Application of Azospirillum brasilense and Humic Substances Improves the Nursery Quality of Olive Seedlings in Pots
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Climatic Characteristics
2.3. Plant Material

2.4. Treatments and Growing Conditions

2.5. Parameters
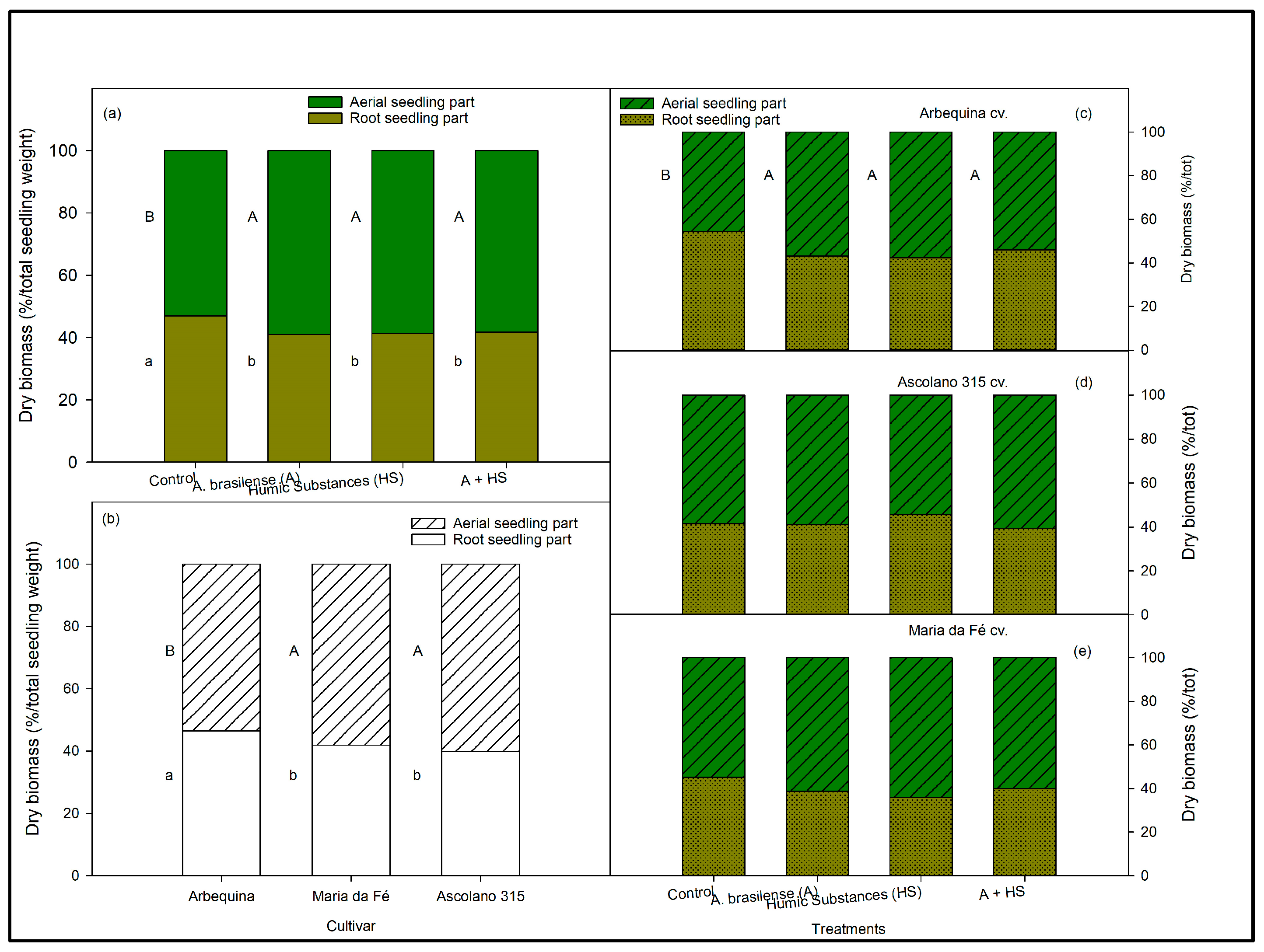
