Abstract
Continuous cropping obstacles (CCOs) lead to a decline in yield and quality under repeated cultivation in the same farmland. Notably, CCOs caused by fusarium wilt, autotoxicity, or imbalance in rhizosphere microbial communities reduce the productivity of watermelons (Citrullus lanatus). Considering the negative environmental impacts of conventional agrochemicals, it is necessary to evaluate the biocontrol efficiency of microorganisms. Therefore, this study aimed to investigate the biocontrol efficiency of Bacillus amyloliquefaciens strain PP19 against CCOs of watermelon so as to develop alternatives to agrochemicals. The inhibitory effect of PP19 on watermelon fusarium wilt was assessed through plate confrontation assays and field trials. The degradation and utilization of autotoxins by PP19 were examined via co-culture experiments. Additionally, 16S rRNA sequencing was employed to analyze the impact of PP19 on the rhizosphere soil microbial community of watermelon. Specifically, we analyzed the PP19 utilization of four phenolic autotoxins secreted by watermelon roots and assessed their effects on microbial diversity in the watermelon rhizosphere. Plant growth assays showed that PP19 improved the weight and quality of watermelon fruit. Although PP19 inhibited the growth of Fusarium oxysporum f. sp. niveum (Fon), the growth inhibitory effect was significantly enhanced by autotoxins produced by watermelon, including mixed phenolic, cinnamic, ferulic, and p-coumaric acids. Additionally, PP19 effectively degraded and utilized the autotoxins, and the autotoxins enhanced PP19’s swimming ability and biofilm formation. Moreover, PP19 treatment significantly enhanced the microbial diversity in watermelon rhizosphere, increased the number of beneficial bacterial genera, and decreased the number of pathogenic genera. Conclusively, these results suggest that B. amyloliquefaciens strain PP19 improves the resistance of watermelon to CCOs by effectively utilizing and degrading autotoxin, altering soil microbial community structure, and inhibiting Fon17 growth, resulting in improved fruit quality. Overall, PP19 possesses potential application as a biological control agent against CCOs in commercial watermelon cultivation.
1. Introduction
Watermelon (Citrullus lanatus), a member of the Cucurbitaceae family, is an economically significant horticultural crop worldwide, with a global planting area of 350 hm2 and a total production of approximately 100 million tons [1]. However, intensive and repeated watermelon cultivation on the same field has exacerbated the occurrence of watermelon continuous cropping obstacles (CCOs). Continuous cropping leads to changes in soil physical and chemical properties, an increase in pathogenic bacteria, a decrease in soil enzyme activities, and the destruction of soil microbial community [2,3]. Collectively, these changes result in a severe decline in fruit yield and quality and hinder sustainable watermelon production [4].
Notably, the primary factors underlying watermelon CCOs are Fusarium wilt, watermelon autotoxicity, and a decrease in beneficial bacteria in the cultivated soil [5,6,7]. Watermelon Fusarium wilt (WFW) is a soil-borne fungal disease caused by Fusarium oxysporum f. sp. niveum (FON), a specialized pathogen of watermelon [8]. FON produces enzymes that degrade the plant cell wall, forming gummy and invasive materials that block the vascular system of the host plant, resulting in wilting due to the plant’s inability to transport water [9,10]. Watermelon CCOs also arise from several autotoxic phenolic acids, including benzoic acid (BA), cinnamic acid (CA), p-coumaric acid (PA), and ferulic acid (FA), produced by the watermelon [11,12]. These autotoxins can directly affect the growth of watermelon roots and reduce yield. As with other continuous cropping diseases, such as replant disease in tobacco, continuous cropping results in soil conditions that prevent the growth of subsequent rotations of the crop. These substances also recruit harmful soil-borne pathogens and can directly or indirectly affect critical processes, such as photosynthesis, ion absorption, and DNA and protein synthesis, thereby inhibiting plant growth [13]. Additionally, watermelon CCOs can be caused by an imbalance in microbial community structure [14].
Currently, the main strategies for addressing CCOs include grafting, crop rotation, intercropping, environmental and plant regulation, along with physical, chemical, and biological controls [15,16,17]. However, grafting is labor-intensive; crop rotation and intercropping affect other crops; environmental regulation is cost-intensive; and chemical control damages the environment. Biological control is an eco-friendly, cost-effective, and economically sustainable alternative to manage CCOs. This can help reduce environmental pollution as required by SDG12 (https://www.un.org/sustainabledevelopment/sustainable-consumption-production/, accessed on 17 September 2025) and promote sustainable development. Notably, several biological agents, such as Bacillus, Pigmentiphaga, Streptomyces, Rhizobium, Corynebacterium, and Pseudomonas, have shown great potential for preventing CCOs [11,18,19,20,21,22,23]. For example. Bacillus subtilis T6-1 enhances the structure of the rhizosphere microbial community, and Bacillus cereus WL08 eliminates autotoxins (butylated hydroxytoluene), thereby alleviating the CCOs of poplar and Pinellia ternata, respectively [21,22]. Additionally, Bacillus subtilis C3 eliminates phenolic acids and improves the composition and structure of the melon rhizosphere microbial community to prevent CCOs.
Over the years, several studies have been conducted to develop effective preventive and management strategies for watermelon CCOs. Notably, the application of novel bioorganic fertilizers [6], urban waste compost [24], Bama pig manure [25], cattle manure and garlic rotation [17], Bacillus subtilis, and Paecilomyces lilacinus [14] can regulate bacterial community composition to inhibit the occurrence of Fusarium wilt under continuous cropping conditions. Root exudates from mycorrhizal watermelon seedlings [26], slightly acidic electrolyzed water [27], and Bacillus sp. XG-1 [28] not only improved the microbial community but also sterilized F. oxysporum. However, studies have yet to investigate the use of autotoxins as potential biocontrol agents. Therefore, this study aimed to investigate whether the biocontrol agent Bacillus amyloliquefaciens PP19 can not only inhibit the growth and improve the microbial community, it can degrade autotoxins and utilize them to promote self-production and enhance biocontrol of watermelon CCOs. Overall, it is anticipated that this study will provide comprehensive information and a practical strategy to prevent and control watermelon CCOs.
2. Materials and Methods
2.1. Plant Materials, Microorganisms, and Autotoxic Solutions
Seeds of the watermelon variety ‘Linglong’ were surface-sterilized as described by Guo et al. [29] and sown in trays filled with potting soil. Seedlings at the three-leaf stage were transplanted to pots (top diameter: 12.8 cm; bottom diameter: 9.3 cm; height: 11 cm) filled with potting soil and incubated under natural conditions.
Bacillus amyloliquefaciens PP19, isolated from lychee fruit peels, was prepared according to the method described by Zheng et al. [30]. Which could inhibite the growth of Peronophthora litchii, Fusarium oxysporum f. sp. niveum and Klebsiella variicola in the preliminary experiment of plate confrontation assay. The WFW pathogen Fusarium oxysporum f. sp. Niveum (Fon17), was kindly provided by Dr. Chunhao Jiang, Nanjing Agricultural University. Technical grade autotoxic substances (cinnamic acid [CA], ferulic acid [FA], benzoic acid [BA], and p-coumaric acid [PA]) were purchased and dissolved in 95% ethanol to prepare 30 mmol/L stock solutions, as listed in Table S1.
2.2. Effects of PP19 on Watermelon Fruit Yield and Quality After Eight Cycles of Continuous Cropping
(1) Plant material cultivation: The efficacy of PP19 against CCOs and its effects on watermelon yield were assessed in a large plastic transparent tent (40 m × 24 m) in the Huadu district of Guangzhou City (Guotai Base, Guangzhou Experimental Station, Chinese Academy of Tropical Agricultural Sciences). The temperature, light, and humidity changed based on weather conditions, and the frequency and amount of watering also depended on weather changes, ensuring that the soil moisture was around 70%. The native soil was composed mostly of clay, and abundant organic matter, such as cow manure, was added before planting watermelon seedlings. In-ground irrigation was installed for manual operation. Watermelon plants were continuously cultivated during four consecutive years. Planting occurred in early April each year; this planting could result in two or three crops. The plant can regrow with top growth cut to 15 cm from the ground after each harvest. The last harvest was in late October each year. The last harvest was in late October each year. After three years of cultivation (eight cycles of continuous cropping), the watermelon fruits were used for yield and quality testing.
