Expression Regulatory Mechanisms of the Key Structural Genes in the Carotenoid Biosynthesis Pathway Under Salt Stress of Lycium barbarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment Methods
2.2. Data Sources and Candidate Gene Screening
2.3. Gene Structure and Chromosomal Localization Analysis
2.4. Protein Domain Prediction and Functional Annotation
2.5. Synteny Analysis
2.6. Promoter cis-Element Prediction
2.7. Expression Profile Analysis and Dynamic Analysis of Stress Response Expression
3. Results
3.1. Analysis of Physicochemical Properties of Carotenoid Biosynthetic Proteins in Wolfberry
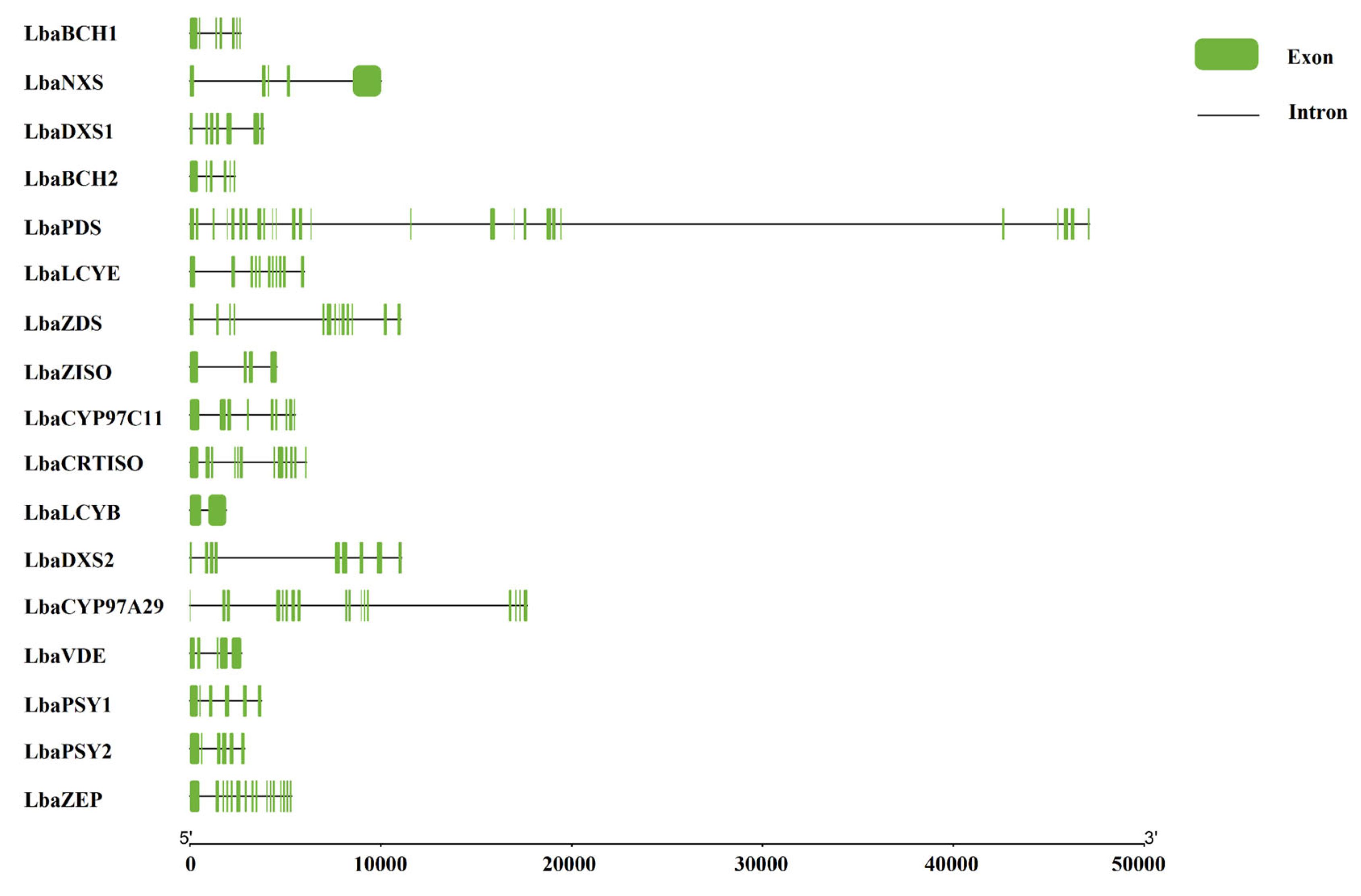
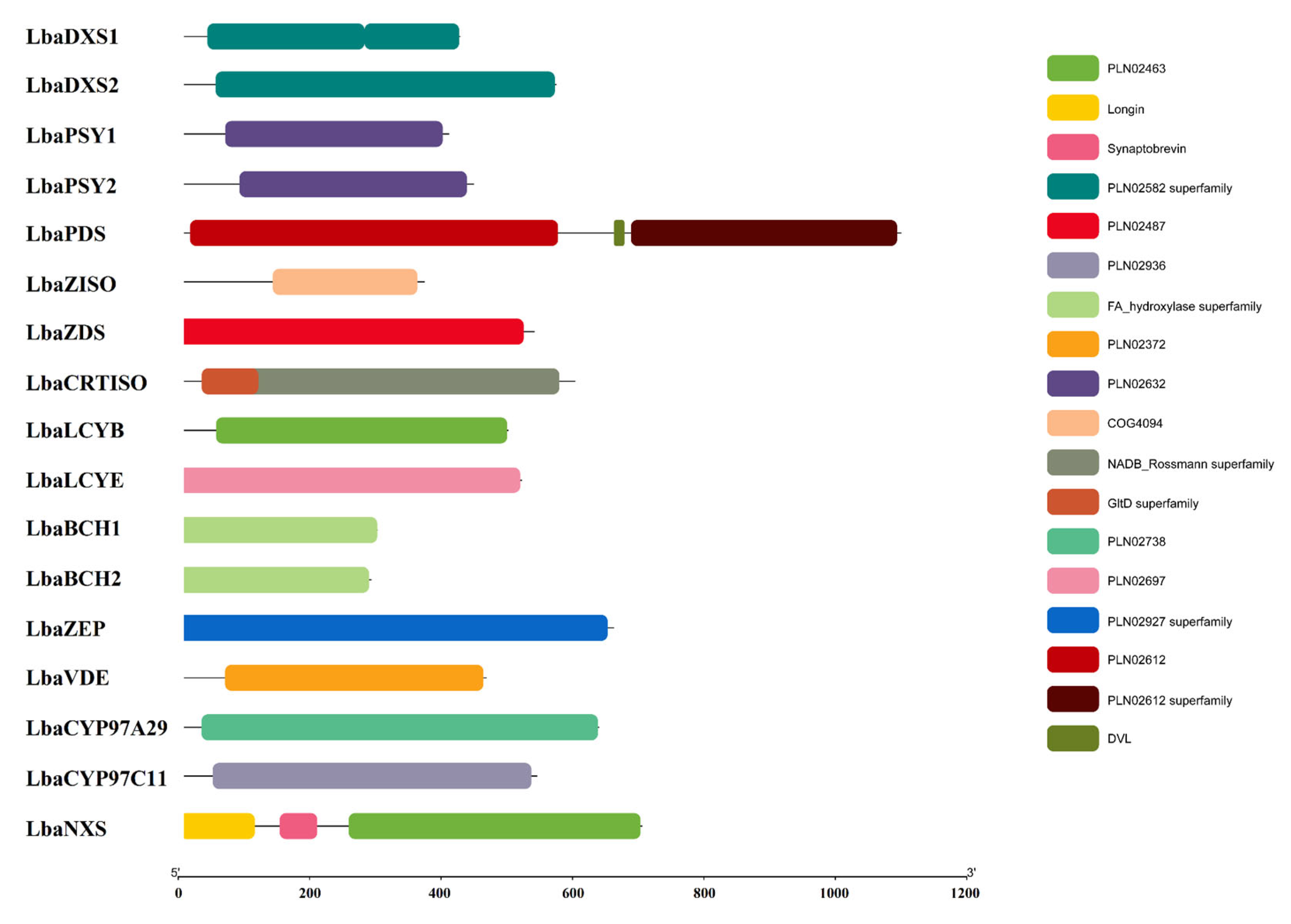
3.2. Structural and Functional Analysis of Carotenoid Biosynthetic Genes
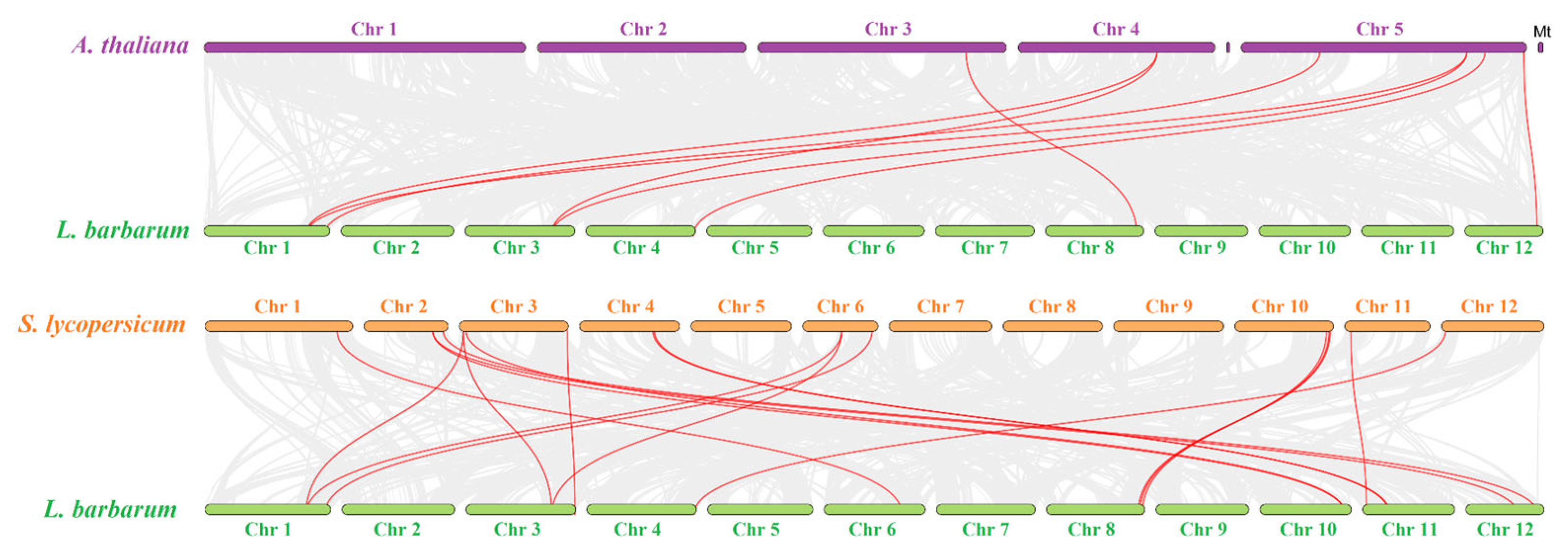
3.3. Genomic Distribution and Evolutionary Analysis of Carotenoid Biosynthetic Genes
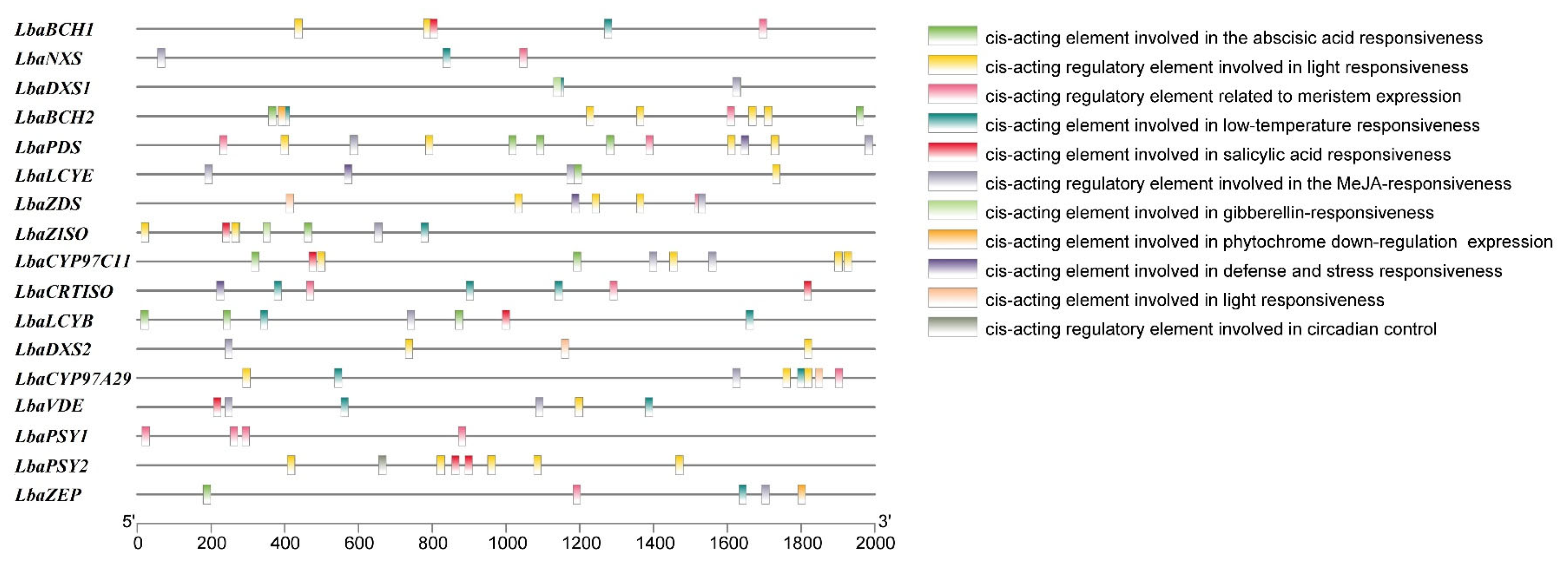
3.4. Analysis of cis-Regulatory Elements of Carotenoid Biosynthetic Genes in L. barbarum
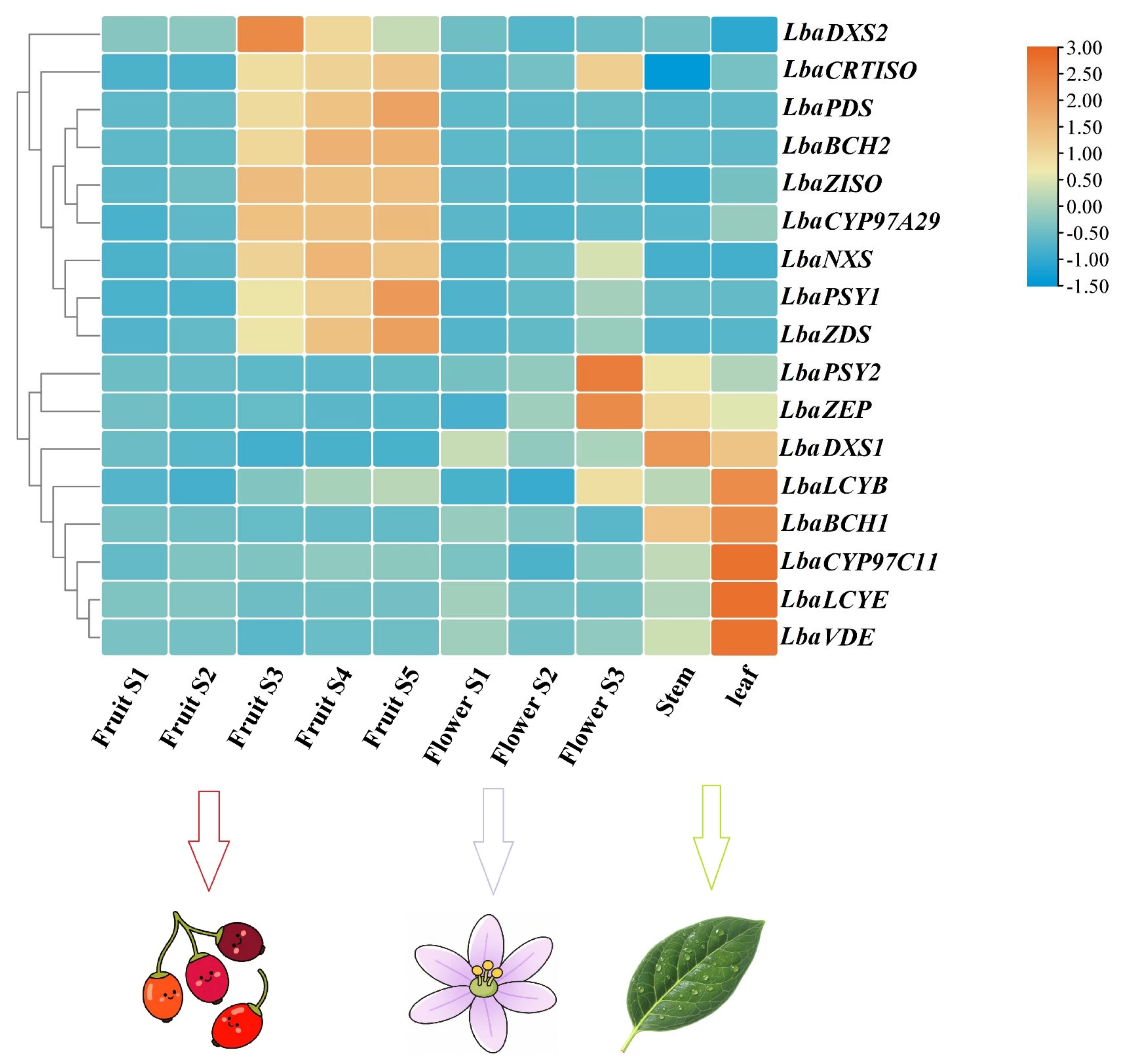
3.5. Tissue-Specific and Developmental Expression Patterns of Carotenoid Biosynthetic Genes
3.6. Expression Dynamics of Carotenoid Synthesis Pathway Genes Under 300 mM NaCl Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.J.; Wei, Y.F.; Wang, Y.Q.; Gao, F.; Chen, Z.G. Lycium barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- Xiong, M.; Peng, J.; Zhou, S.H.; Gao, Q.; Lu, J.; Ou, C.; Song, H.P.; Peng, Q.H. Lycium barbarum L.: A potential botanical drug for preventing and treating retinal cell apoptosis. Front. Pharmacol. 2025, 16, 1571554. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.J.; Zheng, Y.H.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Appl. Sci. 2025, 15, 262. [Google Scholar] [CrossRef]