2.6. Aerial and Root Parts Weight and Distribution
2.7. Experimental Design and Data Analysis
2.8. Seedling Characteristics at the Beginning of Trial
3. Results
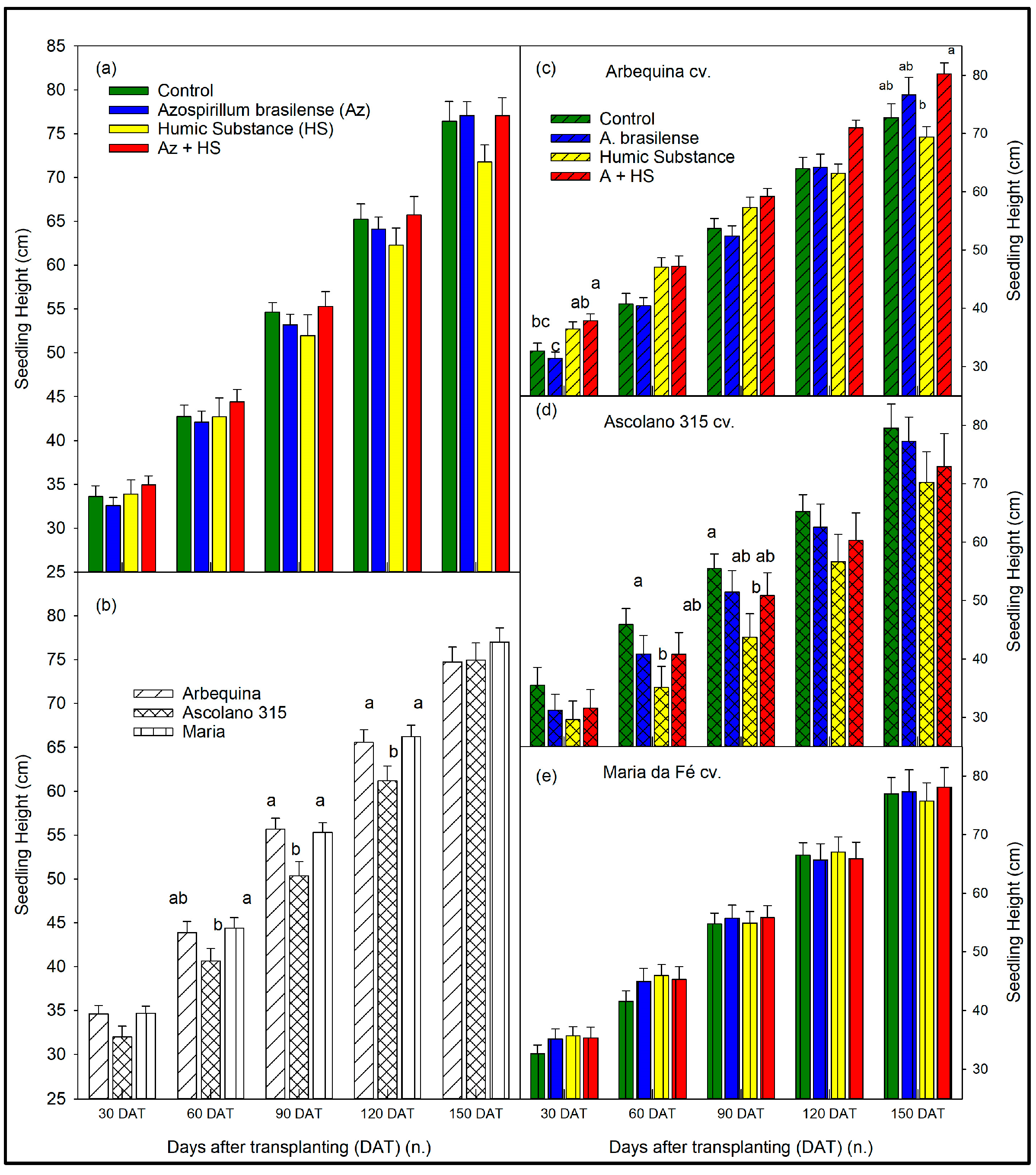
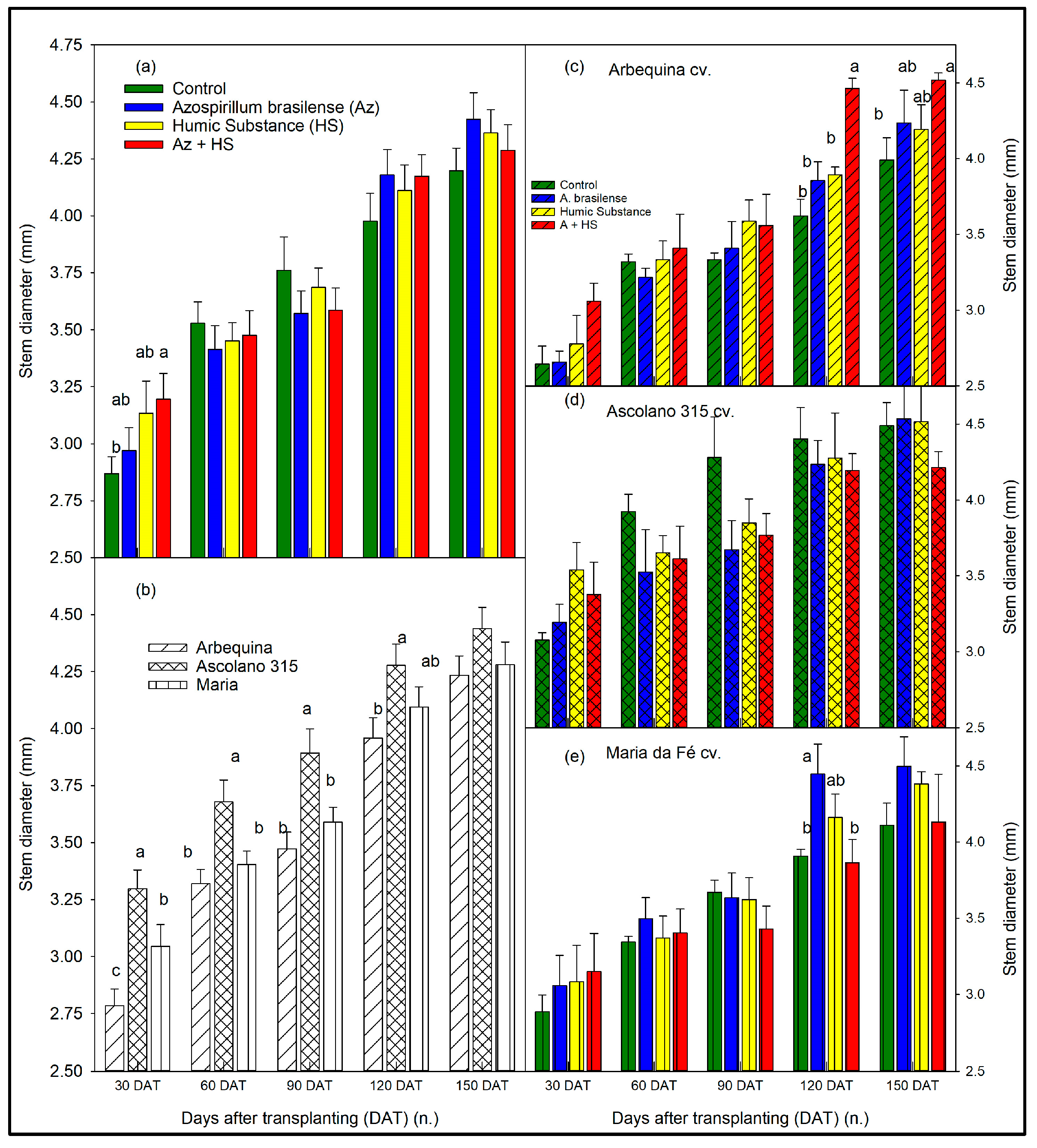
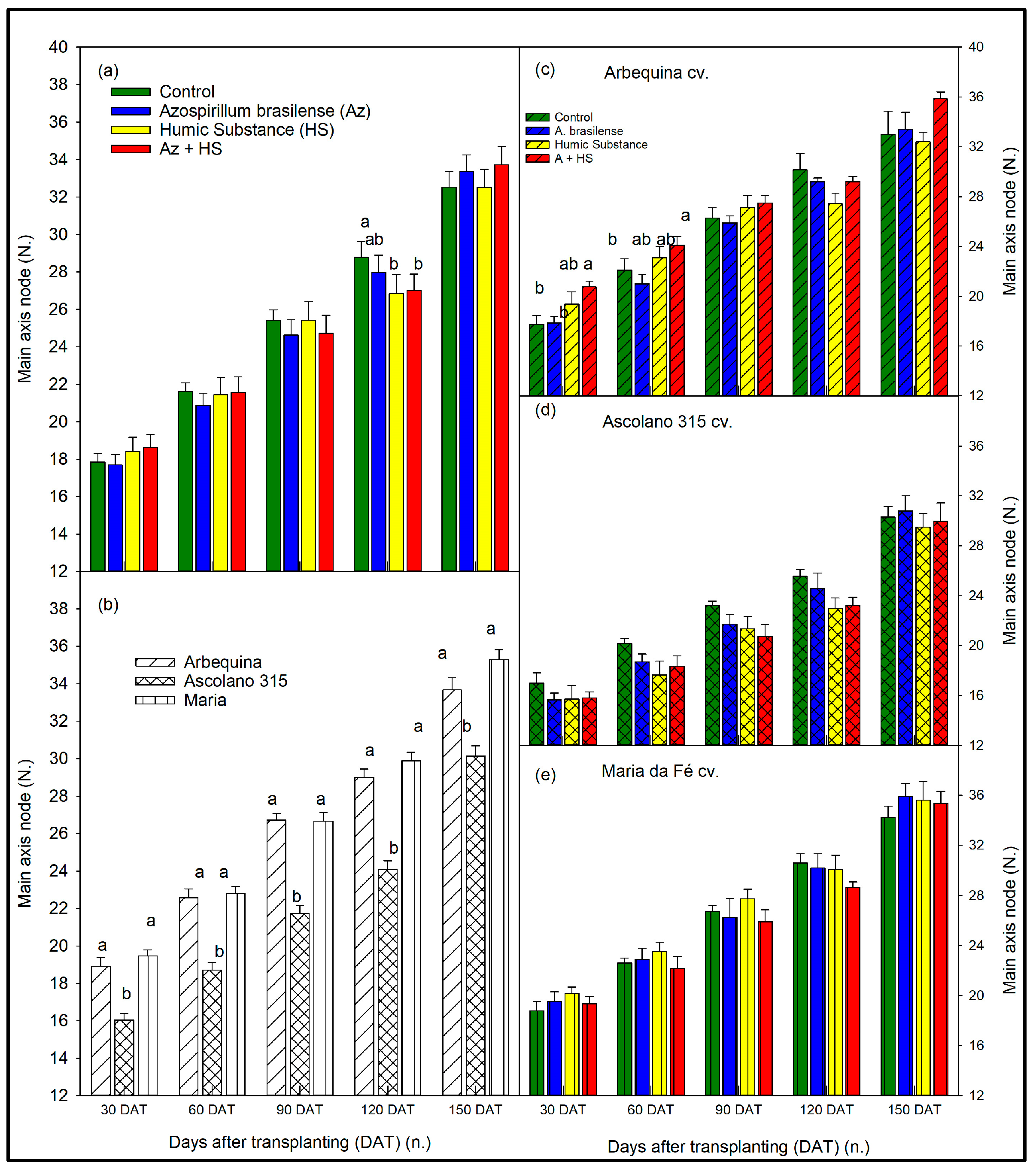
