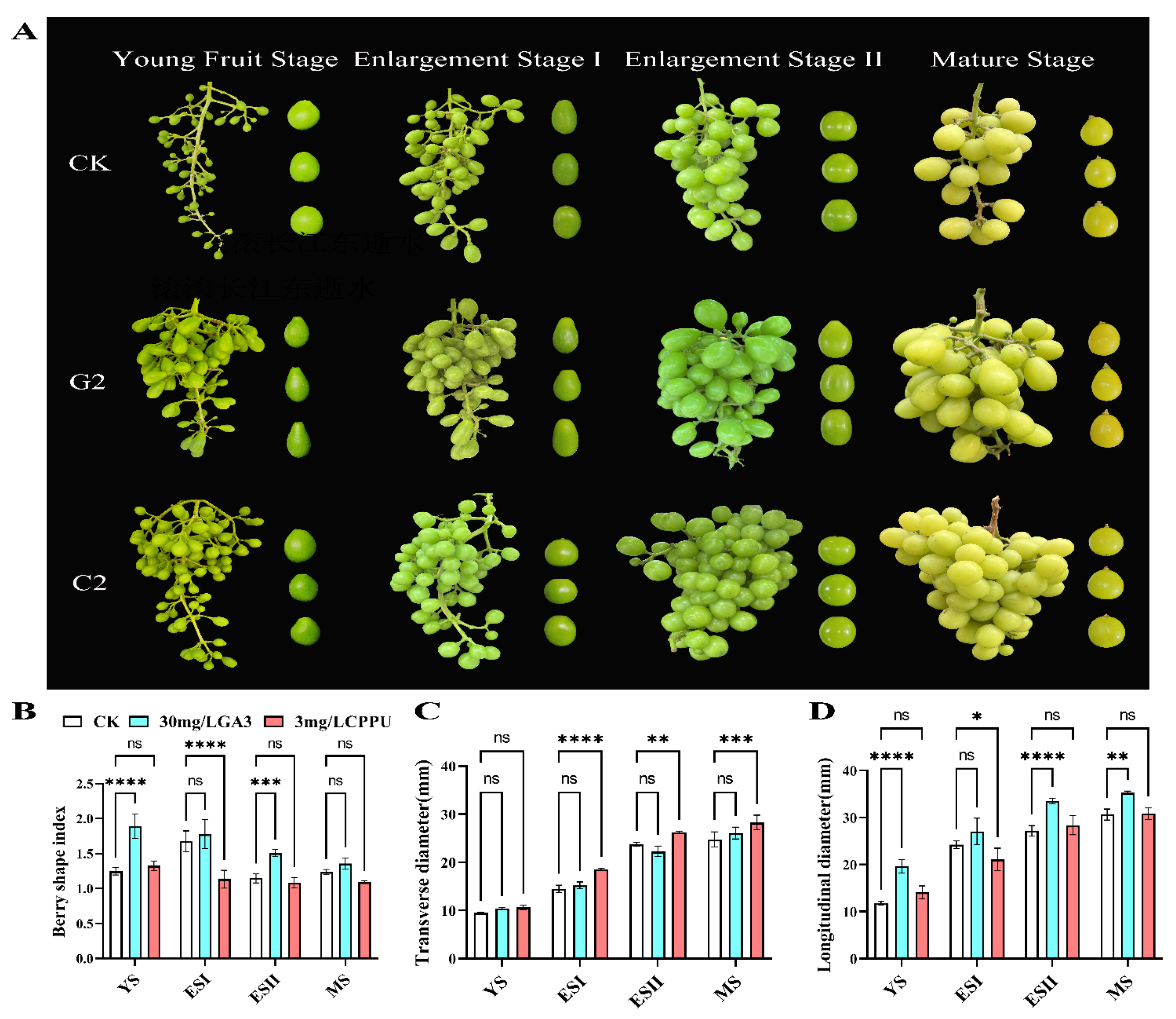
2.3. Determination of Grapevine Berry Growth Index
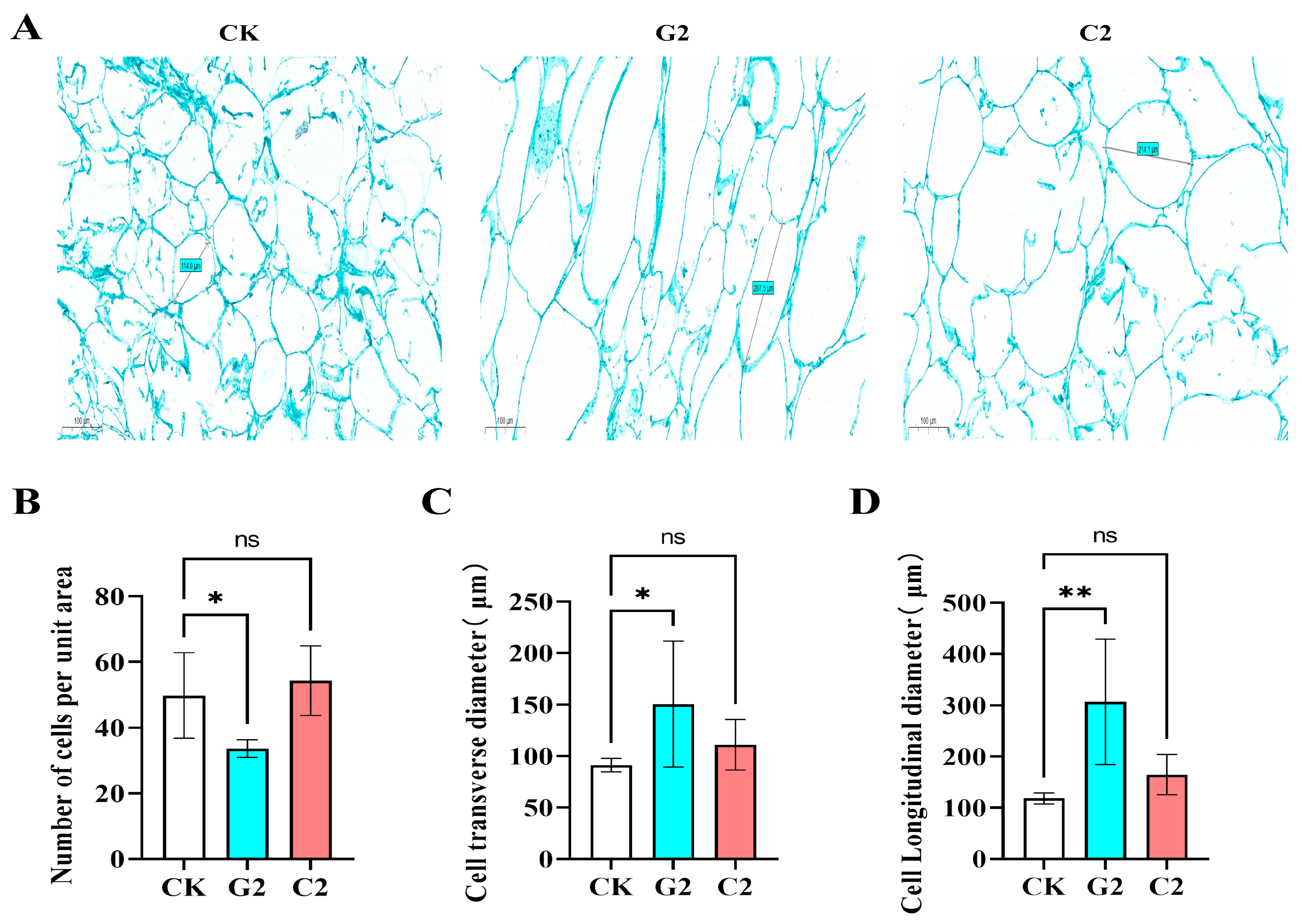
2.4. Microscopic Observation of Grapevine Berry Cell Morphology and Size
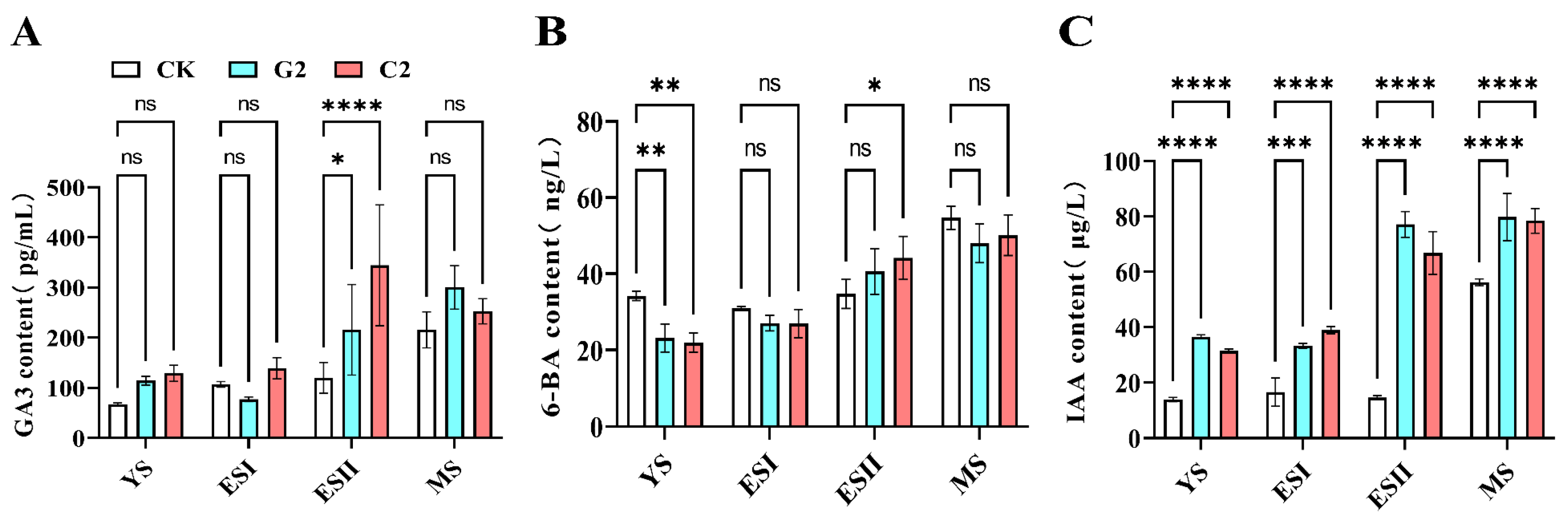
2.5. Determination of Endogenous IAA, GA3 and 6-BA Concentrations in Grapevine Berry
2.6. Transcriptome Analysis
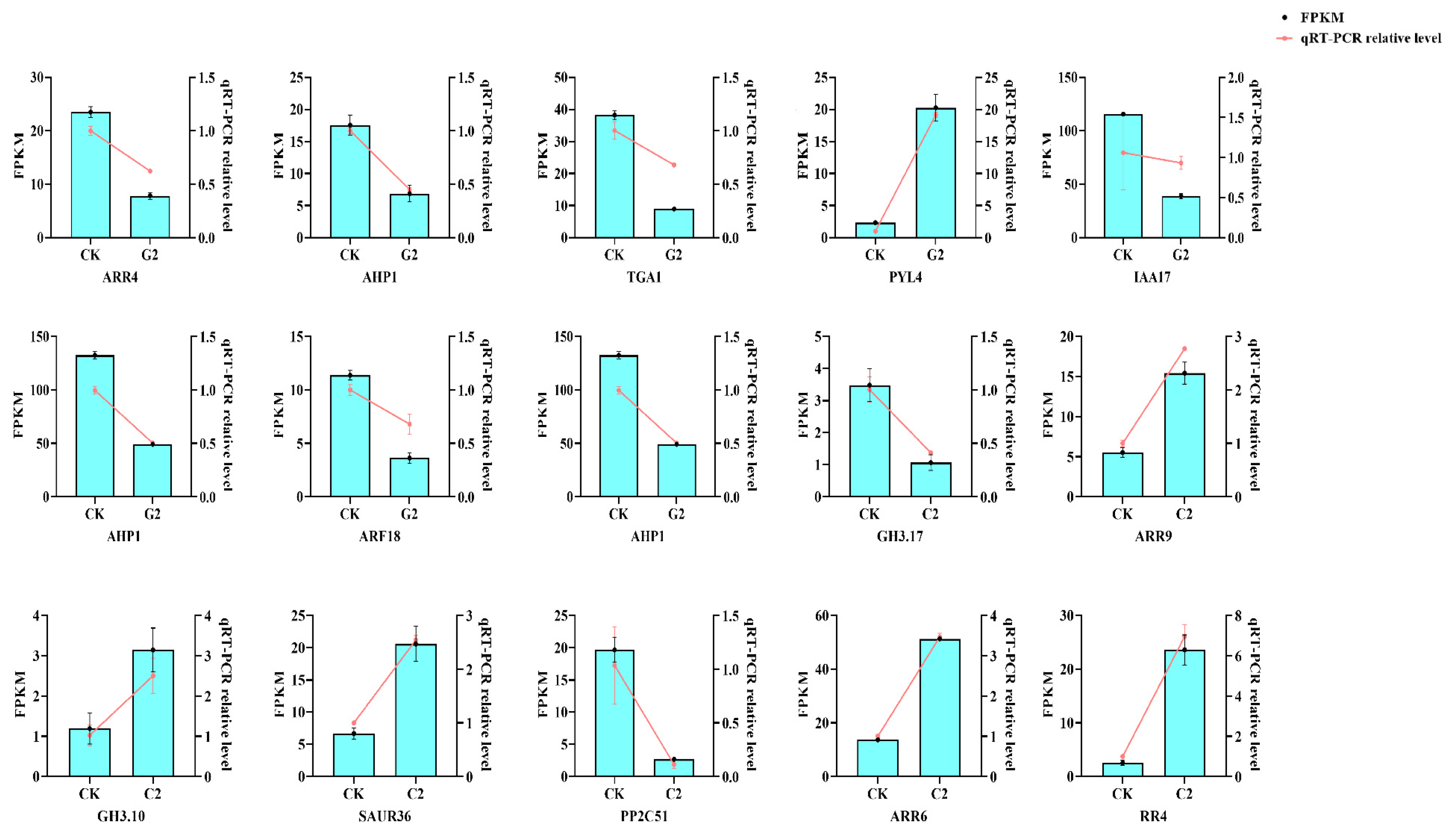
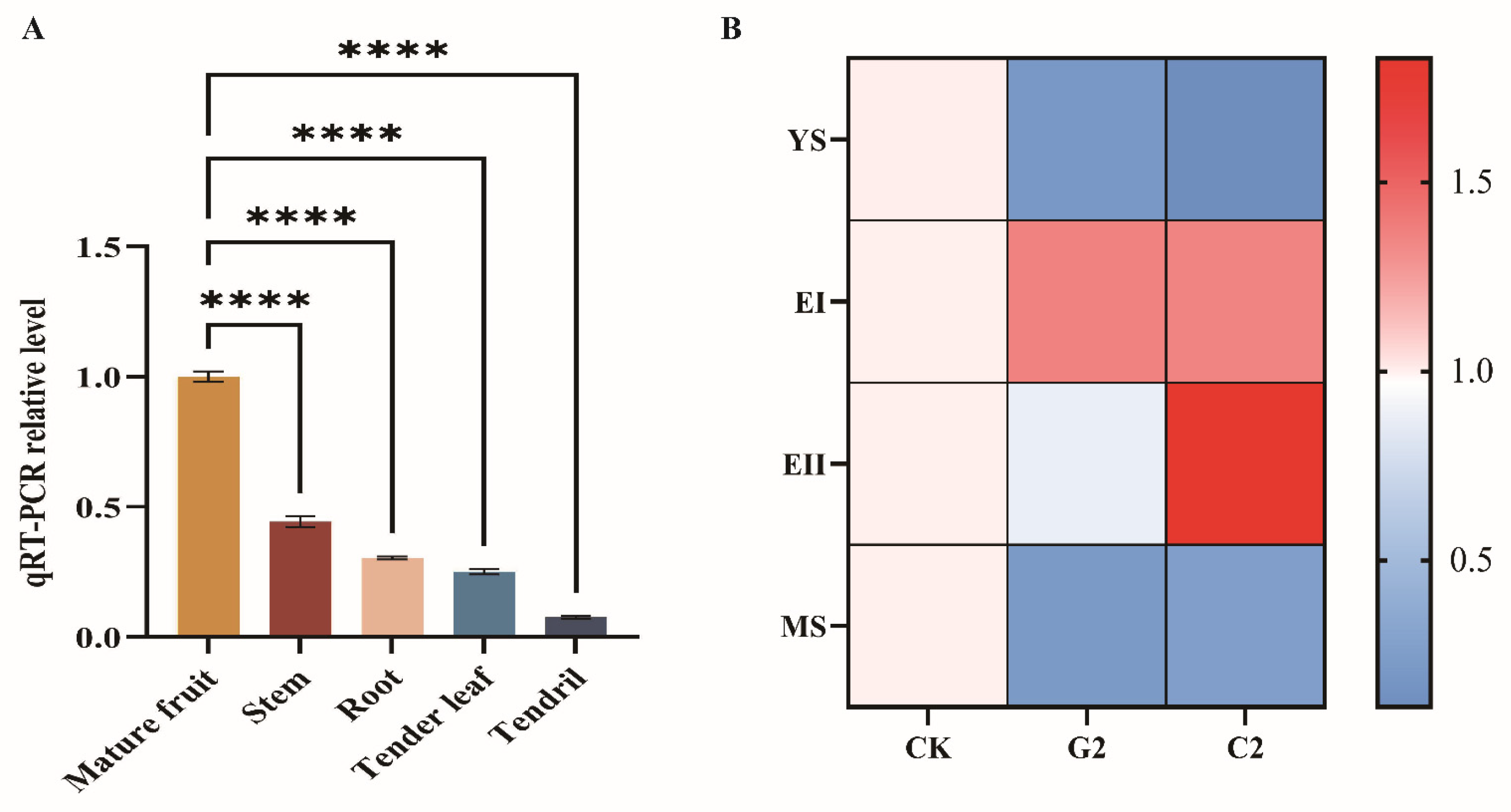
2.7. qRT-PCR Validation Analysis
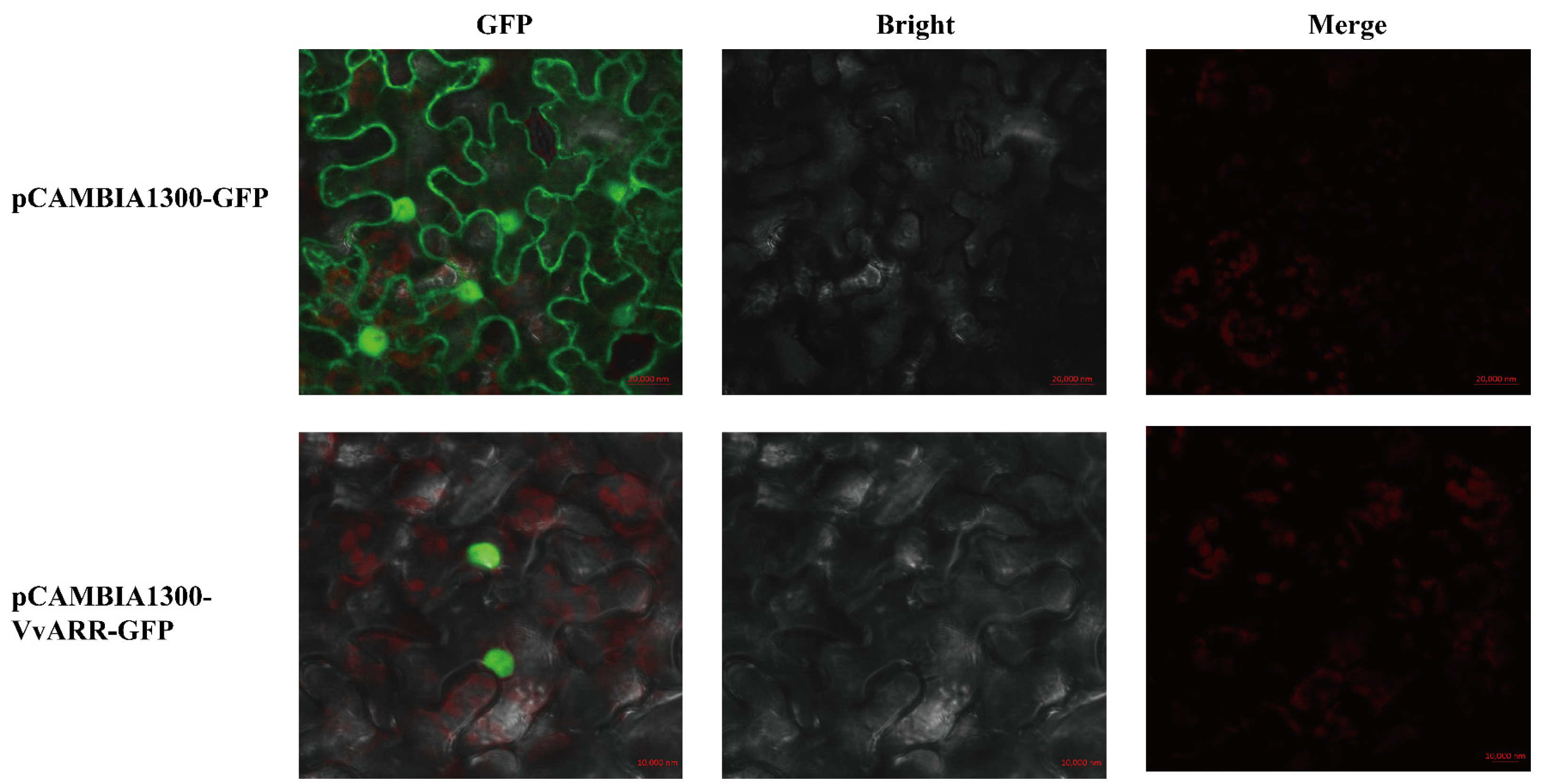
2.8. Subcellular Localization of VvARR: Green Fluorescent Protein Fusion
2.9. Transient Transformation of Grapevine Berry
2.10. Statistical Analysis
3. Results
3.1. Effects of GA3 and CPPU Application on Grape Berry Shape
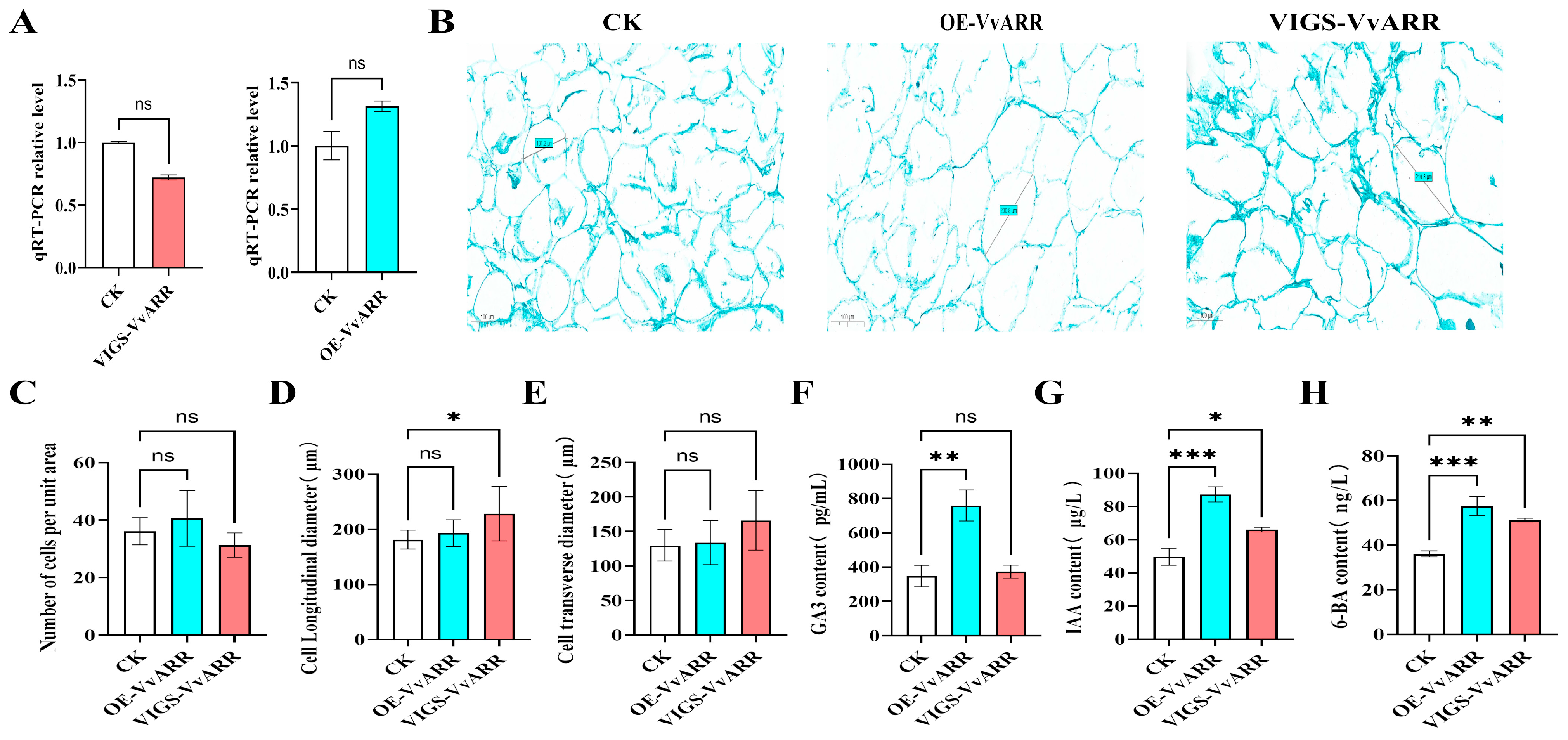
3.2. Effects of GA3 and CPPU Application on Cell Morphology of Grape Berries
3.3. Effects of GA3 and CPPU Application on Endogenous Hormones in Grape
3.4. Transcriptome Data Analysis of ‘Shine Muscat’ After GA3 and CPPU Treatments
3.5. Analysis of Expression Characteristics of Differentially Expressed Genes
3.6. Transient Overexpression and Virus-Induced Gene Silencing of VvARR Result in Changes in the Cellular Morphology of ‘Shine Muscat’
4. Discussion
4.1. Potential Regulation of Plant Hormones on Berry Shape
4.2. Impact of Cell Morphology on Berry Shape
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.; Huang, S.; Yang, X. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef]
- Cong, B.; Liu, J.; Tanksley, S.D. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc. Natl. Acad. Sci. USA 2002, 99, 13606–13611. [Google Scholar] [CrossRef]