(2) Microbial culture and treatment: A stock sample of the biocontrol agent PP19 was taken from a −80 °C freezer, streaked onto Luria–Bertani (LB) medium, and cultivated at 28 °C for 24 h. A single colony was picked and inoculated into 4.0 mL of LB. Cultures were incubated at 28 °C and 180 rpm for 20–22 h, transferred into 100 mL of LB, and incubated at 28 °C and 180 rpm for 36 h. Cultures were then centrifuged at 8000 rpm for 15 min at 4 °C, the supernatant was discarded, the bacterial cells were resuspended in sterile water, and their concentration was adjusted to an optical density at 600 nm (OD600) of 1. At each of the eight cycles, plants, each watermelon seedling was irrigated with 250 mL of a PP19 bacterial suspension (OD600 = 0.1) or sterile water as a control at 0, 14, and 30 days post-transplantation/post-cutting. Each treatment was performed using three biological replicates.
(3) Methods of fruit sampling and quality testing: Watermelon fruits were harvested 55 days after planting, or 70 and 130 days after harvest, and weighed. Each watermelon sample was cut into half, and three positions, including the center and approximately 5 cm to the left or to the right of the center, were sampled to obtain 100 g of watermelon pulpy tissue. The watermelon tissue was blended for approximately 1–2 min until no visible pulpy tissue remained. Approximately 50 mL was transferred and stored in a centrifuge tube (Sangon, Shanghai, China). The extracted juice was stored at −80 °C in a freezer until analysis. Watermelon water content was determined using the drying method [31], protein content was determined using the Kjeldahl method [32], total sugar content was determined using the anthrone method [33], vitamin C content was determined using the 2,4-dinitrophenylhydrazine colorimetric method [34], and free amino acid content was determined using the ninhydrin method [35].
2.3. Determination of the Effect of Autotoxins on Fon17 Growth
Fon17 stored at 4 °C was inoculated onto potato dextrose agar (PDA), followed by incubation at 28 °C for 10 days until fungal growth covered the entire plate. A cork borer was used to remove 5 mm diameter hyphal plugs from the growing edge of mycelia, and each plug was placed into the center of a fresh plate of PDA amended with autotoxins. The plates were incubated at 28 °C, and colony diameter was measured after 10 days. An appropriate concentration series of autotoxins (0.1, 0.5, and 1.0 mmol/L) was selected following Zhao et al. [36]. The treatments included these three different concentrations of each of the four autotoxins (CA, FA, BA, and PA) and one negative control (CK) without any autotoxin, making a total of 13 treatments (Table S2). Each treatment was performed using two technical replicates and six biological replicates (multiple independent experiments). Inhibition rate (%) was calculated as [(control diameter–treatment diameter/control diameter] × 100.
2.4. Determination of Antifungal Activity of PP19 Against Fon17 In Vitro and Biocontrol Efficacy in Pots
A 5 mm diameter hyphal plug of Fon17 was placed in the center of each plate, followed by the addition of 10 µL of PP19 bacterial suspension (OD600 = 0.1) at four equidistant spots (27 mm from the Fon17). After incubation at 28 °C for 10 days, the colony diameter was recorded. This procedure was repeated three times for each treatment.
Each pot containing 100 mL of peat moss potting mix was planted with a single one-month-old seedling and watered with 25 mL of PP19 suspension (107 CFU/mL [OD600 = 0.1]), 0.02% aqueous solution of chlorothalonil (Chl; positive control), or water (negative control), with four replicates per treatment and nine seedlings per replicate. Seedlings in the negative control group were not treated with any chemicals. After 10 days, the treatments were applied again. Overall, the experiment was arranged in a randomized complete block design. Pathogen inoculation occurred at 10 and 20 days after transplanting (DAT), with 50 mL of Fon17 spore suspension (1 × 105 conidiospores/mL) was applied as a root drench to each plant. The incidence of WFW was investigated 18 d after the last pathogen inoculation (dpi).
2.5. Establishment of PP19-Autotoxin-Fon17 Co-Culture System
Three experiments were performed: co-culture of PP19 and autotoxins; co-culture of PP19, autotoxins, and Fon17; and co-culture of autotoxins and Fon17. WA medium (Waksman agar medium; 5 g tryptone, 10 g glucose, 3 g beef extract, 5 g NaCl per liter) [37] containing 0.1 mmol/L of autotoxins (CA, FA, BA, PA, or mixture) was prepared. In the first experiment, 10 μL of PP19 suspension (OD600 = 0.1) was placed at the center of a WA plate containing an autotoxin (30 mmol/L) approximately 27 mm from the center of the plate. In the second experiment, a 5 mm diameter Fon17 hyphal plug was placed at the center of a WA plate containing autotoxins, followed by the addition of 10 μL of PP19 suspension at four points approximately 27 mm from the center. In the third experiment, a 5 mm diameter Fon17 hyphal plug was placed at the center of a WA plate containing autotoxins. WA plates without autotoxins were used as negative controls. All plates were incubated at 28 °C and scored after 14 days by measuring the fungal colony diameter. Each treatment had three biological replicates and two technical replicates. Growth-promotion rate (%) was calculated as follows: [(treatment colony diameter − control colony diameter)/control colony diameter] × 100.
2.6. Autotoxin Degradation and Utilization by PP19
To test for carbon source utilization, Minimal salts medium (MSM; 1.0 g (NH4)2SO4, 1.0 g K2HPO4, 0.2 g NaH2PO4, 0.2 g MgSO4·7H2O, 0.05 g NaCl, 0.05 g CaCl2, 8.3 mg FeCl3·6H2O, 1.4 mg MnCl2·4H2O, 1.17 mg NaMoO4·2H2O, and 1 mg ZnCl2 per liter) [38] containing autotoxins (0.01 mmol/L) was prepared for two experiments. In the first experiment, 800 μL of MSM broth containing the respective autotoxin (CA, FA, BA, PA, or mixture) was inoculated with 100 μL of PP19 cell suspension (OD600 = 0.1), followed by the addition of 100 μL of sterile water. In the second experiment, 800 μL of MSM broth containing each autotoxin was inoculated with 100 μL of PP19 cell suspension, followed by the addition of 100 μL of Fon17 spore suspension. Each experiment consisted of five autotoxins (CA, FA, BA, PA, and a mixture) and a negative control, making a total of 12 treatments. Each treatment was performed with six biological replicates. Treatments were incubated at 28 °C and 180 rpm, and 100 μL samples were collected at 1, 3, 5, and 7 days of mixing. Samples were diluted 106, and 100 μL aliquots were taken from each dilution to determine the number of colony-forming units (CFU) per mL in each sample. To determine the effect of the autotoxins on PP19 in the presence of a particular carbon source, we performed an experiment consisting of a mixture of 800 μL of Luria–Bertani (LB) broth, 100 μL of PP19 cell suspension (OD600 = 0.1), and 100 μL of sterile water. The samples were incubated at 28 °C and 180 rpm, and OD600 was measured at 48 h post-inoculation.
Based on the results of stimulated growth compared to LB alone, we selected two autotoxins (CA and FA) with potent utilization efficacy and examined their degradability by PP19 using liquid chromatography–tandem mass spectrometry (HPLC-MS/MS, Agilent Technologies, Santa Clara, CA, USA). Cultivation was performed using 800 μL of MSM, 100 μL of PP19 cell suspension, and 100 μL of sterile water at 28 °C under constant shaking at 180 rpm for 7 days. The concentration of CA or FA was 0.1 mmol/L. After 7 days of incubation, 800 μL of 1 M NaOH was added, followed by shaking at 4 °C for 16 h and centrifugation at 10,000× g for 5 min to collect the supernatant. The pH of the supernatant was adjusted to 2.0 with HCl, and 500 μL of ethyl acetate was added to extract metabolites for HPLC-MS/MS. The mixture was vortexed for 1 min, and the ethyl acetate phase was collected. After removal of residual water from this phase using 0.1 g of anhydrous MgSO4 and centrifugation at 12,000 rpm for 2 min, the supernatant was collected, dried with nitrogen gas, and dissolved in 500 μL of 1% formic acid. A sample was eluted with 400 μL of methanol, concentrated using nitrogen gas drying, then dissolved in 50 μL of methanol, passed through a 0.22 μm filter membrane, and analyzed using LC-MS/MS to determine the amount of the two autotoxins. A control experiment was conducted using MSM with the same concentration of autotoxins but without PP19. Each treatment was performed in triplicate.
HPLC-MS/MS detection was performed using a Waters BEH C18 (Waters, Milford, MA, USA) chromatographic column (2.1 × 100 mm × 1.7 μm). The mobile phase consisted of A:B = methanol: 0.1% formic acid in water at a flow rate of 300 μL/min, and the liquid phase gradient parameters are as indicated in Table S3. The column temperature was set at 30 °C, and the injection volume was 1 μL. MS detection was performed in negative ion mode with an electrospray ionization source in multiple reaction monitoring mode. The following parameters were used: curtain gas, 15 psi; spray voltage, −4 kV; ionization temperature, 400 °C; nebulizer gas pressure, 65 psi; and auxiliary gas pressure, 70 psi. The degradation rate of autotoxins (%) was calculated as follows: [(control content − treatment content)/control content] × 100.
