Diploid Ancestor Tracing of Allopolyploid Cultivars in Camellia reticulata Based on ITS and RPB2 Sequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Analysis
2.4. Morphological Analysis of Diploid Camellia reticulata
3. Results
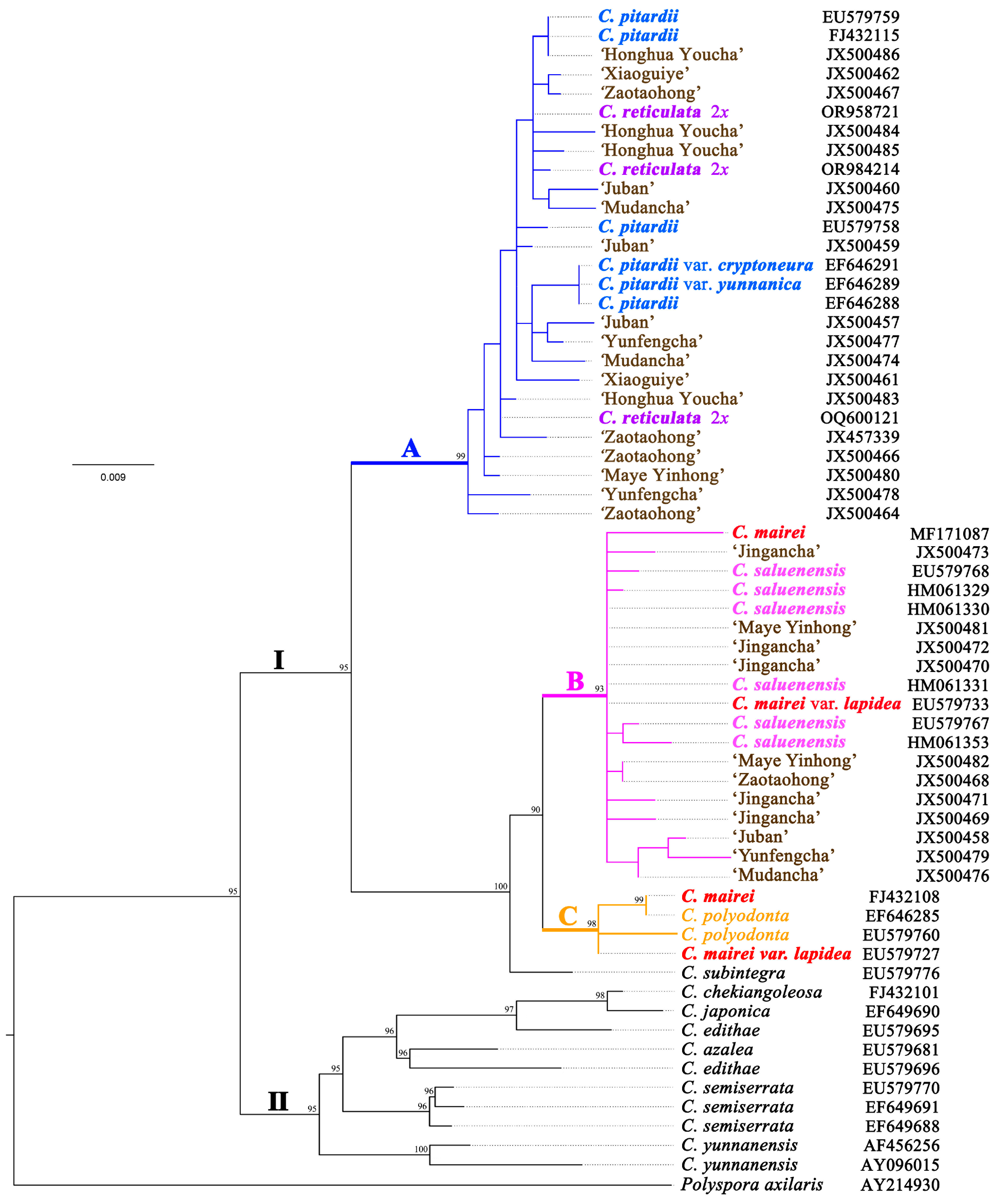
3.1. Analysis of ITS Sequences in Camellia reticulata Cultivars and Related Species
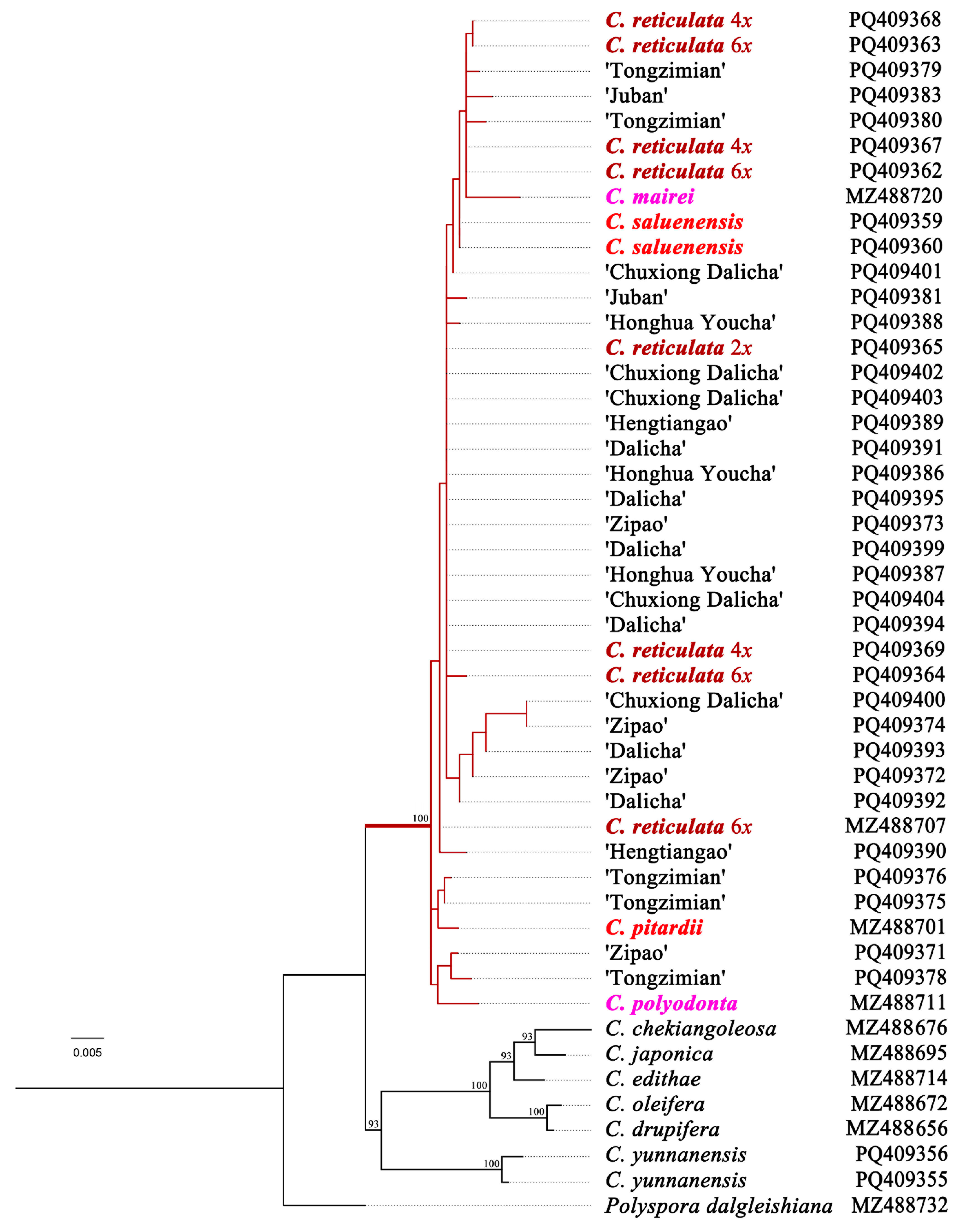
3.2. Analysis of RPB2 Sequences in Camellia reticulata Cultivars and Related Species
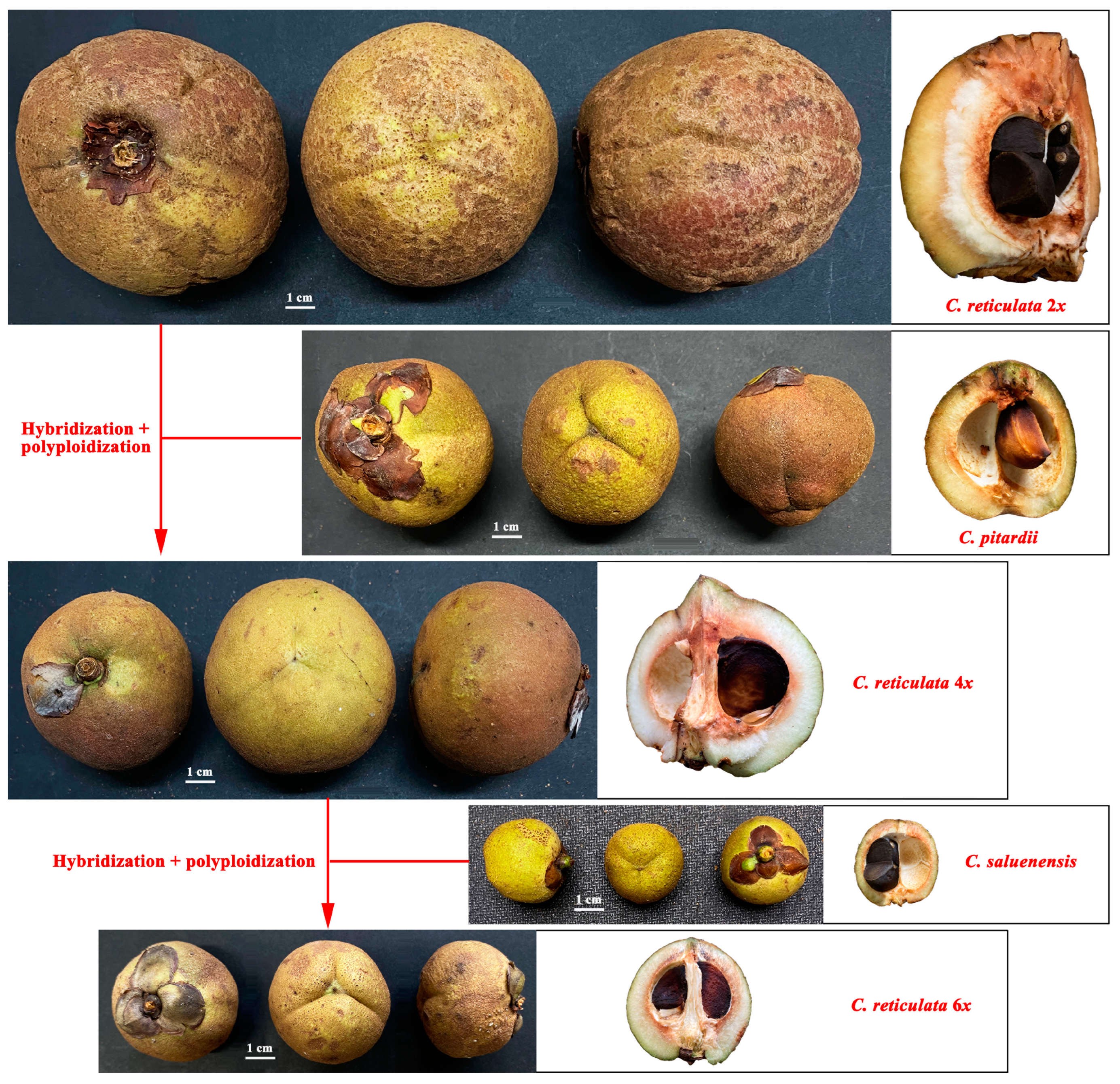
3.3. Fruit Morphology Comparison of Diploid Camellia reticulata and Congeneric Species
4. Discussion
4.1. Diploid Progenitors of Heritage Camellia reticulata Cultivars
4.2. Phylogenetic Position of Diploid Camellia reticulata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.-J. A historical review and future development of Camellia reticulata in Yunnan. Acta Hortic. Sin. 1985, 12, 131–136. [Google Scholar]
- Kunming Association for Science and Technology. Wonderful Flower of Yunnan—Camellia reticulata; Yunnan Science and Technology Press: Kunming, China, 2010. [Google Scholar]
- Wu, G.; Chen, L.; Wei, Q.; Hu, Y.; Xu, J.; Li, D.; Chen, S.; Wang, Z.; Geng, F. Statistics and phenotypic traits analysis of Camellia reticulata registered cultivars. Acta Hortic. Sin. 2023, 50, 2157–2170. [Google Scholar]
- Min, T. The Classification, differentiation and distribution of the genus Camellia Sect. Camellia. Act. Bot. Yunnanica 1998, 20, 127–148. [Google Scholar]
- Xin, T.; Riek, J.; Guo, H.; Jarvis, D.; Ma, L.; Long, C. Impact of traditional culture on Camellia reticulata in Yunnan, China. J. Ethnobiol. Ethnomed. 2015, 11, 74. [Google Scholar] [CrossRef]
- Xin, T.; Huang, W.; De Riek, J.; Zhang, S.; Ahmed, S.; Van Huylenbroeck, J.; Long, C. Genetic diversity, population structure, and traditional culture of Camellia reticulata. Ecol. Evol. 2017, 7, 8915–8926. [Google Scholar] [CrossRef]
- Huang, S.; Hsu, P. Study on the chromosome and application of oil species in genus Camellia. Subtrop. For. Sci. Technol. 1987, 15, 33–39. [Google Scholar]
- Min, T. Monograph of the Genus Camellia; Yunnan Science and Technology Press: Kunming, China, 2000. [Google Scholar]
- Min, T.; Bartholomew, B. Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 12, pp. 368–372. [Google Scholar]