- Tânia, C.S.P.P.; Maria, I.D.; Lillian, B.; Ricardo, C.C.; Maria, J.A.; Celestino, S.B.; Isabel, C.F.R.F. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Yu, Z.L.; Xia, M.Q.; Li, X.P.; Wang, R.; Liu, W.J.; Zheng, R.R.; Wang, Z.T.; Yang, L.; Shi, Y.H. Characterization of carotenoids in Lycium barbarum fruit by using UPC2-PDA-Q-TOF-MSE couple with deep eutectic solvents extraction and evaluation of their 5α-reductase inhibitory activity. Front. Chem. 2022, 10, 1052000. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Ma, Y.N.; Liu, J.; Fan, Y.J.; Zheng, A.R.; Gao, P.Y.; Wang, L.; Liu, D.H. Assessment of the Bioaccessibility of Carotenoids in Goji Berry (Lycium barbarum L.) in Three Forms: In Vitro Digestion Model and Metabolomics Approach. Foods 2022, 11, 3731. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.M.; Badwal, T.S.; Xu, B.J. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Li, T.; Teng, R.M.; Han, M.H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.X.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Bai, L.; Kim, E.H.; DellaPenna, D.; Brutnell, T.P. Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis. Plant J. 2009, 59, 588–599. [Google Scholar] [CrossRef]
- Zhou, X.S.; Rao, S.; Wrightstone, E.; Sun, T.H.; Lui, A.C.W.; Welsch, R.; Li, L. Phytoene Synthase: The Key Rate-Limiting Enzyme of Carotenoid Biosynthesis in Plants. Front. Plant Sci. 2022, 13, 884720. [Google Scholar] [CrossRef]
- Wei, F.; Wan, R.; Shi, Z.G.; Ma, W.L.; Wang, H.; Chen, Y.W.; Bo, J.H.; Li, Y.X.; An, W.; Qin, K.; et al. Transcriptomics and Metabolomics Reveal the Critical Genes of Carotenoid Biosynthesis and Color Formation of Goji (Lycium barbarum L.) Fruit Ripening. Plants 2023, 12, 2791. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, H.Y.; Mi, J.; Qin, X.Y.; Zhao, J.H.; Zhang, D.K.; Guo, C.; He, X.R.; An, W.; Cao, Y.L.; et al. Genome-Wide Identification and Analysis of the BBX Gene Family and Its Role in Carotenoid Biosynthesis in Wolfberry (Lycium barbarum L.). Int. J. Mol. Sci. 2022, 23, 8440. [Google Scholar] [CrossRef]