3.1. Size of the Seedlings over Time According to Biostimulant Treatments and to Cultivars
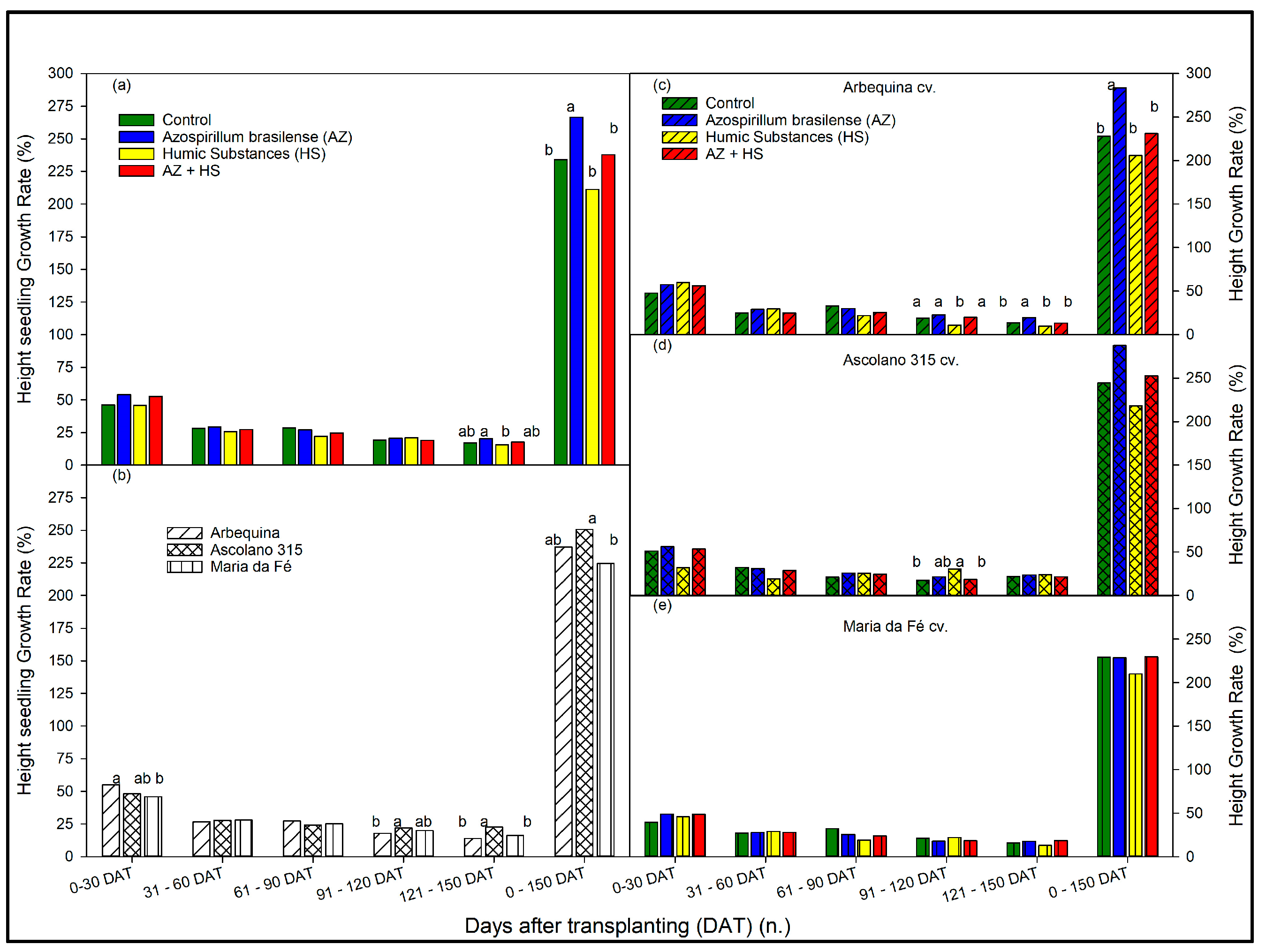
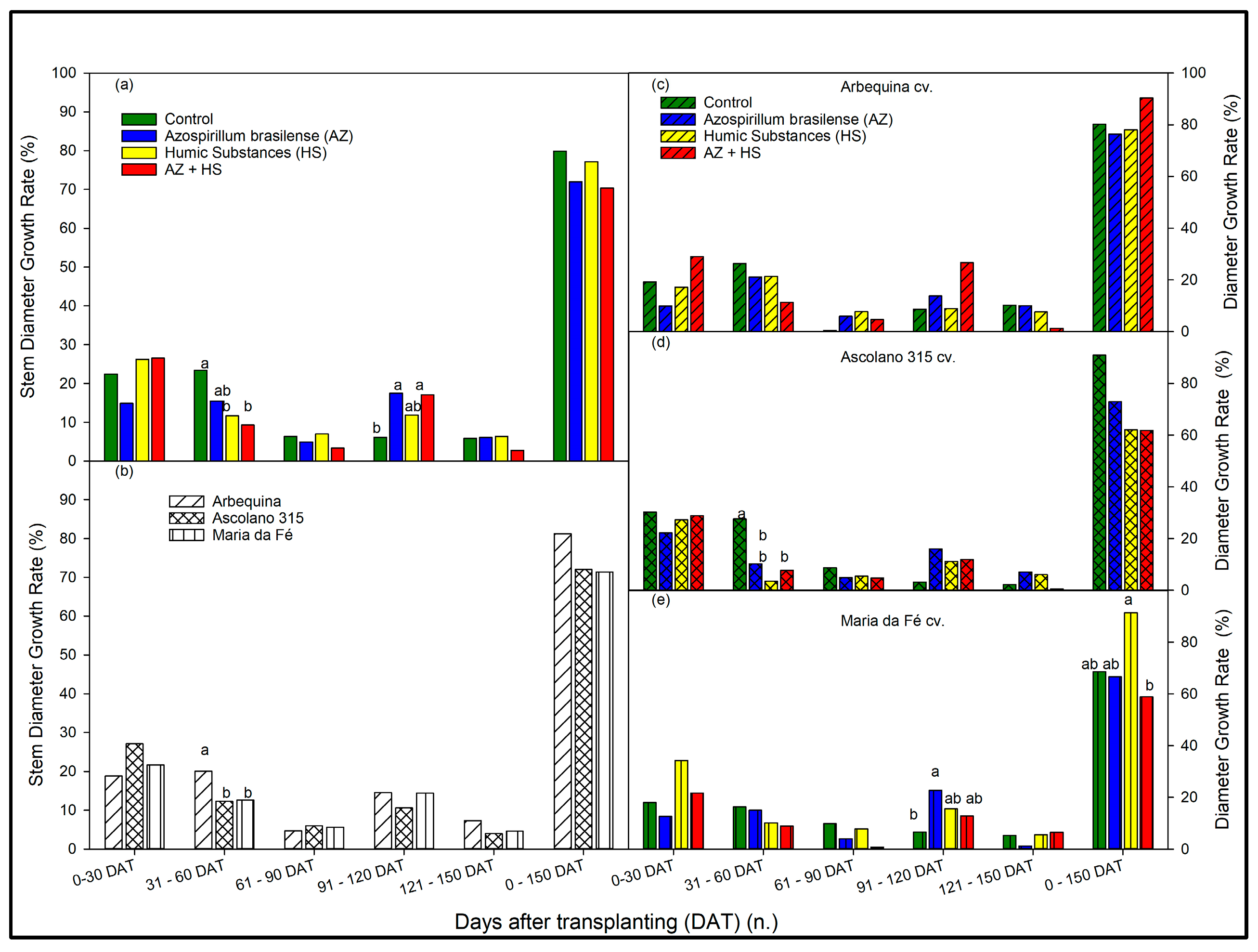
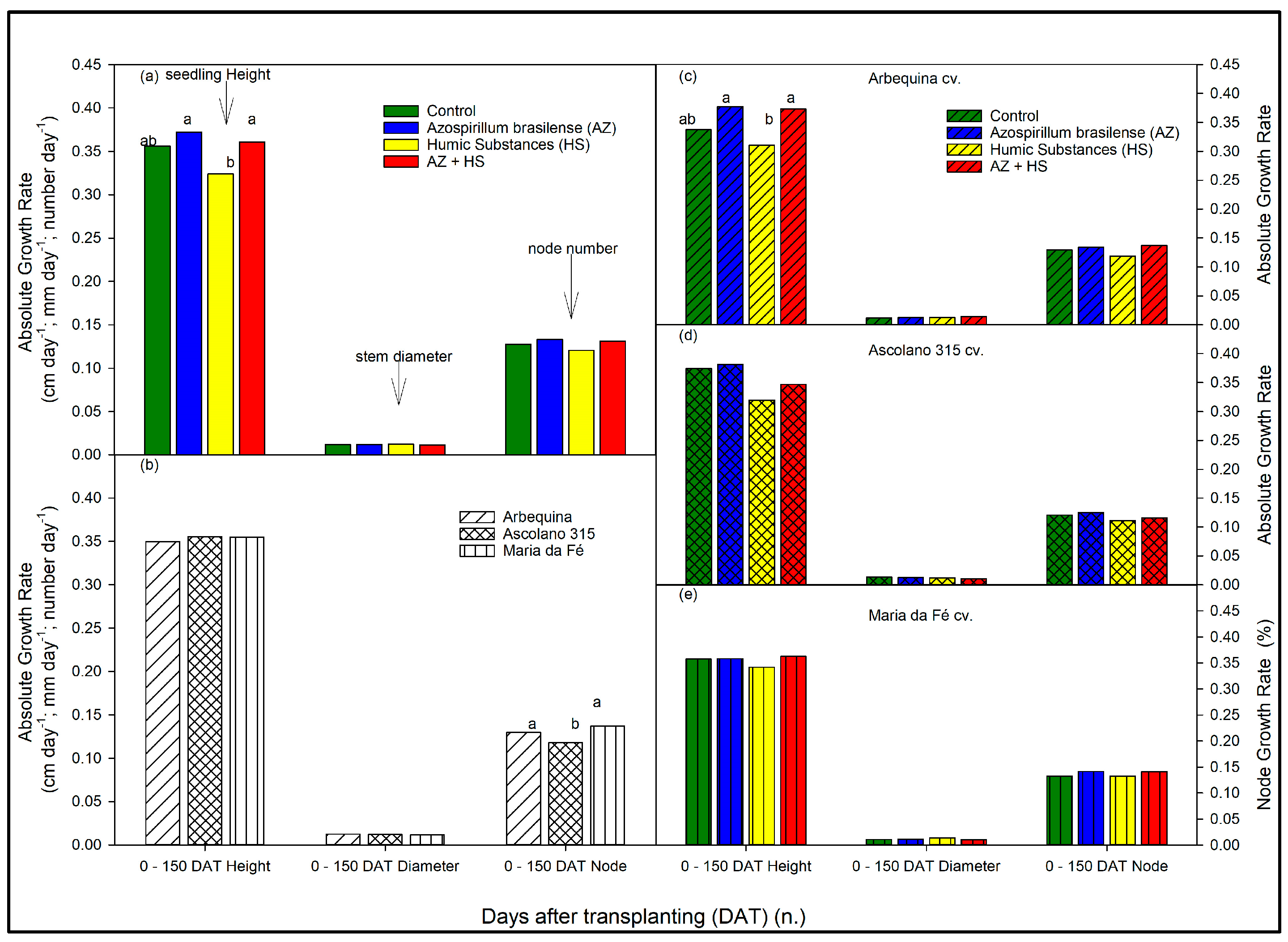
3.2. Growth Rate of the Seedlings over Time According to Biostimulant Treatments and to Cultivars