2.7. Social Behavior Effect of Autotoxins on PP19
In the preliminary experiment, a concentration of 0.1 mmol/L of autotoxins had no effect on the growth of biocontrol and pathogenic microorganisms. Therefore, this concentration was used in swimming, swarming, as well as the co-culture experiments. Two types of motility media containing 0.1 mmol/L each autotoxin (swimming and swarming) were prepared as follows: swimming motility medium (peptone: 5 g/L, sodium chloride: 5 g/L, agar: 5 g/L, and deionized water: 1 L), and swarming motility medium (tryptone: 5 g/L, sodium chloride: 5 g/L, agar: 4 g/L, and deionized water: 1 L). For assessing motility, 20 mL of each medium was poured into 90 mm diameter Petri plates and allowed to solidify. Addition of inoculum followed Pearson [39] with minor modifications: 3 μL of PP19 cell suspension was spotted onto the center of each culture plate, and the plates were sealed with parafilm and incubated at 28 °C. Three biological replicates were used for each treatment group. The PP19 motility range was measured and recorded after 14 h of incubation for swimming motility testing and after 36 h for swarming motility testing.
Biofilm formation was detected using crystal violet staining [40]. SOBG (Salt-Optimized Broth plus Glycerol) medium (20 g tryptone, 5 g yeast extract, 2.4 g MgSO4·7H2O, 0.5 g Na2S, 0.186 g K2S per liter, with 20 mL glycerol/L added just before use) containing autotoxins at a concentration of 0.1 mmol was prepared, and 100 μL of the SOBG medium was added to each well of a 96-well plate. Thereafter, 1 μL of PP19 cell suspension was added to each well, followed by incubation at 28 °C and 180 rpm for 18 h. The culture was discarded, and approximately 150 μL of a 0.1% (w/v) crystal violet solution was added to each well, followed by incubation at 28 °C for 20 min, after which the staining solution was discarded with be careful to avoid the biofilm, suck clean the staining solution at the bottom with the gun head. After three washes with water, the 96-well plates were air-dried. Finally, 200 μL of 95% ethanol was added to each well, and the plates were allowed to stand for 15 min. The absorbance was measured at 595 nm using a Multiskan Go microplate reader (Gen5, Bio-Tek Instruments, Inc., Winooski, VT, USA). The control group consisted of samples that did not contain any autotoxin. Each treatment was performed with eight biological replicates.
To examine the chemotaxic activity PP19 and Fon17 toward autoxins, the chemical solutions (0.1 mmol/L) were prepared following Rivero and Lauffenburger [41]. Glass capillaries (inner diameter 0.3 mm) were used to draw 5 μL of the test solution (CA, BA, FA, PA, mixture, or sterile water) into one end of the capillary, which was sealed with vaseline. The other end of the capillary was inserted into a test tube containing 400 μL of either PP19 bacterial suspension (OD600 = 1) or Fon17 conidial suspension. The test tube was sealed using four layers of gauze, two layers of aluminum foil, and sealed with parafilm, followed by incubation at 28 °C for 90 min. After incubation, the tubes were removed, and any remaining microbial solution was rinsed with sterile water. The upper end of the capillary tube was broken off, and the chemoattractant test solution was transferred into a 1.5 mL centrifuge tube, and 1 mL of sterile water was added. Finally, 100 L of a 103-dilution was plated on Luria–Bertani (LB) agar (PP19) or PDA (Fon17) plates for colony counting. Each treatment was performed in triplicate.
2.8. Analysis of Microbial Communities in Watermelon Potting Soil Pretreated with PP19
After 30 days of cultivation, watermelon seedlings were transplanted, and the roots were irrigated with 25 mL of PP19 bacterial suspension (OD600 = 0.1) or sterile water at 1 and 10 days after transplanting (DAT). At 20 and 30 DAT, the plants were inoculated with a Fon17 suspension (1 × 105 conidiospores/mL), and the disease prevention efficacy was assessed 18 d later. After removing mature plants, the soil was collected for the second planting of watermelon seedlings. On the day of seedling transplantation, 25 mL of root irrigation was applied, and the soil around the root to a depth of 3–10 cm was collected 30 DAT. Soil samples were sieved through a 2 mm mesh sieve to remove soil particles and residual plant debris, sealed in centrifuge tubes, and stored at −80 °C. Each treatment consisted of four biological replicates.
After genomic DNA was extracted from the soil samples, specific primers with barcodes were used to amplify the V3–V4 region of the 16S rDNA to identify soil microorganisms. The primer sequences (5′-to-3′) were: 341F, CCTACGGGNGGCWGCAG; 806R, GGACTACHVGGGTATCTAAT. PCR products were gel-purified and quantified using a fluorometer (QuantiFluorTM, Fremont, CA, USA). Purified products were pooled in equimolar amounts, ligated with sequencing adapters, and used to construct libraries for Illumina PE250 sequencing. The sequencing data generated in this study are deposited in the NCBI SRA database under Bioproject No. PRJNA1070362. Raw reads were obtained and filtered using FASTP, and paired-end reads were merged into tags using FLASH. The tags were filtered to obtain clean tags. Operational taxonomic unit (OTU) clustering and chimeric tag removal were performed using the USEARCH12 software. Species annotation was performed by comparing the sequences with those in the database using RDP Classifier software 2.14 (threshold of 0.8–1). Alpha and beta diversity analyses were performed to obtain information on species richness and evenness within samples. Shared and unique OTUs among different samples were analyzed to explore differences in community structure among different samples. LEfSe analysis was used to identify the specific major microbial groups that differed between the groups.
2.9. Data Analysis
All statistical analyses were performed using SPSS 25.0. Significant differences between more than two groups were determined using one-way ANOVA and Duncan’s multiple comparison test. Statistical significance was set at p < 0.05. Alpha and beta diversity indices were analyzed using R 4.2.2 software, and Principal Coordinates Analysis (PCoA) and Anosim analysis were performed and visualized using the ggplot2 and vegan packages. Other plots were generated using GraphPad Prism 8.0.
3. Results
3.1. Effects of PP19 on Fruit Quality of Watermelon Under Continuous Cropping
At the eighth rotation of watermelon crops with three or four crops per year under a transparent plastic tent, the 8th cropping cycle, PP19 treatment significantly increased the single fruit weight of watermelon compared with that in the control group (Figure 1A,B). Additionally, PP19 treatment significantly increased the total protein, free amino acid, and vitamin C contents of watermelon compared with those in the control group (Figure 1E–G), but did not affect water content (Figure 1C). Although there was no significant difference in total sugar content between the PP19 and control groups, average sugar content was higher in the PP19 group (Figure 1D). Collectively, these results indicate that PP19 treatment may improve the fruit quality of watermelon under continuous cropping and alleviate the CCOs of watermelon.
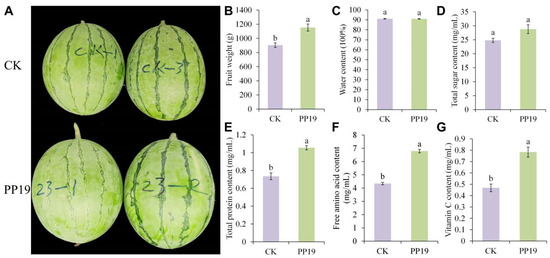
Figure 1.
Effects of Bacillus amyloliquefaciens PP19 on the quality of watermelon fruit under continuous cropping. (A) Fruit photo, (B) fruit weight, and (C) water, (D) total sugar, (E) protein, (F) free amino acid, and (G) vitamin C contents (G) of watermelon treated with PP19 (250 mL by root irrigation) at 0, 14, and 30 d after seedling transplantation. Watermelons were harvested 55 days after transplantation. PP19, B. amyloliquefaciens PP19 (1 × 106 CFU/mL); CK, sterile water control. Data are presented as means ± standard error, and different lowercase letters above the bars indicate significant differences (Duncan’s test, p < 0.05).
3.2. Effects of Autotoxins and PP19 on Fon17 Growth
In the present study, we investigated the inhibitory effects of PP19 and different concentrations of autotoxins on the growth of Fon17 (Figure 2). The production of purple-red pigments by Fon17 resulted in increased growth stress (Figure 2A). At a concentration of 0.1 mmol/L, only BA significantly inhibited Fon17 growth (Figure 2B), with CA, FA, and PA having almost no effect (growth inhibition rate: 0.5, 3.37, and 1.03%, respectively). Notably, the growth inhibitory effects of CA, FA, and PA increase in a concentration-dependent manner, peaking at 1.0 mmol/L (33.4, 35.5, and 39.6%, respectively). Among the autotoxins, BA had the strongest growth inhibitory effect on Fon17, with inhibition rates of 55.3, 62.9, and 45.7% at concentrations of 0.1, 0.5, and 1.0 mmol/L.