- Duduit, J.R.; Kosentka, P.Z.; Miller, M.A.; Blanco-Ulate, B.; Lenucci, M.S.; Panthee, D.R.; Perkins-Veazie, P.; Liu, W.S. Coordinated transcriptional regulation of the carotenoid biosynthesis contributes to fruit lycopene content in high-lycopene tomato genotypes. Hortic. Res. 2022, 9, uhac084. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, M.; Zheng, H.; Jian, Y.; Huang, W.L.; Yuan, Q.; Zheng, A.H.; Chen, Q.; Zhang, Y.T.; Lin, Y.; et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020, 7, 161. [Google Scholar] [CrossRef]
- Arango, J.; Jourdan, M.; Geoffriau, E.; Beyer, P.; Welsch, R. Carotene hydroxylase activity determines the levels of both α-carotene and total carotenoids in orange carrots. Plant Cell 2014, 26, 2223–2233. [Google Scholar] [CrossRef]
- Li, N.N.; Yang, Y.P.; Ye, J.H.; Lu, J.L.; Zheng, X.Q.; Liang, Y.R. Effects of sunlight on gene expression and chemical composition of light-sensitive albino tea plant. Plant Growth Regul. 2016, 78, 253–262. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; He, K.N.; Zhang, T.; Tang, D.; Li, R.J.; Jia, S.F. Physiological responses of Goji berry (Lycium barbarum L.) to saline-alkaline soil from Qinghai region, China. Sci. Rep. 2019, 9, 12057. [Google Scholar] [CrossRef]
- Lin, S.; Zeng, S.H.; A, B.; Yang, X.M.; Yang, T.S.; Zheng, G.Q.; Mao, G.L.; Wang, Y. Integrative Analysis of Transcriptome and Metabolome Reveals Salt Stress Orchestrating the Accumulation of Specialized Metabolites in Lycium barbarum L. Fruit. Int. J. Mol. Sci. 2021, 22, 4414. [Google Scholar] [CrossRef]