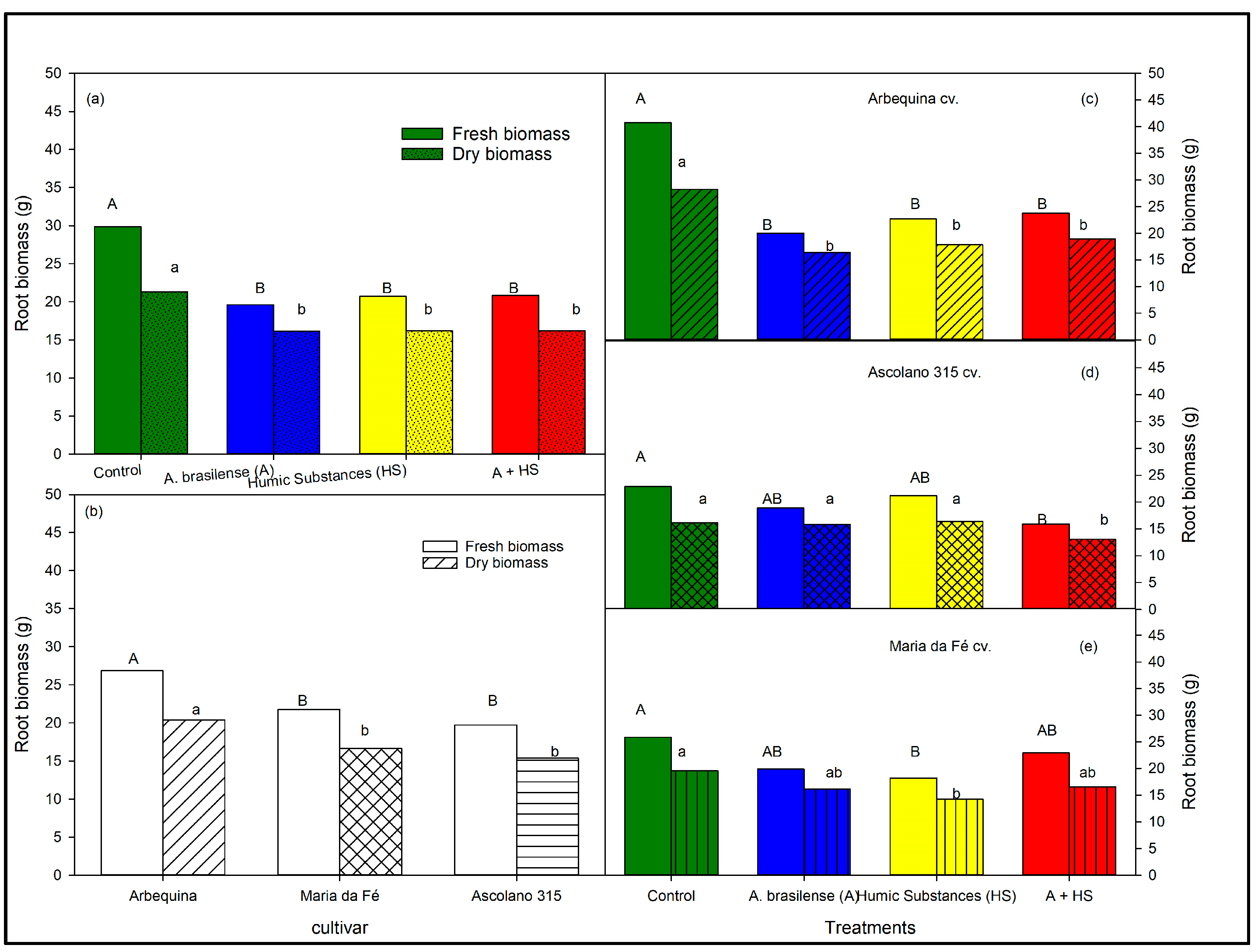
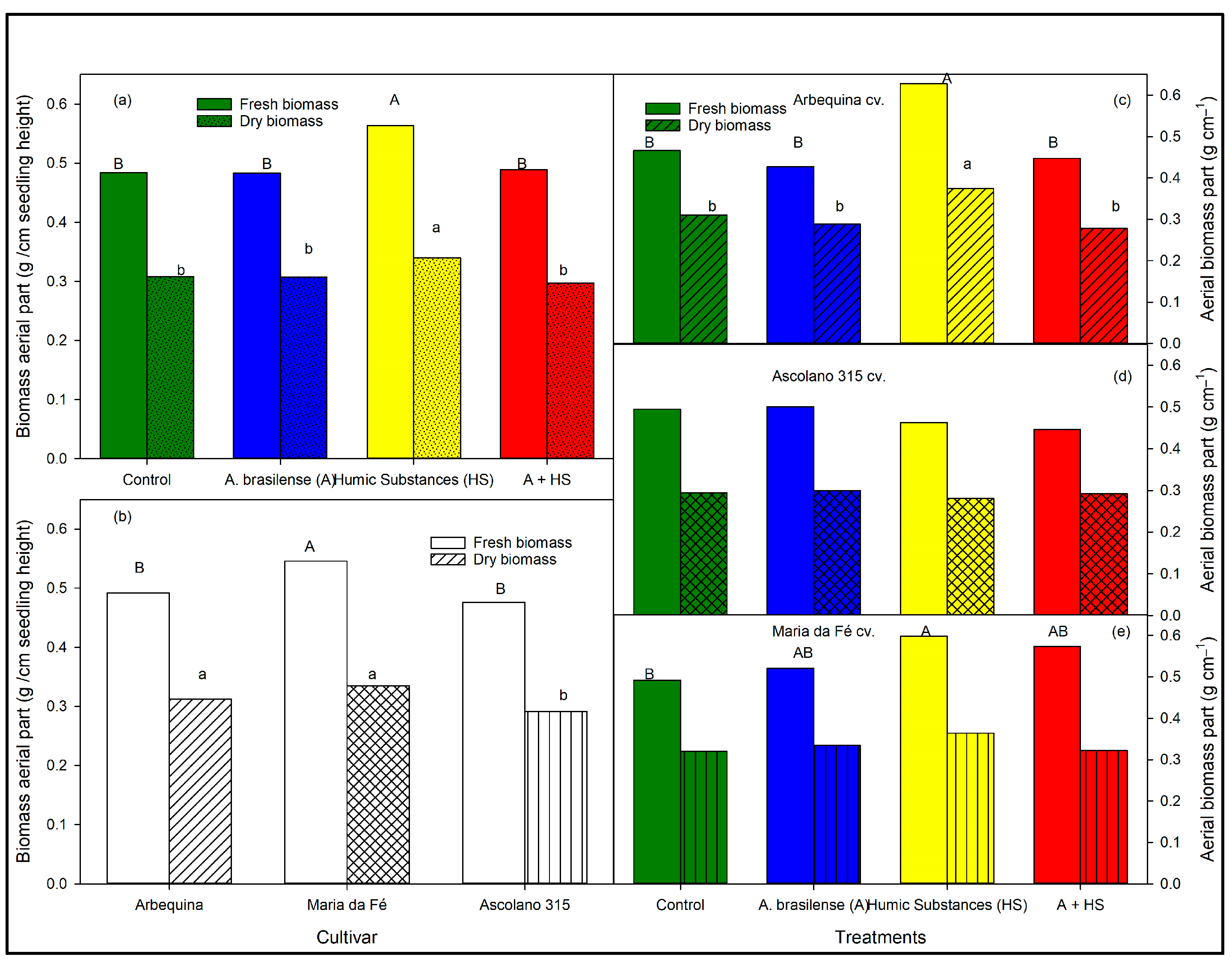
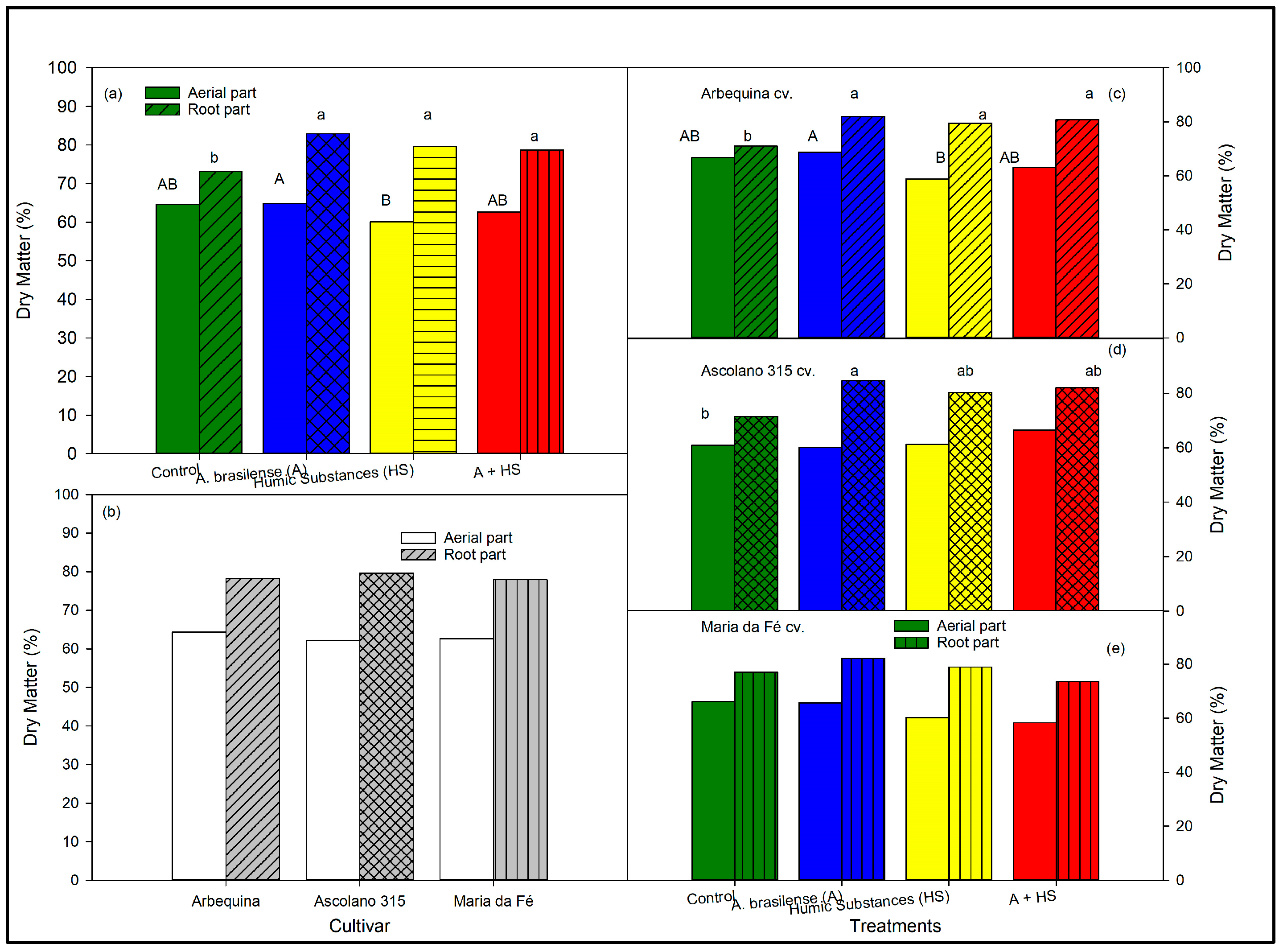
3.3. Aerial and Root Biomasses of the Seedlings at the End of Trial According to Biostimulant Treatments and to Cultivars
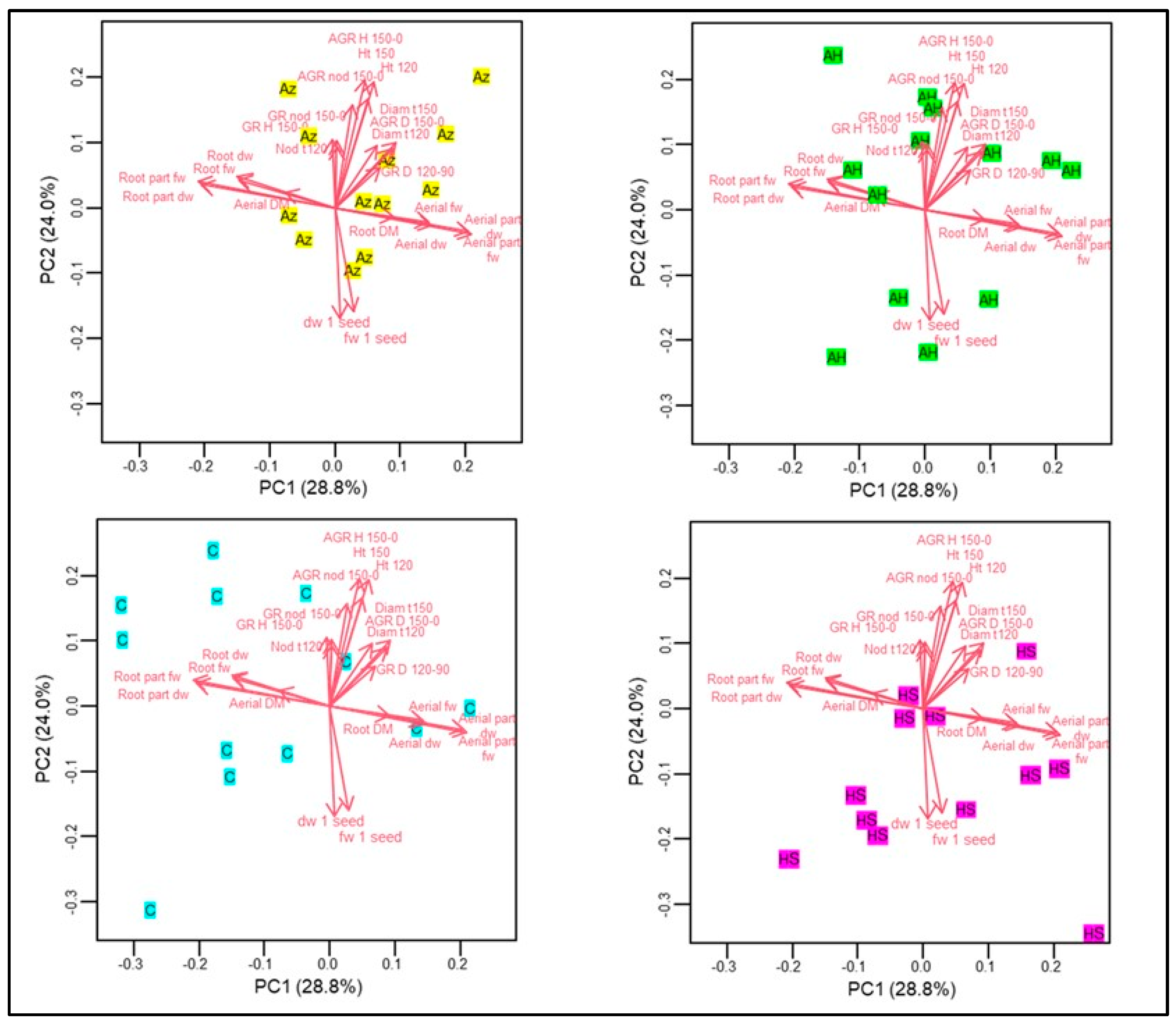
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boeni, M.; Steffen, G.P.K.; Maldaner, J.; Tabaldi, L.A.; Conterato, I.F.; Saldanha, C.W.; Steffen, R.B.; Vieira, F.C.B. Growth-Promoting Microorganisms as a Sustainable Alternative to Optimize the Productive Potential of Olive Plants. Sci. Hortic. 2024, 332, 113167. [Google Scholar] [CrossRef]
- De, D.; Castro, G.; De Sá, F. A Olivicultura e o Azeite No Brasil; Embrapa Agroindústria de Alimentos: Rio de Janeiro, Brazil, 2024; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1165759 (accessed on 29 November 2024).