- Mauxion, J.; Chevalier, C.; Gonzalez, N. Complex cellular and molecular events determining fruit size. Trends Plant Sci. 2021, 26, 1023–1038. [Google Scholar] [CrossRef]
- Wang, C.; Cao, J.; Hao, N.; Wu, T. Genetic and molecular regulation mechanisms in the formation and development of vegetable fruit shape. Appl. Sci. 2022, 12, 1514. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Ma, Z.; Zuo, C.; Chu, M.; Mao, J.; Chen, B. Regulatory mechanism of GA3 application on grape (Vitis vinifera L.) Berry size. Plant Physiol. Biochem. 2024, 210, 108543. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Tan, G.; Zhou, W.; Wang, G. Gibberellin and the plant growth retardant paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Nakata, Y.; Taniguchi, G.; Takazaki, S.; Oda-Ueda, N.; Miyahara, K.; Ohshima, Y. Comparative analysis of cells and proteins of pumpkin plants for the control of fruit size. J. Biosci. Bioeng. 2012, 114, 334–341. [Google Scholar] [CrossRef]
- Wang, G.; Gao, X.; Wang, X.; Liu, P.; Guan, S.L.; Qi, K.; Zhang, S.; Gu, C. Transcriptome analysis reveals gene associated with fruit size during fruit development in pear. Sci. Hortic. 2022, 305, 111367. [Google Scholar] [CrossRef]
- Yamada, M.; Yamane, H.; Sato, A.; Hirakawa, N.; Iwanami, H.; Yoshinaga, K.; Ozawa, T.; Mitani, N.; Shiraishi, M.; Yoshioka, M. New grape cultivar ‘shine muscat’. Bull. Natl. Inst. Fruit Tree Sci. 2008, 7, 21–38. [Google Scholar]
