Seed Germination Inhibitory Activity of Alkaloid Fractions from Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Extraction of Alkaloid Fraction
2.3. Isolation of Alkaloid Standard Lycorine
2.4. Methanolic Extract Preparation from Seedlings of the Target Species
2.5. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
2.5.1. Derivatization of the Methanolic Extracts
2.5.2. Identification of Metabolites
2.6. Assessment of Inhibitory Activity Against Seed Germination
2.7. Data Analysis
3. Results
3.1. Phytochemical Analysis of Alkaloid Fractions
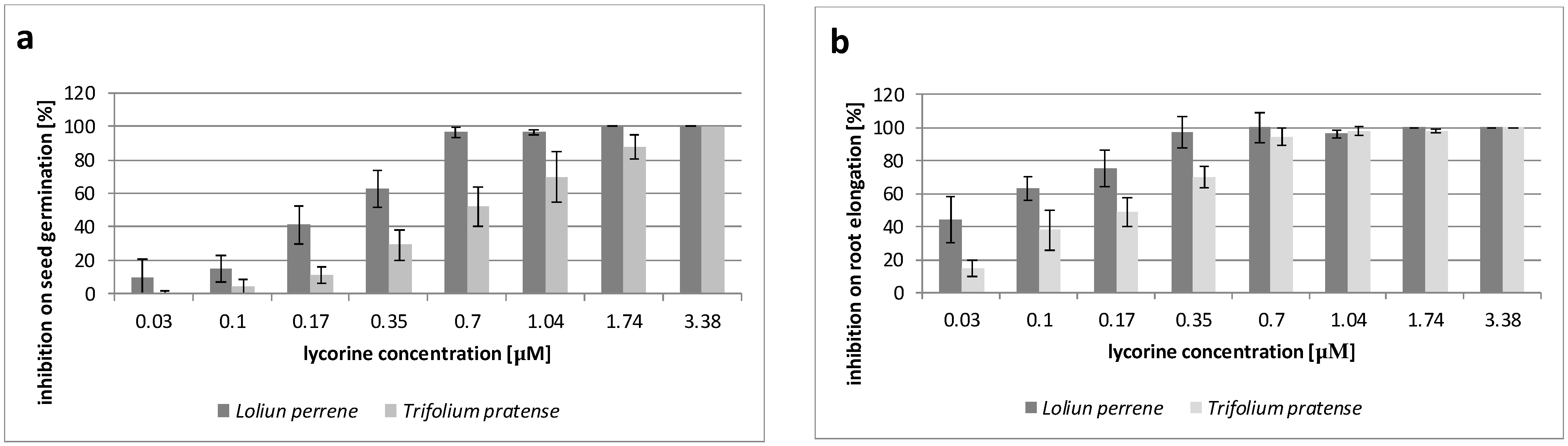
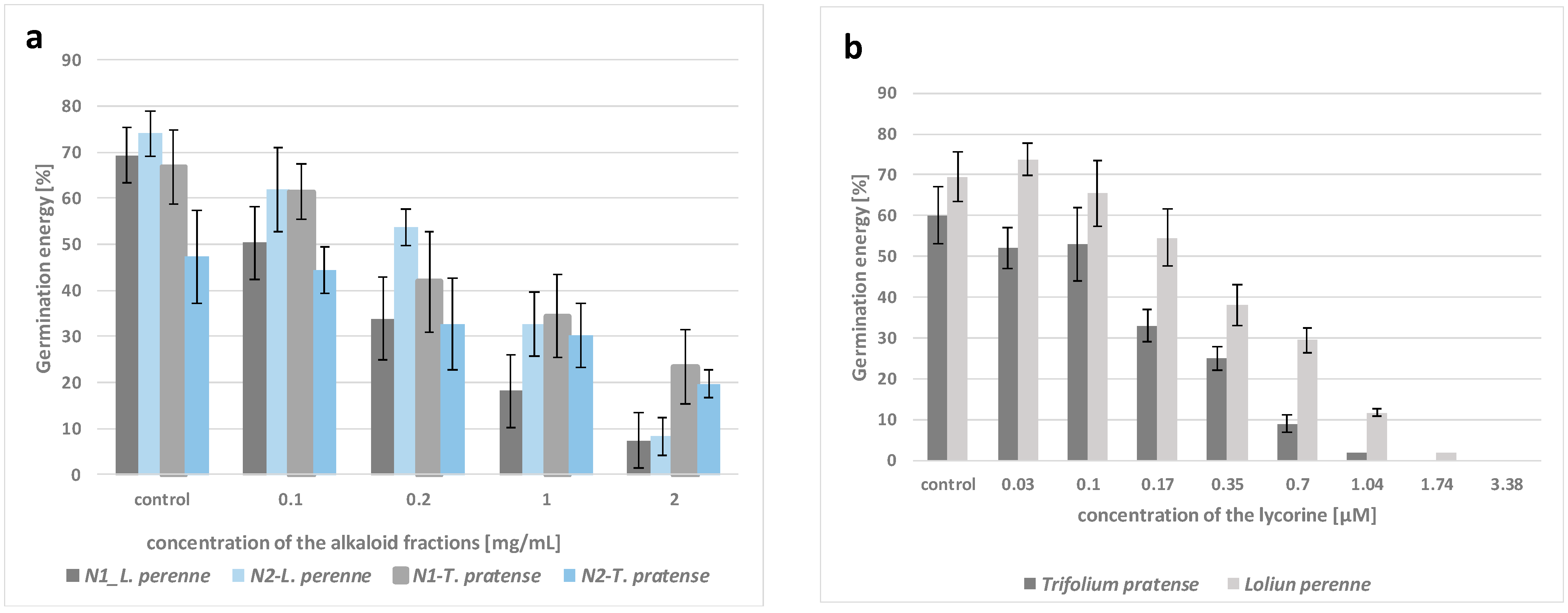
3.2. Inhibition of Seed Germination
3.3. Metabolite Profiles of Target Species Treated with Lycorine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, X.; Gu, M. Bioherbicides in Organic Horticulture. Horticulturae 2016, 2, 3. [Google Scholar] [CrossRef]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential effects of essential oils extracted from Mediterranean aromatic plants on target weeds and soil microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Zaman, K.M.A.; Azzeme, A.M. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 21–29. [Google Scholar] [CrossRef]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and biological aspects of Narcissus alkaloids. Alkaloids Chem. Biol. 2006, 63, 87–179. [Google Scholar] [CrossRef]
- Wahyuni, D.S.C.; Van der Kooy, F.; Klinkhamer, P.G.L.; Verpoorte, R.; Leiss, K. The Use of Bio-Guided Fractionation to Explore the Use of Leftover Biomass in Dutch Flower Bulb Production as Allelochemicals against Weeds. Molecules 2013, 18, 4510–4525. [Google Scholar] [CrossRef]
- Kempthorne, C.J.; Borra, S.; Kumar, M.; Dokuburra, C.B.; Liscombe, D.K.; McNulty, J. Identification of haemanthamine as a phytotoxic alkaloid in Narcissus pseudonarcissus L. (Daffodil) emerging buds. Nat. Prod. Res. 2023, 37, 4232–4238. [Google Scholar] [CrossRef]
