Advancing Industrial Production of White Grifola frondosa: Liquid Inoculum Culture Parameter Optimization and Molecular Insights into Fruiting Body Development
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Fungal Strain
2.2. Single-Factor Experiments
2.3. Orthogonal Experiments
2.4. Cultivation and Collection of G. frondosa for RNA-Seq Analysis
2.5. RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
2.6. Differential Gene Expression Analysis, Functional Enrichment Analysis, and Differential Gene Expression Clustering Analysis
2.7. Real-Time Quantitative PCR (RT-qPCR) Validation
2.8. Statistical Analysis
3. Results
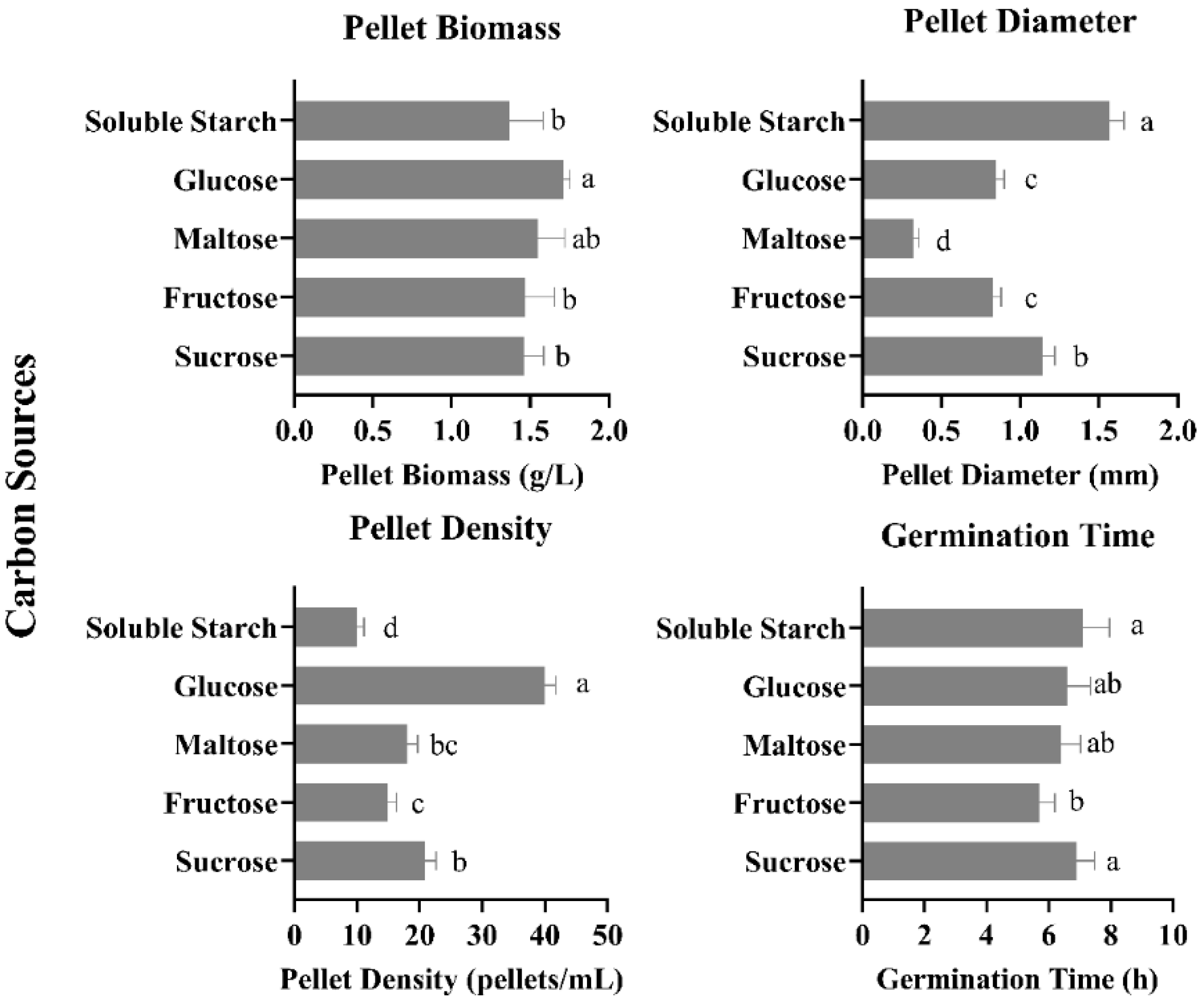