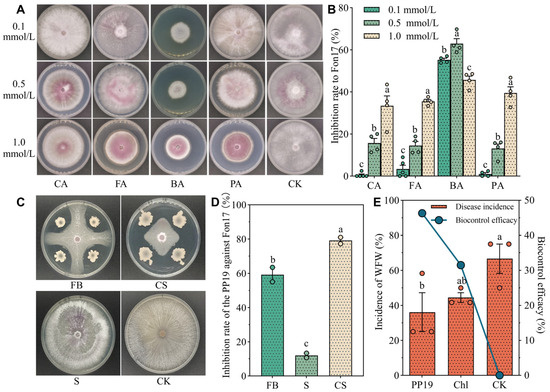
Figure 2.
Effects of watermelon autotoxins and Bacillus amyloliquefaciens (PP19) on the growth of Fusarium oxysporum f. sp. niveum (Fon17) and the biocontrol efficacy of PP19 on WFW in potted watermelon seedlings. (A). Morphology of Fon17 on PDA medium containing autotoxins after growth at 28 °C for 10 days. (B). Inhibition rates of Fon17 growth by the autotoxins. CA: cinnamic acid, FA: ferulic acid, BA: benzoic acid, PA: p-coumaric acid, CK: no autotoxin (control). Each dot represents the measured value. (C) Different components of PP19 and Fon17 cocultured on PDA plates. FB: fermentation broth, CS: cell suspension, S: supernatant. (D) Diameter of Fon17 mycelium, and rate of Fon17 inhibition by PP19. Each dot represents the measured value. (E) Disease incidence and biocontrol efficacy of the treatment against watermelon fusarium wilt (WFW).PP19: PP19 treatment with a concentration of 5 × 106 CFU/mL; Chl: chlorothalonil treatment (1:500); CK: sterile water control. Each orange red dot represents the measured value and each blue dot represents the average value. Data are presented as means ± standard error, and different lowercase letters above bars indicate significant differences at p < 0.05 in Duncan’s test.
Plant root exudates can promote the growth and development of some soil-borne pathogens; however, these organic compounds may become toxic to crop pathogens at high levels [42]. In the present study, treatment with 0.1 mmol/L of autotoxins showed only slight inhibitory effects on Fon17, suggesting that the concentration of autotoxins secreted by watermelon roots in fields affected by CCO is likely <0.1 mmol/L. Moreover, Zhang et al. [43] found that the concentrations of autotoxins (CA, FA, and PA) in watermelon monoculture systems ranged from 0.017 to 0.147 μg/g FW. Therefore, a concentration of 0.1 mmol/L was used in subsequent experiments.
In the plate confrontation assay (Figure 2C) and pot experiment, PP19 treatment (0.1 mmol/L) inhibited the growth of Fon17 by 55.6% (Figure 2D) and effectively prevented WFW in the pots with an efficacy of 46.3% (Figure 2E). Overall, the disease prevention efficacy of PP19 (46.3%) was significantly higher than that of the chemical fungicide Chl (31.5%).
3.3. Watermelon Autotoxins Promote PP19 Growth and Antifungal Activity
Considering that autotoxins may accumulate in the rhizosphere of watermelon under continuous cropping, we investigated the synergistic effects of the autotoxins and PP19 on the growth of Fon17. Notably, the effects of the autotoxin on PP19 growth were examined, followed by the evaluation of its synergistic effect on Fon17 growth. PP19 colonies grown on the WA medium were round and pale yellow in color, with a rough surface, slight elevation, and irregular edges (Figure 3A). Treatment with 0.1 mmol/L of CA, FA, BA, PA, and PAs promoted PP19 growth by 82.6, 19.5, 9.6, 19.7, and 72.5%, respectively (Figure 3B). Notably, the growth-promoting effect of BA was not significant.
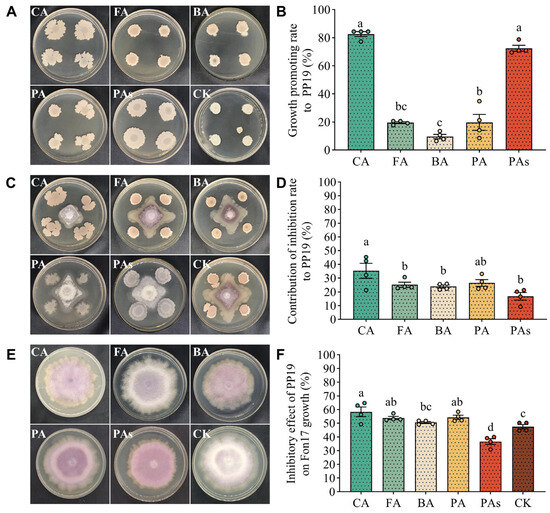
Figure 3.
PP19-autotoxin-Fon17 coculture system. (A). Growth morphology of PP19 when cultured with different autotoxins. (B). Growth-promoting effect of different autotoxins on PP19. Each dot represents the measured value. (C). Plate confrontation assay with PP19 and Fon17 cocultured in WA medium containing 0.1 mmol/L of autotoxins. (D). Enhanced antifungal activity of PP19 against Fon17 growth with different autotoxins. Each dot represents the measured value. (E). Growth morphology of Fon17 when cultured with autotoxin. (F). Inhibitory effect of PP19 on the growth of Fon17. PP19 and Fon17 were cocultured on solid WA medium at 28 °C for 14 days. Each dot represents the measured value. CA: cinnamic acid, FA: ferulic acid, BA: benzoic acid, PA: p-coumaric acid, PAs: mixture of all four acids; CK: no autotoxin. Data are presented as means ± standard error, and different lowercase letters above bars indicate significant differences at p < 0.05 in Duncan’s test.
In the plate confrontation test (Figure 3C), all five treatments affected the growth of Fon17 mycelia. PP19 inhibitory effect on Fon17 growth was stronger in the presence of the autotoxins than in their absence. Co-treatment with CA, FA, BA, PA, and PAs improved the antifungal activity of PP19 by 35.29, 25.11, 23.86, 26.42, and 16.8%, respectively, compared with that in the control group (Figure 3D). Compared with that in the control group (47.5%), the growth inhibitory effect of PP19 on Fon17 was significantly higher (p < 0.05) in the presence of CA, FA, and PA, with inhibition rates >50% (Figure 3E,F). However, there was no significant difference in antifungal activity between the PP19 + BA (50.7%) and CK groups (Figure 3F). Although the antifungal activity decreased in the PP19 + PA group (36.6%), PP19 still exhibited an inhibitory effect on Fon17 growth. Collectively, these results indicate that watermelon autotoxins, particularly CA, FA, and PA, may enhance the growth and antifungal activity of PP19.
3.4. PP19 Can Utilize and Degrade Watermelon Autotoxin
To further clarify the growth characteristics of PP19 and its ability to utilize autotoxins, PP19 was cultured in MSM containing the autotoxins as the sole carbon source (0.01 mmol/L). PP19 survived well in MSM containing only the autotoxins as the sole carbon source (Figure 4A), reaching approximately 108 CFU/mL after 7 days. Notably, the concentration of PP19 did not decrease with the appearance of Fon17, but rather grew normally. Compared with that in the CK group (107.3 CFU/mL), PAs, PA, BA, and CA significantly increased (p < 0.05) the growth of PP19 in LB medium containing a sufficient carbon source at 48 h after culture (Figure 4B), which was consistent with the results in Figure 3A,B. However, there was no significant difference between the PP19 + FA (107.3 CFU/mL) and CK (107.3 CFU/mL) groups (Figure 4B).
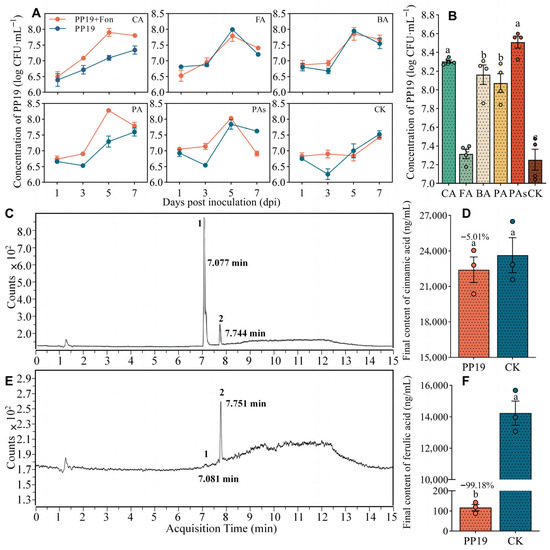
Figure 4.