- Barstow, M.; Beech, E.; Rivers, M.C. Camellia reticulata. In The IUCN Red List of Threatened Species 2018; IUCN Red List: Cambridge, UK, 2018; p. e.T32328A62057403. [Google Scholar] [CrossRef]
- Kondo, K.; Gu, Z.; Na, H.; Xia, L. A cytological study of Camellia reticulata and its related species in Yunnan, China. La Kromosomo II 1986, 43–44, 1405–1419. [Google Scholar]
- Xia, L.-F.; Gu, Z.-J.; Wang, Z.-L.; Xiao, T.-J.; Wang, L.; Katsuhiko, K. Dawn on the origin of Camellia reticulata—The new discovery of its wild diploid in Jinshajiang valley. Act. Bot. Yunnanica 1994, 16, 255–262. [Google Scholar]
- Gu, Z. The discovery of tetraploid Camellia reticulata and its implication in studies on the origin of this species. Acta Phytotaxon Sin. 1997, 35, 107–116. [Google Scholar]
- Liu, L.-Q.; Gu, Z.-J. Genomic in situ hybridization identifies genome donors of Camellia reticulata (Theaceae). Plant Sci. 2011, 180, 554–559. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Yan, Z.; Zheng, Y. Alien DNA introgression and wheat DNA rearrangements in a stable wheat line derived from the early generation of distant hybridization. Sci. China Ser. C Life Sci. Chin. Acad. Sci. 2005, 48, 424–433. [Google Scholar] [CrossRef]
- Wagner, N.D.; Marinček, P.; Pittet, L.; Hörandl, E. Insights into the Taxonomically Challenging Hexaploid Alpine Shrub Willows of Salix Sections Phylicifoliae and Nigricantes (Salicaceae). Plants 2023, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shao, W.; Zheng, W. Ploidy study on polyploidy cultivars of Camellia reticulata. Sci. Silvae Sin. 2018, 54, 44–48. [Google Scholar]
- Zimmer, E.A.; Wen, J. Using nuclear gene data for plant phylogenetics: Progress and prospects II. Next-gen approaches. J. Syst. Evol. 2015, 53, 371–379. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.; Zheng, W. Exploring Genetic Diversity and Phylogenetic Relationships in Camellia reticulata Cultivars Using Novel Low-Copy Nuclear Gene Markers. Horticulturae 2024, 10, 303. [Google Scholar] [CrossRef]
- Geng, F.; Nie, R.; Yang, N.; Cai, L.; Hu, Y.; Chen, S.; Cheng, X.; Wang, Z.; Chen, L. Integrated transcriptome and metabolome profiling of Camellia reticulata reveal mechanisms of flower color differentiation. Front. Genet. 2022, 13, 1059717. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yin, H.; Wang, K.; Gao, H.; Liu, L.; Yao, X. Comparative Genomic Analysis Uncovers the Chloroplast Genome Variation and Phylogenetic Relationships of Camellia Species. Biomolecules 2022, 12, 1474. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, Y.-L.; Mantri, N.; Wang, Y.; Hua, X.-J.; Quan, Y.-P.; Zhou, X.; Jiang, Z.-D.; Qi, Z.-C.; Lu, H.-F. Molecular phylogenetic relationships and taxonomy position of 161 Camellia species in China. Taiwania 2022, 67, 560–570. [Google Scholar]