3.1. Effect of Different Carbon Sources on Pellet Growth
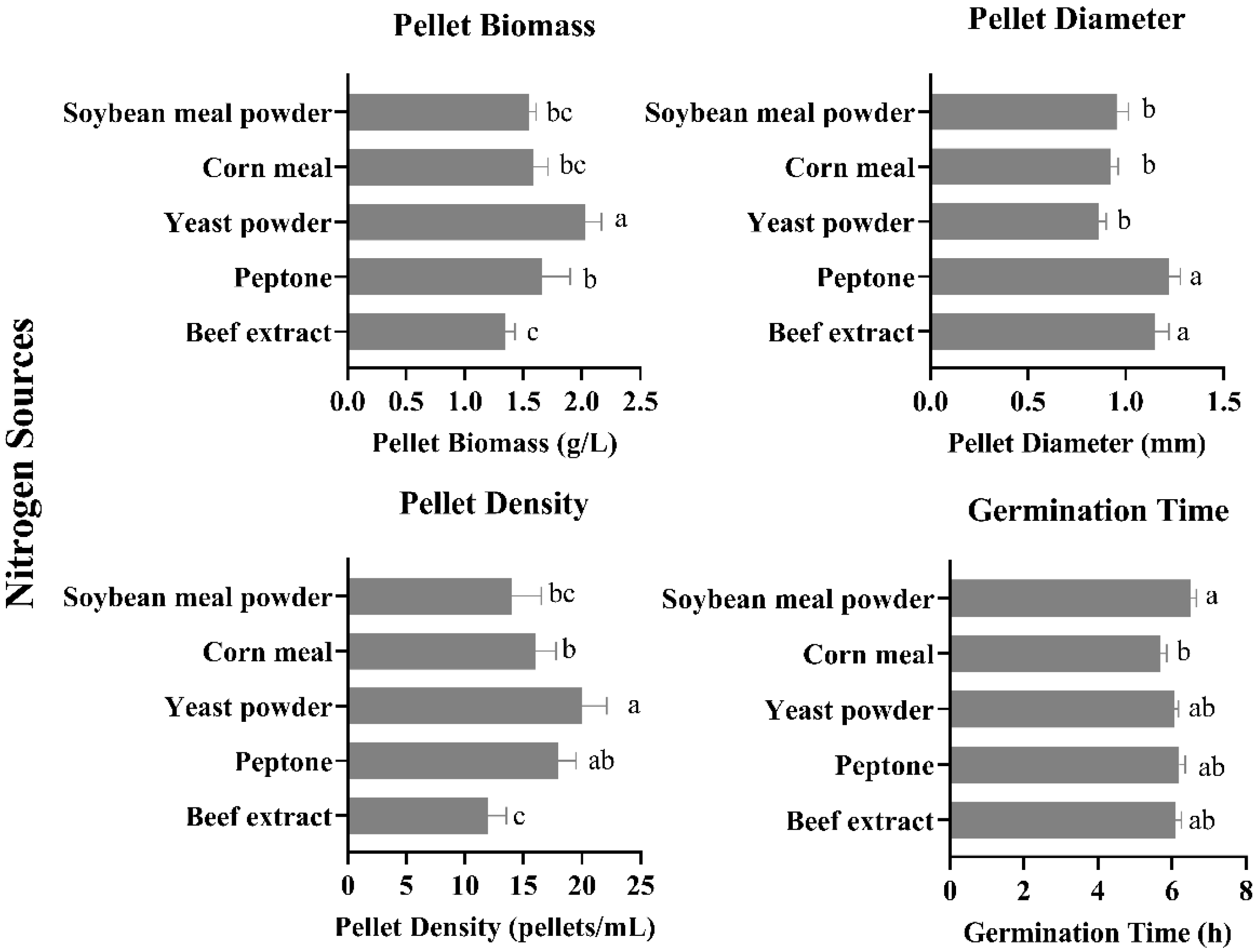
3.2. Effect of Different Nitrogen Sources on Pellet Growth
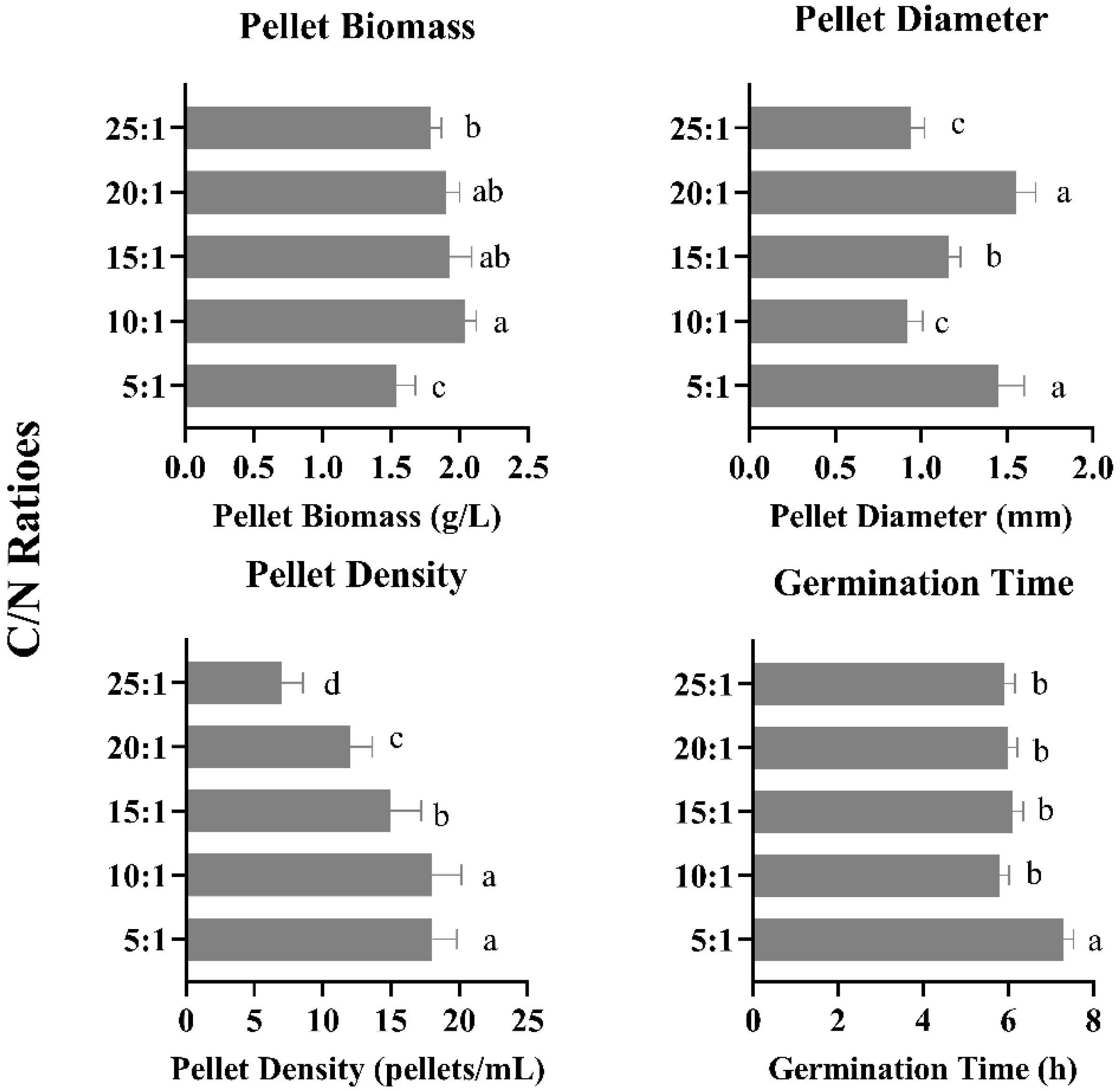
3.3. Effect of Different C/N Ratios on Pellet Growth
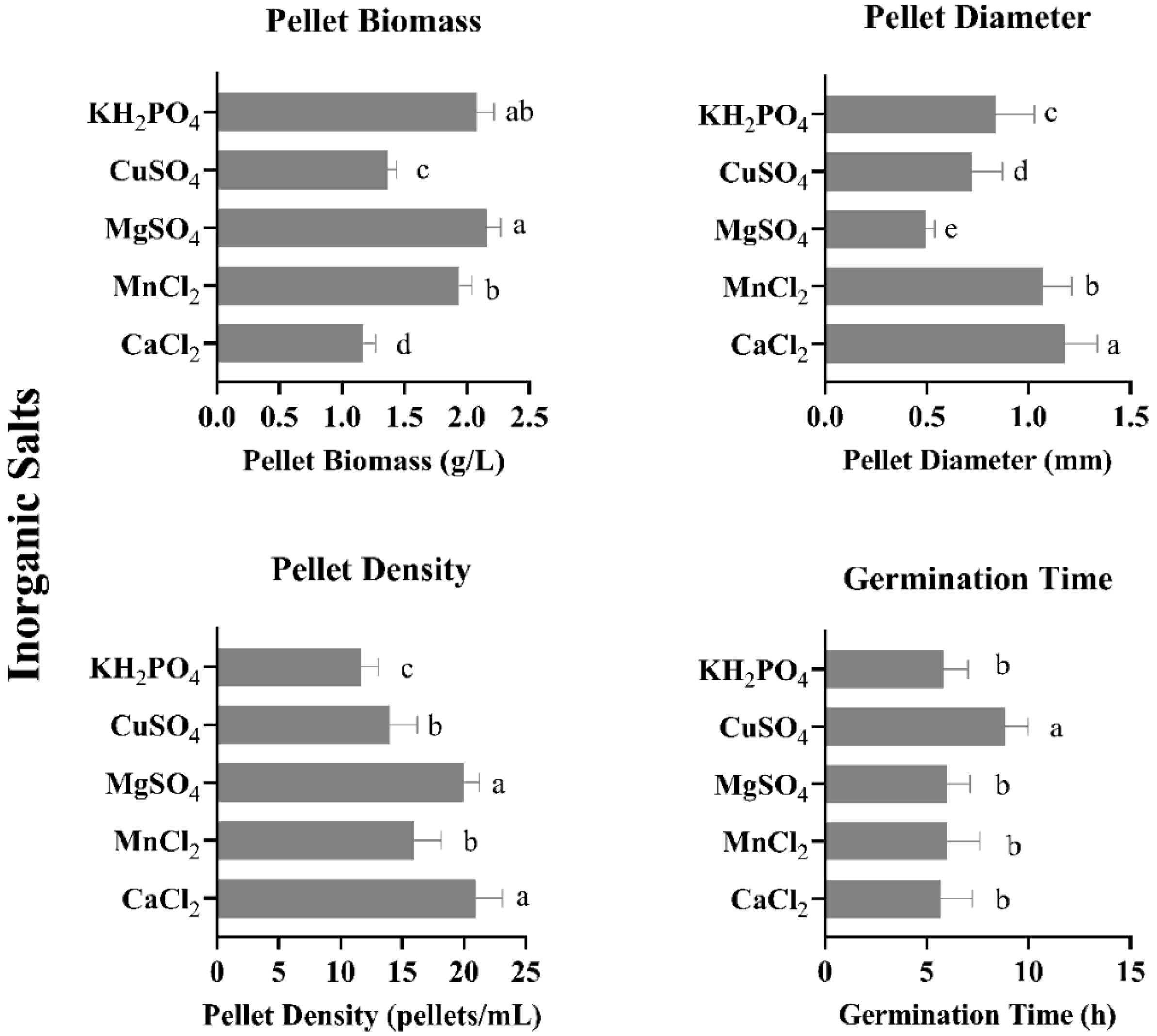
3.4. Effect of Different Inorganic Salts on Pellet Growth