- Chan, Z.; Yokawa, K.; Kim, W.Y.; Song, C.P. Editorial: ROS Regulation during Plant Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, X.J.; Fan, S.J. Meta-analysis of salt-related gene expression profiles identifies common signatures of salt stress responses in Arabidopsis. Plant Syst. Evol. 2017, 303, 757–774. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, X.; Tao, H.; Wang, P. Growth performance and physiological response in the halophyte Lycium barbarum grown at salt-affected soil. Ann. Appl. Biol. 2006, 149, 263–269. [Google Scholar] [CrossRef]
- Cao, Y.L.; Chen, Y.Y.; Li, Y.L.; Li, C.I.; Lin, S.T.; Lee, B.R.; Hsieh, C.L.; Hsiao, Y.Y.; Fan, Y.F.; Luo, Q.; et al. Wolfberry genome database: Integrated genomic datasets for studying molecular biology. Front. Plant Sci. 2024, 15, 1310346. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Gao, H.; Cao, X.Y.; Ma, Y.N.; Qin, X.Y.; Bai, X.R.; Zhang, X.Y.; Xiong, A.S.; Yin, Y.; Zheng, R. Genome-Wide Identification of bZIP Gene Family in Lycium barbarum and Expression During Fruit Development. Int. J. Mol. Sci. 2025, 26, 4665. [Google Scholar] [CrossRef]
- Huang, S.D.; Kang, Z.; Xu, Z.L. Robust deep k-means: An effective and simple method for data clustering. Pattern Recognit. 2021, 117, 107996. [Google Scholar] [CrossRef]
- Othman, R.; Mohd Zaifuddin, F.A.; Hassan, N.M. Carotenoid biosynthesis regulatory mechanisms in plants. J. Oleo Sci. 2014, 63, 753–760. [Google Scholar] [CrossRef]
- Liu, L.H.; Shao, Z.Y.; Zhang, M.; Wang, Q.M. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef]
- Birchler, J.A.; Yang, H. The multiple fates of gene duplications: Deletion, hypofunctionalization, subfunctionalization, neofunctionalization, dosage balance constraints, and neutral variation. Plant Cell 2022, 34, 2466–2474. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shi, T.W.; Luo, Q.; Chen, Y.C.; Guo, S.H.; Tian, W.W.; An, W.; Zhao, J.; Yin, Y.; et al. High-Quality Genome of Black Wolfberry (Lycium ruthenicum Murr.) Provides Insights into the Genetics of Anthocyanin Biosynthesis Regulation. Hortic. Res. 2024, 12, uhae298. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Kim, D.J.; Baek, J.M.; Choi, H.K.; Ellis, L.C.; Küester, H.; McCombie, W.R.; Peng, H.M.; Cook, D.R. Syntenic relationships between Medicago truncatula and Arabidopsis reveal extensive divergence of genome organization. Plant Physiol. 2003, 131, 1018–1026. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B 2019, 193, 18–30. [Google Scholar] [CrossRef]
- Welsch, R.; Maass, D.; Voegel, T.; DellaPenna, D.; Beyer, P. Transcription factor RAP2.2 and its role in the regulation of phytoene synthase gene expression during light-induced carotenoid biosynthesis. Plant Physiol. 2003, 15, 122–134. [Google Scholar] [CrossRef]
- Lyu, D.X.; Wang, Z.H.; Yang, L.E.; Hu, C.M.; Lu, S.; Deng, Y.Y. Diurnal rhythm of carotenoid metabolism in the intertidal red algal seaweed Neoporphyra haitanensis. Algal Res. 2025, 85, 103846. [Google Scholar] [CrossRef]
- Li, Y.P.; Su, L.Y.; Huang, T.; Liu, H.; Tan, S.S.; Deng, Y.J.; Wang, Y.H.; Xiong, A.S. The telomere-to-telomere genome of Pucai (Typha angustifolia L.): A distinctive semiaquatic vegetable with lignin and chlorophyll as quality characteristics. Hortic. Res. 2025, 12, uhaf079. [Google Scholar] [CrossRef]
- Hu, Z.H.; Sun, M.Z.; Yang, K.X.; Zhang, N.; Chen, C.; Xiong, J.W.; Yang, N.; Chen, Y.; Liu, H.; Li, X.H.; et al. High-Throughput Transcriptomic Analysis of Circadian Rhythm of Chlorophyll Metabolism under Different Photoperiods in Tea Plants. Int. J. Mol. Sci. 2024, 25, 9270. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, P.Z.; Liu, H.; Zhang, R.R.; Liang, Y.; Xu, Z.S.; Li, X.J.; Luo, Q.; Tan, G.F.; Wang, G.L.; et al. Telomere-to-telomere carrot (Daucus carota) genome assembly reveals carotenoid characteristics. Hortic. Res. 2023, 10, uhad103. [Google Scholar] [CrossRef]