- Beheiry, H.R.; Awad, A.A.M.; Hussein, H.A.Z. Response of Multi-Stressed Olea europaea Trees to the Adjustment of Soil PH by Acidifying Agents: Impacts on Nutrient Uptake and Productivity. Agronomy 2023, 13, 539. [Google Scholar] [CrossRef]
- Figueiredo, M.C.d.S.; Rosseto, V.; Barão, C.F.; Nunes, R.S.G.; Duarte, I.d.L.; Weber, M.A.; Vieira, F.C.B. Correlations among Nutrient Contents in Soil, Leaf, and Fruit Tissues and Yield of Olea europaea L. Cv. Arbequina in the South of Brazil. Res. Soc. Dev. 2022, 11, e489111033114. [Google Scholar] [CrossRef]
- Silva, B.S.; Schmiele, M. From Olive to Olive Oil: A General Approach. Res. Soc. Dev. 2021, 10, e32210313408. [Google Scholar] [CrossRef]
- da Croce, D.M.; Brugnara, E.C.; de Oliveira, V.P.; Dias, C.R. Avaliação Da Produção e Do Rendimento de Azeite Das Oliveiras ‘Arbequina’, ‘Arbosana’ e ‘Koroneiki’ Em Santa Catarina. Agropecuária Catarin. 2017, 29, 54–57. [Google Scholar] [CrossRef]
- Vergara, J.; da Fonseca, A.; Fiss, C.; Maidana, R.; Wulff, M. Vegetative Development Seedling of Olive Trees ‘Arbequina’ on Different Substrates. Manglar 2020, 17, 141–146. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Mazeh, M.; Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Tucci, M.; Lodolini, E.M.; Rosati, A.; Famiani, F. Use of an Organic Fertilizer Also Having a Biostimulant Action to Promote the Growth of Young Olive Trees. Agriculture 2021, 11, 593. [Google Scholar] [CrossRef]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The Effects of Biostimulants on Induced Plant Defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Baldotto, L.E.B. Ácidos Húmicos. Rev. Ceres 2014, 61, 856–881. [Google Scholar] [CrossRef]
- Rosa, D.M.; Nóbrega, L.H.P.; Mauli, M.M.; de Lima, G.P.; Pacheco, F.P. Humic Substances in Soil Cultivated with Cover Crops Rotated with Maize and Soybean. Rev. Ciência Agronômica 2017, 48, 221–230. [Google Scholar] [CrossRef]
- Olivares, F.L.; Busato, J.G.; de Paula, A.M.; da Silva Lima, L.; Aguiar, N.O.; Canellas, L.P. Plant Growth Promoting Bacteria and Humic Substances: Crop Promotion and Mechanisms of Action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Motok, J.; Salazar, S.M.; Ragout, A.L.; Mentel, M.I.; Tortora, M.L.; Guerrero-Molina, M.F.; Winik, B.C.; Díaz-Ricci, J.C. Growth-Promotion of Strawberry Plants Inoculated with Azospirillum brasilense. World J. Microbiol. Biotechnol. 2010, 26, 265–272. [Google Scholar] [CrossRef]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key Physiological Properties Contributing to Rhizosphere Adaptation and Plant Growth Promotion Abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything You Must Know about Azospirillum and Its Impact on Agriculture and Beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the Plant Growth-Promoting Bacterium Azospirillum brasilense: An Environment-Friendly Component in the Reclamation of Degraded Pastures in the Tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131. [Google Scholar] [CrossRef]
- Carrillo-Flores, E.; Arreola-Rivera, J.; Pazos-Solís, D.M.; Bocanegra-Mondragón, M.; Fierro-Romero, G.; Mellado-Rojas, M.E.; Beltrán-Peña, E. Participation of Auxin Transport in the Early Response of the Arabidopsis Root System to Inoculation with Azospirillum brasilense. Phyton 2022, 91, 2383–2401. [Google Scholar] [CrossRef]
- Costa, S.M.L.; Melloni, R. Relação de Fungos Micorrízicos Arbusculares e Rizobactérias No Crescimento de Mudas de Oliveira (Olea europaea). Ciência Florest. 2019, 29, 169–180. [Google Scholar] [CrossRef]
- de Góes, G.B.; Vilvert, J.C.; de Araújo, N.O.; de Medeiros, J.F.; Aroucha, E.M.M. Application Methods of Biostimulants Affect the Production and Postharvest Conservation of Yellow Melon. Biosci. J. 2021, 37, e37075. [Google Scholar] [CrossRef]
- Zamljen, T.; Grohar, M.C.; Slatnar, A. Effects of Pre- and Post-Transplantation Humic Acid Biostimulant Treatment and Harvest Date on Yield Quantity and Quality Parameters of Sweet Peppers (Capsicum annuum L.). Sci. Hortic. 2024, 338, 113747. [Google Scholar] [CrossRef]
- Verma, I.; Soni, S.K.; Singh, P.C. Trichoderma Produces Methyl Jasmonate-Rich Metabolites in the Presence of Fusarium, Showing Biostimulant Activity and Wilt Resistance in Tomatoes. Plant Physiol. Biochem. 2024, 215, 108953. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding Impact of Azospirillum brasilense Strains Ab-V5 and Ab-V6 on the Brazilian Agriculture: Lessons That Farmers Are Receptive to Adopt New Microbial Inoculants. Rev. Bras. Cienc. Solo 2021, 45, e0200128. [Google Scholar] [CrossRef]
- Prando, A.M.; Barbosa, J.Z.; de Oliveira, A.B.; Nogueira, M.A.; Possamai, E.J.; Hungria, M. Benefits of Soybean Co-Inoculation with Bradyrhizobium spp. and Azospirillum brasilense: Large-Scale Validation with Farmers in Brazil. Eur. J. Agron. 2024, 155, 127112. [Google Scholar] [CrossRef]
- Condori, T.; Alarcón, S.; Huasasquiche, L.; García-Blásquez, C.; Padilla-Castro, C.; Velásquez, J.; Solórzano, R. Inoculation with Azospirillum brasilense as a Strategy to Reduce Nitrogen Fertilization in Cultivating Purple Maize (Zea mays L.) in the Inter-Andean Valleys of Peru. Microorganisms 2024, 12, 2107. [Google Scholar] [CrossRef]
- de Oliveira, A.J.M.; Cavalcanti, V.P.; Rodrigues, F.A.; dos Santos, V.L.; Julio, A.D.L.; Molina, Y.C.; Pasqual, M.; Martins, A.D.; Fufato, L.; Dória, J. Application Frequency and Colonization of the Rhizosphere of Strawberry (Fragaria x Ananassa Duchesne) Plants by Azospirillum brasilense. J. Plant Growth Regul. 2024, 43, 986–997. [Google Scholar] [CrossRef]
- Rosa, D.D.; Villa, F.; da Silva, D.F.; Corbari, F. Rooting of Semihardwood Cuttings of Olive: Indolbutyric Acid, Calcium and Azospirillum brasilense. Comun. Sci. 2018, 9, 34–40. [Google Scholar] [CrossRef][Green Version]