Utilization and degradation of autotoxins by B. amyloliquefaciens PP19. (A). Concentration of PP19 in MSM medium containing 0.1 mmol/L of the indicated autotoxin as the sole carbon source. (B). The concentration of PP19 in LB medium containing 0.1 mmol/L of autotoxin, recorded at 48 h post-inoculation. Each dot represents the measured value. (C,E) Analysis of solutions containing FA and CA without (C) and with (E) PP19. (D,F) CA (D) and FA (F) contents after 7 days of treatment at 28 °C, 180 rpm. Peak 1: FA, Peak 2: CA. Each dot represents the measured value. CA: cinnamic acid, FA: ferulic acid, BA: benzoic acid, PA: p-coumaric acid, PAs: mixture of all four acids, CK: no autotoxin (control). Data are presented as means ± standard error, and different lowercase letters above bars indicate significant differences at p < 0.05 in Duncan’s test.
Based on the above results, we analyzed the ability of PP19 to degrade CA and FA using LC-MS/MS. After 7 days of culturing PP19 in MSM containing 0.1 mmol/L of CA or FA as the sole carbon source, the concentration of FA decreased significantly; however, CA concentration was unaffected (Figure 4C,D). Notably, the final concentrations of CA and FA were 22,405 and 116.64 ng/mL, representing decreases of 5.01 and 99.18%, respectively, compared with those in the CK group (Figure 4E,F).
3.5. Watermelon Autotoxins Can Improve the Social Behavior of PP19
To investigate the effect of the autotoxins on the motility of PP19 in culture, we measured the diameters of PP19 colonies on media (containing 0.1 mmol/L of autotoxin) designed to facilitate swarming (Figure 5A) and swimming (Figure 5C). PP19 swarming ability was higher in all the treatments than in the CK group, with a significant increase (p < 0.05) observed in the BA group (Figure 5B). Similarly, PP19 swimming ability was significantly higher in medium containing CA (7.37 cm), FA (7.38 cm), and BA (6.93 cm) than in the CK group (3.51 cm; Figure 5D). Overall, these results suggest that autotoxins produced by watermelon may improve the social behavior of PP19.
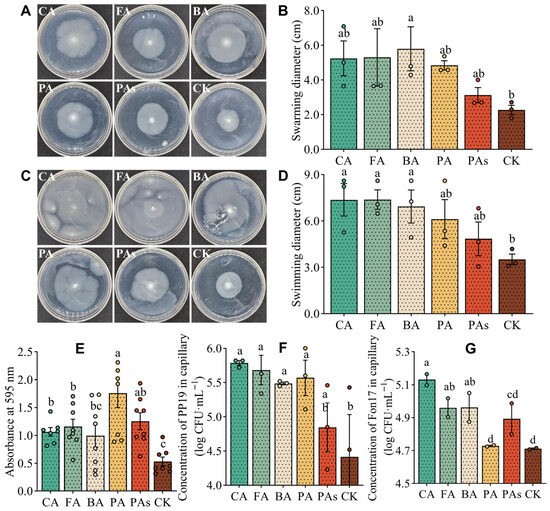
Figure 5.
Effects of different autotoxins on motility, biofilm formation, and chemotaxis of B. amyloliquefaciens PP19. (A) Swarming and (C) swimming morphology of PP19 in plates containing different autotoxins. Swarming diameters (B) of PP19 cultured at 28 °C for 14 h and the swimming diameters (D) of PP19 cultured at 28 °C for 36 h in the different autotoxins. (E) Crystal violet staining was performed to determine biofilm formation by PP19. (F,G) Concentrations of PP19 (F) and Fon17 (G) in capillaries containing autotoxins (see Materials and Methods). After 90 min of horizontal incubation at 28 °C, the solution in the capillary was diluted 1000-fold, and 100 μL was spread on an LB (PP19) or PDA (Fon17) plate. CA, cinnamic acid; FA, ferulic acid; BA, benzoic acid; PA, p-coumaric acid; PAs, mixture of the four acids; CK, no autotoxin (control). Each dot in (B,D–G) represents the measured value. Data are presented as means ± standard error, and different lowercase letters above the bars indicate significant differences (Duncan’s test, p < 0.05).
Additionally, we examined the effects of the autotoxins on biofilm formation by PP19 using crystal violet staining. Compared with that in the control group, all autotoxins (except BA) significantly promoted (p < 0.05) biofilm formation by PP19, with PA (OD595 = 1.761) having the highest effect (Figure 5E).
Furthermore, we performed a capillary assay to investigate the chemotactic effects of the autotoxins (0.1 mmol/L) on PP19 and Fon17. All four autotoxins (CA, FA, BA, and PA) had significant (p < 0.05) chemotactic effects on PP19 growth (Figure 5F), as evidenced by an increase in the concentrations of PP19 in capillaries containing CA, FA, BA, and PA (105.8, 105.7, 105.5, and 105.6 CFU/mL, respectively) compared with that in the CK group (104.4 CFU/mL). However, there was no significant difference between the PAs (104.8 CFU/mL) and the CK group. Additionally, CA, FA, and BA exhibited significant chemotactic effects on Fon17 (Figure 5G), as evidenced by an increase in the concentrations of Fon17 in capillaries containing CA, FA, and BA (105.1, 105.0, and 105.0 CFU/mL, respectively). However, there were no significant differences among PA (104.7 CFU/mL), PAs (104.9 CFU/mL), and CK (104.7 CFU/mL) groups. Collectively, these results suggest that the autotoxins are important nutritional components necessary for the growth of PP19 and Fon17 in the watermelon rhizosphere.
3.6. Effects of PP19 Pretreatment on Microbial Community Diversity and Structure in Rhizosphere Soil of Potted Watermelon
In the present study, we performed 16S rDNA sequencing of eight soil samples from the two treatments to elucidate the effect of PP19 on microbial community diversity and structure. In total, we identified 4832 bacterial OTUs belonging to 36 phyla, 86 classes, 135 orders, 162 families, and 218 genera. Notably, the major bacterial phyla in the potted watermelon soils were Proteobacteria, Acidobacteria, and Actinobacteria, accounting for more than 70% of the relative abundance in both the PP19 and CK samples (Figure 6A). Additionally, the dominant bacterial genera were Rhodoplanes, Candidatus_Solibacter, and Opitutus, but their combined relative abundances were relatively low (<20%) in both treatments (Figure 6B).
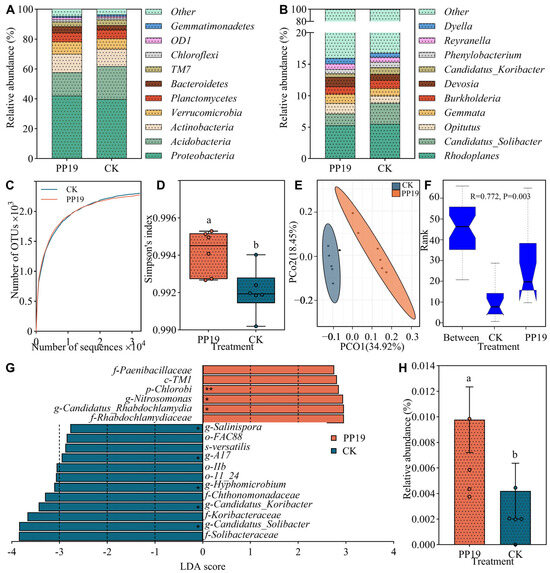
Figure 6.
Bacterial community structure and diversity in rhizosphere soil of potted watermelon. Bacterial community structure at phylum (A) and genus (B) levels. Data are presented for the top 10 most abundant species in each sample, and all other species and unclassified tags are grouped into the “Other” category. (C,D) Alpha diversity dilution curve (C) and Simpson’s index (D) of the bacterial community in the rhizosphere soil. Each dot represents the measured value. (E) Principal coordinate analysis (PCoA) based on Bray–Curtis distances between samples. (F) Beta diversity differences in the rhizosphere soil bacterial community (ANOSIM analysis, R = 0.772, p = 0.003). When the rank of “Between” was higher than that of other groups, it means that the difference between groups was greater than that within groups. (G) Linear analysis of rhizosphere soil bacteria. p, phylum (**); c, class; o, order; f, family; g, genus (*); s, species. (H) The relative abundance of PP19 in the rhizosphere soil of potted watermelon plants under different treatments. Each dot represents the measured value. Different lowercase letters above the bars indicate significant differences at p < 0.05, Duncan’s test.