- Thakur, P.; Misra, R.L.; Misra, S. Narcissus. In Commercial Ornamental Crops Cut Flowers; Misra, R.L., Misra, S., Eds.; Kruger Brentt Publishers UK LTD: Edgware, UK, 2017; pp. 373–388. [Google Scholar]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. Alkaloids Chem. Biol. 2020, 83, 113–185. [Google Scholar] [CrossRef]
- Schrader, K.K.; Andolfi, A.; Cantrell, C.L.; Cimmino, A.; Duke, S.O.; Osbrink, W.; Wedge, D.E.; Evidente, A. A Survey of Phytotoxic Microbial and Plant Metabolites as Potential Natural Products for Pest Management. Chem. Biodivers. 2010, 7, 2261–2280. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC–MS combined with k-means cluster analysis. Ind. Crops Prod. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Singh, A.; Desgagné-Penix, I. Transcriptome and metabolome profiling of Narcissus pseudonarcissus ‘King Alfred’ reveal components of Amaryllidaceae alkaloid metabolism. Sci. Rep. 2017, 7, 17356. [Google Scholar] [CrossRef]
- Nikolova, M.; Yankova-Tsvetkova, E.; Stefanova, T.; Dimitrova, M.; Berkov, S. Bioherbicidal potential of leaves of Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus. In Memorias del VIII Congreso de la Sociedad Latinoamericana de Plantas Medicinales; Flores, E.N.Q., Echeverri, L.F.L., Cazar, M.E.R., Eds.; Solaplamed: La Paz, Bolivia, 2020; p. 67. [Google Scholar]
- Petkova, M.; Bozhanski, B.; Iliev, M.; Bozhanska, T. Economic Significance and Application of Perennial Ryegrass (Lolium perenne L.). J. Mt. Agric. Balk. 2021, 24, 175–194. [Google Scholar]
- Berkov, S.; Bastida, J.; Viladomat, F.; Codina, C. Development and validation of a GC-MS method for rapid determination of galanthamine in Leucojum aestivum and Narcissus ssp.: A metabolomic approach. Talanta 2011, 83, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Pechlivanova, D.; Denev, R.; Nikolova, M.; Georgieva, L.; Sidjimova, B.; Bakalov, D.; Tafradjiiska, R.; Stoynev, A.; Momekov, G.; et al. GC-MS analysis of Amaryllidaceae and Sceletium-type alkaloids in bioactive fractions from Narcissus cv. Hawera. Rapid Commun. Mass Spectrom. 2021, 35, e9116. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef]
