Abstract
As the only harvest organ of pepper, fruit size is an important yield determinant. To elucidate the molecular mechanisms underlying pepper fruit size, we performed histological, physiological, and transcriptomic analyses on the pepper varieties QB6 (large fruit) and CXJ82 (small fruit). High contents of auxin and cytokinin in the early stage of fruit development promoted the rapid division of fruit cells in both varieties, which provided sufficient cells for subsequent fruit enlargement. High gibberellin accumulation induced the elongation and expansion of QB6 pericarp cells. Transcriptome analysis showed that genes related to cell division, cell wall polysaccharide degradation, and photosynthesis were highly expressed in QB6 fruit, likely contributing to its larger size. In the hormone–signal transduction factor–gene interaction network, GID6, GID1, IAA12, MYC30, and SAUR36 exhibited high correlations with numerous genes related to cell division, the cell wall, and photosynthesis, emerging as key signal transduction factors for the hormone-mediated regulation of pepper fruit size. Weighted gene co-expression network analysis identified the transcription factors OFP20, HD-ZIP6, and HD-ZIP13 as fundamental for pepper fruit size regulation. Our results expand the understanding of hormone regulation of pepper fruit size, providing a foundation for the breeding and improvement of excellent pepper varieties.
1. Introduction
Pepper (Capsicum annum L.) belongs to the Solanaceae family, which originated in Latin America and is one of the most widely cultivated vegetables and spice crops globally [1,2]. According to the United Nations Food and Agriculture Organization statistics, the cultivated area of pepper reached 206.99 million hectares in 2020, and the annual output is more than 3600 million tons [3]. The fruit is the only edible part of pepper, and fruit size is an important trait for yield determination and appearance quality, directly affecting consumers’ purchasing intentions and market competitiveness.
Solanaceae crops mainly comprise plants with fleshy fruits. Fruit development starts from ovule fertilization, which can usually be divided into three major stages: fruit setting, fruit growth, and fruit maturity stages [4,5]. Fruit setting refers to the transformation of ovules to fruits after fertilization. The fruit growth period can be further divided into two stages, namely the cell division stage and the cell expansion stage. Fruit growth begins with a very active cell division period inside the ovary, and the frequency of cell division or the duration of the cell cycle phase is likely to determine the ultimate size and weight of the fruit [6,7]. The second stage of fruit growth is related to cell expansion, which begins within a few days after fruit setting, accompanied by cell division and a continued increase in fruit size. After completion of the cell division and cell expansion stages, the fruit enters the mature stage when various nutrients begin to accumulate, accompanied by a slow increase in fruit size [7].
The plant cell wall is mainly composed of polysaccharides such as cellulose, hemicellulose, pectin, and lignin. The main function of the cell wall is to withstand the expansion pressure of the cell and to maintain its overall mechanical strength and rigidity along with other structures of the cell; this function therefore largely determines the ultimate size of the cell [8,9]. Expansin (EXP) is a typical non-enzymatic protein in the cell wall, which affects its ductility by destroying the cross-linking between the cell wall polysaccharide components, thereby regulating the growth and size of cells during fruit development [10,11]. Inhibiting the expression of tomato SlEXP1 reduced the depolymerization of cell wall polysaccharide components [12]. In pepper, silencing CaEXP4 resulted in smaller fruit epidermal cells with a more orderly arrangement [13]. Cell cycle regulation is essential for plant growth and development. Cyclins and cyclin-dependent protein kinases are the main regulators of the cell cycle [14]. Previous studies have shown that cyclins are highly expressed in the early stages of tomato and cucumber fruit development [15,16].
Plant hormones play an important role in fruit growth and development, especially auxin, gibberellin (GA), and cytokinin [17]. Auxin can regulate fruit size by participating in physiological processes such as cell division and cell enlargement [18]. Auxin response factor (ARF) is an important transcription factor in the auxin signaling pathway, and its function in the fruit size regulation of horticultural plants has been widely reported. SlARF9 is highly expressed in the ovule, placenta, and peel of pollinated tomatoes; overexpression of SlARF9 resulted in a decrease in tomato fruit size, while SlARF9-inhibition lines had larger fruits [19]. Moreover, the transcription factor SlARF7 negatively regulates the tomato fruit-setting process [20]. GA plays an essential role in the fruit enlargement of various horticultural crops. In tomato, the two peaks of GA content are consistent with the stages of fruit cell division and expansion on the 8th and 15th day after pollination, respectively [21]. Exogenous GA treatment leads to the longitudinal elongation of woodland strawberry fruit [22]. Other studies have shown that exogenous GA can promote the division and expansion of grape pulp cells along with an increase in fruit weight [21]. Cytokinin is another main regulator of fruit development, playing key roles in the regulation of cell division and differentiation. The content of cytokinin in tomato fruit is higher at the cell division stage but then decreases rapidly at the fruit-ripening stage [23]. A recent study demonstrated that exogenous cytokinin treatment activated cell division at the early stage of tomato fruit development, leading to the formation of parthenocarpic fruit [24]. Furthermore, a large number of studies emphasized that the crosstalk among auxin, GA, cytokinin, and other plant hormones synergistically regulates fruit size [17,25].
Fruit size is a typical quantitative trait that is regulated by multiple genes. Genetic linkage and association analyses have identified the major genes and quantitative trait loci (QTLs) related to fruit size in a variety of horticultural crops [4,21]. Loci such as FW2.2 (CNR), FW3.2, FW11.3 (CSR), SUN, OVATE, LC, and FAS were identified to regulate the size or shape of tomato fruit [4,21]. Through population genomic analysis, the loci Qfwt2-1 and Qfwt3 were identified to play an important role in watermelon domestication and the evolution of its fruit size [26]. In pepper, seven QTLs related to fruit weight were identified, among which BEL1-LIKE HOMEODOMAIN PROTEIN 2 (QTL FWe-P2.4) was identified as a key candidate gene for regulating fruit weight [27]. Furthermore, two QTLs, qFW2.1 and qFW3.1 on chromosomes 2 and 3 of pepper, were significantly associated with fruit weight, which could explain 12.28% and 15.50% of the phenotypic variation, respectively [28]. Pepper and tomato both belong to the Solanaceae family, exhibiting high genetic similarity. The tomato homologous gene CaOvate negatively regulates pepper fruit elongation [29]. However, the IQ67-domain protein CaIQD1 can interact with the Ovate family protein CaOFP20 to synergistically regulate pepper fruit elongation [30]. CaKLUH/CYP78A5 was reported to play a role in the regulation of pepper fruit weight [31]. Moreover, physalis organ size 1 (CaPOS1), a homologous gene of FW11.3 (CSR), was reported to affect the size of pepper fruit by positively regulating cell expansion [32].
Although a large number of genes or loci related to fruit size and shape have been identified in horticultural crops to date, only a small number of loci controlling fruit size have been identified in pepper, and there is an overall lack of functional verification of these genes. Furthermore, research on the regulation of fruit size by plant hormones in pepper is sparse, leaving gaps in understanding the mechanisms by which hormone signals cooperate with other pathways to regulate pepper fruit development. To address these gaps, in this study, two recombinant pepper lines differing in fruit size were selected as materials to analyze the molecular mechanism underlying pepper fruit size differences from the perspectives of histology, physiology, and transcriptomics. By uncovering these mechanisms along with the identification of candidate key genes regulating pepper fruit size, our results will contribute to the quality breeding and improvement of excellent pepper varieties.
2. Materials and Methods
2.1. Plant Material and Sampling
Two pepper varieties with large differences in fruit size, QB6 (large-fruit type) and CXJ82 (small-fruit type), provided by Hunan Vegetable Research Institute (Changsha, China), were identified as the research materials for this study. After standardized seedling raising, the two pepper varieties were transplanted in the biological garden of Hengyang Normal University (Hengyang City, Hunan Province, China) for routine cultivation and management in March 2024. The peppers were sampled at 10, 20, and 30 days after anthesis (DAA); 30 DAA corresponds to the green ripe stage, when fruit enlargement is completed. Each sample consisted of six pepper pericarps derived from three plants (two pericarps per plant). The collected samples, each with three biological replicates, were immediately frozen in liquid nitrogen for subsequent phytohormone, transcriptome, and quantitative real-time polymerase chain reaction (RT-qPCR) analyses. Furthermore, the single-fruit weight was measured using an analytical balance, and the fruit length, fruit diameter, and flesh thickness were measured with a vernier scale at the three fruit development stages.