3.5. Effect of Different pH Values on Pellet Growth
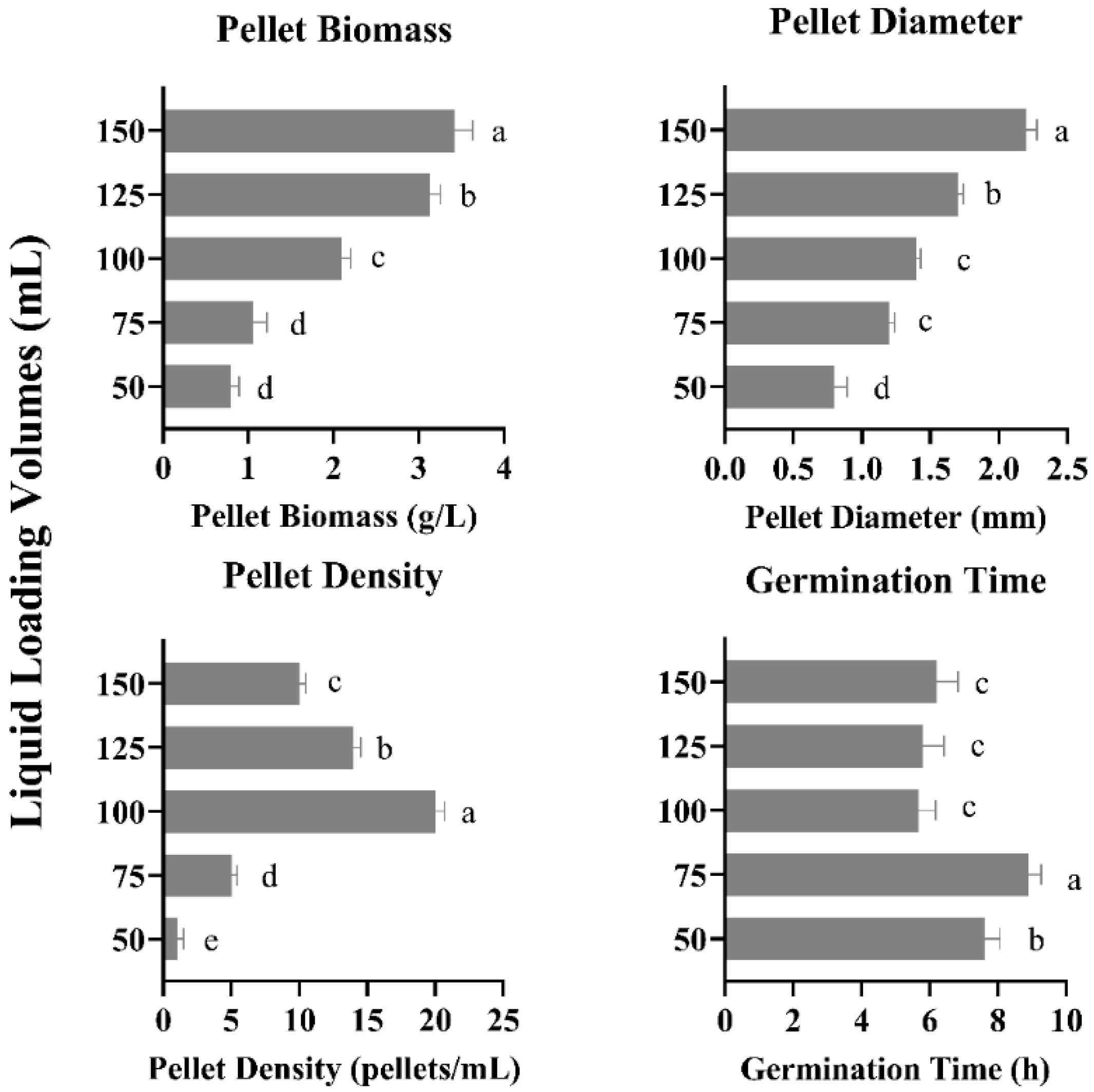
3.6. Effect of Different Flask Filling Volumes on Pellet Growth
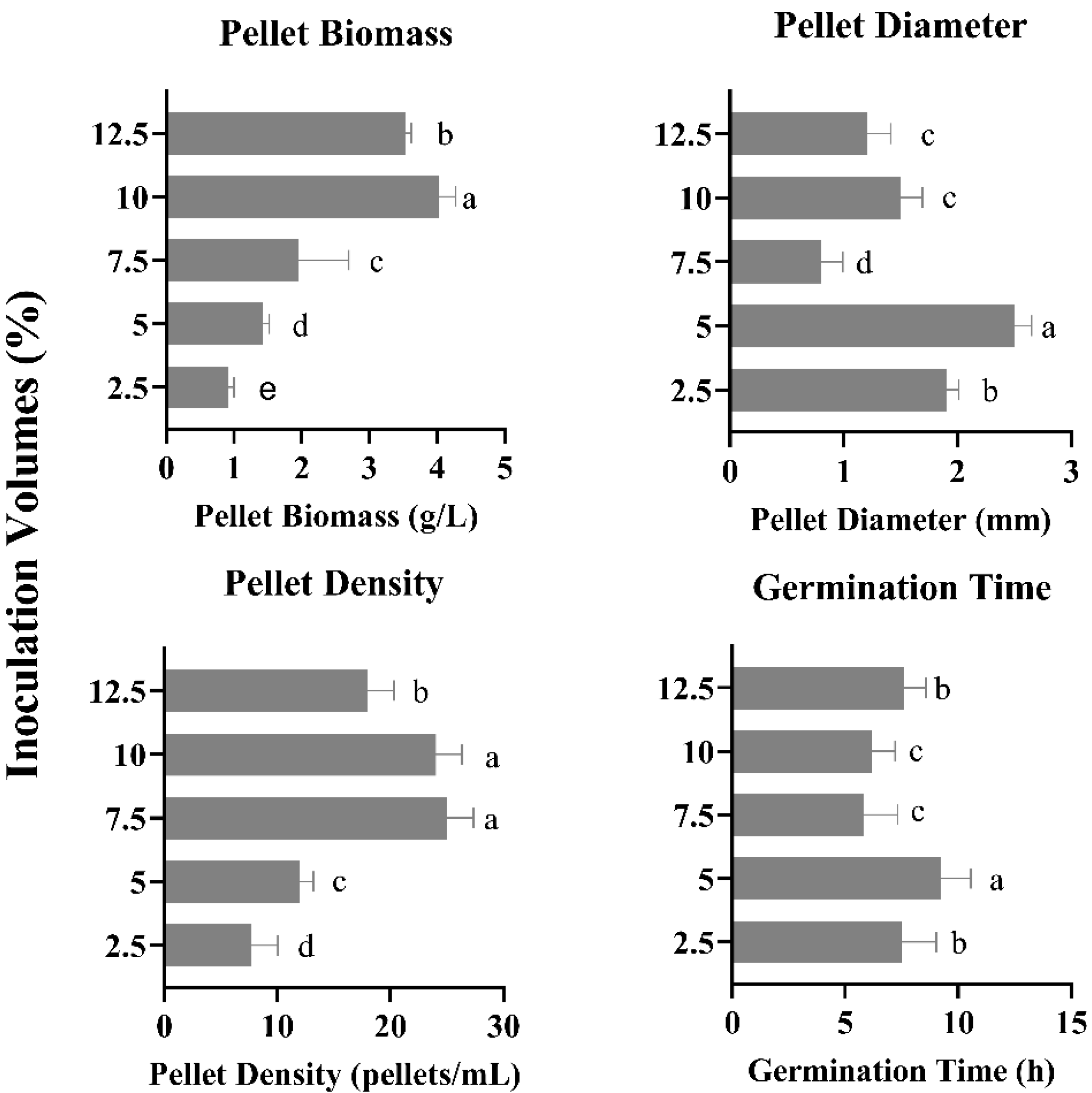
3.7. Effect of Different Inoculation Volumes on Pellet Growth
3.8. Orthogonal Experiment Analysis