Alpha diversity analysis showed that the dilution curve of the soil samples gradually flattened with an increase in sequence number (Figure 6C), confirming that the amount of sequencing performed should have covered all species in the sample. Simpson index was significantly higher (p < 0.05) in the PP19 group than in the CK group (Figure 6D). The PCoA results based on the Bray–Curtis distance between the samples showed that the two groups of samples formed two distinct clusters (Figure 6E), and there was a significant difference (p < 0.05) in beta diversity between the groups (Figure 6F). Overall, these results show that B. amyloliquefaciens PP19 significantly improves bacterial diversity in the rhizosphere soil of potted watermelons.
LEfSe analysis showed that the treatment significantly affected 18 taxa at different taxonomic levels (Figure 6G), including one phylum, one class, three orders, five families, seven genera, and one species. At the phylum level (**), Chlorobi was the signature taxon, and at the genus level (*), Nitrosomonas, Candidatus_Rhabdochlamydia, Salinispora, A17, Hyphomicrobium, Candidatus_Koribacter, and Candidatus_Solibacter were the signature taxa. Additionally, we compared the 16S rDNA sequence of PP19 with representative sequences of OTUs obtained via microbiome analysis using BLAST 2.15.0. PP19 was identified as OTU1196 in the soil microbiome. Notably, the PP19 treatment group had a significantly higher (p < 0.05) relative abundance of OTU1196 than the CK group (Figure 6H).
4. Discussion
In the present study, we investigated the mechanism and effectiveness of B. amyloliquefaciens PP19 in alleviating watermelon CCOs. PP19 treatment improved the quality of watermelon fruit under continuous cropping and indirectly inhibited the growth of Fon17. Additionally, PP19 effectively utilized and degraded watermelon autotoxins (BA, CA, PA, and FA), which promoted its swarming and swimming abilities and biofilm formation. Moreover, PP19 treatment improved the bacterial diversity in the rhizosphere soil of watermelons. Collectively, these results indicate that PP19 may control watermelon CCO by inhibiting Fon17 growth, utilizing autotoxins, and enhancing microbial community structure in the rhizosphere.
Root exudates are important signals for plant–environment communication and key regulators of rhizosphere microbial ecology [44]. Phenolic acids, the main components of watermelon root exudates, can cause autotoxicity and inhibit crop growth [4]. Continuous cropping over a long period of time may cause the accumulation of these autotoxic substances in the soil [45]. The biological control of autotoxins mainly depends on soil microorganisms that perform autotoxin biodegradation [46]. Inoculation with Trichoderma harzianum can degrade 80% of the autotoxins produced by plant roots, including hydroxybenzoic acid, vanillic acid, and FA [47]. Pseudomonas putida and P. hunanensis can effectively degrade phthalic acid FA, p-hydroxybenzoic acid, and syringic acid and promote plant growth [12]. PP19 has a strong ability to utilize autotoxins to promote growth (Figure 3A,B and 4). Considering that 0.1 mmol/L of CA, FA, and PA had almost no inhibitory effect on Fon17 (0.5, 3.37, and 1.03%, respectively; Figure 2), we examined the synergistic effects of 0.1 mmol/L of the autotoxins and PP19 on Fon17. PP19 showed a better antagonistic effect against Fon17 in the presence of CA, FA, BA, and PA (Figure 3), suggesting that autotoxins can be utilized and degraded by PP19. The ability to colonize roots is a prerequisite for biocontrol agents to exert their disease-suppressing functions, and chemotaxis and biofilm formation are critical for effective colonization [48]. The stable, long-term biocontrol efficacy of PP19 was closely related to the induction of biofilm formation by autotoxins (Figure 5E), which enhanced its adhesion to the soil medium. Notably, BLAST sequence alignment showed that the PP19 strain was also detected in the soil rhizosphere of the CK group (Figure 6H), indicating that autotoxins act as chemical signals (Figure 5F) that attract PP19 to stably colonize the watermelon rhizosphere.
B. amyloliquefaciens is an excellent agent for plant disease control, as it can inhibit the growth of pathogens. For example, B. amyloliquefaciens WS-10 suppressed the incidence of tobacco bacterial wilt disease by reducing the Ralstonia solanacearum population through biofilm formation and secretion of hydrolytic enzymes and exopolysaccharides [49]. Treatment with a mixture of B. subtilis and B. amyloliquefaciens demonstrated antagonistic activity against Rhizoctonia solani and decreased potato black scurf index from 40.9% to 12.0% [50]. Additionally, B. amyloliquefaciens LZN01 inhibited Fon growth and reproduction, with its functional component myriocin exhibiting even stronger activity against Fon [51]. In the present study, PP19 inhibited the growth of Fon17 (Figure 2A,C,D) and suppressed the occurrence of WFW (Figure 2E). Additionally, PP19 treatment improved the microbial diversity in the watermelon rhizosphere (Figure 6D,F). In addition to preventing diseases, biocontrol agents also have a promoting effect on plant growth. Bacillus cereus and Bacillus thuringiensis improved pepper growth [52], and Bacillus subtilis YB-04 displayed pronounced growth promotion of cucumber seedlings [53]. PP19 promotes watermelon seedling growth and, more importantly, enhances fruit quality, laying the foundation for the development and promotion of future biocontrol products.
The composition of rhizosphere microbiota is an important indicator of healthy plant growth [54]. Biodiversity is related to functional diversity, and a richer population indicates a higher functional diversity of the microbial community in the system, indicating that the plant has stronger resistance to disease and stress [55]. In this study, PP19 treatment significantly increased the bacterial diversity in watermelon rhizosphere under continuous cropping (Figure 6), which was consistent with a previous finding that showed an increase in bacterial diversity in cucumber rhizosphere following B. amyloliquefaciens B1408 treatment [56]. Collectively, these findings showed that PP19 improves the ability of watermelon plants to resist pathogens and autotoxic stress by altering the microbial diversity in the rhizosphere. Moreover, the dominant bacterial groups detected in the watermelon rhizosphere under continuous cropping at both the phylum (Proteobacteria, Acidobacteria, Actinobacteria, etc.) and genus levels (Rhodoplanes, Candidatus_Solibacter, and Opitutus) were almost the same as those found in a previous study [57]. PP19 treatment significantly increased the relative abundance of the phylum Chlorobi p and the genera Nitrosomonas and Candidatus_Rhabdochlamydia, but significantly decreased the relative abundance of Salinispora, A17, Hyphomicrobium, Candidatus_Koribacter, and Candidatus_Solibacter (Figure 6G). Chlorobi, a phylum of photosynthetic chemoautotrophic green sulfur bacteria that contain CO2 fixation enzymes, can undergo organoheterotrophic growth under both aerobic and anaerobic conditions and fix N2 and oxidize sulfides, providing sources of usable nitrogen and energy [58]. Nitrosomonas is a chemoautotrophic ammonia-oxidizing bacterium that converts ammonia to nitrite for efficient absorption by plant roots [59]. Therefore, we speculated that an increase in the relative abundance of these two beneficial bacteria has a potential role in improving soil environments under continuous cropping. Notably, PP19 treatment significantly decreased the relative abundance of members of two potentially pathogenic genera, Candidatus_Koribacter and Candidatus_Solibacter. Bacteria species belonging to Candidatus_Koribacter and Candidatus_Solibacter genera have low resource utilization efficiency, accumulate in acid-polluted soils, and increase over time during continuous cropping [60].
5. Conclusions
In this study, we demonstrated that B. amyloliquefaciens PP19 has a strong antagonistic effect against Fon17, altered the bacterial community structure in watermelon rhizosphere, increased the abundance of beneficial bacteria, and decreased the abundance of pathogenic bacteria. Additionally, PP19 effectively utilized and degraded autotoxins produced by watermelon. Additionally, watermelon autotoxins promoted PP19 growth, motility, biofilm formation, and watermelon root colonization, thereby enhancing the antifungal effects of PP19 against Fon17. Overall, PP19 treatment increased the yield and quality of watermelon fruit under continuous cropping. PP19 effectively degraded watermelon autotoxins while utilizing them to enhance its growth, motility, biofilm formation, and root colonization. This autotoxin-mediated enhancement improved PP19’s antifungal activity against Fon17. Field trials demonstrated that PP19 treatment increased the yield and fruit quality in continuous watermelon cropping systems. Conclusively, these results suggest that B. amyloliquefaciens PP19 may be effective in the biocontrol of watermelon CCOs (Figure 7). However, further studies are necessary to evaluate the biocontrol efficacy of PP19 in other crop species and to identify the optimal formulation to maximize PP19’s biocontrol performance for sustainable application in continuous cropping systems.
