Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Site Description
2.2. Experimental Design
2.2.1. Plant 3D Phenotyping
2.2.2. Morphological Indicators
- (1)
- Plant Height: The height was determined by measuring from the base of the seedling to its highest growth point.
- (2)
- Stem Diameter: Measurements were taken 1 cm above the ground using a vernier caliper.
- (3)
- Leaf Count: Leaves longer than 2 cm were counted visually.
- (4)
- Leaf Area: All leaves were washed with distilled water and laid flat for measurement using the WinRHIZO Arabidopsis 2021 system.
- (5)
- Leaf Thickness: This was measured with a vernier caliper.
- (6)
- Fresh Weight: Plant material was quickly cut and weighed using an analytical balance.
- (7)
- Dry Weight: The lettuce was placed in an oven and dried at 105 °C for one and a half hours, after which it was weighed. Each treatment involved selecting 10 plants for measurement.
2.2.3. Photosynthetic Characteristics
2.2.4. Chlorophyll Content
2.2.5. Root Traits
2.2.6. Chlorophyll Fluorescence Parameters
2.2.7. Data Processing Methods
3. Results and Discussion
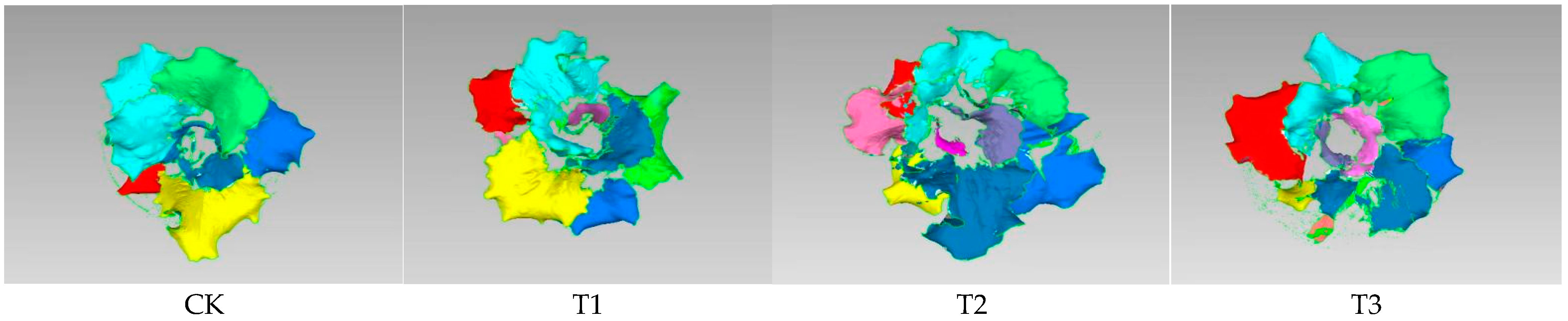
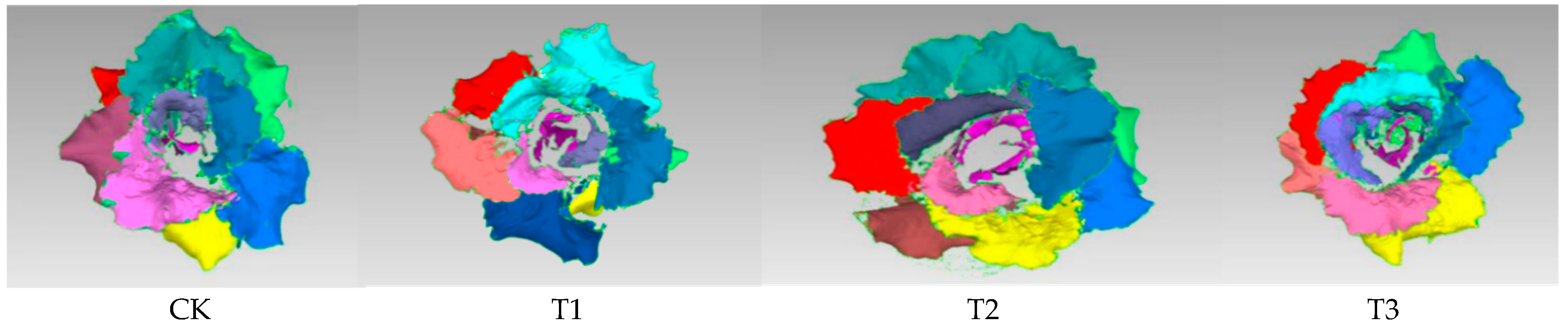
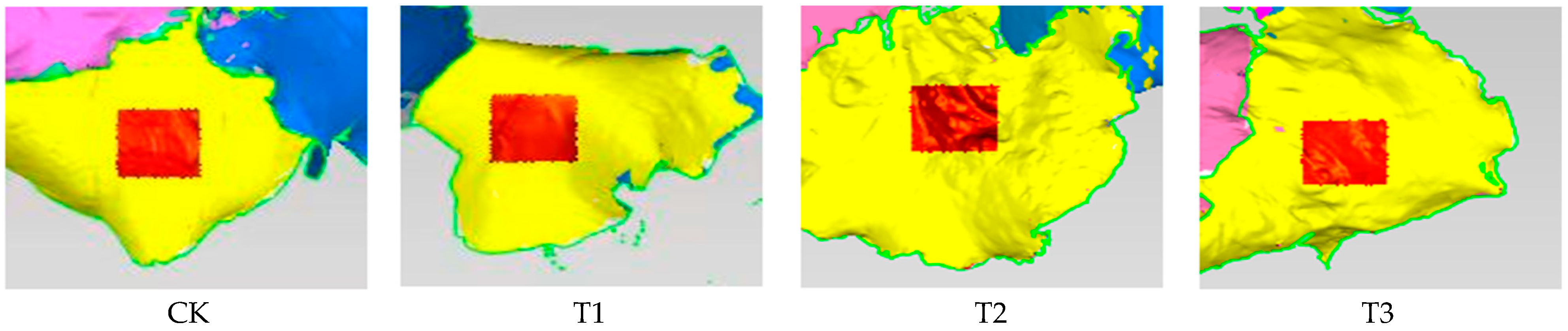
3.1. Effects of Different Treatments on Lettuce Phenotype
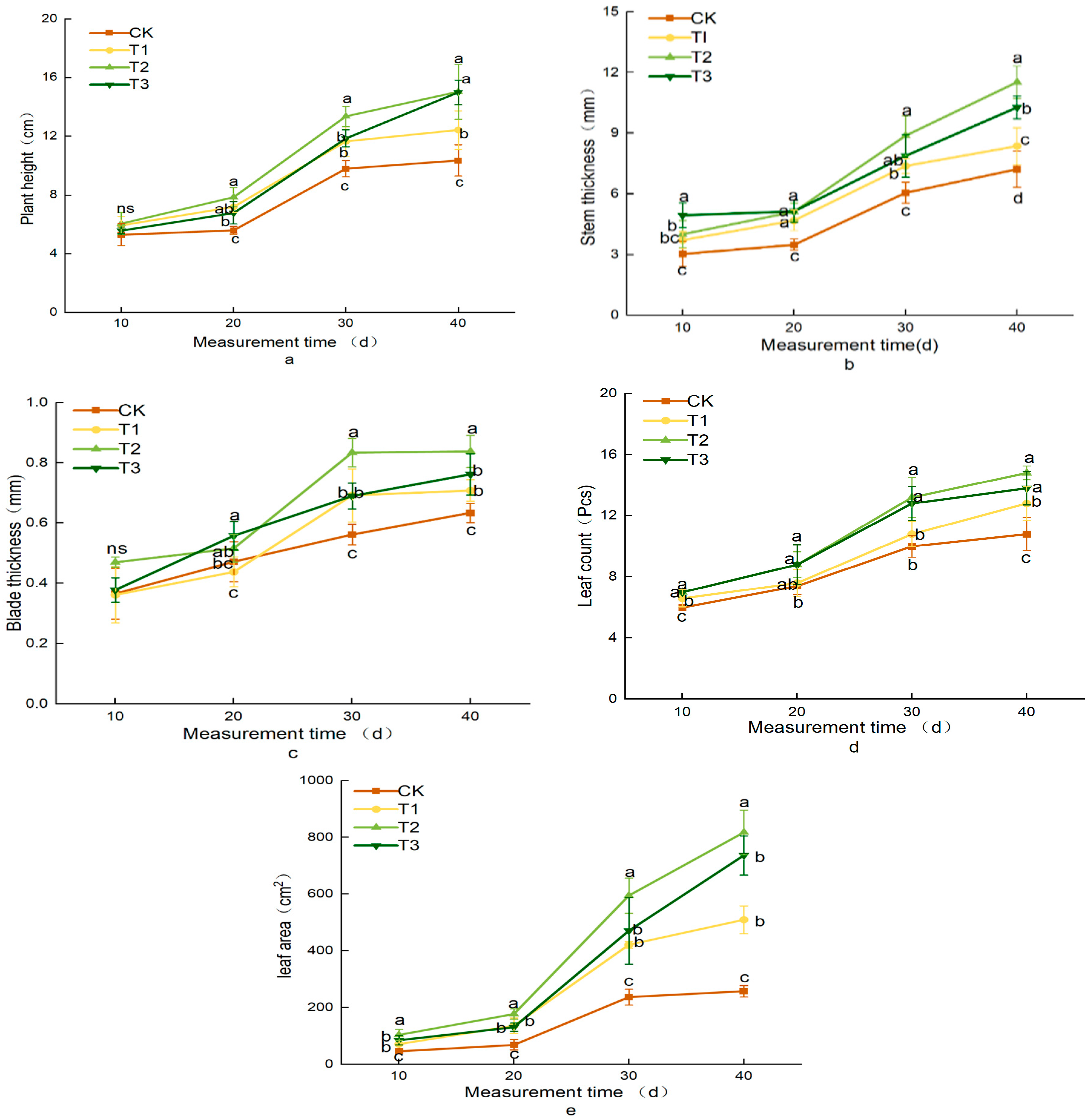
3.2. Effects of Different Treatments on Lettuce Growth Indicators
3.3. Effects of Different Treatments on Photosynthetic Characteristics of Lettuce
3.4. Effects of Different Treatments on Chlorophyll Content and Photosynthetic Pigments in Lettuce
3.5. Effects of Different Treatments on Lettuce Root Traits