- Atak, M.; Mavi, K.; Uremis, I. Bio-herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11159. [Google Scholar]
- Pawłat, J.; Starek-Wójcicka, A.; Kopacki, M.; Terebun, P.; Kwiatkowski, M.; Sujak, A.; Pascuzzi, S.; Santoro, F.; Andrejko, D. Germination Energy, Germination Capacity and Microflora of Allium cepa L. Seeds after RF Plasma Conditioning. Energies 2022, 15, 7687. [Google Scholar] [CrossRef]
- Tang, D.; Wei, F.; Qin, S.; Khan, A.; Kashif, M.H.; Zhou, R. Polyethylene glycol induced drought stress strongly influences seed germination, root morphology and cytoplasm of different kenaf genotypes. Ind. Crops Prod. 2019, 137, 180–186. [Google Scholar] [CrossRef]
- Cahlíková, L.; Ločárek, M.; Benešová, N.; Kučera, R.; Chlebek, J.; Novák, Z.; Opletal, L. Isolation and Cholinesterase Inhibitory Activity of Narcissus Extracts and Amaryllidaceae Alkaloid. Nat. Prod. Commun. 2013, 8, 781–785. [Google Scholar] [CrossRef]
- Berkov, S.; Denev, R.; Sidjimova, B.; Zarev, Y.; Shkondrov, A.; Torras-Claveria, L.; Viladomat, F.; Bastida, J. Gas chromatography-mass spectrometry of some homolycorine-type Amaryllidaceae alkaloids. Rapid Commun. Mass. Spectrom. 2023, 37, e9506. [Google Scholar] [CrossRef] [PubMed]
- Shanmugalingam, S.; Umarukatha, J.B. Review on Use of Plant Extracts in Weed Control. Curr. Trends Biomed. Eng. Biosci. 2019, 18, 555993. [Google Scholar] [CrossRef]
- Ben Kaab, S.; Lins, L.; Hanafi, M.; Bettaieb Rebey, I.; Deleu, M.; Fauconnier, M.L.; Ksouri, R.; Jijakli, M.H.; Clerck, C. Cynara cardunculus Crude Extract as a Powerful Natural Herbicide and Insight into the Mode of Action of Its Bioactive Molecules. Biomolecules 2020, 10, 209. [Google Scholar] [CrossRef]