- Li, X.; Cai, Z.; Liu, X.; Wu, Y.; Han, Z.; Yang, G.; Li, S.; Xie, Z.; Liu, L.; Li, B. Effects of gibberellic acid on soluble sugar content, organic acid composition, endogenous hormone levels, and carbon sink strength in shine muscat grapes during berry development stage. Horticulturae 2024, 10, 346. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Zhong, R.; Tang, J.; Pervaiz, T.; Zhou, S.; Liu, J.; Wang, B.; Jia, H. Brassinolide and gibberellin promote grape fruit development and quality. Sci. Hortic. 2024, 338, 113619. [Google Scholar] [CrossRef]
- Xue, H.; Sekozawa, Y.; Sugaya, S. Effects of gibberellin and abscisic acid on fruit quality and aromatic compound formation during fruit development in ‘kyoho’ grapes (Vitis vinifera L. × Vitis labrusca L.). Environ. Control Biol. 2022, 60, 67–78. [Google Scholar] [CrossRef]
- Gao, X.T.; Wu, M.H.; Sun, D.; Li, H.Q.; Chen, W.K.; Yang, H.Y.; Liu, F.Q.; Wang, Q.C.; Wang, Y.Y.; Wang, J. Effects of gibberellic acid (GA3) application before anthesis on rachis elongation and berry quality and aroma and flavour compounds in Vitis vinifera L. ‘cabernet franc’ and ‘cabernet sauvignon’ grapes. J. Sci. Food. Agric. 2020, 100, 3729–3740. [Google Scholar] [CrossRef]
- Marzouk, H.A.; Kassem, H.A. Improving yield, quality, and shelf life of thompson seedless grapevine by preharvest foliar applications. Sci. Hortic. 2011, 130, 425–430. [Google Scholar] [CrossRef]
- Peppi, M.C.; Fidelibus, M.W. Effects of forchlorfenuron and abscisic acid on the quality of ‘flame seedless’ grapes. Hortscience 2008, 43, 173–176. [Google Scholar] [CrossRef]
- Stern, R.; Shargal, A.; Flaishman, M. Thidiazuron increases fruit size of ‘spadona’and ‘coscia’pear (Pyrus communis L.). J. Horticult. Sci. Biotechnol. 2003, 78, 51–55. [Google Scholar] [CrossRef]