- Wu, Q.; Tong, W.; Zhao, H.; Ge, R.; Li, R.; Huang, J.; Li, F.; Wang, Y.; Mallano, A.I.; Deng, W.; et al. Comparative transcriptomic analysis unveils the deep phylogeny and secondary metabolite evolution of 116 Camellia plants. Plant J. 2022, 111, 406–421. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Folk, R.A.; Zhao, J.-L.; Zamora, N.A.; Yang, S.-X.; Soltis, D.E.; Soltis, P.S.; Gao, L.-M.; Peng, H.; et al. Phylotranscriptomics of Theaceae: Generic-level relationships, reticulation and whole-genome duplication. Ann. Bot. 2022, 129, 457–471. [Google Scholar] [CrossRef]
- Zan, T.; He, Y.-T.; Zhang, M.; Yonezawa, T.; Ma, H.; Zhao, Q.-M.; Kuo, W.-Y.; Zhang, W.-J.; Huang, C.-H. Phylogenomic analyses of Camellia support reticulate evolution among major clades. Mol. Phylogenetics Evol. 2023, 182, 107744. [Google Scholar] [CrossRef]
- Zhang, W.; Min, T. A cytogeographical study of Camellia, Sect. Camellia. Act. Bot. Yunnanica 1998, 20, 321–328. [Google Scholar]
- Huang, H.; Tong, Y.; Zhang, Q.-J.; Gao, L.-Z. Genome Size Variation among and within Camellia Species by Using Flow Cytometric Analysis. PLoS ONE 2013, 8, e64981. [Google Scholar] [CrossRef]
- Yang, J.-B.; Yang, S.-X.; Li, H.-T.; Yang, J.; Li, D.-Z. Comparative Chloroplast Genomes of Camellia Species. PLoS ONE 2013, 8, e73053. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, W.; Wen, J. The complete chloroplast genome of the long blooming and critically endangered Camellia azalea. Conserv. Genet. Resour. 2018, 10, 5–7. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Yu, Z.; Jia, X. Complete Chloroplast Genome Sequence of the Long Blooming Cultivar Camellia ‘Xiari Qixin’: Genome Features, Comparative and Phylogenetic Analysis. Genes 2023, 14, 460. [Google Scholar] [CrossRef]