- Gedik, O.; Kaya, A.; Erol, A.; Khan, M.A.; Tassever, M.N. Allelopathic Effects of Flower Extract of Oleander ( Nerium oleander ) on the Germination of Seed and Seedling Growth of Lolium multiflorum. J. Inst. Sci. Tech. 2018, 8, 309–317. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Al-Humaid, A.I. Searching for natural herbicides in methanol extracts of eight plant species. Bull. Natl. Res. Cent. 2019, 43, 22. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- El-Sobki, A.E.; Saad, A.M.; El-Saadony, M.T.; El-Tahan, A.M.; Taha, A.E.; Aljuaid, B.S.; El-Shehawi, A.M.; Salem, R.E.M.E. Fluctuation in amino acids content in Triticum aestivum L. cultivars as an indicator on the impact of post-emergence herbicides in controlling weeds. Saudi J. Biol. Sci. 2021, 11, 6332–6338. [Google Scholar] [CrossRef]
- Orcaray, L.; Zulet, A.; Zabalza, A.; Royuela, M. Impairment of carbon metabolism induced by the herbicide glyphosate. J. Plant Physiol. 2012, 169, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wu, Z.; Li, X.; Yang, M.; Han, J.; Lu, B.; Lu, B.; Wang, J. Effects of nicosulfuron on plant growth and sugar metabolism in sweet maize (Zea mays L.). PLoS ONE 2022, 17, e0276606. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Scognamiglio, M.; Fiumano, V.; Esposito, A.; Choi, Y.H.; Verpoorte, R.; Fiorentino, A. Plant bioassay to assess the effects of allelochemicals on the metabolome of the target species Aegilops geniculata by an NMR-based approach. Phytochemistry 2013, 93, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Walkers, J. Commercial production and showing daffodils. Comb. Proc. IPPS 2019, 69, 394–396. [Google Scholar]
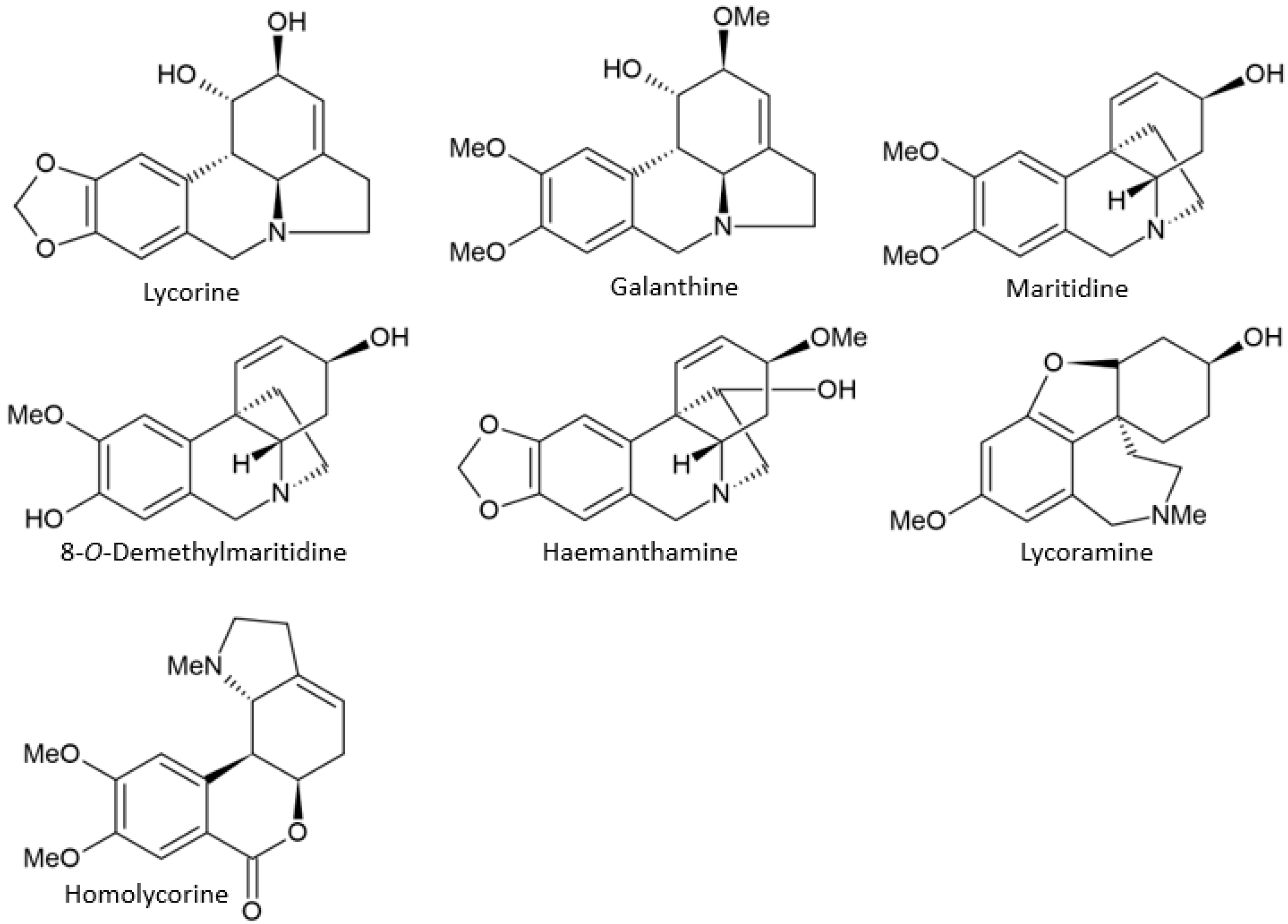
| Alkaloids * | RT | N. pseudonarcissus | N. poeticus |
|---|---|---|---|
| cv. Carlton | |||
| Miscellaneous type | |||
| UA1 ** | 19.24 | 0.11 | |
| UA2 | 19.53 | 0.53 | |
| Trisphaeridine | 23.81 | 0.16 | 0.32 |
| 0.16 | 0.97 | ||
| Galanthamine type | |||
| Galanthamine | 25.41 | 7.58 | 8.03 |
| Lycoramine | 25.78 | 21.70 | 0.59 |
| Lycoraminone | 26.09 | 1.23 | |
| Norlycoramine | 26.44 | 0.33 | |
| Narwedine | 26.67 | 0.47 | 0.13 |
| 31.31 | 8.75 | ||
| Haemanthamine type | |||