2.2. Histological Analysis of Fruit Pericarp Tissue
The pericarp tissue of pepper fruits sampled at 10, 20, and 30 DAA was transversely cut into blocks (0.6 cm × 0.4 cm × 0.3 cm) and placed in 70% FAA (ethanol/acetic acid = 3:1, v/v) as a fixative for more than 24 h. The samples were dehydrated with gradient concentrations of ethanol (70%, 85%, 95%, 100%). Subsequently, the blocks were immersed in paraffin, embedded, sectioned with a slicer RM2016 (Leica, Wetzlar, Germany), and stained using the method proposed by Marinov et al. [33]. After the slices were dried naturally, they were observed and photographed under an optical microscope (NIKON Eclipse E100, Nikon, Tokyo, Japan) with 20-fold magnification. Three biological replicates were performed on two pepper varieties at each fruit development stage. ImageJ software (https://imagej.net/ij/, NIH, Bethesda, ML, USA) was used to calculate the length, area, and number of cells per unit area with six replicates.
2.3. Hormone Quantification
The freeze-dried pericarp was ground into a powder using a mixer mill (MM 400, Retsch, Haan, Germany) for 1 min at 30 Hz. Fifty milligrams of powder was weighed and placed in a 2-mL plastic tube. Fruit samples were extracted with 1 mL of a mixed solution of methanol, water, and formic acid (15:4:1, v/v/v), followed by the addition of 10 μL of an internal standard mixed solution (100 ng/mL). The mixture was vortexed for 10 min and then centrifuged for 5 min (12,000 rpm, 4 °C). The supernatant was transferred to a clean centrifuge tube, followed by evaporation to dryness. Finally, the remaining solids were re-dissolved in 100 μL of 80% methanol (v/v) and filtered through a 0.22-μm membrane. The plant hormones were extracted and analyzed using the liquid chromatography–tandem mass spectrometry method previously described [34].
2.4. RNA Sequencing Analysis
Total RNA from the frozen peel was isolated using the Magen kit (Magen, Guangzhou, China) according to the manufacturer’s instructions. The extracted RNA was subjected to electrophoresis on 1% denaturing agarose gels to analyze its integrity and determine the presence of DNA contamination. An Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) was used to accurately determine RNA integrity and the total amount of RNA extracted. The qualified RNA was used to construct a sequencing library, which was sequenced on an Illumina HiseqTM 2500 platform (Illumina, San Diego, CA, USA). After sequencing, the adapters and low-quality sequences were removed by Fastp software (HaploX, Shenzhen, China), and the clean reads were mapped to the pepper (‘Zunla’) reference genome using HISAT2 software (JHU, Washington, DC, USA) [35,36]. Subsequently, the expected number of fragments per kilobase of transcript sequence per million base pairs sequenced (FPKM) value of each gene was calculated to determine the expression level of corresponding genes. Finally, differential expressions among the sample groups were analyzed. Genes satisfying the criteria of |log2 fold change| ≥ 1 and false discovery rate < 0.05 were defined as differentially expressed genes (DEGs), which were subjected to Gene Ontology (GO) enrichment analysis [37].
2.5. Integrative Phytohormone and Transcriptome Analysis
To analyze the network of phytohormones regulating pepper fruit size, the Pearson correlation coefficients (PCCs) between hormone and transcriptome data were calculated by the cited method [1]. Previous studies have shown that physiological processes related to hormone signal transduction, cell division, cell wall structure formation, and photosynthesis play key roles in fruit size determination [21,38]. Therefore, we focused on the PCCs for DEGs related to hormone signal transduction, cell division, cell wall structure, and photosynthesis with hormones. To accurately obtain the key genes regulating the fruit size of pepper, DEGs with an FPKM value < 10 in all samples were filtered out. |PCC values| > 0.80 were selected, and the relationships between the DEGs and hormones were visualized using Cytoscape software (version 3.7.1, Cytoscape Team).
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA)
To improve the accuracy of network construction, genes with low expression levels in all samples were removed. Referring to previous methods, the WGCNA package was used for co-expression network analysis with default parameters [39,40]. Finally, the co-expression network was visualized using Cytoscape software.
2.7. RT-qPCR Analysis
Nine genes related to fruit size were selected for RT-qPCR analysis, with the pepper β-actin gene used as the reference gene to normalize gene expression levels. Gene-specific primers for qPCR were identified by Primer 5 software, which are listed in Supplementary Table S1. The total RNA was isolated from the peels of the QB6 and CXJ82 pepper varieties according to the Magen kit (Majorbio, Guangzhou, China) instructions. The purified RNA was reverse-transcribed into first-strand cDNA using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). The synthesized cDNA was then used as a template for qPCR validation using the Real Master Mix (SYBR Green) kit (Vazyme, China). The RT-qPCR method was referenced by the cited method [34]. Three replicates were performed for each sample. The relative expression levels of target genes were calculated following the 2−ΔΔCT method.
3. Results
3.1. Physiology and Tissue Structure of Pepper Fruit
The single-fruit weight of QB6 pepper was 5.49, 8.33, and 9.13 times higher than that of CXJ82 pepper at the three fruit development stages (10, 20, and 30 DAA), respectively. Moreover, the longitudinal and transverse diameters of QB6 fruit were significantly higher than those of CXJ82; however, there was no significant difference in flesh thickness between the two varieties (Figure 1A,B). Histological analysis of the peel showed no significant difference in the area of fruit cells between the two varieties at the early stage of fruit development; however, the area of fruit cells in QB6 was significantly higher than that in CXJ82 at 30 DAA. Furthermore, the area of the first, sixth, and ninth layers of cells under the epidermis of QB6 fruit was, respectively, 2.55, 1.64, and 2.77 times the corresponding values for CXJ82 at 30 DAA. Similarly, the length of QB6 fruit cells was significantly higher than that of CXJ82 at 30 DAA (Figure 2A,B). The cell number per unit area in the pericarp of the two pepper varieties changed greatly during fruit development. The cell number in the pericarp of QB6 was significantly higher than that of CXJ82 at 10 DAA, whereas the CXJ82 pericarp showed a higher number of cells than found in QB6 at 30 DAA (Figure 2C). In summary, the change in cell number and cell area during fruit development may be the major contributor to the difference in fruit size between the two pepper varieties.
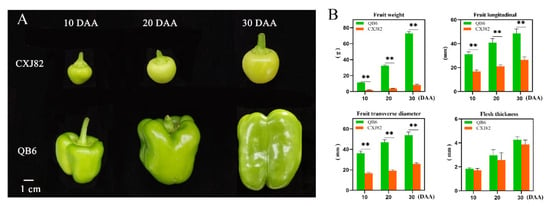
Figure 1.
Phenotype and agricultural traits of two pepper varieties. (A) Phenotypes of fruits of two pepper varieties at 10, 20, and 30 days after anthesis (DAA). (B) The fruit weight, fruit longitudinal, fruit transverse diameter, and flesh thickness of the two pepper varieties at each developmental stage. **, p < 0.01.
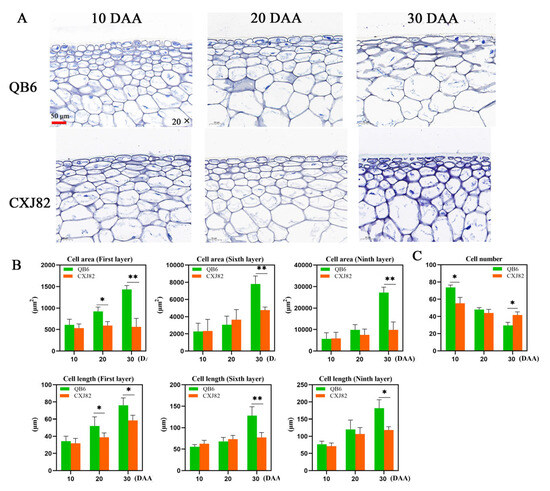
Figure 2.
Anatomical structure and cell size of QB6 and CXJ82 pepper fruits. (A) Histological cross-section images of fruit pericarp. (B) The area and length of the first, sixth and ninth layers of cells under the fruit epidermis. (C) The number of cells per unit area (0.1 mm2) of QB6 and CXJ82 fruits. *, p < 0.05; **, p < 0.01.
3.2. Overview of Transcriptomic Data
A total of 18 libraries were constructed, yielding 142.29 Gb (6.90–9.02 Gb per sample) of clean reads. These clean reads were mapped to the reference pepper genome with match ratios in the range of 94.16–96.35% (Table S2). A total of 39,885 genes (including 11,797 novel genes) predicted from the genome were found to be expressed in at least one sample (with FPKM > 0) (Table S3). To further screen the genes related to fruit size, differential expression analysis was performed. Overall, 3672 (1822 upregulated and 1850 downregulated), 4224 (2054 upregulated and 2170 downregulated), and 3796 (1883 upregulated and 1913 downregulated) DEGs were detected between QB6 and CXJ82 at 10, 20, and 30 DAA, respectively (Figure S1A). After eliminating redundancy, a total of 6484 genes differentially expressed in at least one developmental stage were identified between the two varieties, 1936 of which were differentially expressed at all three stages of fruit development (Figure S1B).