- Bhujbal, B.G.; Ranawade, D.B.; Jagtap, K.B. Effect of CPPU on quality of grapes cv. Tas-a-ganesh. J. Maharashtra Agric. Univ. 2002, 27, 13–14. [Google Scholar]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Akhiyarova, G. Special issue “phytohormones 2022–2023”. Biomolecules 2024, 14, 1146. [Google Scholar] [CrossRef]
- Yang, B.; Wendrich, J.R.; De Rybel, B.; Weijers, D.; Xue, H.W. Rice microtubule-associated protein IQ67-DOMAIN14 regulates grain shape by modulating microtubule cytoskeleton dynamics. Plant Biotechnol. J. 2020, 18, 1141–1152. [Google Scholar] [CrossRef]
- Kumari, P.; Dahiya, P.; Livanos, P.; Zergiebel, L.; Kölling, M.; Poeschl, Y.; Stamm, G.; Hermann, A.; Abel, S.; Müller, S. IQ67 DOMAIN proteins facilitate preprophase band formation and division-plane orientation. Nat. Plants 2021, 7, 739–747. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Calcium: The missing link in auxin action. Plants 2013, 2, 650–675. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Boualem, A.; Berthet, S.; Devani, R.S.; Camps, C.; Fleurier, S.; Morin, H.; Troadec, C.; Giovinazzo, N.; Sari, N.; Dogimont, C. Ethylene plays a dual role in sex determination and fruit shape in cucurbits. Curr. Biol. 2022, 32, 2390–2401. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.; Feng, J.; Khalil-Ur-Rehman, M.; Tao, J. Transcriptome sequencing analyses reveals mechanisms of eliminated russet by applying GA3 and CPPU on ‘shine muscat’ grape. Sci. Hortic. 2019, 250, 94–103. [Google Scholar] [CrossRef]
- Xin, S.P.; Liu, S.; Yu, Y.; Nie, S.Q.; Gao, Z.H.; Tao, J.M. Effects of GA, and CPPU on grape fruit adjacent leaf photosynthesis and fruit quality. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2015, 26, 1814–1820. [Google Scholar]