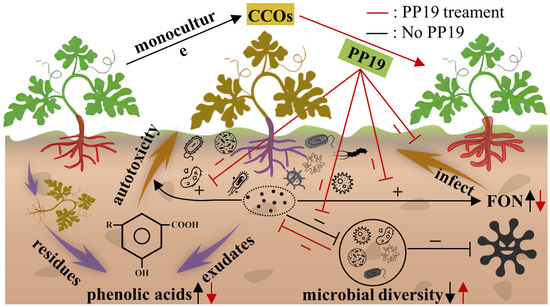
Figure 7.
Schematic diagram of the mechanism by which B. amyloliquefaciens PP19 alleviates watermelon continuous cropping obstacle (CCOs).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11101155/s1. Table S1: Preparation of stock solutions of autotoxic substances; Table S2: Compositions of solid PDA media containing autotoxic substances; Table S3: Gradient Parameters of HPLC.
Author Contributions
L.Z.: data curation, funding acquisition, methodology, investigation, writing—original draft. J.H.: data curation, methodology, software, writing—original draft. G.L.: methodology, formal analysis, software, writing—original draft. Q.C.: data curation, validation. T.H.: supervision, project administration, writing—review and editing. X.C.: conceptualization, funding acquisition, project administration, writing—review and editing. S.H.: conceptualization, data curation, formal analysis, funding acquisition, project administration, resources, supervision, validation, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Academy of Tropical Agricultural Sciences Basic Research Funds Independent Project and High-tech (Grant No. 1630112019004), the Science and Technology Projects in Guangzhou (Grant No. 202002030278), the Science and Technology Innovation Strategy Special Fund “Climbing Plan” project of Guangdong Province (Grant No. pdjh2020b0288), the Huizhou Kaisa Poverty Alleviation Project Operation Co., Ltd. “Shangdong watermelon-passion fruit green three-dimensional agricultural scientific research and industrial upgrading project”, and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630112021006).
Data Availability Statement
The 16S raw sequence data for this study can be found in the Sequence Read Archive data in the National Genomics Data Center (https://www.ncbi.nlm.nih.gov/sra, accessed on 28 February 2025; SRA: SRX23463975-SRX23463986). The original contributions presented in the study are included in the article/Supplementary Materials.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors. We thank the students JH Qiu, JX Li, YP Zhou, YH Huang, SM He, JH Chen, CX Guo, QY Gao, YW Xu, and XH Zhou at Zhongkai University of Agriculture and Engineering for their help on partial trials.
Conflicts of Interest
Author Mr. Quan Chen was employed by the company China Traditional Chinese Medicine Holdings Co., Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of Interest. The authors declare that this study was funded by Huizhou Kaisa Poverty Alleviation Project Operation Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
References
- Xie, X.; Huang, C.; Cai, Z.; Chen, Y.; Dai, C. Targeted acquisition of Fusarium oxysporum f.sp. niveum toxin-deficient mutant and its effects on watermelon. Fusarium wilt. J. Agric. Food Chem. 2019, 67, 8536–8547. [Google Scholar] [CrossRef] [PubMed]
- Seifert, C.A.; Roberts, M.J.; Lobell, D.B. Continuous corn and soybean yield penalties across hundreds of thousands of fields. Agron. J. 2017, 109, 541–548. [Google Scholar] [CrossRef]
- He, D.; Yao, X.; Zhang, P.; Liu, W.; Huang, J.; Sun, H.; Wang, N.; Zhang, X.; Wang, H.; Zhang, H.; et al. Effects of continuous cropping on fungal community diversity and soil metabolites in soybean roots. Microbiol. Spectr. 2023, 11, e0178623. [Google Scholar] [CrossRef]
- Yu, J.Q. Autotoxic potential of cucurbit crops: Phenomenon, chemicals, mechanisms and means to overcome. J. Crop Prod. 2001, 4, 335–348. [Google Scholar] [CrossRef]
- Blok, W.J.; Bollen, G.J. The role of autotoxins from root residues of the previous crop in the replant disease of asparagus. Eur. J. Plant Pathol. 1993, 99, 29–40. [Google Scholar] [CrossRef]
- Huang, L.; Song, L.; Xia, X.; Mao, W.; Shi, K.; Zhou, Y.; Yu, J. Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Gu, X.; Yang, N.; Zhao, Y.; Liu, W.; Li, T. Long-term watermelon continuous cropping leads to drastic shifts in soil bacterial and fungal community composition across gravel mulch fields. BMC Microbiol. 2022, 22, 189. [Google Scholar] [CrossRef]
- Martyn, R.D. Fusarium wilt of watermelon: 120 years of research. Hortic. Rev. 2014, 42, 349–442. [Google Scholar]
- Michielse, C.B.; Van, W.R.; Reijnen, L.; Manders, E.M.; Boas, S.; Olivain, C.; Alabouvette, C.; Rep, M. The nuclear protein Sgel of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009, 5, e1000637. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in saprophytic and plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shao, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zhang, Z.; Wang, W.; Xu, S.; He, X. Isolation, identification and characterization of phenolic acid-degrading bacteria from soil. J. Appl. Microbiol. 2021, 131, 208–220. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.; Sun, J.; Xiang, H.; Yang, Y.; Chen, S.; Yu, J.; Yang, C. Mitigation of tobacco bacteria wilt with microbial degradation of phenolic allelochemicals. Sci. Rep. 2022, 12, 20716. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, J.; Li, M.; Fang, F.; Hu, J.; Sun, Z.; Zhang, A.; Gao, X.; Li, J. Synergistic effect of Bacillus subtilis and Paecilomyces lilacinus in alleviating soil degradation and improving watermelon yield. Front. Microbiol. 2023, 13, 1101975. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Salerno, A.; Rea, E. The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ. Exp. Bot. 2010, 68, 283–291. [Google Scholar] [CrossRef]
- Mao, L.G.; Wang, Q.X.; Yan, D.D.; Xie, H.W.; Li, Y.; Guo, M.X.; Cao, A.C. Evaluation of the combination of 1,3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest Manag. Sci. 2012, 68, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mo, Y.; Liu, C.; Wang, Y.; Ma, J.; Zhang, Y.; Li, H.; Zhang, X. The effects of cattle manure and garlic rotation on soil under continuous cropping of watermelon (Citrullus lanatus L.). PLoS ONE 2016, 11, e0156515. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Miller, W.; Sikora, R.; Lindow, S. Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology 2001, 91, 415–422. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Lai, H.; Guo, Q.; Xue, Q. Effects of two strains of Streptomyces on root-zone microbes and nematodes for biocontrol of root-knot nematode disease in tomato. Appl. Soil Ecol. 2017, 112, 34–41. [Google Scholar] [CrossRef]
- Ku, Y.; Li, W.; Mei, X.; Yang, X.; Cao, C.; Zhang, H.; Cao, L.; Li, M. Biological control of melon continuous cropping obstacles: Weakening the negative effects of the vicious cycle in continuous cropping soil. Microbiol. Spectr. 2022, 10, e01776-22. [Google Scholar] [CrossRef]
- Sui, J.; Yu, Q.; Yang, K.; Yang, J.; Li, C.; Liu, X. Effects of Bacillus subtilis T6-1 on the rhizosphere microbial community structure of continuous cropping poplar. Biology 2022, 11, 791. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Luo, M.; Wang, Q.; Wu, X. Bacillus cereus WL08 immobilized on tobacco stem charcoal eliminates butylated hydroxytoluene in soils and alleviates the continuous cropping obstacle of Pinellia ternata. J. Hazard. Mater. 2023, 450, 131091. [Google Scholar] [CrossRef]
- Xiong, J.X.; Du, L.S.; Li, N.N.; Wu, X.T.; Xiang, Y.; Li, S.; Zou, L.; Liu, D.; Huang, D.; Xie, Z.F.; et al. Pigmentiphaga kullae CHJ604 improved the growth of tobacco by degrading allelochemicals and xenobiotics in continuous cropping obstacles. J. Hazard. Mater. 2024, 465, 133466. [Google Scholar] [CrossRef]