- Tanikawa, N.; Onozaki, T.; Nakayama, M.; Shibata, M. Maternal Origin of ‘Tarokaja’ and Other Wabisuke Camellia Cultivars Indicated by Chloroplast DNA Variation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 77–83. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Zheng, W.; Harris, A.J.; Wang, W.; Shao, W.; Wen, J. Assessing the maternal origin in the polyploid complex of Camellia reticulata based on the chloroplast rpl16 intron sequences: Implications for camellia cross breeding. Mol. Breed. 2018, 38, 123. [Google Scholar] [CrossRef]
- Álvarez, I.; Wendel, J.F. Ribosomal ITS sequences and plant phylogenetic inference. Mol. Phylogenetics Evol. 2003, 29, 417–434. [Google Scholar] [CrossRef]
- Eickbush, T.H.; Eickbush, D.G. Finely Orchestrated Movements: Evolution of the Ribosomal RNA Genes. Genetics 2007, 175, 477–485. [Google Scholar] [CrossRef]
- Wei, S.-J.; Lu, Y.-B.; Ye, Q.-Q.; Tang, S.-Q. Population Genetic Structure and Phylogeography of Camellia flavida (Theaceae) Based on Chloroplast and Nuclear DNA Sequences. Front. Plant Sci. 2017, 8, 00718. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Shi, S.; Zhong, Y.; Wang, Y. Phylogenetic relationships of golden camellias (sect. Chrysantha, Camellia) in China: Evidence from ITS sequences of nuclear ribosomal DNA. Guihaia 2004, 24, 488–492. [Google Scholar]
- Yang, J.; Li, H.; Yang, S.; Li, D.; Yang, Y. The application of four DNA sequences to studying molecular phylogeny of Camellia (Theaceae). Act. Bot. Yunnanica 2006, 28, 108–114. [Google Scholar]
- Tian, M.; Li, J.; Ni, S.; Fan, Z.; Li, X. Phylogenetic study on section Camellia based on ITS sequences data. Acta Hortic. Sin. 2008, 35, 1685–1688. [Google Scholar]
- Vijayan, K.; Tsou, C.-H. Technical report on the molecular phylogeny of Camellia with nrITS: The need for high quality DNA and PCR amplification with Pfu-DNA polymerase. Bot. Stud. 2008, 49, 177–188. [Google Scholar]
- Vijayan, K.; Zhang, W.-J.; Tsou, C.-H. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Am. J. Bot. 2009, 96, 1348–1360. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.-B.; Yang, S.-X.; Li, D.-Z. Phylogeny and taxonomy of the Pyrenaria complex (Theaceae) based on nuclear ribosomal ITS sequences. Nord. J. Bot. 2011, 29, 780–787. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.-X.; Ji, P.-Z.; Gao, L.-Z. Phylogeography of Camellia taliensis (Theaceae) inferred from chloroplast and nuclear DNA: Insights into evolutionary history and conservation. BMC Evol. Biol. 2012, 12, 92. [Google Scholar] [CrossRef]
- Su, M.-H.; Hsieh, C.-F.; Tsou, C.H. The confirmation of Camellia formosensis (Theaceae) as an independent species based on DNA equence analyses. Bot. Stud. 2009, 50, 477–485. [Google Scholar]