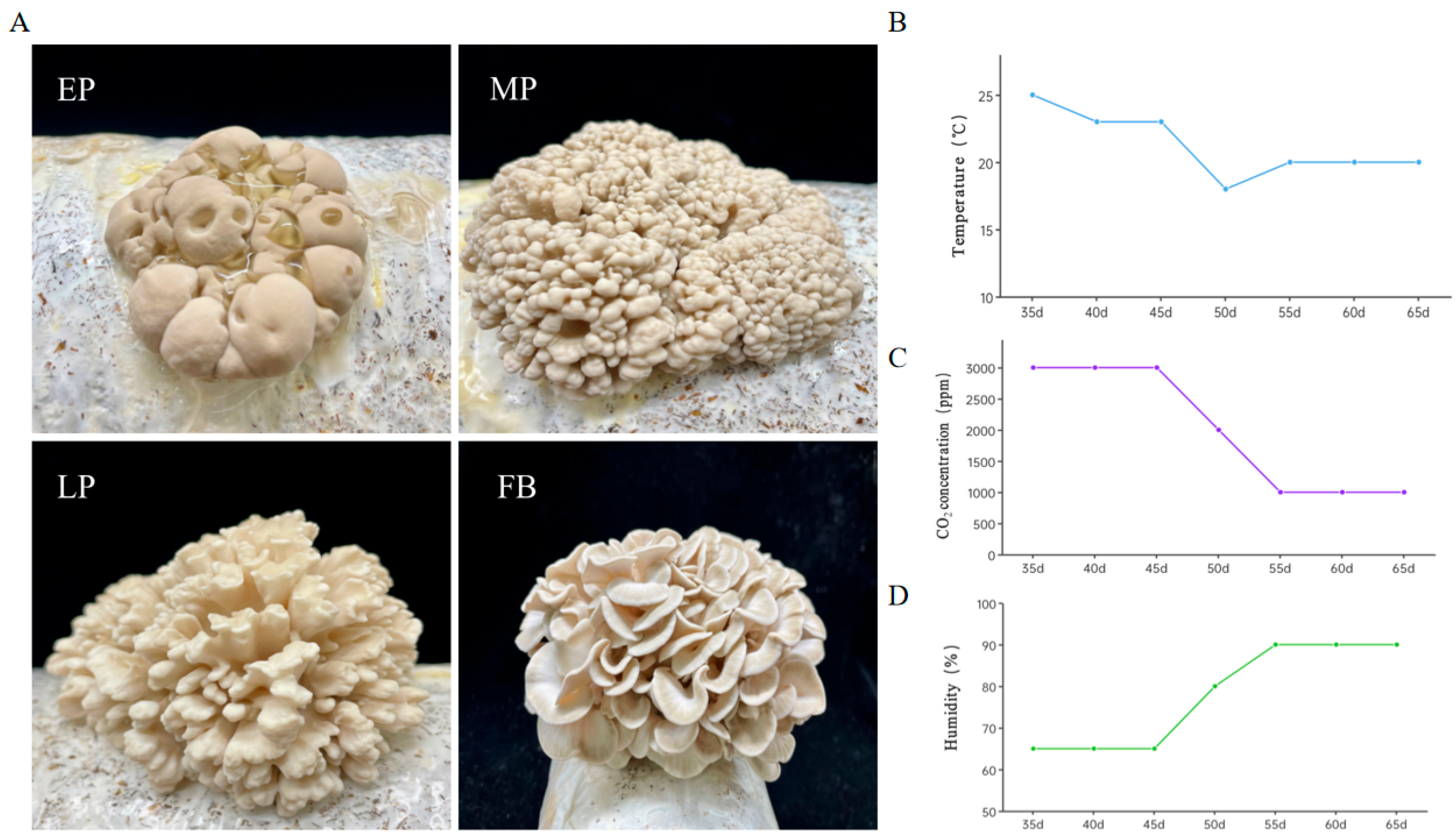
3.9. Morphological Characteristics of White G. frondosa
3.10. Global Transcriptomic Analysis
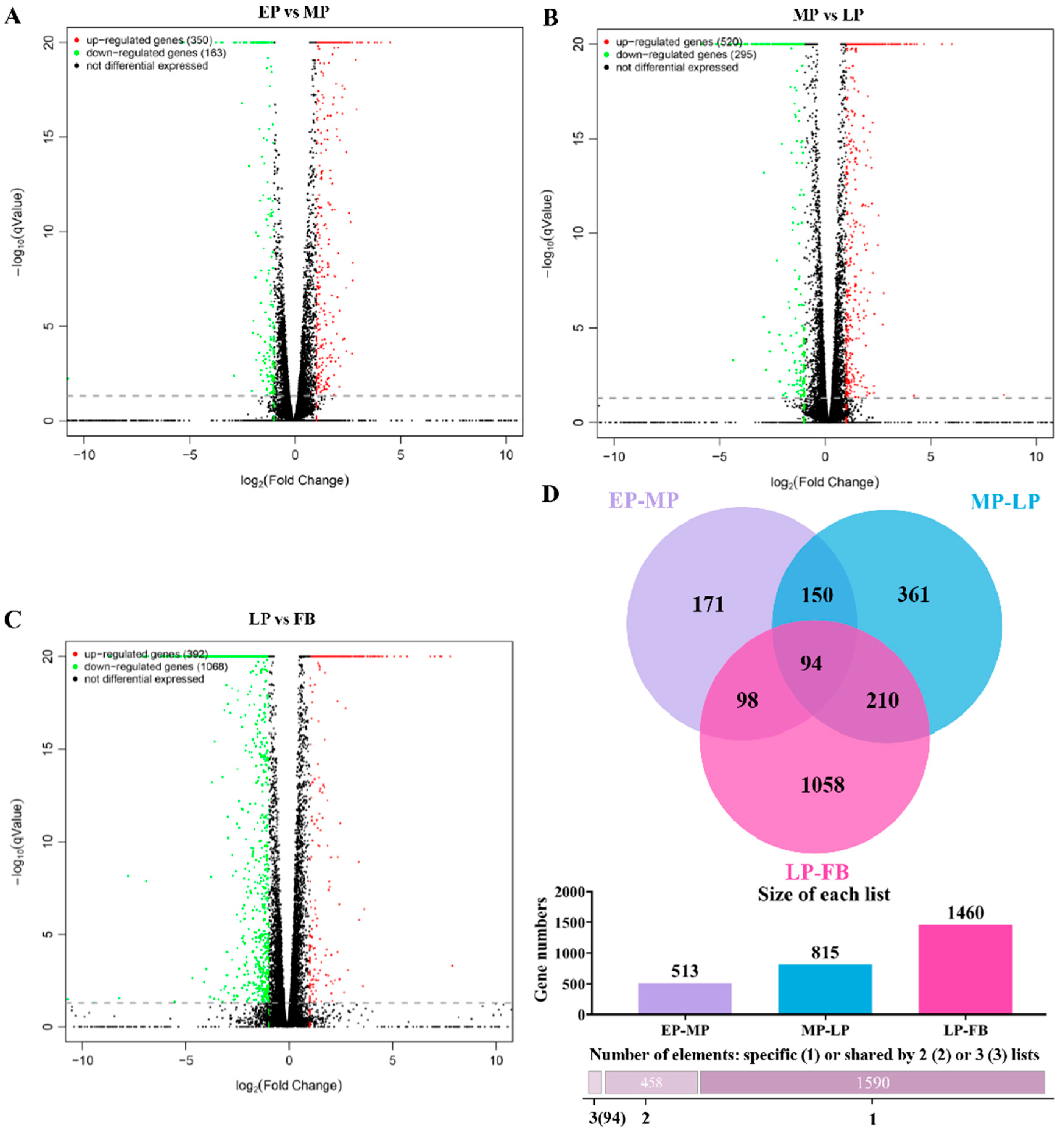
3.11. Differential Gene Expression Analysis Across Developmental Transitions
3.12. Cluster-Based Analysis of Differential Gene Expression Across Developmental Stages