To confirm the accuracy of DEG analysis, nine genes related to cell cycle, photosynthesis, cell wall polysaccharide metabolism, and hormone signal transduction were selected for RT-qPCR analysis. The results showed that the expression patterns of the nine genes were consistent with the RNA-seq results. For instance, the expression levels of auxin response protein 12 (IAA12, Capana06g001308) and gibberellin receptor GID6 (Capana02g001365) genes in the two varieties gradually increased during fruit development. Furthermore, their expression levels in CXJ82 fruit were higher than those in QB6 fruit, which was consistent with the transcriptome data (Figure S2). Similar results from RT-qPCR and RNA-seq analyses indicated that the RNA-seq data were reproducible and reliable.
3.3. Functional Enrichment Analysis of DEGs
The DEGs were widely distributed in the three main GO functional groups of biological processes, molecular functions, and cellular components. In the biological process group, the DEGs were mainly enriched in “plant-type cell wall organization or biogenesis (GO:0071669),” “cellular response to auxin stimulus (GO:0071365),” “secondary metabolite biosynthetic process (GO:0044550),” “auxin-activated signaling pathway (GO:0009734),” “cellular polysaccharide metabolic process (GO:0044264),” and “regulation of hormone levels (GO:0010817)” categories (Figure 3A and Figure S3). The DEGs were mainly enriched in the cellular component categories “microtubule cytoskeleton (GO:0015630),” “photosystem (GO:0009521),” “extracellular space (GO:0005615),” “plant-type cell wall (GO:0009505),” and “cell wall (GO:0005618)” (Figure 3A and Figure S3). The DEGs associated with the “aspartic-type endopeptidase activity (GO:0004190),” “aspartic-type peptidase activity (GO:0070001),” “DNA polymerase activity (GO:0034061),” “RNA-directed DNA polymerase activity (GO:0003964),” “endoribonuclease activity (GO:0004521),” and “glucosyltransferase activity (GO:0046527)” categories were dominant in the molecular function group (Figure 3A and Figure S3). A large number of DEGs were enriched in categories related to plant hormones, the cell wall, the photosynthetic system, and basal metabolic enzymes, suggesting that physiological processes such as hormone signal transduction, cell wall formation, photosynthesis, and genetic material replication/synthesis are closely related to the size or weight of pepper fruit.
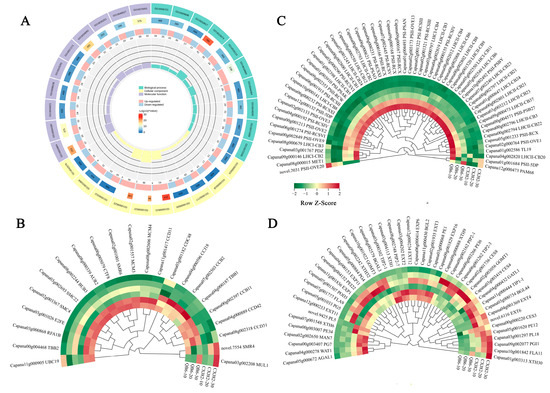
Figure 3.
GO enrichment analysis and expression profile of differentially expressed genes (DEGs). (A) GO analysis of DEGs between QB6 and CXJ82 varieties at 10 DAA. The heat maps of DEGs related to cell division (B), photosynthesis, (C) and cell wall polysaccharide metabolism (D).
3.4. DEGs Related to Fruit Size
To accurately determine the genes related to pepper fruit size, the expression profiles of the identified DEGs were further analyzed. In addition to cyclin-dependent protein kinase inhibitor (novel.7554) and E3 ubiquitin-protein ligase (Capana03g002208) genes, the transcription levels of 20 other DEGs in the GO cell division category, including cyclin, cytoskeletal tubulin, DNA replication licensing factor, structural maintenance of chromosomes protein, and E2F transcription factor genes, were significantly higher in QB6 fruit than in CXJ82 fruit, especially at 10 and 20 DAA (Figure 3B). The transcription abundance of the cyclin-B1-1 gene (Capana00g002397) in QB6 fruit was 29.38, 32.47, and 28.82 times greater than that of CXJ82 at the three developmental stages, respectively (Figure 3B). Moreover, 57 DEGs related to photosynthesis were detected, which were mainly annotated as photosystem reaction center subunit (15 members) and light-harvesting complex chlorophyll a/b binding protein (25 members). The expression pattern of photosynthesis-related genes was similar to that of genes in the cell division category. That is, photosynthesis-related genes at the three fruit development stages exhibited higher transcription levels in QB6 fruits than in CXJ82 fruits (Figure 3C).
Forty-six DEGs were found to be involved in cell wall metabolism. Some genes related to cell wall polysaccharide degradation, including xyloglucan endotransglucosylase/hydrolase (XET/XTH, Capana03g004113, Capana07g001548), mannan endo-1,4-beta-mannosidase 7 (MAN7, Capana02g002650), pectinesterase (PE, Capana03g000068, Capana00g003007), and polygalacturonase 7 (PG7, Capana00g003407), were highly expressed in the QB6 variety, especially at the 10 DAA and 20 DAA stages (Figure 3D). However, the corresponding polygalacturonase inhibitor gene (Capana09g002077) exhibited higher transcriptional abundance in CXJ82 fruit (Figure 3D). In addition, some genes involved in the synthesis and modification of cell wall polysaccharides were also highly expressed in CXJ82 fruits, such as cellulose synthase (Capana02g001736, Capana00g000220, and Capana03g003419), glucuronoxylan 4-O-methyltransferase (Capana02g003056, Capana10g001922), galacturonosyltransferase (Capana04g000432), and peroxidase (Capana07g001507) (Figure 3D). EXP and extensin (EXT) are two important structural proteins in the plant cell wall. EXP mainly promotes relaxation of the cell wall, whereas EXT enhances the stability of the cell wall cross-linking network through glycosylation modification and maintains the dynamic balance of the cell wall with EXP [13,41]. Four differentially expressed EXP genes and six differentially expressed EXT genes were identified between the two cultivars. Among these, the expression levels of EXP6 (Capana06g000414) and EXP16 (Capana09g001829) in QB6 were significantly higher than those in CXJ82, whereas EXT4 (Capana04g001369) and EXT6 (novel.6116) were only expressed in CXJ82 fruits (Figure 3D).
Transcription factors are a class of proteins that regulate gene expression and play an important role in plant growth and development. A total of 277 differentially expressed transcription factors were detected between the two pepper varieties. Among them, B3, MYB, AP2/ERF, basic helix-loop-helix (bHLH), C2C2, HD-ZIP, and LOB were the most highly enriched transcription factor families, with 28, 22, 21, 19, 12, 12, and 12 DEGs, respectively (Table S4). Further expression analysis showed that the transcription factor genes OFP20 (Capana10g001230), MYC30 (Capana02g001781), HD-ZIP5 (Capana03g001081), HD-ZIP6 (Capana12g002675), MBF1 (Capana00g004275), and TGA7 (Capana04g001165) were highly expressed in CXJ82 fruit (Figure S4 and Table S4). However, three members of the bHLH family (Capana00g001573, Capana03g002644, and Capana02g002899), along with one HD-ZIP4 (Capana06g001620) and one NFYC2 (Capana11g000869) gene, exhibited relatively higher transcriptional abundance in QB6 fruit (Figure S4 and Table S4).