- Ding, S.; Zhou, D.; Wei, H.; Wu, S.; Xie, B. Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 2020, 262, 128387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Zhang, X.; Sun, J.; Dong, L.; Han, H.; Chen, Z. Bama pig manure organic fertilizer regulates the watermelon rhizosphere bacterial community to inhibit the occurrence of Fusarium wilt under continuous cropping conditions. Curr. Microbiol. 2022, 79, 364. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.Y.; Zhu, C.S.; Guo, S.X.; Li, M. Control effect of root exudates from mycorrhizal watermelon seedlings on Fusarium wilt and the bacterial community in continuously cropped soil. Front. Plant Sci. 2023, 14, 1225897. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, C.; Lu, D.; Wu, Y.; Ye, Z.; Xia, L.; Sun, Y.; Bao, E.; Ye, L.; Tang, Y.; et al. Variation of soil microbial community and sterilization to Fusarium oxysporum f.sp. niveum play roles in slightly acidic electrolyzed water-alleviated watermelon continuous cropping obstacle. Front. Microbiol. 2022, 13, 837121. [Google Scholar]
- Zhang, H.; Hua, Z.W.; Liang, W.Z.; Niu, Q.H.; Wang, X. The prevention of bio-organic fertilizer fermented from cow manure compost by Bacillus sp. XG-1 on watermelon continuous cropping barrier. Int. J. Environ. Res. Public Health 2020, 17, 5714. [Google Scholar] [CrossRef]
- Guo, J.H.; Qi, H.Y.; Guo, Y.H.; Ge, H.L.; Gong, L.Y.; Zhang, L.X.; Sun, P.H. Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol. Control 2004, 29, 66–72. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, S.; Hsiang, T.; Yu, G.; Guo, D.; Jiang, Z.; Li, J. Biocontrol using Bacillus amyloliquefaciens PP19 against litchi downy blight caused by Peronophythora litchii. Front. Microbiol. 2021, 11, 619423. [Google Scholar] [CrossRef]
- Proietti, S.; Rouphael, Y.; Colla, G.; Cardarelli, M.; De Agazio, M.; Zacchini, M.; Rea, E.; Moscatello, S.; Battistelliet, A. Fruit quality of mini-watermelon as affected by grafting and irrigation regimes. J. Sci. Food Agr. 2008, 88, 1107–1114. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zeng, W.; Luo, X.; Liao, C. Determination of total protein content by Coomassie brilliant blue method. Chin. J. Biol. 2000, 13, 118–119. [Google Scholar]
- Wang, Y.; Hu, J.; Dai, Z.; Li, J.; Huang, J. In vitro assessment of physiological changes of watermelon (Citrullus lanatus) upon iron oxide nanoparticles exposure. Plant Physiol. Biochem. 2016, 108, 353–360. [Google Scholar] [CrossRef]
- Castelli, A.; Martorana, G.E.; Frasca, A.M.; Meucci, E. Colorimetric determination of plasma Vitamin C: Comparison between 2, 4-dinitrophenylhydrazine and phosphotungstic acid methods (author’s transl). Acta Vitaminol. Enzymol. 1981, 3, 103–110. [Google Scholar] [PubMed]
- Lie, S. The EBC-ninhydrin method for determination of free alpha amino nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, Y.; Ma, Y.; Chen, C.; Xu, F.; Dong, X. Role of phenolic acids from the rhizosphere soils of Panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules 2018, 23, 819. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Fritze, A.; Roskot, N.; Smalla, K. Evaluation of potential biocontrol rhizobacteria from different host plants of Verticillium dahliae Kleb. J. Appl. Microbiol. 2001, 91, 963–971. [Google Scholar] [CrossRef]
- Ogata, Y.; Goda, S.; Toyama, T.; Sei, K.; Ike, M. The 4-tert-butylphenol-utilizing bacterium sphingobium fuliginis omi can degrade bisphenols via phenolic ring hydroxylation and meta-cleavage pathway. Environ. Sci. Technol. 2013, 47, 1017–1023. [Google Scholar] [CrossRef]
- Pearson, M.M. Methods for studying swarming and swimming motility. Methods Mol. Biol. 2019, 2021, 15–25. [Google Scholar]
- Sun, P.; Hui, C.; Wang, S.; Wan, L.; Zhang, X.; Zhao, Y. Bacillus amyloliquefaciens biofilm as a novel biosorbent for the removal of crystal violet from solution. Colloids Surf. B Biointerfaces 2016, 139, 164–170. [Google Scholar] [CrossRef]
- Rivero-Hudec, M.; Lauffenburger, D.A. Quantification of bacterial chemotaxis by measurement of model parameters using the capillary assay. Biotechnol. Bioeng. 1986, 28, 1178–1190. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Wang, J.; Zhu, Q.; Lin, S.; Xu, J.; Zheng, C.; Chen, J.; Qin, X.; Fang, C.; et al. Mixed phenolic acids mediated proliferation of pathogens Talaromyces helicus and Kosakonia sacchari in continuously monocultured Radix pseudostellariae rhizosphere soil. Front. Microbiol. 2016, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, R.; Wu, P.; Ren, L.; Xu, G. Response of root exudates to watermelon/aerobic rice intercropping oriented to alleviate watermelon Fusarium wilt. Acta Pedol. Sin. 2014, 51, 585–593. [Google Scholar]
- Balasubramanian, V.; Sur, A.; Nayak, K.K.; Singh, R.K. Plant root exudates as determinant of Rhizomicrobiome. In Rhizosphere Microbes: Soil and Plant Functions; Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Sharma, S.K., Eds.; Springer: Singapore, 2020; Volume 23, pp. 105–126. [Google Scholar]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.; Zhang, L.; Li, J.; Zheng, Y.; Yang, W.; Deng, L.; Gao, Q.; Mi, Q.; Li, X.; et al. Autotoxins in continuous tobacco cropping soils and their management. Front. Plant Sci. 2023, 14, 1106033. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres ofcontinuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, M.; Chen, L.; Zhou, J.; Zhang, L.; Li, Y.; Xue, Q.; Lai, H. The biocontrol agent Streptomyces pactum increases Pseudomonas koreensis populations in the rhizosphere by enhancing chemotaxis and biofilm formation. Soil Biol. Biochem. 2020, 144, 107755. [Google Scholar] [CrossRef]
- Ahmed, W.; Dai, Z.; Zhang, J.; Li, S.; Ahmed, A.; Munir, S.; Liu, Q.; Tan, Y.; Ji, G.; Zhao, Z.; et al. Plant-microbe interaction: Mining the impact of native Bacillus amyloliquefaciens WS-10 on tobacco bacterial wilt disease and rhizosphere microbial communities. Microbiol. Spectr. 2022, 10, e0147122. [Google Scholar] [CrossRef]
- Maslennikova, V.S.; Tsvetkova, V.P.; Shelikhova, E.V.; Selyuk, M.P.; Alikina, T.Y.; Kabilov, M.R.; Dubovskiy, I.M. Bacillus subtilis and Bacillus amyloliquefaciens mix suppresses rhizoctonia disease and improves rhizosphere microbiome, growth and yield of potato (Solanum tuberosum L.). J. Fungi 2023, 9, 1142. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Lv, Z.; Shi, Y.; Wang, Z. Antifungal activity and functional components of cell-free supernatant from Bacillus amyloliquefaciens LZN01 inhibit Fusarium oxysporum f.sp. niveum growth. Biotechnol. Biotechnol. Equip. 2019, 33, 1042–1052. [Google Scholar] [CrossRef]
- Hernández-Huerta, J.; Tamez-Guerra, P.; Gomez-Flores, R.; Delgado-Gardea, M.C.E.; Robles-Hernández, L.; Gonzalez-Franco, A.C.; Infante-Ramirez, R. Pepper growth promotion and biocontrol against Xanthomonas euvesicatoria by Bacillus cereus and Bacillus thuringiensis formulations. PeerJ 2023, 11, e14633. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Yang, F.; Xie, X.; Goodwin, P.H.; Deng, X.; Tian, B.; Yang, L. Evaluation and genome analysis of Bacillus subtilis YB-04 as a potential biocontrol agent against Fusarium wilt and growth promotion agent of cucumber. Front. Microbiol. 2022, 13, 885430. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Wang, Z.Y.; Li, N.; Wang, Y.H.; Feng, J.T.; Zhang, X. Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Appl. Soil Ecol. 2019, 136, 55–66. [Google Scholar] [CrossRef]
- Ling, N.; Deng, K.; Song, Y.; Wu, Y.; Zhao, J.; Raza, W.; Huang, Q.; Shen, Q. Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Microbiol. Res. 2014, 169, 570–578. [Google Scholar] [CrossRef]
- Liu, Z.; Frigaard, N.U.; Vogl, K.; Lino, T.; Ohkuma, M.; Overmann, J.; Bryant, D.A. Complete genome of Ignavibacterium album, a metabolically versatile, flagellated, facultative anaerobe from the phylum Chlorobi. Front. Microbiol. 2012, 3, 185. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Guo, C.; Bao, Y.; Lu, G.; Reinfelder, J.R.; Zhi, D. Bacterial, archaeal, and fungal community responses to acid mine drainage-laden pollution in a rice paddy soil ecosystem. Sci. Total Environ. 2018, 616, 107–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).