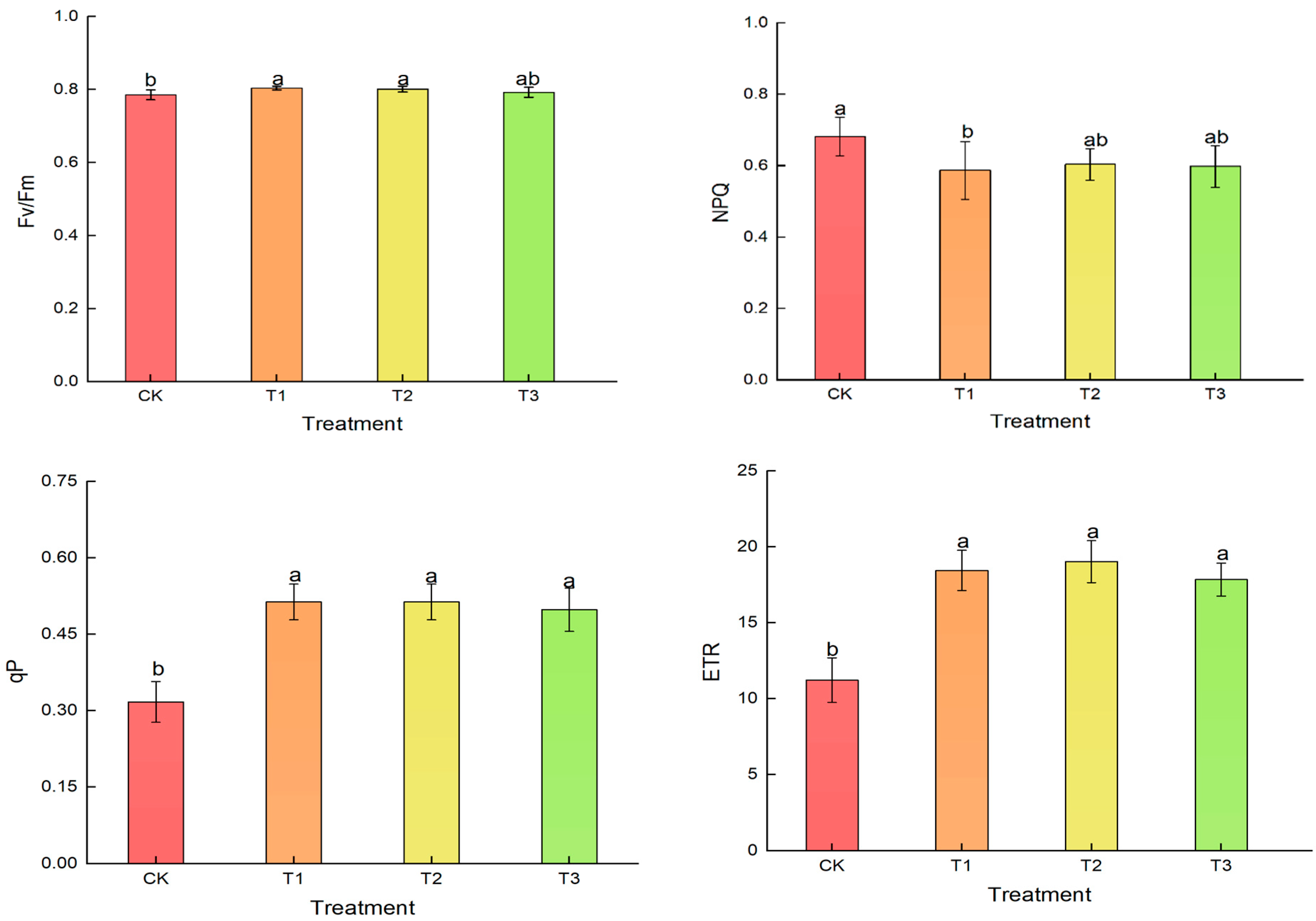
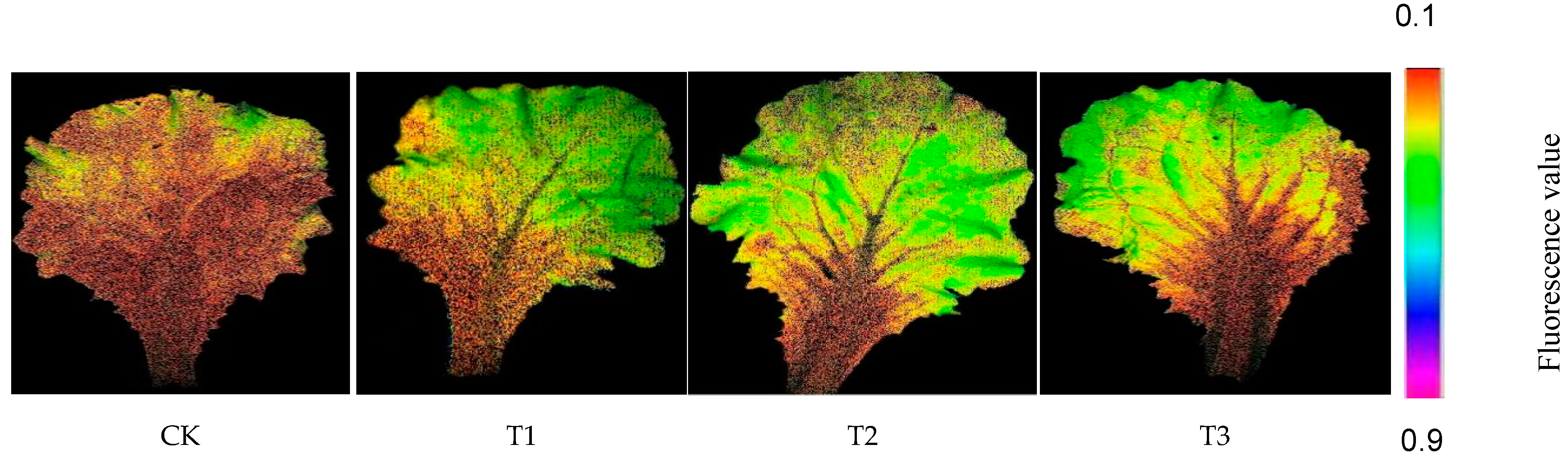
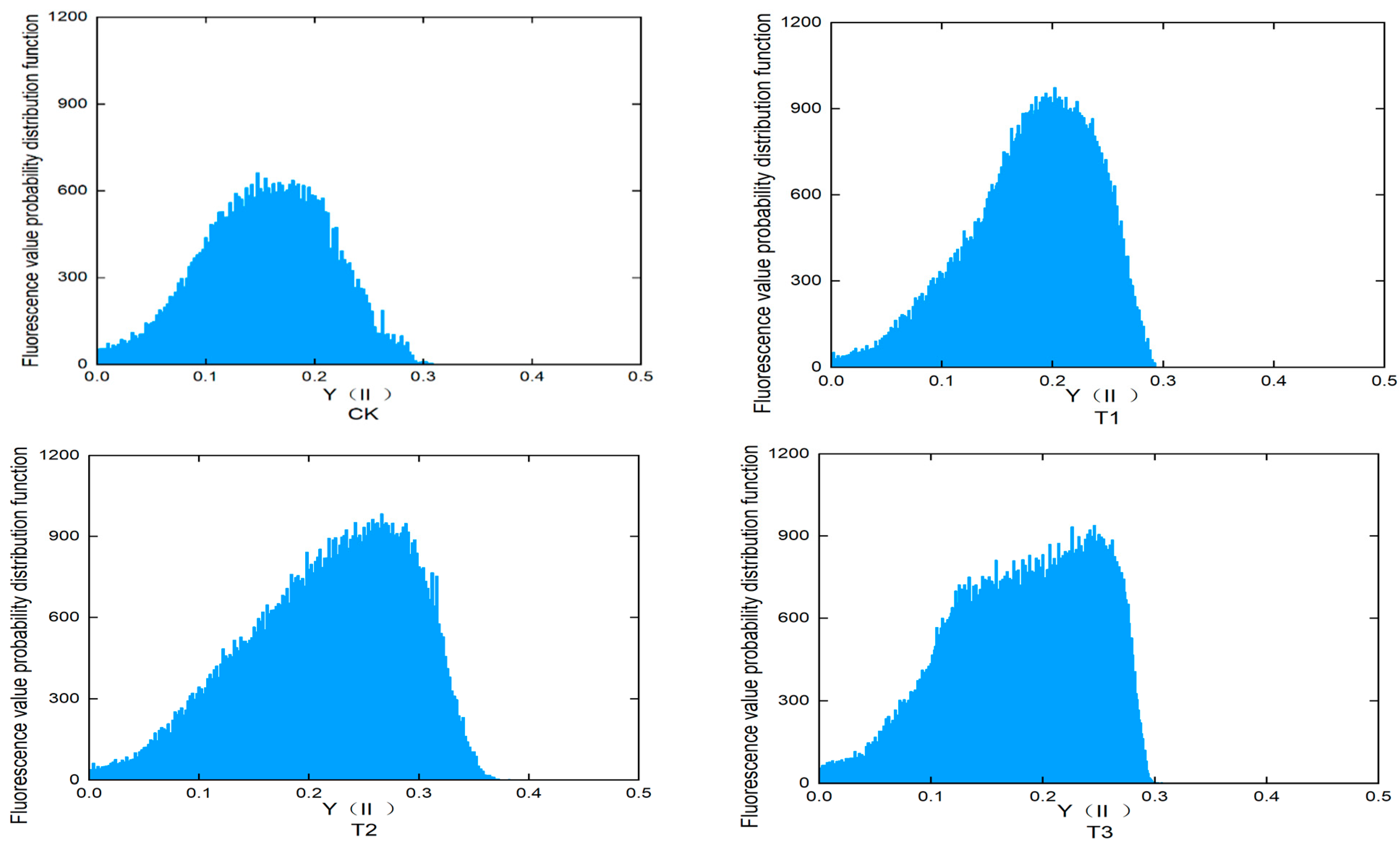
3.6. Effects of Different Treatments on Chlorophyll Fluorescence Parameters in Lettuce
4. Discussion
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Razzak, M.A.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Effects of Supplementing Green Light to Red and Blue Light on the Growth and Yield of Lettuce in Plant Factories. Sci. Hortic. 2022, 305, 111429. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.-X.; Yang, Q.-C. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. South Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of Green Light on Nitrate Reduction and Edible Quality of Hydroponically Grown Lettuce (Lactuca sativa L.) under Short-Term Continuous Light from Red and Blue Light-Emitting Diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf Photosynthetic Rate, Growth, and Morphology of Lettuce under Different Fractions of Red, Blue, and Green Light from Light-Emitting Diodes (Leds). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F. Improving Light Management in Lettuce Crops. Nat. Food 2021, 2, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Sipos, L.; Boros, I.F.; Csambalik, L.; Székely, G.; Jung, A.; Balázs, L. Horticultural Lighting System Optimalization: A Review. Sci. Hortic. 2020, 273, 109631. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light Regulation of Horticultural Crop Nutrient Uptake and Utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light Intensity and Photoperiod Influence the Growth and Development of Hydroponically Grown Leaf Lettuce in a Closed-Type Plant Factory System. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Kozai, T.; Fujiwara, K.; Runkle, E.S. Led Lighting for Urban Agriculture; Springer: Singapore, 2016. [Google Scholar]
- Viršilė, A.; Olle, M.; Duchovskis, P. Led Lighting in Horticulture. In Light Emitting Diodes for Agriculture: Smart Lighting; Gupta, S.D., Ed.; Springer: Singapore, 2017; pp. 113–147. [Google Scholar]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant Responses to Red and Far-Red Lights, Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Kumar, B.; Angulo, Y.; Smita, K.; Cumbal, L.; Debut, A. Capuli Cherry-Mediated Green Synthesis of Silver Nanoparticles under White Solar and Blue Led Light. Particuology 2016, 24, 123–128. [Google Scholar] [CrossRef]
- Mitchell, C.; Dzakovich, M.; Gomez, C.; Lopez, R.; Burr, J.; Hernández, R.; Kubota, C.; Currey, C.; Meng, Q.; Runkle, E.; et al. Light-Emitting Diodes in Horticulture. In Horticultural Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–87. [Google Scholar]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulturae 2022, 8, 435. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of Supplemental Lighting with Light-Emitting Diodes (Leds) on Tomato Yield and Quality of Single-Truss Tomato Plants Grown at High Planting Density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F.; et al. Supplementary Led Interlighting Improves Yield and Precocity of Greenhouse Tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hayashi, Y. Chapter Three—New Insights into the Regulation of Stomatal Opening by Blue Light and Plasma Membrane H+-Atpase. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 89–115. [Google Scholar]