3.5. Changes in Auxin and Cytokinin Contents and Expression Profiles of Their Related Genes
Twelve auxins or their derivatives were quantitatively analyzed. Among these, L-tryptophan, 3-indole acetamide, indole, and 3-indoleacetonitrile highly accumulated in QB6 fruit, whereas indole-3-acetic acid (IAA), methyl indole-3-acetate, indole-3-carboxylic acid, N-(3-indolylacetyl)-L-alanine, and indole-3-carboxaldehyde exhibited higher contents in CXJ82 fruit (Figure 4A). Since plant hormones often regulate plant growth and development through downstream signaling pathways, the expression patterns of genes in the auxin signal transduction pathway were also analyzed. Sixteen DEGs were enriched in the auxin signal transduction pathway. The upstream genes in the auxin signaling pathway, such as transport inhibitor response 1 (TIR1, Capana06g003085), auxin-responsive protein IAA (Capana03g003343, Capana06g001308, and Capana06g001465), and auxin response factor (ARF, Capana09g001199, Capana00g004126, and novel.5712), were more highly expressed in CXJ82 fruit (Figure 4B). However, the downstream genes in the auxin signaling pathway, including Gretchen Hagen 3 (GH3, Capana10g000405) and small auxin-up RNA protein (SAUR, Capana08g002650, Capana08g001103, Capana08g002648, and Capana01g000511), exhibited higher expression levels in QB6 fruit (Figure 4B). Moreover, some genes related to auxin synthesis were also differentially expressed between the two varieties, such as indole-3-acetamide amidohydrolase (amiE, Capana11g000470, Capana08g002686, Capana08g002687, and Capana00g000045), tryptophan 2-monooxygenase (iaaM, Capana09g001148), and nitrilase (NIT, Capana00g000314, and Capana00g000549) genes (Figure 4A). The large differences in auxin content and expression patterns of genes in auxin signal transduction pathways between the two varieties suggest that auxin plays an important role in regulating pepper fruit size.
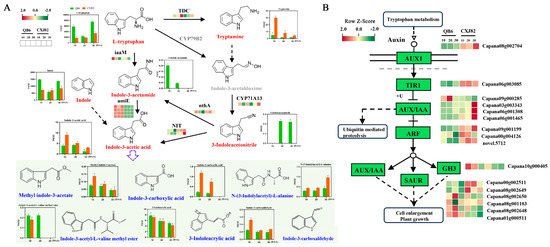
Figure 4.
Changes in auxin contents and expression profile of genes related to auxin synthesis and signal transduction. (A) Auxin levels and heat map of auxin synthesis genes. (B) Comparative analyses of transcription levels in auxin signal-related genes. iaaM, tryptophan 2-monooxygenase; amiE, indole-3-acetamide amidohydrolase; TDC, L-tryptophan decarboxylase; CYP71A13, indoleacetaldoxime dehydratase; nthA, nitrile hydratase subunit alpha; NIT, nitrilase; AUX1, auxin influx carrier; TIR1, transport inhibitor response 1, IAA, auxin-induced protein; ARF, auxin response factor; GH3, Gretchen Hagen 3; SAUR, small auxin-up RNA protein.
Cytokinins can directly participate in plant growth and development by promoting cytokinesis. Accordingly, we performed quantitative analysis of 14 cytokinins or their derivatives. With the development of fruits, the contents of N6-isopentenyladenine, trans-zeatin, cis-zeatin, and dihydrozeatin were significantly different between the two varieties. The contents of trans-zeatin, cis-zeatin, and dihydrozeatin in CXJ82 were significantly higher than those in QB6 at both 10 and 20 DAA (Figure 5A). Compared with those in QB6, the contents of trans-zeatin and cis-zeatin in CXJ82 fruit, respectively, increased by 38.02% and 29.19% at 10 DAA and by 8.63% and 39.20% at 20 DAA. In addition, some cytokinin derivatives such as cis-zeatin riboside, N6-benzyladenine-7-glucoside, and N-6-iso-pentenyladenosine-5′-monophosphate showed higher accumulation in CXJ82 fruit than in QB6 (Figure 5A). Subsequently, we analyzed the expression patterns of genes in the cytokinin metabolic pathway and the cytokinin signal transduction pathway. The expression levels of adenosine phosphate-isopentenyltransferase (IPT, Capana01g002558), cytokinin trans-hydroxylase (CTH, Capana02g001818), and two-component response regulator ARR-B family (Capana07g000238) genes were higher in CXJ82 than those in QB6 fruits at all three fruit development stages (Figure 5B). Furthermore, seven differentially expressed zeatin O-glucosyltransferase (ZOGT) genes were identified, including three ZOGT genes (Capana11g000203, novel.11134, and Capana05g000788) with higher transcriptional abundance in CXJ82 fruit (Figure 5B).
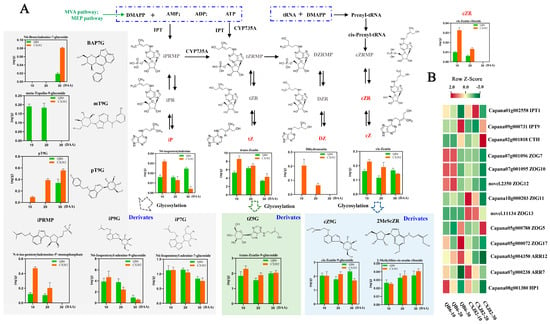
Figure 5.
Changes in cytokinin contents, and expression profile of genes related to cytokinin synthesis and signal transduction. (A) The content of cytokinin in two pepper varieties during fruit development. (B) The heat maps of genes related to cytokinin synthesis and signal transduction. cZR, cis-zeatin riboside; iP, N6-isopentenyladenine; tZ, trans- zeatin; DZ, dihydrozeatin; cZ, cis-zeatin; pT9G, 4-[[(9-beta-D-Glucopyranosyl-9H-purin-6-yl) amino] methyl] phenol.
3.6. Changes in the Contents of GA and Other Hormones and Expression Profiles of Their Related Genes
The content of GA3 in QB6 was significantly higher than that in CXJ82 at the three fruit development stages, which increased by 43.49%, 37.73%, and 76.63% at 10, 20, and 30 DAA, respectively. However, GA19 exhibited higher accumulation in CXJ82 fruit during fruit development (Figure 6A). Ten genes involved in GA synthesis and its signal transduction pathway were differentially expressed. Among these, the phytochrome-interacting factor (PIF, Capana01g003548, and Capana03g000296), DELLA protein (Capana08g001582), and gibberellin receptor 1 (GID1, Capana01g002745) genes were more highly expressed in QB6, whereas three GID genes (Capana02g001365, Capana05g001932, Capana01g002747) were more highly expressed in CXJ82 (Figure 6B).

Figure 6.
Changes in gibberellin contents and expression profile of genes related to gibberellin synthesis and signal transduction. (A) The content of gibberellin in two pepper varieties during fruit development. (B) The heat maps of genes related to gibberellin synthesis and signal transduction. *, p < 0.05; **, p < 0.01.
The contents of jasmonic acid, cis (+)-12-oxophytodienoic acid, and 3-oxo-2-(2-(Z) -pentenyl) cyclopentane-1-hexanoic acid in QB6 were significantly lower than those in CXJ82 during fruit development (Figure S5A). Three genes in the jasmonate signal transduction pathway, including jasmonate ZIM domain-containing protein (Capana03g000749, Capana07g000750, and Capana03g000117) genes, and MYC transcription factor genes (Capana02g001781, Capana02g002899, and Capana03g002644) were differentially expressed; among these, the transcription level of MYC30 (Capana02g001781) in CXJ82 fruit was significantly higher than that of QB6 (Figure S5B). Moreover, the content of abscisic acid in CXJ82 fruit was higher than that in QB6 fruit at the early stage of fruit development, but there were no significant differences in the contents of salicylic acid or its derivatives between the two varieties (Figure S6A and Figure S7A). Notably, some DEGs involved in abscisic acid and salicylic acid signal transduction were also identified, including TGACG sequence-specific DNA-binding protein (TGA, Capana09g000329, Capana05g000330, and Capana04g001165) and abscisic acid receptor PYR/PYL family (Capana05g002062, Capana06g001547) genes (Figure S6B and Figure S7B).
3.7. Transcriptome and Hormone Correlation Analysis to Determine the Key Genes Regulating Fruit Size
The differential accumulation of auxin, cytokinin, GA, jasmonic acid, or their derivatives between the two varieties with different fruit sizes suggests that these hormones play an important role in the development of pepper fruit. To further determine the molecular mechanism by which these hormones regulate pepper fruit size, we performed correlation analyses between the quantitative changes in hormones, DEGs in hormone signal transduction pathways, and DEGs related to fruit size in the QB6 and CXJ82 pepper varieties. The 29 hormones or derivatives analyzed showed strong correlations with 146 DEGs (|PCC| > 0.8), including 57 photosynthesis-, 44 cell wall-, 26 signal transduction-, and 19 cell division-related genes (Figure 7). Among the 29 hormone derivatives, IAA, gibberellin A19, trans-zeatin, meta-topolin-9-glucoside, 3-indole acetamide, and jasmonic acid showed high correlations with more than 15 individual genes (Figure 7), suggesting that auxin, cytokinin, GA, and jasmonic acid may regulate pepper fruit size by affecting the expression of cell wall-, cell proliferation-, and photosynthesis-related genes.