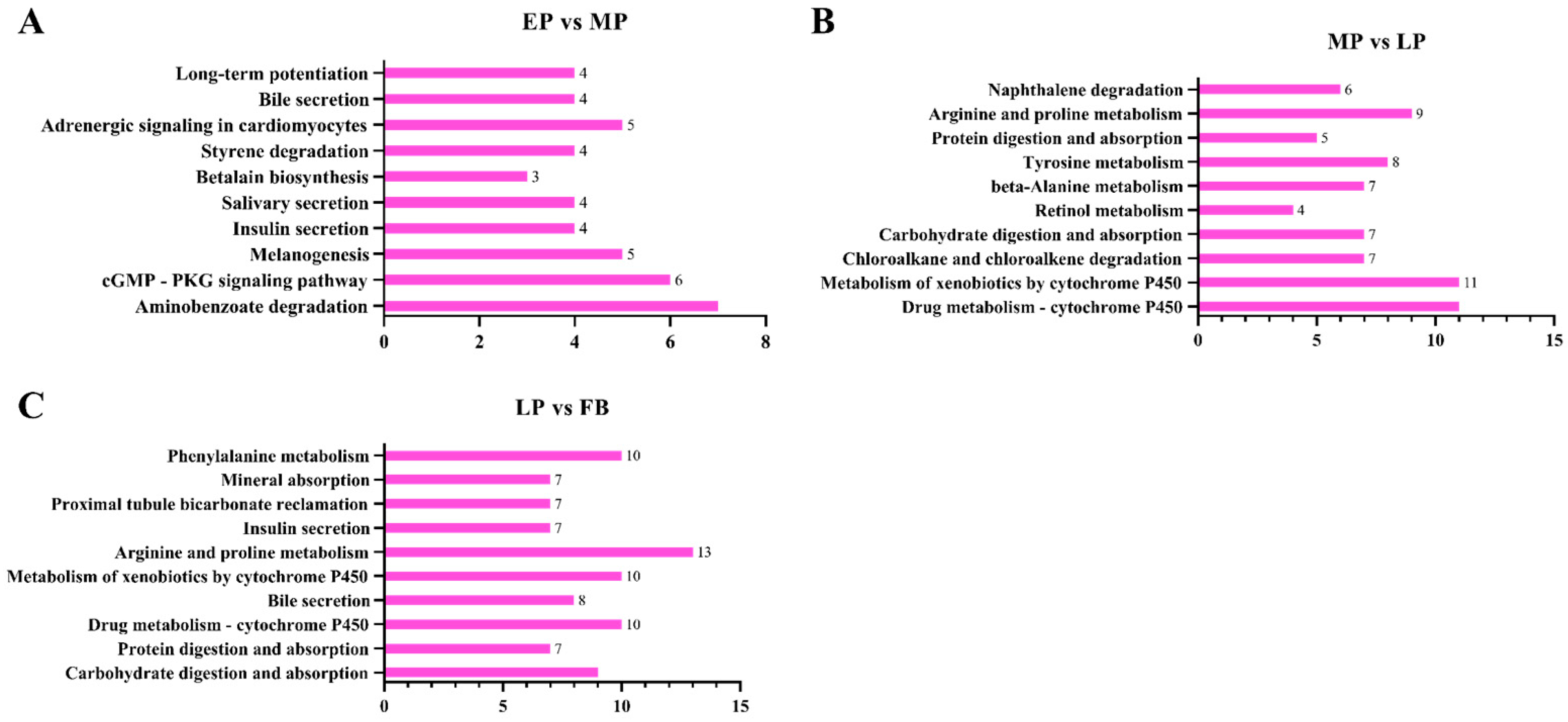
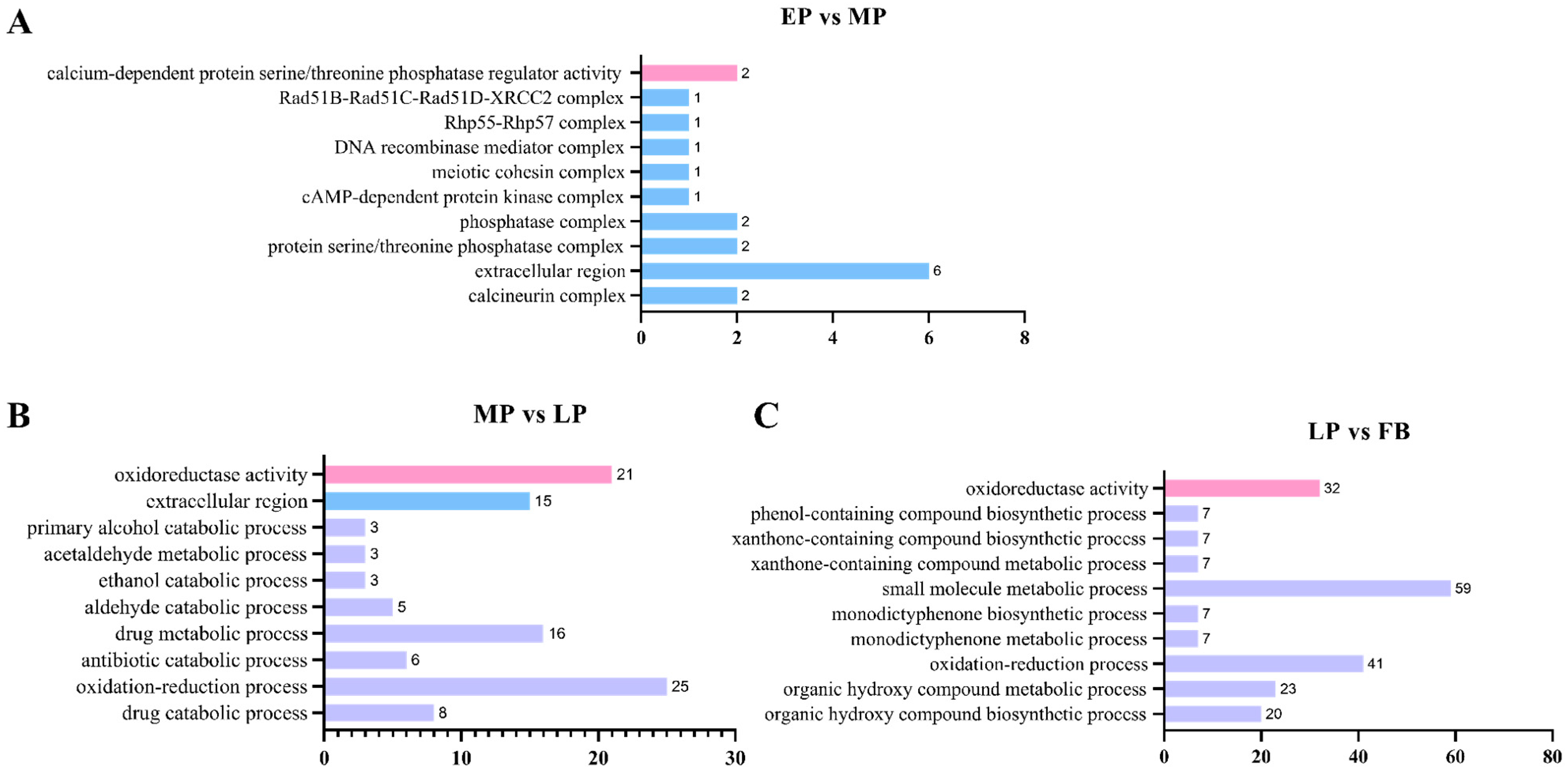
3.13. Functional Categorization of Differentially Expressed Genes
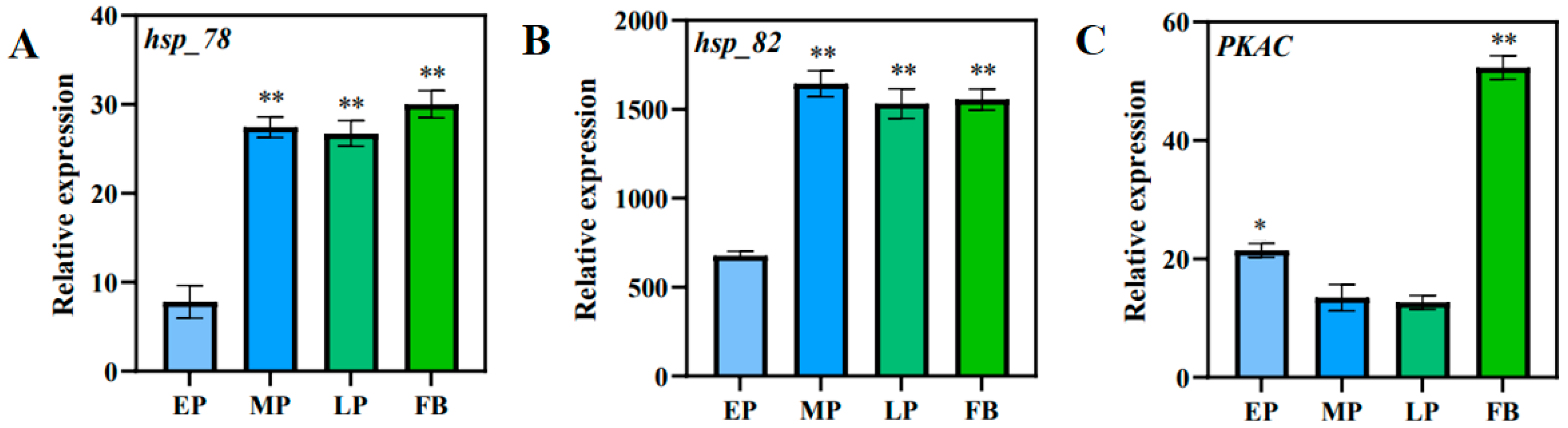
3.14. Validation of Transcriptomic Data with RT-qPCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI: Wallingford, UK, 2008; pp. 1–784.