- Xu, Y.; Xu, J.; Gao, J.; Zhang, W. Polymorphism of the internal transcribed spacer of rDNA in Camellia—An escape from concerted evolution. Chin. Bull. Bot. 2011, 46, 162–169. [Google Scholar]
- Zhou, A.T.; Yue, L.-L.; Li, M.; Liu, D.-Q.; Ding, Y.-M. Intra-genomic Polymorphism in the nrDNA ITS Sequence of Camellia reticulata. Plant Sci. J. 2013, 31, 1–10. [Google Scholar] [CrossRef]
- Větrovský, T.; Kolařík, M.; Žifčáková, L.; Zelenka, T.; Baldrian, P. The rpb2 gene represents a viable alternative molecular marker for the analysis of environmental fungal communities. Mol. Ecol. Resour. 2016, 16, 388–401. [Google Scholar] [CrossRef]
- Oxelman, B.; Yoshikawa, N.; McConaughy, B.L.; Luo, J.; Denton, A.L.; Hall, B.D. RPB2 gene phylogeny in flowering plants, with particular emphasis on asterids. Mol. Phylogenetics Evol. 2004, 32, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yoshikawa, N.; Hodson, M.C.; Hall, B.D. Duplication and paralog sorting of RPB2 and RPB1 genes in core eudicots. Mol. Phylogenetics Evol. 2007, 44, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-W.; Hodkinson, T.R.; Parnell, J.A.N. Phylogenetics of global Camellia (Theaceae) based on three nuclear regions and its implications for systematics and evolutionary history. J. Syst. Evol. 2023, 61, 356–368. [Google Scholar] [CrossRef]
- Doyle, J.J.T.; Doyle, J.L. Isolation of Plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Wen, Q.; Zhu, H.; Ye, J.; Xu, L.; Xu, L.; Jiang, X. Authentication of Camellia chekiangoleosa and its close species using RPB2 gene sequences. Mol. Plant Breed. 2015, 13, 2559–2565. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Solís-Lemus, C.; Ané, C. Inferring Phylogenetic Networks with Maximum Pseudolikelihood under Incomplete Lineage Sorting. PLoS Genet. 2016, 12, e1005896. [Google Scholar] [CrossRef]
- Fukushima, E.; Iwasa, S.; Endo, N.; Yoshinari, T. Cytogenetic studies in Camellia I. Chromosome survey in some Camellia species. J. Jpn. Soc. Hortic. Sci. 1966, 35, 413–421. [Google Scholar] [CrossRef][Green Version]
- Ackerman, W.L.; Kondo, K. Pollen Size and Variability as Related to Chromosome Number and Speciation in the Genus Camellia. Jpn. J. Breed. 1980, 30, 251–259. [Google Scholar] [CrossRef]