- Ferreira, G.M.d.R.; Silva, L.F.d.O.; Pasqual, M.; Melloni, R.; Magno Queiroz Luz, J.; Dória, J. Microbial Biostimulants as Alternatives for the Rooting of Olive Tree Cuttings. Biosci. J. 2022, 38, e38091. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Nitsche, P.R.; Caramori, P.H.; Ricce, W.D.S.; Pinto, L.F.D. Atlas Climático do Estado do Paraná; Instituto Agronômico do Paraná: Londrina, Brazil, 2019. [Google Scholar]
- Traini, C.; Facchin, S.L.; Brigante, R.; Vinci, A.; Persichetti, S.; Meneghini, M.; Micheli, M.; Famiani, F.; Portarena, S.; Dradi, G.; et al. Field Performance of Grafted, Micropropagated, and Own-Rooted Plants of Three Italian Hazelnut Cultivars during the Initial Four Seasons of Development. Front. Plant Sci. 2024, 15, 1412170. [Google Scholar] [CrossRef]
- Benincasa, M.M.P. Análise de Crescimento de Plantas Noções Básicas, 2nd ed.; Funep: Sao Paulo, Brazil, 2003. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Bazakos, C.; Alexiou, K.G.; Ramos-Onsins, S.; Koubouris, G.; Tourvas, N.; Xanthopoulou, A.; Mellidou, I.; Moysiadis, T.; Vourlaki, I.; Metzidakis, I.; et al. Whole Genome Scanning of a Mediterranean Basin Hotspot Collection Provides New Insights into Olive Tree Biodiversity and Biology. Plant J. 2023, 116, 303–319. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; García-López, M.C.; Vidoy, I.; de la O Leyva-Pérez, M.; Fernández-Ocaña, A.; Barroso, J.B.; Barceló, A.; Beuzón, C.R.; de la Rosa, R.; Luque, F. Transcriptional Analysis of Adult Cutting and Juvenile Seedling Olive Roots. Tree Genet. Genomes 2015, 11, 77. [Google Scholar] [CrossRef]
- Ritter, G.; Villa, F.; da Silva, D.F.; Eberling, T.; Guimarães, V.F.; da Silva, E.C.; Dória, J. Substâncias Húmicas e Formas de Aplicação No Enraizamento de Estacas Semilenhosas de Oliveira. Comun. Sci. 2023, 14, e3700. [Google Scholar] [CrossRef]
- Arrobas, M.; de Almeida, S.F.; Raimundo, S.; da Silva Domingues, L.; Rodrigues, M.Â. Leonardites Rich in Humic and Fulvic Acids Had Little Effect on Tissue Elemental Composition and Dry Matter Yield in Pot-Grown Olive Cuttings. Soil Syst. 2022, 6, 7. [Google Scholar] [CrossRef]
- Souza, A.d.G.; Smiderle, O.J.; Maia, S.d.S.; Dias, T.J. Do Azospirillum brasilense Application Methods and Doses Influence the Quality of Cordia Alliodora Seminal Seedlings? Sci. For. 2023, 51, e3971. [Google Scholar] [CrossRef]
- Lopes, J.I.; Correia, C.M.; Gonçalves, A.; Silva, E.; Martins, S.; Arrobas, M.; Rodrigues, M.Â. Arbuscular Mycorrhizal Fungi Inoculation Reduced the Growth of Pre-Rooted Olive Cuttings in a Greenhouse. Soil Syst. 2021, 5, 30. [Google Scholar] [CrossRef]
- Iqbal, R.; Valipour, M.; Ali, B.; Zulfiqar, U.; Aziz, U.; Zaheer, M.S.; Sarfraz, A.; Javed, M.A.; Afridi, M.S.; Ercisli, S.; et al. Maximizing Wheat Yield through Soil Quality Enhancement: A Combined Approach with Azospirillum brasilense and Bentonite. Plant Stress 2024, 11, 100321. [Google Scholar] [CrossRef]
- Luciani, E.; Palliotti, A.; Tombesi, S.; Gardi, T.; Micheli, M.; Berrios, J.G.; Zadra, C.; Farinelli, D. Mitigation of Multiple Summer Stresses on Hazelnut (Corylus avellana L.): Effects of the New Arbuscular Mycorrhiza Glomus iranicum tenuihypharum sp. Nova. Sci. Hortic. 2019, 257, 108659. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Brugnoli, E.; Battistelli, A.; Proietti, S.; Moscatello, S.; Famiani, F.; Tombesi, S.; Zadra, C.; Farinelli, D. Carbon Allocation Strategies and Water Uptake in Young Grafted and Own-Rooted Hazelnut (Corylus avellana L.) Cultivars. Tree Physiol. 2022, 42, 939–957. [Google Scholar] [CrossRef]

| A | P | OM | pH | H + Al | Al3+ | K+ | Ca2+ | Mg2+ | SB | CEC | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg dm−3 | g dm−3 | ----------cmolc dm−3---------- | % | |||||||||
| A1 | 443.04 | 39.63 | 6.32 | 2.91 | 0.09 | 1.30 | 9.98 | 3.96 | 15.24 | 15.33 | 83.96 | 0.50 |
| A2 | 298.44 | 42.38 | 6.37 | 2.79 | 0.00 | 2.42 | 7.73 | 5.72 | 15.87 | 18.66 | 85.05 | 0.00 |
| A | Sand (%) | Silt (%) | Clay (%) | |||||||||
| A1 | 23.79 | 13.51 | 62.70 | |||||||||
| Cultivar | Seedling Height (cm) | Stem Diameter (mm) | Seedling Node (n.) |
|---|---|---|---|
| Arbequina | 22.33 ± 0.35 ab | 2.35 ± 0.05 b | 14 ± 0.32 a |
| Ascolano 315 | 21.68 ± 0.43 b | 2.64 ± 0.10 a | 12 ± 0.34 b |
| Maria da Fé | 23.78 ± 0.30 a | 2.53 ± 0.09 ab | 15 ± 0.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, G.; de Vargas, R.J.; Farinelli, D.; Cinosi, N.; Traini, C.; Facchin, S.L.; Hiromi Kiahara, L.; da Silva, D.F.; Portarena, S.; Villa, F. Application of Azospirillum brasilense and Humic Substances Improves the Nursery Quality of Olive Seedlings in Pots. Horticulturae 2025, 11, 48. https://doi.org/10.3390/horticulturae11010048
Ritter G, de Vargas RJ, Farinelli D, Cinosi N, Traini C, Facchin SL, Hiromi Kiahara L, da Silva DF, Portarena S, Villa F. Application of Azospirillum brasilense and Humic Substances Improves the Nursery Quality of Olive Seedlings in Pots. Horticulturae. 2025; 11(1):48. https://doi.org/10.3390/horticulturae11010048
Chicago/Turabian StyleRitter, Giovana, Rodrigo José de Vargas, Daniela Farinelli, Nicola Cinosi, Chiara Traini, Simona Lucia Facchin, Larissa Hiromi Kiahara, Daniel Fernandes da Silva, Silvia Portarena, and Fabiola Villa. 2025. "Application of Azospirillum brasilense and Humic Substances Improves the Nursery Quality of Olive Seedlings in Pots" Horticulturae 11, no. 1: 48. https://doi.org/10.3390/horticulturae11010048
APA StyleRitter, G., de Vargas, R. J., Farinelli, D., Cinosi, N., Traini, C., Facchin, S. L., Hiromi Kiahara, L., da Silva, D. F., Portarena, S., & Villa, F. (2025). Application of Azospirillum brasilense and Humic Substances Improves the Nursery Quality of Olive Seedlings in Pots. Horticulturae, 11(1), 48. https://doi.org/10.3390/horticulturae11010048