Figure 7.
The correlation networks of phytohormone (auxin, cytokinin, gibberellin and jasmonic acid) and differentially expressed genes associated with fruit size. Triangle, phytohormone; Rhombus, hormone signal transduction factor; Light green ellipse, photosynthesis-related genes; Pink ellipse, cell wall polysaccharide metabolism-related genes; Purple blue ellipse, cell division-related gene.
Plant hormones activate downstream signaling pathways by binding to cell-surface receptors, thereby regulating plant growth and development. In the hormone–signal transduction factor–gene interaction network, 30 hormone signal transduction factors showed high correlations with 121 fruit size-related genes (Figure 7). Among them, the signal transduction factors MYC93 (Capana02g002899), MYC30, GID1, GID13 (Capana01g002747), GID6 (Capana02g001365), GID5 (Capana05g001932), GH3, JAZ6 (Capana03g000749), TIR1, ARF7 (Capana09g001199), SAUR36 (Capana01g000511), and IAA27 (Capana09g000285) showed high correlations with more than 35 fruit size-related genes (101 non-redundant genes), including 25 light-harvesting complex I/II chlorophyll a/b-binding protein, 16 photosystem I/II reaction center subunit, 6 photosystem II oxygen-evolving enhancer protein, 6 cyclins (Capana11g001417, Capana04g000889, Capana00g002397, Capana08g002318, Capana07g002503, and Capana04g000596), 4 EXT (Capana03g004202, Capana04g001369, novel.6116, and Capana02g001935), and 3 pectinesterase (Capana00g003007, Capana05g001620, and Capana03g000068) genes (Figure 7). In summary, MYC, GID, SAUR36, ARF7, and IAA27 are identified as hub genes (highly connected genes) in the hormone–gene interaction network. These genes may affect physiological processes such as cell wall structure formation, cell division, and photosynthesis by mediating hormone signal transduction, thereby regulating pepper fruit size/weight.
3.8. WGCNA
WGCNA was performed to screen the co-expression gene modules related to pepper fruit size. A total of 27,919 genes were divided into 18 co-expression modules, with the number of genes in each module ranging from 59 to 8589 (Figure S8). Among them, genes in the blue (4006 genes) and brown (3188 genes) modules were highly expressed in QB6 fruit, while genes in the black (1890) and green (2822) modules exhibited higher transcriptional abundance in CXJ82 fruit (Figure 8C,D and Figure 9C,D). To further obtain the core genes regulating pepper fruit size, we used Cytoscape software to construct the interaction network of genes in the modules. In the blue module, 11 highly expressed transcription factors (FPKM > 40) were identified, including NFYA7 (Capana02g001381), ERF92 (Capana05g001701), HD-ZIP4, ethylene-insensitive like protein (EIL, Capana08g001175), and MADS-MIKC (Capana10g000710), which showed high correlations with 34 nucleotide metabolism-, 24 amino acid/protein metabolism-, 14 ribosome assembly-, 10 cell wall structure-, 4 cell cycle-, and 3 aquaporin-related genes (Figure 8A). The transcription factors bHLH137 (Capana11g001506), IAA16 (Capana08g001238), NFYC1 (Capana06g000871), B3 (Capana08g002375), TCP21 (Capana03g001891), and GATA4 (Capana10g002303) emerged as key nodes in the interaction network of the brown module, and these genes exhibited the highest correlation with genes related to fruit size (Figure 8B). The interaction network of the green module was composed of 8 transcription factors [MYC30, TGA7, OFP20, HD-ZIP6, MADS23 (Capana12g000846), LUG (Capana06g000177), WRKY22 (Capana04g000568), and SAUR50 (Capana08g002649)], 10 signal transduction-, 6 ribosome assembly-, 8 transporter-, 16 amino acid/protein metabolism-, 15 nucleic acid metabolism-, and 3 cell wall metabolism-related genes (Figure 9A). In the interaction network of the black module, 11 transcription factors, including bZIP44 (Capana04g000305), ARF5 (Capana00g004127), IAA21 (Capana03g000311), HD-ZIP13 (Capana11g001647), YABBY2 (Capana06g000658), and C2H2-9 (Capana04g001932), showed high correlations with cell wall-, ribosome assembly-, cell division-, nucleic acid metabolism-, hormone synthesis-, and amino acid/protein metabolism-related genes (Figure 9B). In summary, the expression patterns of the transcription factors in the above modules were significantly different between the two pepper varieties, showing high correlations with multiple genes related to cell wall structure, cell division, ribosome assembly, and nucleic acid metabolism. Therefore, these transcription factors are candidate gene targets for regulating pepper fruit size.

Figure 8.
The interaction network of highly expressed transcription factors in the blue (A) and brown (B) modules related to fruit weight; The expression pattern of genes in blue (C) and brown (D) modules. Rhombus, transcription factor; Purple ellipse, cell wall polysaccharide metabolism-related genes; Green ellipse, ribosome-related genes; Yellow ellipse, cell division-related genes; Cyan ellipse, nucleic acid metabolism-related genes; Purple blue ellipse, amino acid/protein metabolism-related genes; Light pink ellipse, cell structure-related genes.

Figure 9.
The interaction network of highly expressed transcription factors in the green (A) and black (B) modules related to fruit weight; The expression pattern of genes in green (C) and black (D) modules. Purple ellipse, cell wall polysaccharide metabolism-related genes; Cyan ellipse, nucleic acid metabolism-related genes; Purple blue ellipse, amino acid/protein metabolism-related genes; Green ellipse, ribosome-related genes; Orange ellipse, hormone-related genes; Sky blue ellipse, transporter genes; Yellow ellipse, cell division-related genes.
4. Discussion
Fruit development is regulated by cell division and cell proliferation, which are important factors affecting fruit size [38]. In this study, the cell division of two pepper varieties with different fruit sizes was found to be relatively vigorous at the early stage of fruit development; however, with the extension of fruit development time, the number of cells per unit area of fruits gradually decreased in both varieties. Moreover, the number of cells per unit area of QB6 fruit was significantly lower than that of CXJ82 at 30 DDA. Combined with the higher single-fruit weight and cell area of QB6, we speculate that cell division in the early stage of fruit development mainly provides the material basis/cell number for fruit enlargement, while cell enlargement is the key determinant of the size of pepper fruit.
Cell division and cell proliferation are mainly regulated by cyclins and cyclin-dependent kinases, which phosphorylate excessive substrates by interacting to ensure orderly cell cycle progression [42]. Two cyclins, c54419.graph_c0 and c50340.graph _c0, were reported to be highly expressed in large-fruited bottle gourd and were positively correlated with fruit weight [42]. In this study, six differentially expressed cyclins (Capana00g002397, Capana11g001417, Capana08g002318, Capana04g000889, Capana07g002503, Capana04g000596) were identified, all of which were more highly expressed in the large-fruit variety. Moreover, 14 DEGs enriched in the cell division category, including DNA replication licensing factor (Capana08g002606), E2F transcription factor (Capana03g001026), structural maintenance of chromosomes protein (Capana03g002693, Capana02g003367), and tubulin beta chain (Capana04g000187, Capana00g004468) genes, showed higher transcription levels in QB6. Therefore, the high expression of these cell division-related genes promoted the division of cells in the early stage of fruit development, which ensured the material basis/cell number for the subsequent expansion of QB6 fruit.
The modification of cell wall polysaccharide components is one of the most important factors for reducing the rigidity of the cell wall, improving the ductility and elongation of cells. Xyloglucan endotransglucosylase/hydrolase (XET/XTH) is a bifunctional enzyme that participates in the expansion and remodeling of cell walls, regulating the relaxation of cell walls by hydrolyzing and reconnecting xyloglucan to polysaccharides such as cellulose and hemicellulose [43]. Recently, it was found that two members of the XTH family, DkXTH6 and DkXTH7, showed opposite expression patterns in persimmon fruits, accompanied by functional differences during fruit softening [44]. Consistent with these previous findings, we identified five XTH genes that were differentially expressed between the two pepper varieties. Among them, XTH6 (Capana07g001548) and XTH1 (Capana03g004113) were more highly expressed in QB6, whereas XTH32 (Capana09g002242), XTH30 (Capana01g003313), and XTH9 (Capana09g000688) exhibited higher transcriptional abundance in CXJ82. We speculate that these five XTH genes may have functional differentiation: XTH6 and XTH1 are mainly involved in cell wall remodeling, whereas XTH32, XTH30, and XTH9 play important roles in cell wall assembly; however, this hypothesis needs further verification.