| Maritidine | 27.36 | 0.31 | 15.37 |
| 8-O-Demethylmaritidine | 27.81 | 28.90 | |
| Haemanthamine | 29.19 | 7.16 | 5.38 |
| UA3 | 29.74 | 0.30 | |
| UA4 | 29.97 | 0.33 | |
| UA5 | 30.33 | 1.49 | |
| UA6 | 31.39 | 2.72 | |
| UA7 | 32.09 | 3.88 | |
| 7.47 | 58.37 | ||
| Lycorine type | |||
| Kirkine | 27.92 | 1.94 | 0.06 |
| Pluvine | 28.08 | 0.86 | |
| Assoanine | 28.30 | 2.00 | 1.98 |
| 9-O-Demethylpluviine | 28.59 | 0.44 | |
| 11,12-Didehydroassoanine | 29.76 | 1.48 | 2.33 |
| Lycorine | 30.01 | 0.89 | |
| Galanthine | 30.18 | 39.66 | 3.06 |
| 8-O-Methylpseudolycorine | 31.33 | 1.50 | |
| Tortuosine | 31.85 | 1.35 | |
| 11,12-Didehydrotortuosine | 33.58 | 3.95 | |
| 2-Methoxypratosine | 35.59 | 0.17 | |
| 53.36 | 8.32 | ||
| Pretazettine type | |||
| Tazettine | 29.38 | 4.24 | 1.10 |
| 4.24 | 1.10 | ||
| Homolycorine type | |||
| Lycorenine | 26.16 | 1.88 | |
| Homolycorine | 29.54 | 18.41 | |
| 8-O-Demethylhomolycorine | 31.71 | 3.44 | 2.30 |
| 3.44 | 22.59 | ||
| Monthanine type | |||
| Pancracine | 28.65 | 0.55 | |
| 0.55 |
| Narcissus Species | Target Species | Inhibition of Seed Germination [%] * | |||
|---|---|---|---|---|---|
| Concentration [mg/mL] | |||||
| 0.1 | 0.5 | 1 | 2 | ||
| N. pseudonarcissus | L. perenne | 18 ± 10 a | 55 ± 15 b | 98 ± 2 c | 100 ± 0 c |
| T. pratense | 12 ± 9 a | 23 ± 11 a | 75 ± 14 b | 92 ± 7 c | |
| N. poeticus | L. perenne | 14 ± 9 a | 40 ± 11 b | 77 ± 14 c | 91 ± 6 d |
| T. pratense | 11 ± 5 a | 32 ± 12 b | 50 ± 9 c | 89 ± 7 d | |
| Compounds | RT | Trifolium pratense | Lolium perenne | ||
|---|---|---|---|---|---|
| Control | Treated | Control | Treated | ||
| Amino acids | |||||
| L-Alanine | 5.95 | 27.73 ± 3.7 | 51.11 ± 11.7 | 2.30 ± 0.3 | 7.21 ± 1.8 |
| Glycine | 6.85 | 7.69 ± 0.2 | 12.01 ± 3.6 | ||
| L-Valine | 8.23 | 39.83 ± 1.7 | 67.32 ± 15.1 | 2.05 ± 0.3 | 4.89 ± 1.4 |
| L-Leucine | 9.60 | 5.93 ± 0.6 | 21.31 ± 6.1 | 1.67 ± 0.1 | 5.91 ± 0.8 |
| L-Isoleucine | 10.04 | 30.82 ± 2.6 | 60.56 ± 16.3 | ||