- Gu, Z.; Xiao, T.; Xia, L.; Kondo, K. A comparative study in Giesma C-banded karyotypes of four species of Camellia, section Camellia. Rev. Espaola De Pediatría 1990, 9, 471–504. [Google Scholar]
- Lu, T.; Liao, H. Karyotypeanalysis of Camellia polyodonta How. Guihaia 1986, 6, 111–115. [Google Scholar]
- Huang, S.; Zhao, Z.; Zhuang, R.; Thu, B.; Zhou, Q.; Qiu, G. Karyotype analysis in Camellia polyodonta How et Hu. Guihaia 1986, 6, 107–110. [Google Scholar]
- Gu, Z.; Kondo, K.; Na, H.; Xia, L. A karyomorphological study in four species of Camellia Sect. Camellia. La Kromosomo 1988, 49, 1575–1582. [Google Scholar]
- Jia, W.Q. Fundamental Study on Reproductive Biology and Ploidy Breeding of Camellia Flower. Doctoral Dissertation, Chinese Academy of Forestry, Beijing, China, 2015. [Google Scholar]
- Yan, C.; Sun, G. Multiple origins of allopolyploid wheatgrass Elymus caninus revealed by RPB2, PepC and TrnD/T genes. Mol. Phylogenetics Evol. 2012, 64, 441–451. [Google Scholar] [CrossRef]
- Du, N. The Genomic In Situ Hybridization Studies On The Complex Polyploid of Camellia reticulata Lindle. Master’s Thesis, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China, 2003. [Google Scholar]
- Liu, L.Q. Molecular cytogenetic study on the origin and evolution of polyploid complex of Camellia reticulata. Doctoral Dissertation, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China, 2009. [Google Scholar]
- Chang, H.T. New Camellia from Jingshajiang valley. Acta Sci. Nat. Univ. Sunyatseni 1989, 28, 50–58. [Google Scholar]
- Sealy, J.R. A Revision of the Genus Camellia; The Royal Horticultural Society: London, UK, 1958. [Google Scholar]
- Wang, B.Y.; Ruan, Z.Y. Genetic diversity and differentiation in Camellia reticulata (Theaceae) polyploid complex revealed by ISSR and ploidy. Genet. Mol. Res. 2012, 11, 503–511. [Google Scholar] [CrossRef]


| Code | Cultivars and Species | Cultivation Site | ITS GenBank Accessions | RPB2 GenBank Accessions |
|---|---|---|---|---|
| 1 | ‘Xiaoguiye’ (6x) | Kunming, China | JX500461-2 | - |
| 2 | ‘Zaotaohong’ (6x) | Kunming, China | JX457339, JX500464, JX500466-8 | - |
| 3 | ‘Dalicha’ (6x) | Kunming, China | - | PQ409391-5, PQ409399 |
| 4 | ‘Juban’ (8x) | Kunming, China | JX500457-60 | PQ409381, PQ409383 |
| 5 | ‘Mudancha’ (8x) | Kunming, China | JX500474-6 | - |
| 6 | ‘Yunfengcha’ (8x) | Kunming, China | JX500477-9 | - |
| 7 | ‘Maye Yinhong’ (8x) | Kunming, China | JX500480-2 | - |