- Xie, X.J.; Wu, F.; Li, S.M.; Vlasák, J.; Zhang, X.; Tian, J.; Li, M.; Li, G. Revision of the scientific name for edible and medicinal fungus huishuhua (maitake) in China. Mycosystema 2024, 43, 230303. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, X.; Liao, W.; Fang, J.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, L-fuco-D-manno-1,6-α-D-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohydr. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Ohno, N.; Adachi, Y.; Suzuki, I.; Sato, K.; Oikawa, S.; Yadomae, T. Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. 1986, 34, 1709–1715. [Google Scholar] [CrossRef]
- Suzuki, I.; Hashimoto, K.; Oikawa, S.; Sato, K.; Osawa, M.; Yadomae, T. Antitumor and immunomodulating activities of a beta-glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. 1989, 37, 410–413. [Google Scholar] [CrossRef]
- Bu, Q.M.; Wang, S.F.; Dong, H.X.; Huang, Q.R.; Lai, G.S. Study on the optimal conditions of liquid fermentation of Grifola frondosa. Hubei Agric. Sci. 2003, 22, 23–24+39. (In Chinese) [Google Scholar] [CrossRef]
- Deng, G.C.; Zhao, H.; Gong, L.A.; Li, Y.B.; Ma, Y.; Li, J.L.; Li, J. Optimization of liquid fermentation conditions for Grifola frondosa mycelium. Jiang Su Agric. Sci. 2013, 41, 216–218. (In Chinese) [Google Scholar] [CrossRef]
- Feng, L.G.; Xu, N.; Huang, X.H.; Deng, Z.L.; Xia, Y.L.; Zhou, N. Optimization of liquid seed medium for Grifola frondosa. J. Agric. Sci. Tech.-Iran 2020, 21, 40–44. [Google Scholar] [CrossRef]
- Shen, X.; Lui, H.N. Effects of the extracts of traditional Chinese medicines on the extracellular polysaccharide production of Grifola frondosa fermentation system. J. Fungal. Res. 2021, 19, 115–121. (In Chinese) [Google Scholar] [CrossRef]
- Yan, J.; Tong, Z.; Han, X.; Gan, Y.; Liu, Y.; Chen, J.; Duan, X.; Lin, J.; Gan, B.; Xie, B. Transcriptome profiling reveals candidate genes related to stipe gradient elongation of Flammulina filiformis. J. Fungi 2022, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Du, F.; Zou, Y.; Hu, Q. Transcriptomics analysis of primordium formation in Pleurotus eryngii. Genes 2021, 12, 1863. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Liu, Q.; Li, Q.; Qian, Z.; Zhang, X.; Li, K.; Li, W.; Dong, C. Developmental transcriptomics of Chinese cordyceps reveals gene regulatory network and expression profiles of sexual development-related genes. BMC Genom. 2019, 20, 337. [Google Scholar] [CrossRef]
- Nie, W.Q.; Wu, T.X.; Zhong, M.; Lu, H.Y. Transcriptome sequencing and analysis of Grifola frondosa mycelia. Food Sci. 2017, 38, 6–11. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.K.; Zhou, Z.F.; Chen, Q.; Yuan, W.D. Analysis of differentially expressed genes between mycelium and primordium of Grifola frondos. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2016, 34, 74–80. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.S.; Li, X.; Li, G.J.; Huang, Q.; Tian, J.H.; Wang, J.L.; Li, M.; Li, S.M. Genetic and molecular evidence of a tetrapolar mating system in the edible mushroom Grifola frondosa. J. Fungi 2023, 9, 959. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Hayashi, M.; Chen, F.C.; Shimomura, N.; Yamaguchi, T.; Aimi, T. Genetic analyses of causal genes of albinism (white fruiting body) in Grifola frondosa. J. Wood Sci. 2019, 65, 32. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Hayashi, M.; Nakano, S.; Shimomura, N.; Yamaguchi, T.; Aimi, T. Expression of tyrosinase genes associated with fruiting body formation and pigmentation in Grifola frondosa. Mycoscience 2019, 60, 262–269. [Google Scholar] [CrossRef]
- Chen, Y.L. Product Development and Functional Analysis of Solid State Fermentation Products of Grifola frondosa. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2022; pp. 1–94. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Li, S.M.; Ren, H.H.; Zhang, J.; Gao, S.P.; Chen, Z.X.; Li, G.J.; Tian, J.; Wang, J.; Li, M.; Li, X.; et al. Chitin synthases in Cordyceps militaris: Genome-wide gene identification, evolutionary insights, and life cycle transcript profiling. Horticulturae 2024, 10, 494. [Google Scholar] [CrossRef]
- Xie, H.Y.; Wan, L.Z.; Han, J.D.; Huang, C.Y.; Li, J.; Yang, P.; Zhang, Y.; Gong, Z.; Yu, H. TMT-based proteomic and transcriptomic analysis reveal new insights into heat stress responsive mechanism in edible mushroom Grifola frondosa. Sci. Hortic-Amsterdam. 2024, 323, 112542. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sakamoto, Y. Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol. Rev. 2018, 32, 236–248. [Google Scholar] [CrossRef]
- Wang, G.Z.; Li, M.; Zhang, C.H.; Sun, C.Y.; Li, T.H.; Deng, W.Q. Dissecting the regulation network of high temperature inhibiting primordial formation of Cordyceps guangdongensis based on transcriptomic data. Mycosystema 2021, 40, 2940–2952. (In Chinese) [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, C.H.; Huang, Q.J.; Huang, H.; Li, T.H.; Wang, G.Z.; Deng, W.Q. unction of the heat shock protein 40 gene CgDnaJ05 in Cordyceps guangdongensis analysed by RNAi. Mycosystema 2022, 41, 1786–1795. (In Chinese) [Google Scholar] [CrossRef]