EXP and EXT are two major modifier proteins in the plant cell wall. EXP can enhance the ductility of the cell wall by destroying the non-covalent bonds between the cell wall polysaccharide components, while EXT enhances the stability of the cell wall cross-linking network through glycosylation modification [13,41]. A previous study identified four EXP genes that were highly expressed in large-fruited bottle gourd during fruit development [42]. Furthermore, previous studies have shown that reducing the expression level of EXP genes can lead to a decrease in the volume of pepper peel cells along with a more orderly and compact arrangement of cells [13]. The insertion mutation in the coding region of the extensin gene was identified to be one of the main reasons for the difference in fruit size of pepper [45]. In this study, EXP6 and EXP16 were highly expressed in QB6 fruit, while EXT4 and EXT6 were only expressed in CXJ82 fruit. Moreover, some genes related to cell wall polysaccharide degradation, such as PE1 (Capana03g000068), PG7, and MAN7, also showed higher transcriptional abundance in QB6 than in CXJ82. The high expression of cell wall polysaccharide metabolism-related genes such as PE, PG, MAN, and EXP in QB6 fruit might promote the hydrolysis of cell wall polysaccharide substances, soften the cell wall (i.e., ductility enhancement), and contribute to cell elongation and expansion. This finding is consistent with the observations of tissue sections, indicating that the flesh cells of QB6 were longer and larger than those of CXJ82.
Auxin and cytokinin are key hormones regulating fruit size by regulating cell division and cell expansion [21]. The increase in auxin content after fruit setting leads to the acceleration of cell division in young cucumber fruit [46]. In this study, the contents of auxin and cytokinin in the fruits of the two pepper varieties were high at the early stage of fruit development and then gradually decreased with the progression of fruit development. Consistent with these changes, the number of cells per unit area decreased gradually during the fruit development of the two pepper varieties, indicating that the high contents of auxin and cytokinin in the early stage of fruit development promoted the division of pepper fruit cells. Notably, the contents of auxin and cytokinin in CXJ82 fruit were higher than those in QB6 at all three fruit development stages, and the number of cells per unit area in CXJ82 fruit was significantly higher than that in QB6 at 30 DAA. However, the single-fruit weight of CXJ82 was lower than that of QB6 throughout the fruit development period. These results suggest that the main function of auxin and cytokinin might be in promoting the division of cells, providing the material basis/cell number for fruit enlargement, whereas the regulation of cell enlargement is dominated by other hormones. Moreover, the continuous high content of auxin in a fixed cell life cycle may lead to the prolongation of cell division time (continuous division) of CXJ82 peel cells, which in turn compresses the time of the cell expansion process and is not conducive to cell expansion, contributing to the smaller fruit of CXJ82.
GA is a diterpene carboxylic acid organic compound. More than 130 GAs have been identified to date, among which GA1, GA3, GA4, and GA7 exhibit biological activity and can regulate plant growth and development [47]. A high content of GA promotes the division and expansion of tomato fruit cells [21]. Exogenous GA treatment led to the longitudinal elongation of woodland strawberry fruit and ultimately increased the fruit weight [22]. In this study, the content of GA3 in QB6 fruit was significantly higher than that in CXJ82 at the three fruit development stages, suggesting that a high content of GA3 may be the main factor promoting the expansion of QB6 fruit cells, leading to the large-fruit phenotype of QB6 pepper. Furthermore, the significant down-regulation of auxin and cytokinin, along with the high accumulation of GA3 in QB6 fruit, strongly suggests that auxin, cytokinin, and GA might synergistically regulate physiological processes such as cell proliferation and cell expansion, thereby controlling pepper fruit size.
Auxin mainly regulates fruit growth and development through downstream signaling pathways, in which the ARF and Aux/IAA transcription factors play a key role. ARF can directly bind to the TGTCTC cis-element in the auxin-responsive gene promoter to regulate gene expression [48]. In the early stage of fruit development, the transcription factor SlARF9 regulates tomato fruit size by negatively controlling cell division. The SlARF9-overexpression lines formed smaller fruits, while the fruits of the SlARF9-silenced lines showed the opposite phenotype [19]. Another study showed that BnaA9.ARF18 negatively regulates fruit and seed size by limiting the elongation of silique wall cells [49]. Four ARF transcription factors (ARF6-like, ARF8-like, ARF11, and ARF19-like) were down-regulated during pepper fruit elongation [50]. Moreover, ARF106 was strongly linked to a QTL for fruit weight and was identified as a potential gene regulating apple size [51]. In this study, three differentially expressed ARF transcription factors, ARF5, ARF7, and ARF8, were identified and highly expressed in CXJ82 fruit. These three ARF genes were in the key nodes of the hormone–signal transduction factor–gene regulatory network, indicating that these transcription factors may be involved in the regulation of pepper fruit size. IAA protein is an important inhibitor in the auxin signal transduction process, which can bind to ARF to form a dimer, thereby inhibiting the activity of the transcription factor [17]. SlIAA17 negatively regulates cell expansion, and SlIAA17-silenced tomato lines exhibit larger fruits and thicker pericarp tissues [52]. In this study, four differentially expressed IAA genes were identified, including IAA22 (Capana03g003343), IAA12 (Capana06g001308), and IAA29 (Capana06g001465), with higher transcriptional abundance in CXJ82; these genes also exhibited high correlations with genes related to cell wall metabolism. Combining the observations of tissue sections, hormone measurements, and previous research results, we speculate that IAA22, IAA12, and IAA29 might negatively regulate the expression of genes related to cell wall polysaccharide metabolism by interacting with ARF transcription factors, thereby inhibiting the fruit enlargement process of pepper.
DELLA is a subfamily of the GRAS transcription factor gene family, which forms the GA signal transduction center and negatively regulates the GA signal transduction process [25]. In Arabidopsis, knockout of five DELLA genes (GAI, RGA, RGL1–RGL3) resulted in reduced plant fertility, seed number per silique, and silique length [53]. Decreasing the expression level of GRAS2 resulted in insufficient active GA levels, smaller ovary cells, and lower fruit weight in tomato [54]. GA-insensitive receptors can bind to GA and then regulate the activity of DELLA protein through the SCF complex (SKP1–CUL1–F-box) [25]. The combined mutants of three GA receptor genes (GID1A, GID1B, and GID1C) showed GA-insensitive phenotypes, including semi-dwarfing, short siliques, reduced male fertility, and reduced seed number per silique in Arabidopsis [55,56]. In this study, nine DEGs in the GA signal transduction pathway were identified, including five GA receptor genes [GID1, GID5, GID6, GID13, and GID9 (Capana01g001719)] and two DELLA genes (Capana08g001582, Capana07g001537). Moreover, five GID genes and DELLA32 (Capana07g001537) showed high correlations with multiple genes related to the cell cycle, cell wall metabolism, and photosynthesis, and these genes were at the key nodes of the hormone–signal transduction factor–gene regulatory network. These findings suggest that GA signal transduction genes play an important role in regulating pepper fruit size.
bHLH is one of the most important transcription factors in plants, playing key roles in many physiological processes. An insertion mutation of the ABORTED MICROSPORES (AMS, bHLH family) gene resulted in male sterility of pollen and abnormal development of siliques in Arabidopsis [57]. Both IND and ALC belong to the bHLH family, and these genes have been reported to be involved in regulating fruit development and fruit cracking in Arabidopsis [58]. In this study, 19 differentially expressed bHLH transcription factors were identified. Among them, bHLH105 (Capana00g001573), bHLH51 (Capana03g002644), and bHLH93 (Capana02g002899) showed higher transcriptional abundance in QB6 throughout fruit development, whereas MYC30 was more highly expressed in CXJ82. Moreover, MYC30 expression showed a strong correlation with the expression levels of multiple cell wall metabolism- and cell division-related genes, and this gene was a key node in the green module regulatory network. Therefore, these bHLH transcription factors may be involved in the regulation of fruit development.