| L-Serine | 11.83 | 72.48 ± 2.6 | 170.11 ± 24 | 2.89 ± 0.5 | 7.51 ± 2.8 |
| L-Threonine | 12.12 | 32.58 ± 3.0 | 38.72 ± 8.7 | 1.90 ± 0.2 | 6.23 ± 2.2 |
| L-Aspartic acid | 16.52 | 12.61 ± 7.2 | 16.98 ± 3.1 | 1.68 ± 0.1 | 1.96 ± 0.5 |
| L-Glutamic acid | 18.95 | 9.42 ± 2.8 | 27.97 ± 9.2 | 3.69 ± 0.4 | 8.45 ± 2.1 |
| Pyroglutamic acid | 19.33 | 3.82 ± 0.4 | 4.59 ± 2.0 | 3.08 ± 0.7 | 9.97 ± 3.9 |
| L-Phenylalanine | 20.23 | 82.76 ± 9.2 | 66.88 ± 14.8 | ||
| L-Tyrosine | 26.00 | 20.22 ± 1.1 | 20.49 ± 4.1 | ||
| L-Tryptophan | 32.50 | 5.60 ± 0.6 | 133.04 ± 47 | ||
| Organic acids | |||||
| Phosphoric acid | 11.01 | 47.41 ± 4.9 | 97.22 ± 13.2 | 4.82 ± 0.4 | 22.90 ± 4.8 |
| Glyceric acid | 11.31 | 26.06 ± 5.0 | 7.82 ± 3.8 | ||
| Succinic acid | 12.74 | 3.28 ± 0.02 | 2.33 ± 0.8 | 2.50 ± 0.6 | 6.19 ± 2.5 |
| Malic acid | 15.83 | 11.19 ± 0.3 | 13.49 ± 7.8 | 14.33 ± 3.2 | 11.05 ± 3 |
| Sugars and sugar alcohols | |||||
| Glycerol | 8.93 | 4.05 ± 0.2 | 4.16 ± 1.2 | 2.69 ± 0.8 | 14.43 ± 5.3 |
| Fructose 1 | 19.84 | 0.43 ± 0.1 | 6.43 ± 2.4 | 80.67 ± 21 | 66.44 ± 18 |
| Fructose 2 | 20.13 | 0.77 ± 0.3 | 31.06 ± 9.2 | 33.78 ± 11 | 160.03 ± 60 |
| Fructose 3 | 20.40 | 90.51 ± 19 | 124.66 ± 34.6 | 90.44 ± 30 | 338.47 ± 90 |
| Quinic acid | 21.68 | 27.12 ± 7.5 | 52.55 ± 8.7 | ||
| Monosaccharide | 22.27 | Trace | 6.43 ± 2.4 | ||
| Galactose | 22.60 | 23.35 ± 1.7 | 31.06 ± 9.1 | 132.59 ± 40 | 442.06 ± 90 |
| β-D-Glucopyranose | 24.18 | 9.17 ± 3.9 | 27.31 ± 21.8 | 254.1 ± 70 | 769.60 ± 105 |
| Disaccharide | 31.77 | 1.43 ± 0.4 | 26.52 ± 12 | 17.46 ± 3.7 | 455 ± 177 |
| Sucrose | 32.19 | 6.77 ± 3.4 | 343.78 ± 107 | 77.80 ± 16 | 1127 ± 139 |
| Disaccharide | 32.39 | 2.10 ± 0.4 | 20.64 ± 9 | 9.24 ± 4.2 | 232.1 ± 65 |
| Disaccharide | 32.67 | 31.72 ± 9 | 794.5 ± 140 | ||
| Disaccharide | 32.93 | 2.15 ± 0.9 | 99.11 ± 9.8 | 35.04 ± 8.2 | 809.1 ± 109 |
| myo-Inositol | 24.98 | 0.4 ± 0.2 | 2.37 ± 1.2 | 1.38 ± 0.1 | 4.65 ± 2 |
| Fatty acids | |||||
| Hexadecanoic acid | 27.71 | 9.63 ± 0.5 | 11.58 ± 9.0 | 3.01 ± 0.7 | 6.14 ± 1.8 |