- Matthews, J.; Vialet-Chabrand, S.; Lawson, T. Role of Blue and Red Light in Stomatal Dynamic Behaviour. J. Exp. Bot. 2019, 71, 2253–2269. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Barbaresi, A.; Barbanti, L.; Mandrone, M.; Poli, F.; Torreggiani, D.; Trenta, M.; Tassinari, P. Effects of Led Supplemental Lighting on the Growth and Metabolomic Profile of Taxus Baccata Cultivated in a Smart Greenhouse. PLoS ONE 2022, 17, e0266777. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of Green Light Wavelength and Intensity on Photomorphogenesis and Photosynthesis in Lactuca Sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Mickens, M.A.; Torralba, M.S.; Robinson, S.A.; Spencer, L.E.; Romeyn, M.W.; Massa, G.D.; Wheeler, R.M. Growth of Red Pak Choi under Red and Blue, Supplemented White, and Artificial Sunlight Provided by Leds. Sci. Hortic. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Saito, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. The Effect of Light Quality on Growth of Lettuce. IFAC Proc. Vol. 2010, 43, 294–298. [Google Scholar] [CrossRef]
- Trouwborst, G.; Schapendonk, A.H.C.M.; Rappoldt, K.; Pot, S.; Hogewoning, S.W.; van Ieperen, W. The Effect of Intracanopy Lighting on Cucumber Fruit Yield—Model Analysis. Sci. Hortic. 2011, 129, 273–278. [Google Scholar] [CrossRef]