HD-ZIP is another important transcription factor regulating fruit development. In tomato, the HD-ZIP family gene VAHOX1 negatively regulates fruit ripening by affecting the expression of genes related to ethylene biosynthesis, signal transduction, and cell wall metabolism [59]. GhHB12 in the HD-ZIP family can inhibit plant growth by regulating auxin signal transduction and cell wall expansion in cotton [60]. In this study, the expression levels of HD-ZIP6, HD-ZIP5, and HD-ZIP13 in the large-fruit pepper variety were significantly higher than those in the small-fruit variety. Ovate family protein (OFP) has been reported to be involved in the regulation of fruit development in several horticultural crops. OFP20 can interact with tonneau1 recruitment motif 5 (TRM5) to form a TRM–OFP complex that regulates cell division patterns, thereby affecting the shape of tomato fruit [61]. The present study showed that the homologous gene, OFP20, of tomato was highly expressed in the small-fruit pepper variety, and this gene showed high correlations with cell wall- and cell cycle-related genes in the co-expression network of the green module. Earlier, it was found that OFP20 negatively regulated the elongation of pepper fruit [62]. Therefore, OFP20 might inhibit the elongation of pepper fruit by negatively regulating cell expansion and division, thereby affecting fruit size. The consistency of our results with those mentioned above further confirmed the reliability of the gene co-expression network constructed in this study [62]. In the next step, functional validation of transcription factors and hormone signal transduction factors in the regulatory network will be conducted, which will include gene silencing and complementation analysis, to determine their roles in regulating fruit growth and fruit size.
5. Conclusions
We used histological, endogenous hormones and transcriptomic analyses to investigate the molecular mechanisms of hormones in the mediation of pepper fruit size. Based on our results, we proposed a molecular model for the enlargement of QB6 fruit. High levels of auxin and cytokinin at the early stage of fruit development promoted the division of fruit cells in two pepper varieties. The auxin content was significantly reduced in QB6 fruit at 30 DAA, resulting in a lower number of cells per unit area, whereas a high content of gibberellin (GA3) contributed to the elongation and expansion of QB6 fruit cells. Transcriptome analysis demonstrated that genes related to cell division and photosynthesis were highly expressed in QB6 fruit, which may be the main reason for the vigorous cell division in this variety at the early stage of fruit development. Simultaneously, the high expression of cell wall polysaccharide degradation and expansion genes in QB6 fruit might promote the degradation of cell wall polysaccharide components, resulting in the weakening of cell wall rigidity, which is beneficial for the expansion of QB6 fruit cells. In the hormone–signal transduction factor–gene interaction network, a large number of hormones and signal transduction factors showed high correlations with cell wall polysaccharide metabolism- and cell division-related genes. Among them, GID6, GID1, IAA12, and SAUR36 were located at key nodes of the network, and these genes were identified as key signal transduction factors for auxin/GA to regulate cell division and expansion. Moreover, the genes in the blue, black, green, and brown co-expression modules showed strong correlations with pepper fruit size; among these, the OPF20, HD-ZIP6, and HD-ZIP13 transcription factors were identified as core genes regulating pepper fruit size. Overall, our study provides a basis for further exploring the molecular regulation mechanism of pepper fruit growth and size (Figure 10).
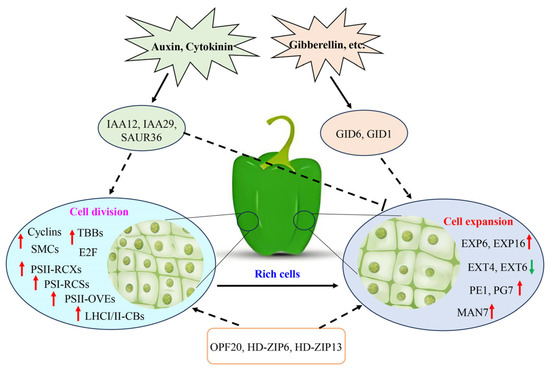
Figure 10.
Molecular model of hormone regulating pepper fruit size. Arrows beside genes indicate increases (upward) or decreases (downward) in gene expression. The dotted arrows represent speculative regulatory relationships.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11101150/s1. Figure S1: Statistics of the identified DEGs between QB6 and CXJ82 varieties. (A) The number of differentially expressed genes between QB6 and CXJ82 varieties at 10, 20, and 30 days after anthesis (DAA). (B) Venn diagrams of DEGs identified between QB6 and CXJ82 varieties at 10, 20, and 30 days after anthesis (DAA); Figure S2: Expression analysis of DEGs related to fruit size by qRT-PCR; Figure S3: GO analysis of DEGs at 20 (A) and 30 (B) DAA; Figure S4: Heatmap of differentially expressed transcription factors with high expression (FPKM > 40); Figure S5: Changes in jasmonic acid or derivatives contents, and expression profile of genes related to jasmonic acid synthesis and signal transduction. OPC-6, 3-oxo-2-(2-(Z)-pentenyl) cyclopentane-1-hexanoic acid. Figure S6: Changes in abscisic acid contents, and expression profile of genes related to abscisic acid synthesis and signal transduction; Figure S7: Changes in salicylic acid or derivatives contents, and expression profile of genes related to salicylic acid synthesis and signal transduction. MeSAG, 2-methoxycarbonylphenyl beta-D-glucopyranoside; Figure S8: The correlation analysis of different modules with fruit samples; Table S1: Primers for RT-qPCR analysis; Table S2: Statistics of transcriptome sequencing data; Table S3: Genes expressed in at least one pepper sample during fruit development (FPKM > 0); Table S4: Differentially expressed transcription factors between the two varieties.
Author Contributions
Formal analysis, S.T. and Y.L. (Xin Liu); Funding acquisition, J.Z. and Y.L.; Investigation, X.F., Q.N., X.L. (Xin Liu) and X.L. (Xingtian Long); Methodology, Z.O. and W.L.; Project administration, J.Z. and Y.L.; Resources, W.L.; Writing—original draft, S.T. and Z.O.; Writing—review and editing, J.Z. and Y.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 32302528), the Scientific Research Fund of Hunan Provincial Education Department (Grant No. 24C0868), The Foundation of Hunan Key Laboratory for Conservation and Utilization of Biological Resources in the Nanyue Mountainous Region (Grant No. 2023HSKFJJ004), and College Students’ Innovation Entrepreneurship Training Plan Program (S202510546045 and S202410546056).
Data Availability Statement
Transcriptomic data from pepper fruits is available from NCBI BioProject ID: PRJNA1308277.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Liu, Y.; Zhou, J.; Yi, C.; Chen, F.; Liu, Y.; Liao, Y.; Zhang, Z.; Liu, W.; Lv, J. Integrative analysis of non-targeted metabolome and transcriptome reveals the mechanism of volatile formation in pepper fruit. Front. Genet. 2023, 14, 1290492. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Zapata, P.J.; García-Pastor, M.E. A Comprehensive Review on Characterization of Pepper Seeds: Unveiling Potential Value and Sustainable Agrifood Applications. Foods 2025, 14, 1969. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, Q.; Mao, L.; Wu, Z.; Liu, Z.; Zou, X.; Yang, B. Comparative Transcriptome Analysis Identified Genes Associated with Fruit Size in Pepper (Capsicum annuum L.). Horticulturae 2023, 9, 1009. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Jenike, K.M.; Satterlee, J.W.; Ramakrishnan, S.; Gentile, I.; Hendelman, A.; Passalacqua, M.J.; Suresh, H.; Shohat, H.; Robitaille, G.M.; et al. Solanum pan-genetics reveals paralogues as contingencies in crop engineering. Nature 2025, 640, 135–145. [Google Scholar] [CrossRef]
- Mu, Q.; Huang, Z.; Chakrabarti, M.; Illa-Berenguer, E.; Liu, X.; Wang, Y.; Ramos, A.; van der Knaap, E. Fruit weight is controlled by Cell Size Regulator encoding a novel protein that is expressed in maturing tomato fruits. PLoS Genet. 2017, 13, e1006930. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Wang, C.; Hao, N.; Zhang, X.; Liu, M.; Wu, T. Research Progress on the Leaf Morphology, Fruit Development and Plant Architecture of the Cucumber. Plants 2022, 11, 2128. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Li, Y.; Jones, L.; McQueen-Mason, S. Expansins and cell growth. Curr. Opin. Plant Biol. 2003, 6, 603–610. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, S.Y.; Liu, G.T.; Jia, X.Y.; ul Haq, S.; Deng, Z.J.; Luo, D.X.; Li, R.; Gong, Z.H. Morphological, physiochemical, and transcriptome analysis and CaEXP4 identification during pepper (Capsicum annuum L.) fruit cracking. Sci. Hortic. 2022, 297, 110982. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, S.; Yang, W.; Li, Y.; Xia, M.; Chen, Z.; Wang, Q.; Yan, L.; Song, X.; Liu, R.; et al. Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber (Cucumis sativus L.). Sci. Rep. 2015, 5, 8031. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Carr, K.M.; Grumet, R. Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genom. 2012, 13, 518. [Google Scholar] [CrossRef] [PubMed]