- Choi, S.; Ban, S.; Choi, C. The impact of plant growth regulators and floral cluster thinning on the fruit quality of ‘shine muscat’ grape. Horticulturae 2023, 9, 392. [Google Scholar] [CrossRef]
- Coombe, B.G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Leng, F.; Cao, J.; Wang, S.; Jiang, L.; Li, X.; Sun, C. Transcriptomic analyses of root restriction effects on phytohormone content and signal transduction during grape berry development and ripening. Int. J. Mol. Sci. 2018, 19, 2300. [Google Scholar] [CrossRef]
- Xie, J.; He, C.; Li, Z.; Li, M.; He, S.; Qian, J.; Tan, B.; Zheng, X.; Cheng, J.; Wang, W. A rapid and efficient agrobacterium-mediated transient transformation system in grape berries. Protoplasma 2024, 261, 819–830. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef]
- Ali, S.; Baloch, A.M. Overview of sustainable plant growth and differentiation and the role of hormones in controlling growth and development of plants under various stresses. Recent Pat. Food Nutr. Agric. 2020, 11, 105–114. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Fan, S.; Du, L.; Shen, Y.; Xing, L.; Li, Y.; Ma, J.; Han, M. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘fuji’ apple (Malus domestica borkh.). Plant Physiol. Biochem. 2016, 107, 178–186. [Google Scholar] [CrossRef]
- Zheng, H.; Dong, Y.; Nong, H.; Huang, L.; Liu, J.; Yu, X.; Zhang, Y.; Yang, L.; Hong, B.; Wang, W. VvSUN may act in the auxin pathway to regulate fruit shape in grape. Hortic. Res. 2022, 9, uhac200. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Guan, P.; Hu, J.; Sun, L. Interaction of VvDELLA2 and VvCEB1 mediates expression of expansion-related gene during GA-induced enlargement of grape fruit. Int. J. Mol. Sci. 2023, 24, 14870. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Abd El-Wahed, M.S. Effect of exogenous salicylic acid or indole acetic acid on their endogenous levels, germination, and growth in maize. Bull. Natl. Res. Cent. 2020, 44, 167. [Google Scholar] [CrossRef]
- Nan, X.; Xie, M.; Li, W.; Chen, L.; Jiang, S.; Zhao, Y.; Ma, Z. Analysis of the regulatory mechanisms of morphogenesis in grape berries with different fruit shapes based on transcriptome. Plant Stress 2025, 15, 100792. [Google Scholar] [CrossRef]
- He, J.; Yu, S.; Ma, C. Effects of plant growth regulator on endogenous hormone levels during the period of the red globe growth. J. Agric. Sci. 2009, 1, 92. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, L.; Zhang, W.; Liu, J.; Nong, H.; Wang, X.; Zheng, H.; Tao, J. Transcriptomic and functional analysis reveals that VvSAUR43 may be involved the elongation of grape berries. Sci. Hortic. 2023, 318, 112119. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Xu, X.; Wang, R.; Liu, Y.; Huang, S.; Wei, H.; Wei, Z. Molecular mechanisms of diverse auxin responses during plant growth and development. Int. J. Mol. Sci. 2022, 23, 12495. [Google Scholar] [CrossRef]
- Chai, L.; Li, Y.; Chen, S.; Perl, A.; Zhao, F.; Ma, H. RNA sequencing reveals high resolution expression change of major plant hormone pathway genes after young seedless grape berries treated with gibberellin. Plant Sci. 2014, 229, 215–224. [Google Scholar] [CrossRef]
- Hayata, Y.; Li Xinxian, L.X.; Osajima, Y. Pollination and CPPU treatment increase endogenous IAA and decrease endogenous ABA in muskmelons during early development. J. Am. Soc. Hortic. Sci. 2002, 127, 908–911. [Google Scholar] [CrossRef]