- Xiao, L.; Shibuya, T.; Kato, K.; Nishiyama, M.; Kanayama, Y. Effects of Light Quality on Plant Development and Fruit Metabolism and Their Regulation by Plant Growth Regulators in Tomato. Sci. Hortic. 2022, 300, 111076. [Google Scholar] [CrossRef]
- Ieperen, W.; Savvides, A.; Fanourakis, D. Red and Blue Light Effects During Growth on Hydraulic and Stomatal Conductance in Leaves of Young Cucumber Plants. Acta Hortic. 2012, 956, 223–230. [Google Scholar] [CrossRef]
- Lu, N.; Mitchell, C.A. Supplemental Lighting for Greenhouse-Grown Fruiting Vegetables. In Led Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer: Singapore, 2016; pp. 219–232. [Google Scholar]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gianquinto, G.; Orsini, F. Effect of Supplementary Red and Blue Led Interlighting on Greenhouse-Grown Tomatoes During Spring Season in the Mediterranean. Acta Hortic. 2022, 1337, 31–38. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Zhang, Y.-T.; Liu, H.-C.; Li, Y.-M.; Liu, Y.-L.; Hao, Y.-W.; Lei, B.-F. Supplemental Blue Light Increases Growth and Quality of Greenhouse Pak Choi Depending on Cultivar and Supplemental Light Intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Klein, R.M. Effects of Green Light on Biological Systems. Biol. Rev. Camb. Philos. Soc. 1992, 67, 199–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, H. Sensing the Light Environment: The Functions of the Phytochrome Family. In Photomorphogenesis in Plants; Kendrick, R.E., Kronenberg, G.H.M., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 377–416. [Google Scholar]