- Joubès, J.; Walsh, D.; Raymond, P.; Chevalier, C. Molecular characterization of the expression of distinct classes of cyclins during the early development of tomato fruit. Planta 2000, 211, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef]
- de Jong, M.; Wolters-Arts, M.; Schimmel, B.C.; Stultiens, C.L.; de Groot, P.F.; Powers, S.J.; Tikunov, Y.M.; Bovy, A.G.; Mariani, C.; Vriezen, W.H.; et al. Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J. Exp. Bot. 2015, 66, 3405–3416. [Google Scholar] [CrossRef]
- de Jong, M.; Wolters-Arts, M.; García-Martínez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2011, 62, 617–626. [Google Scholar] [CrossRef]
- Zhao, X.; Muhammad, N.; Zhao, Z.; Yin, K.; Liu, Z.; Wang, L.; Luo, Z.; Wang, L.; Liu, M. Molecular regulation of fruit size in horticultural plants: A review. Sci. Hortic. 2021, 288, 110353. [Google Scholar] [CrossRef]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, e11542–e11550. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, X.; Peng, F.; Zhou, T.; Tian, F. Changes of CTK and Few Nitrogen Index During Development of Flower and Fruit in Zhanhua Jujube. Acta Agric. Boreali-Sin. 2007, 22, 97–100. [Google Scholar]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef]
- He, H.; Yamamuro, C. Interplays between auxin and GA signaling coordinate early fruit development. Hortic. Res. 2022, 9, uhab078. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef]
- McLeod, L.; Barchi, L.; Tumino, G.; Tripodi, P.; Salinier, J.; Gros, C.; Boyaci, Y.C.; Ozalp, R.; Borovsky, Y.; Schafleitner, R.; et al. Multi-environment association study highlights candidate genes for robust agronomic quantitative trait loci in a novel worldwide Capsicum core collection. Plant J. 2023, 116, 1508–1528. [Google Scholar] [CrossRef]
- Guan, C.; Jin, Y.; Zhang, Z.; Cao, Y.; Wu, H.; Zhou, D.; Shao, W.; Yang, C.; Ban, G.; Ma, L.; et al. Fine Mapping and Candidate Gene Analysis of Two Major Quantitative Trait Loci, qFW2.1 and qFW3.1, Controlling Fruit Weight in Pepper (Capsicum annuum). Genes 2024, 15, 1097. [Google Scholar] [CrossRef]
- Tsaballa, A.; Pasentsis, K.; Darzentas, N.; Tsaftaris, A.S. Multiple evidence for the role of an Ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 2011, 11, 46. [Google Scholar] [CrossRef]
- Mao, L.; Shen, Y.; Cui, Q.; Huang, Y.; Zhang, X.; Lv, J.; Xing, W.; Zhang, D.; Fang, N.; Chen, D.; et al. The IQ67-domain protein IQD1 regulates fruit shape through complex multiprotein interactions in pepper (Capsicum annuum L.). Plant Biotechnol. J. 2025, 23, 2651–2666. [Google Scholar] [CrossRef]
- Chunthawodtiporn, J.; Hill, T.; Stoffel, K.; Van Deynze, A. Quantitative Trait Loci Controlling Fruit Size and Other Horticultural Traits in Bell Pepper (Capsicum annuum). Plant Genome 2018, 11, 160125. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Li, Q.; Xu, N.; He, C. A lineage-specific arginine in POS1 is required for fruit size control in Physaleae (Solanaceae) via gene co-option. Plant J. 2022, 111, 183–204. [Google Scholar] [CrossRef]
- Marinov, O.; Nomberg, G.; Sarkar, S.; Arya, G.C.; Karavani, E.; Zelinger, E.; Manasherova, E.; Cohen, H. Microscopic and metabolic investigations disclose the factors that lead to skin cracking in chili-type pepper fruit varieties. Hortic. Res. 2023, 10, uhad036. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, L.; Yang, B.; Cui, Q.; Dai, Y.; Li, X.; Chen, Y.; Dai, X.; Zou, X.; Ou, L. A multi-omics approach identifies bHLH71-like as a positive regulator of yellowing leaf pepper mutants exposed to high-intensity light. Hortic. Res. 2023, 10, uhad098. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Shi, J.; Scheben, A.; Zhan, J.; Wang, X.; Liu, G.; Yan, G.; King, G.J.; Edwards, D.; Wang, H. Genetic and signalling pathways of dry fruit size: Targets for genome editing-based crop improvement. Plant Biotechnol. J. 2020, 18, 1124–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Lamport, D.T.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, J.; Zhang, M.; Huang, S.; Chen, X. Comparative Transcriptomic Analysis of Two Bottle Gourd Accessions Differing in Fruit Size. Genes 2020, 11, 359. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Y.; Jiang, Y.; Li, J.; Liu, H.; Duan, X.; Song, L. Differential expression of litchi XET genes in relation to fruit growth. Plant Physiol. Bioch 2006, 44, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ban, Q.; Hou, Y.; Meng, K.; Suo, J.; Rao, J. Isolation and Characterization of Two Persimmon Xyloglucan Endotransglycosylase/Hydrolase (XTH) Genes That Have Divergent Functions in Cell Wall Modification and Fruit Postharvest Softening. Front. Plant Sci. 2016, 7, 624. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Aiese Cigliano, R.; Cardi, T.; Tripodi, P. Whole-genome resequencing reveals genomic footprints of Italian sweet and hot pepper heirlooms giving insight into genes underlying key agronomic and qualitative traits. BMC Genom. Data 2022, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Z.; Cui, L.; Zhang, T.; Guo, Q.; Xu, J.; Jia, L.; Lou, Q.; Huang, S.; Li, Z.; et al. Transcriptome comparison of global distinctive features between pollination and parthenocarpic fruit set reveals transcriptional phytohormone cross-talk in cucumber (Cucumis sativus L.). Plant Cell Physiol. 2014, 55, 1325–1342. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Liu, J.; Hua, W.; Hu, Z.; Yang, H.; Zhang, L.; Li, R.; Deng, L.; Sun, X.; Wang, X.; Wang, H. Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl. Acad. Sci. USA 2015, 112, E5123–E5132. [Google Scholar] [CrossRef]
- Zeng, J.; Li, P.; Duan, J.; Huang, F.; Hou, J.; Zou, X.; Ou, L.; Liu, Z.; Yang, S. Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.). Horticulturae 2025, 11, 1025. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J.; Ross, J.J.; Hallett, I.C.; Gunaseelan, K.; Dayatilake, G. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Bassa, C.; Audran, C.; Mila, I.; Cheniclet, C.; Chevalier, C.; Bouzayen, M.; Roustan, J.P.; Chervin, C. The auxin Sl-IAA17 transcriptional repressor controls fruit size via the regulation of endoreduplication-related cell expansion. Plant Cell Physiol. 2014, 55, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Ljung, K.; Sorefan, K.; Alvey, E.; Harberd, N.P.; Østergaard, L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 2012, 24, 3982–3996. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, C.; Li, H.; Zhang, J.; Ye, Z. Silencing GRAS2 reduces fruit weight in tomato. J. Integr. Plant Biol. 2018, 60, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.P. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef]
- Livne, S.; Weiss, D. Cytosolic activity of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1A. Plant Cell Physiol. 2014, 55, 1727–1733. [Google Scholar] [CrossRef]
- Sorensen, A.M.; Kröber, S.; Unte, U.S.; Huijser, P.; Dekker, K.; Saedler, H. The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J. 2003, 33, 413–423. [Google Scholar] [CrossRef]
- Ballester, P.; Ferrándiz, C. Shattering fruits: Variations on a dehiscent theme. Curr. Opin. Plant Biol. 2017, 35, 68–75. [Google Scholar] [CrossRef]
- Li, F.; Fu, M.; Zhou, S.; Xie, Q.; Chen, G.; Chen, X.; Hu, Z. A tomato HD-zip I transcription factor, VAHOX1, acts as a negative regulator of fruit ripening. Hortic. Res. 2023, 10, uhac236. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, L.; Zhang, X.; Xin, H. The HD-Zip transcription factor GhHB12 represses plant height by regulating the auxin signaling in cotton. J. Integr. Agr. 2023, 22, 2015–2024. [Google Scholar]
- Zhang, B.; Li, Q.; Keyhaninejad, N.; Taitano, N.; Sapkota, M.; Snouffer, A.; van der Knaap, E. A combinatorial TRM-OFP module bilaterally fine-tunes tomato fruit shape. New Phytol. 2023, 238, 2393–2409. [Google Scholar] [CrossRef]
- Borovsky, Y.; Raz, A.; Doron-Faigenboim, A.; Zemach, H.; Karavani, E.; Paran, I. Pepper fruit elongation is controlled by Capsicum annuum ovate family protein 20. Front. Plant Sci. 2022, 12, 815589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).