- Zhu, K.J.; Zheng, X.J.; Ye, J.L.; Huang, Y.; Chen, H.Y.; Mei, X.H.; Xie, Z.Z.; Cao, L.X.; Zeng, Y.L.; Larkin, R.M.; et al. Regulation of carotenoid and chlorophyll pools in hesperidia, anatomically unique fruits found only in Citrus. Plant Physiol. 2021, 187, 829–845. [Google Scholar] [CrossRef]
- Giuliano, G.; Bartley, G.E.; Scolnik, P.A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell 1993, 5, 379–387. [Google Scholar] [CrossRef][Green Version]
- Pecker, I.; Gabbay, R.; Cunningham, F.X.; Hirschberg, J. Cloning and characterization of the cDNA for lycopene beta-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Mol. Biol. 1996, 30, 807–819. [Google Scholar] [CrossRef]
- Gao, M.; Qu, H.; Gao, L.; Chen, L.; Sebastian, R.S.; Zhao, L. Dissecting the mechanism of Solanum lycopersicum and Solanum chilense flower colour formation. Plant Biol. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Janik, E.; Bednarska, J.; Zubik, M.; Sowinski, K.; Luchowski, R.; Grudzinski, W.; Matosiuk, D.; Gruszecki, W.I. The xanthophyll cycle pigments, violaxanthin and zeaxanthin, modulate molecular organization of the photosynthetic antenna complex LHCII. Arch. Biochem. Biophys. 2016, 592, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Telegina, T.A.; Vechtomova, Y.L.; Aybush, A.V.; Buglak, A.A.; Kritsky, M.S. Isomerization of carotenoids in photosynthesis and metabolic adaptation. Biophys. Rev. 2023, 15, 887–906. [Google Scholar] [CrossRef]
- Hao, Z.D.; Liu, S.Q.; Hu, L.F.; Shi, J.S.; Chen, J.H. Transcriptome analysis and metabolic profiling reveal the key role of carotenoids in the petal coloration of Liriodendron tulipifera. Hortic. Res. 2020, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.Y.; Sha, H.Y.; Nie, J.Y.; Wang, Y.Z.; Yuan, Y.B.; Jia, D.J. An apple (Malus domestica) AP2/ERF transcription factor modulates carotenoid accumulation. Hortic. Res. 2021, 8, 223. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Deng, Y.J.; Wang, Y.H.; Lou, Y.R.; He, L.F.; Liu, H.; Li, T.; Yan, Z.M.; Zhuang, J.; Xiong, A.S. Changes in Carotenoid Concentration and Expression of Carotenoid Biosynthesis Genes in Daucus carota Taproots in Response to Increased Salinity. Horticulturae 2022, 8, 650. [Google Scholar] [CrossRef]
- Leiva-Ampuero, A.; Agurto, M.; Matus, J.T.; Hoppe, G.; Huidobro, C.; Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Stange, C.; Canessa, P.; Vega, A. Salinity impairs photosynthetic capacity and enhances carotenoid-related gene expression and biosynthesis in tomato (Solanum lycopersicum L. cv. Micro-Tom). PeerJ 2020, 8, e9742. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Yin, Y.; Zhang, Y.J.; An, W.; Mu, Z.X. Genetic Variation in Salt Stress Response and Germplasm Resource Evaluation among Lycium Accessions. Am. J. Plant Sci. 2020, 11, 1765–1783. [Google Scholar] [CrossRef]


| Gene Name | Gene Number | Number of Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| LbaBCH1 | Lba01g01319 | 304 | 33,996.51 | 9.22 | 46.69 | 82.17 | 0.001 | Plasma membrane |
| LbaNXS | Lba01g02622 | 706 | 80,304.38 | 9.28 | 46.66 | 88.5 | −0.196 | Chloroplast |
| LbaDXS1 | Lba02g01426 | 429 | 46,494.11 | 8.25 | 45.19 | 95.71 | −0.092 | Cytosol |
| LbaBCH2 | Lba03g01505 | 292 | 32,712.94 | 8.89 | 48.89 | 88.9 | −0.03 | Chloroplast |
| LbaPDS | Lba03g03127 | 1099 | 122,847.17 | 8.15 | 47.79 | 92.98 | −0.153 | Vacuolar membrane |
| LbaLCYE | Lba04g02855 | 523 | 58,534.68 | 7.03 | 44.31 | 90.21 | −0.043 | Plasma membrane |
| LbaZDS | Lba06g01695 | 543 | 59,423.04 | 7.52 | 39.33 | 87.29 | −0.14 | Chloroplast |
| LbaZISO | Lba07g02021 | 373 | 42,133.81 | 9.1 | 30.7 | 100.64 | 0.157 | Plasma membrane |
| LbaCYP97C11 | Lba08g01705 | 546 | 60,951.73 | 6.19 | 35.27 | 93.75 | −0.169 | Chloroplast |
| LbaCRTISO | Lba08g01811 | 604 | 66,427.4 | 7.86 | 33.53 | 91.99 | −0.095 | Cytosol |