| 9,12-Octadecadienoic acid | 30.07 | 7.20 ± 0.3 | 13.16 ± 4.3 | 1.38 ± 0.4 | 1.72 ± 0.5 |
| 9-Octadecenoic acid | 30.40 | 5.69 ± 0.2 | 2.82 ± 2.8 | 3.47 ± 0.7 | 4.04 ± 0.9 |
| 9,12,15-Octadecatrienoic acid | 30.93 | 3.78 ± 0.2 | 4.66 ± 1.2 | 7.14 ± 0.9 | 8.43 ± 1.2 |
| Phenolic acids | |||||
| Caffeic acid | 29.70 | 2.67 ± 1.6 | 4.44 ± 4.6 | 3.28 ± 1.9 | 1.83 ± 0.9 |
| Ferulic acid | 30.31 | 0.16 ± 0.1 | 0.22 ± 0.1 | 0.08 ± 0.04 | 0.34 ± 0.3 |
| Chlorogenic acid | 37.19 | 9.69 ± 3.2 | 8.71 ± 5.2 | ||
| Sterols | |||||
| Campestrol | 38.90 | 0.50 ± 0.1 | 0.56 ± 0.3 | 0.89 ± 0.2 | 0.91 ± 0.2 |
| Stigmasterol | 40.16 | 1.80 ± 0.1 | 1.37 ± 0.4 | 0.37 ± 0.1 | 0.47 ± 0.1 |
| β-Sitosterol | 40.88 | 5.70 ± 0.7 | 4.37 ± 1.3 | 4.90 ± 1.1 | 6.76 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, M.; Yankova-Tsvetkova, E.; Sijimova, B.; Denev, R.; Berkov, S. Seed Germination Inhibitory Activity of Alkaloid Fractions from Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus Leaves. Horticulturae 2025, 11, 1154. https://doi.org/10.3390/horticulturae11101154
Nikolova M, Yankova-Tsvetkova E, Sijimova B, Denev R, Berkov S. Seed Germination Inhibitory Activity of Alkaloid Fractions from Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus Leaves. Horticulturae. 2025; 11(10):1154. https://doi.org/10.3390/horticulturae11101154
Chicago/Turabian StyleNikolova, Milena, Elina Yankova-Tsvetkova, Boriana Sijimova, Rumen Denev, and Strahil Berkov. 2025. "Seed Germination Inhibitory Activity of Alkaloid Fractions from Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus Leaves" Horticulturae 11, no. 10: 1154. https://doi.org/10.3390/horticulturae11101154
APA StyleNikolova, M., Yankova-Tsvetkova, E., Sijimova, B., Denev, R., & Berkov, S. (2025). Seed Germination Inhibitory Activity of Alkaloid Fractions from Narcissus pseudonarcissus cv. Carlton and Narcissus poeticus Leaves. Horticulturae, 11(10), 1154. https://doi.org/10.3390/horticulturae11101154