- De Jong, M.; Wolters Arts, M.; Feron, R.; Mariani, C.; Vriezen, W.H. The solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant. J. 2009, 57, 160–170. [Google Scholar] [CrossRef]
- Upadhyay, A.; Maske, S.; Jogaiah, S.; Kadoo, N.Y.; Gupta, V.S. GA 3 application in grapes (Vitis vinifera L.) Modulates different sets of genes at cluster emergence, full bloom, and berry stage as revealed by RNA sequence-based transcriptome analysis. Funct. Integr. Genom. 2018, 18, 439–455. [Google Scholar] [CrossRef]
- Wang, L.; Xue, J.; Yan, J.; Liu, M.; Tang, Y.; Wang, Y.; Zhang, C. Expression and functional analysis of VviABCG14 from Vitis vinifera suggest the role in cytokinin transport and the interaction with VviABCG7. Plant Physiol. Biochem. 2020, 153, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Liu, X.; Fan, X.; Zhang, Y.; Liu, C.; Sun, L. Structure and function of Vitis vinifera arabidopsis response regulator 1 (VvARR1) protein provide insights into the regulatory mechanism of grape fruit shape through gibberellin-cytokinin crosstalk. Int. J. Biol. Macromol. 2025, 319, 145246. [Google Scholar] [CrossRef]
- Sweetman, C.; Wong, D.C.; Ford, C.M.; Drew, D.P. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genom. 2012, 13, 691. [Google Scholar] [CrossRef]
- Marowa, P.; Ding, A.; Kong, Y. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sha, G.; Gao, R.; Pang, J.; Zhai, R.; Yang, C.; Wang, Z.; Xu, L. PbGIF1 promoting cell-proliferation in pear fruit is transcriptionally activated by PbRR1. Hortic. Plant J. 2024, 10, 689–697. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Guo, Y.; Hu, H.; Fang, C.; Wang, L.; Hu, L.; Lin, Z.; Zhang, D.; Yang, Z.; Wu, Y. Regulation of Cell Metabolism and Changes in Berry Shape of Shine Muscat Grapevines Under the Influence of Different Treatments with the Plant Growth Regulators Gibberellin A3 and N-(2-Chloro-4-Pyridyl)-N′-Phenylurea. Horticulturae 2025, 11, 1160. https://doi.org/10.3390/horticulturae11101160
Chen J, Guo Y, Hu H, Fang C, Wang L, Hu L, Lin Z, Zhang D, Yang Z, Wu Y. Regulation of Cell Metabolism and Changes in Berry Shape of Shine Muscat Grapevines Under the Influence of Different Treatments with the Plant Growth Regulators Gibberellin A3 and N-(2-Chloro-4-Pyridyl)-N′-Phenylurea. Horticulturae. 2025; 11(10):1160. https://doi.org/10.3390/horticulturae11101160
Chicago/Turabian StyleChen, Jiangbing, Yanfei Guo, Haichao Hu, Congling Fang, Liru Wang, Lingling Hu, Zhihao Lin, Danyidie Zhang, Zhongyi Yang, and Yueyan Wu. 2025. "Regulation of Cell Metabolism and Changes in Berry Shape of Shine Muscat Grapevines Under the Influence of Different Treatments with the Plant Growth Regulators Gibberellin A3 and N-(2-Chloro-4-Pyridyl)-N′-Phenylurea" Horticulturae 11, no. 10: 1160. https://doi.org/10.3390/horticulturae11101160
APA StyleChen, J., Guo, Y., Hu, H., Fang, C., Wang, L., Hu, L., Lin, Z., Zhang, D., Yang, Z., & Wu, Y. (2025). Regulation of Cell Metabolism and Changes in Berry Shape of Shine Muscat Grapevines Under the Influence of Different Treatments with the Plant Growth Regulators Gibberellin A3 and N-(2-Chloro-4-Pyridyl)-N′-Phenylurea. Horticulturae, 11(10), 1160. https://doi.org/10.3390/horticulturae11101160