| 8 | ‘Honghua Youcha’ (8x) | Kunming, China | JX500483-6 | PQ409386-8 |
| 9 | ‘Jing’ancha’ (8x) | Kunming, China | JX500469-73 | - |
| 10 | ‘Zipao’ (8x) | Kunming, China | - | PQ409371-4 |
| 11 | ‘Tongzimian’ (8x) | Kunming, China | - | PQ409375-6, PQ409378-80 |
| 12 | ‘Chuxiong Dalicha’ (10x) | Kunming, China | - | PQ409400-4 |
| 13 | ‘Hentiangao’ (10x) | Kunming, China | - | PQ409389-90 |
| 14 | C. reticulata (2x) | Huaping and Yanbian, China | OQ600121, OR958721, OR984214 | PQ409365 |
| 15 | C. reticulata (4x) | Yanbian, China | - | PQ409367-9 |
| 16 | C. reticulata (6x) | Kunming, China | HM061380-401 | PQ409362-4, MZ488707 |
| 17 | C. pitardii | SW China | EF646288, EU579758-9, FJ432115 | MZ488701 |
| 18 | C. pitardii var. cryptoneura | SW China | EF646291 | - |
| 19 | C. pitardii var. yunnanica | SW China | EF646289 | - |
| 20 | C. saluenensis | SW China | EU579767-8, HM061329-31, HM061353 | PQ409359-60 |
| 21 | C. mairei | SW and S China | MF171087, FJ432108 | MZ488720 |
| 22 | C. mairei var. lapidea | SW and S China | EU579733, MF171087 | - |
| 23 | C. polyodonta | C and S China | EF646285, EU579760 | MZ488711 |
| 24 | C. subintegra | C and S China | EU579776 | - |
| 25 | C. chekiangoleosa | SE China | FJ432101 | MZ488676 |
| 26 | C. japonica | S and E China, S Japan | EF649690 | MZ488695 |
| 27 | C. edithae | SE China | EU579695-6 | MZ488714 |
| 28 | C. azalea | S China | EU579681 | - |
| 29 | C. semiserrata | S China | EF649688, EU579770, EF649691 | - |
| 30 | C. oleifera | S China, SE Asia | - | MZ488672 |
| 31 | C. drupifera | S China | - | MZ488656 |
| 32 | C. yunnanensis | SW China | AY096015, AF456256 | PQ409355-6 |
| 33 | Polyspora dalglieshiana | Laos, Thailand | - | MZ488732 |
| 34 | Polyspora axillaris | SW China | AY214930 | - |
| Taxon | Location | Longitude | Latitude | Altitude |
|---|---|---|---|---|
| C. reticulata 2x | Huaping, Yunnan | E 101°09′37″ | N 26°38′01″ | 1830 |
| C. reticulata 2x | Yanbian, Sichuan | E 101°18′40″ | N 27°06′63″ | 2110 |
| C. reticulata 4x | Xichang, Sichuan | E 102°21′11″ | N 27°43′30″ | 2423 |
| C. reticulata 6x | Panlong, Yunnan | E 102°43′13″ | N 25°23′30″ | 2426 |
| C. pitardii | Fuyuan, Yunnan | E 104°25′17″ | N 25°52′05″ | 2144 |
| C. saluenensis | Songming, Yunnan | E 102°44′13″ | N 25°13′27″ | 2420 |