- Bahn, Y.S.; Mühlschlegel, F.A. CO2 sensing in fungi and beyond. Curr. Opin. Microbiol. 2006, 9, 572–578. [Google Scholar] [CrossRef]
- Li, W.D.; Zhang, W.B.; Hua, J.; Wang, Z.J. Study on liquid culture characteristics and extracellular enzyme activities of Grifola frondosa. Edible Fungi. 2019, 41, 11–13. (In Chinese) [Google Scholar]
- Zhou, F.Z.; Chang, L.; Chen, X.F.; Li, Y.F.; Li, F. A priliminary study on Grifola frondosa liquid culture and its extracellular polysaccharides. Henan Sci. 2012, 30, 577–579. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, L. Optimization of Fermentation Conditions for Grifola frondosa Using Traditional Chinese Medicine-Based Substrates; Gui Zhou University: Guizhou, China, 2008. (In Chinese) [Google Scholar]
- Sun, H.J.; Chen, J.W.; Yan, J.; Wang, J.; Xie, Y.; Wang, W.Z.; Jiang, F.Y.; Wu, F.L. Optimization of carbon-to-nitrogen ratio in culture medium for maximum mycelial growth of Grifola frondosa. Edible Fungi. 2019, 41, 18–20. (In Chinese) [Google Scholar]
- Duan, C.; Yao, L.; Lv, J.H.; Jia, C.W.; Tian, F.H.; Li, C.T. Systematic analysis of changes across different developmental stages of the mushroom Sarcomyxa edulis. Gene 2022, 824, 146450. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, J.; Wang, H.; Wang, Q.; Chen, M.; Juan, J.; Feng, Z.; Chen, H. Comparative transcriptome analysis reveals potential fruiting body formation mechanisms in Morchella importuna. AMB Express 2019, 9, 103. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, A.; Li, M.J.; Cao, P.F.; Chen, T.X.; Zhang, G.; Shi, L.; Jiang, A.L.; Zhao, M.W.; Brakhage, A.A. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via Cytosolic Ca2+. Appl. Environ. Microbiol. 2016, 82, 4112–4125. [Google Scholar] [CrossRef]
- Lu, Y.; Lian, L.; Guo, L.; Xie, B.; Wang, W.; Chen, B.; van Peer, A.F.; Li, S.; Wu, T.; Xie, B. The accordant trend of both parameters (rgs expression and cAMP content) follows the pattern of development of fruiting body in Volvariella volvacea. Curr. Microbiol. 2015, 71, 579–584. [Google Scholar] [CrossRef]
- Lengeler, K.B.; Davidson, R.C.; D’souza, C.; Harashima, T.; Shen, W.C.; Wang, P.; Pan, X.; Waugh, M.; Heitman, J. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 2000, 64, 746–785. [Google Scholar] [CrossRef]


| Level | Factor | |||
|---|---|---|---|---|
| Carbon Source | Nitrogen Source | C–N Ratio | Inorganic Salt | |
| 1 | Fructose | Peptone | 10:1 | MnCl2 |
| 2 | Maltose | Yeast powder | 15:1 | KH2PO4 |
| 3 | Glucose | Corn flour | 20:1 | MgSO4 |
| Level | Factor | Pellet Biomass (g/L) | |||
|---|---|---|---|---|---|
| Carbon Source | Nitrogen Source | C–N Ratio | Inorganic Salts | ||
| 1 | 1 | 1 | 1 | 1 | 1.4867 |
| 2 | 1 | 2 | 3 | 2 | 1.8333 |
| 3 | 1 | 3 | 2 | 3 | 1.3200 |
| 4 | 2 | 1 | 3 | 3 | 0.6870 |
| 5 | 2 | 2 | 2 | 1 | 1.1133 |
| 6 | 2 | 3 | 1 | 2 | 0.8133 |
| 7 | 3 | 1 | 2 | 2 | 1.3600 |
| 8 | 3 | 2 | 1 | 3 | 2.3333 |
| 9 | 3 | 3 | 3 | 1 | 1.3333 |
| K1 | 1.547 | 1.178 | 1.544 | 1.311 | |
| K2 | 0.871 | 1.760 | 1.264 | 1.335 | |
| K3 | 1.675 | 1.155 | 1.284 | 1.446 | |
| R | 0.804 | 0.604 | 2.280 | 0.135 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-H.; Zhang, J.-Y.; Wang, J.-Y.; Xiao, S.-S.; Liu, S.-Y.; Sun, B.-Y.; Li, S.-M.; Li, M.; Wen, Z.-Q.; Li, X. Advancing Industrial Production of White Grifola frondosa: Liquid Inoculum Culture Parameter Optimization and Molecular Insights into Fruiting Body Development. Horticulturae 2025, 11, 1151. https://doi.org/10.3390/horticulturae11101151
Ren H-H, Zhang J-Y, Wang J-Y, Xiao S-S, Liu S-Y, Sun B-Y, Li S-M, Li M, Wen Z-Q, Li X. Advancing Industrial Production of White Grifola frondosa: Liquid Inoculum Culture Parameter Optimization and Molecular Insights into Fruiting Body Development. Horticulturae. 2025; 11(10):1151. https://doi.org/10.3390/horticulturae11101151
Chicago/Turabian StyleRen, Hui-Hui, Jia-Ye Zhang, Jia-Yuan Wang, Shang-Shang Xiao, Su-Ya Liu, Bao-Yue Sun, Shou-Mian Li, Ming Li, Zhi-Qiang Wen, and Xiao Li. 2025. "Advancing Industrial Production of White Grifola frondosa: Liquid Inoculum Culture Parameter Optimization and Molecular Insights into Fruiting Body Development" Horticulturae 11, no. 10: 1151. https://doi.org/10.3390/horticulturae11101151
APA StyleRen, H.-H., Zhang, J.-Y., Wang, J.-Y., Xiao, S.-S., Liu, S.-Y., Sun, B.-Y., Li, S.-M., Li, M., Wen, Z.-Q., & Li, X. (2025). Advancing Industrial Production of White Grifola frondosa: Liquid Inoculum Culture Parameter Optimization and Molecular Insights into Fruiting Body Development. Horticulturae, 11(10), 1151. https://doi.org/10.3390/horticulturae11101151