| LbaLCYB | Lba08g02019 | 501 | 56,609.62 | 7.98 | 35.9 | 89.46 | −0.15 | Chloroplast |
| LbaDXS2 | Lba10g00254 | 576 | 62,241.74 | 8.39 | 42.95 | 99.91 | −0.034 | Chloroplast |
| LbaCYP97A29 | Lba10g01149 | 639 | 71,390.93 | 5.61 | 46.13 | 89.11 | −0.184 | Cytosol |
| LbaVDE | Lba10g01158 | 470 | 53,558.04 | 6.02 | 43.35 | 82.34 | −0.454 | Chloroplast |
| LbaPSY1 | Lba11g02324 | 412 | 46,499.07 | 8.09 | 45.71 | 84.51 | −0.312 | Chloroplast |
| LbaPSY2 | Lba12g00874 | 449 | 50,938.21 | 9.2 | 55.28 | 81.92 | −0.379 | Chloroplast |
| LbaZEP | Lba12g02412 | 663 | 72,198.58 | 7.14 | 31.52 | 88.97 | −0.156 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.-H.; Wang, L.-X.; Zhang, N.; Chen, C.; Zhuang, J.; Yin, Y.; Xiong, A.-S. Expression Regulatory Mechanisms of the Key Structural Genes in the Carotenoid Biosynthesis Pathway Under Salt Stress of Lycium barbarum. Horticulturae 2025, 11, 1149. https://doi.org/10.3390/horticulturae11101149
Hu Z-H, Wang L-X, Zhang N, Chen C, Zhuang J, Yin Y, Xiong A-S. Expression Regulatory Mechanisms of the Key Structural Genes in the Carotenoid Biosynthesis Pathway Under Salt Stress of Lycium barbarum. Horticulturae. 2025; 11(10):1149. https://doi.org/10.3390/horticulturae11101149
Chicago/Turabian StyleHu, Zhi-Hang, Li-Xiang Wang, Nan Zhang, Chen Chen, Jing Zhuang, Yue Yin, and Ai-Sheng Xiong. 2025. "Expression Regulatory Mechanisms of the Key Structural Genes in the Carotenoid Biosynthesis Pathway Under Salt Stress of Lycium barbarum" Horticulturae 11, no. 10: 1149. https://doi.org/10.3390/horticulturae11101149
APA StyleHu, Z.-H., Wang, L.-X., Zhang, N., Chen, C., Zhuang, J., Yin, Y., & Xiong, A.-S. (2025). Expression Regulatory Mechanisms of the Key Structural Genes in the Carotenoid Biosynthesis Pathway Under Salt Stress of Lycium barbarum. Horticulturae, 11(10), 1149. https://doi.org/10.3390/horticulturae11101149