| Species | C. pitardii | C. reticulata 2x | C. subintegra | C. saluenensis | C. polyodonta | C. semiserrata | C. azalea | C. edithae | C. chekiangoleosa | C. japonica |
|---|---|---|---|---|---|---|---|---|---|---|
| C. pitardii | - | 98.64 | 92.66 | 92.83 | 92.97 | 91.08 | 90.90 | 89.71 | 89.76 | 89.64 |
| C. reticulata 2x | 9 | - | 92.78 | 92.94 | 92.80 | 91.33 | 91.14 | 89.96 | 89.72 | 89.60 |
| C. subintegra | 49 | 48 | - | 95.73 | 95.43 | 92.13 | 92.38 | 89.99 | 91.64 | 91.52 |
| C. saluenensis | 48 | 47 | 28 | - | 97.11 | 93.31 | 91.95 | 88.86 | 89.91 | 89.79 |
| C. polyodonta | 47 | 48 | 30 | 19 | - | 91.86 | 90.65 | 89.39 | 88.88 | 90.33 |
| C. semiserrata | 60 | 58 | 52 | 44 | 54 | - | 94.58 | 91.45 | 92.01 | 92.03 |
| C. azalea | 62 | 60 | 51 | 54 | 63 | 36 | - | 93.00 | 92.84 | 92.86 |
| C. edithae | 70 | 70 | 67 | 75 | 71 | 57 | 47 | - | 94.12 | 94.57 |
| C. chekiangoleosa | 69 | 69 | 55 | 67 | 67 | 53 | 48 | 39 | - | 98.62 |
| C. japonica | 70 | 70 | 56 | 68 | 64 | 53 | 48 | 36 | 9 | - |
| Species | C. pitardii | C. reticulata 2x | C. saluenensis | C. polyodonta | C. edithae | C. japonica |
|---|---|---|---|---|---|---|
| C. pitardii | - | 99.00 | 98.49 | 98.65 | 94.46 | 94.16 |
| C. reticulata 2x | 10 | - | 98.70 | 98.65 | 94.07 | 93.77 |
| C. saluenensis | 15 | 13 | - | 97.94 | 93.56 | 93.47 |
| C. polyodonta | 14 | 14 | 21 | - | 94.02 | 93.72 |
| C. edithae | 56 | 60 | 65 | 61 | - | 98.70 |
| C. japonica | 59 | 63 | 66 | 64 | 13 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Zheng, W.; Yan, C.; Xu, X. Diploid Ancestor Tracing of Allopolyploid Cultivars in Camellia reticulata Based on ITS and RPB2 Sequences. Horticulturae 2025, 11, 85. https://doi.org/10.3390/horticulturae11010085
Fan Z, Zheng W, Yan C, Xu X. Diploid Ancestor Tracing of Allopolyploid Cultivars in Camellia reticulata Based on ITS and RPB2 Sequences. Horticulturae. 2025; 11(1):85. https://doi.org/10.3390/horticulturae11010085
Chicago/Turabian StyleFan, Zhifeng, Wei Zheng, Chengmin Yan, and Xiaodan Xu. 2025. "Diploid Ancestor Tracing of Allopolyploid Cultivars in Camellia reticulata Based on ITS and RPB2 Sequences" Horticulturae 11, no. 1: 85. https://doi.org/10.3390/horticulturae11010085
APA StyleFan, Z., Zheng, W., Yan, C., & Xu, X. (2025). Diploid Ancestor Tracing of Allopolyploid Cultivars in Camellia reticulata Based on ITS and RPB2 Sequences. Horticulturae, 11(1), 85. https://doi.org/10.3390/horticulturae11010085