- Pan, T.; Ding, J.; Qin, G.; Wang, Y.; Xi, L.; Yang, J.; Li, J.; Zhang, J.; Zou, Z. Interaction of Supplementary Light and CO2 Enrichment Improves Growth, Photosynthesis, Yield, and Quality of Tomato in Autumn through Spring Greenhouse Production. HortSci. Horts 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Tang, Z.; Wu, Y.; Xiao, X.; Zhang, G.; Hu, L.; Liu, Z.; Lyu, J.; Yu, J. Red and Blue Led Light Supplementation in the Morning Pre-Activates the Photosynthetic System of Tomato (Solanum lycopersicum L.) Leaves and Promotes Plant Growth. Agronomy 2022, 12, 897. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Azad, M.O.K.; Park, C.H. Effect of Different Combinations of Red and Blue Led Light on Growth Characteristics and Pigment Content of in Vitro Tomato Plantlets. Agriculture 2019, 9, 196. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of Light on Secondary Metabolites in Selected Leafy Greens: A Review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of Yield, Lycopene, and Lutein Content in Tomatoes Grown under Continuous Par Spectrum Led Lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef]
- Ji, T.; Du, Y.; Wei, M.; Gu, D.; Li, J.; Wang, H.; Yang, F. Adding Different Proportions of Red/Blue = 3/1 to White Light Affects Eggplant Seedling Quality by Regulating Leaf Morphology and Photosynthetic System. Plant Growth Regul. 2023, 100, 593–607. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and B of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-Red Light: A Regulator of Plant Morphology and Photosynthetic Capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Wassenaar, M.L.J.; van Ieperen, W.; Driever, S.M. Low Red to Far-Red Ratio Increases Resistance to CO2 Diffusion and Reduces Photosynthetic Efficiency in Low Light Grown Tomato Plants. Environ. Exp. Bot. 2022, 200, 104918. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, C.; Li, Z.; Li, X.; Cheng, F.; Lin, D.; Yang, Y. Supplementary White, Uv-a, and Far-Red Radiation Differentially Regulates Growth and Nutritional Qualities of Greenhouse Lettuce. Plants 2023, 12, 3234. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light Added with Red Leds Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, K.-H.; Oh, M.-M. Increase in Biomass and Bioactive Compounds in Lettuce under Various Ratios of Red to Far-Red Led Light Supplemented with Blue Led Light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Kushunina, M.A.; Zhigalova, T.V. Effects of Red and Blue Led Light on the Growth and Photosynthesis of Barley (Hordeum vulgare L.) Seedlings. J. Plant Growth Regul. 2023, 42, 1804–1820. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, Y.; Fan, P.; Wang, Y.; Xu, X.; Wang, L.; Li, S.; Duan, W.; Liang, Z.; Dai, Z. Far-Red Light Modulates Grapevine Growth by Increasing Leaf Photosynthesis Efficiency and Triggering Organ-Specific Transcriptome Remodelling. BMC Plant Biol. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Panigrahi, K.C.S. Light and Auxin Signaling Cross-Talk Programme Root Development in Plants. J. Biosci. 2019, 44, 26. [Google Scholar] [CrossRef]
- Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The Role of Light-Regulated Auxin Signaling in Root Development. Int. J. Mol. Sci. 2023, 24, 5253. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Lin, C.-C. Red Light-Emitting Diode Light Irradiation Improves Root and Leaf Formation in Difficult-to-Propagate Protea Cynaroides L. Plantlets in Vitro. HortSci. Horts 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, K.; Wang, Y.; Wang, H.; Ding, S.; Song, D.; Shen, J.; Li, H.; Song, Y.; Han, X.; et al. Red and Blue Light Affect the Formation of Adventitious Roots of Tea Cuttings (Camellia sinensis) by Regulating Hormone Synthesis and Signal Transduction Pathways of Mature Leaves. Front. Plant Sci. 2022, 13, 943662. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. Led Technology Applied to Plant Development for Promoting the Accumulation of Bioactive Compounds: A Review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of Different Led Sources on the Growth and Nitrogen Metabolism of Lettuce. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Wang, D.; Mao, Z.; Fahlman, B.D. Phosphors with a 660-Nm-Featured Emission for Led/Ld Lighting in Horticulture. In Phosphors, up Conversion Nano Particles, Quantum Dots and Their Applications; Liu, R.-S., Ed.; Springer: Singapore, 2016; Volume 2, pp. 83–117. [Google Scholar]
- Finlayson, S.A.; Hays, D.B.; Morgan, P.W. Phyb-1 Sorghum Maintains Responsiveness to Simulated Shade, Irradiance and Red Light: Far-Red Light. Plant Cell Env. 2007, 30, 952–962. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Li, Y.; Li, S.; Zhang, Y. Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda Salsa in Coastal Wetlands. Remote Sens. 2021, 13, 2066. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortSci. Horts 2015, 50, 1128–1135. [Google Scholar] [CrossRef]

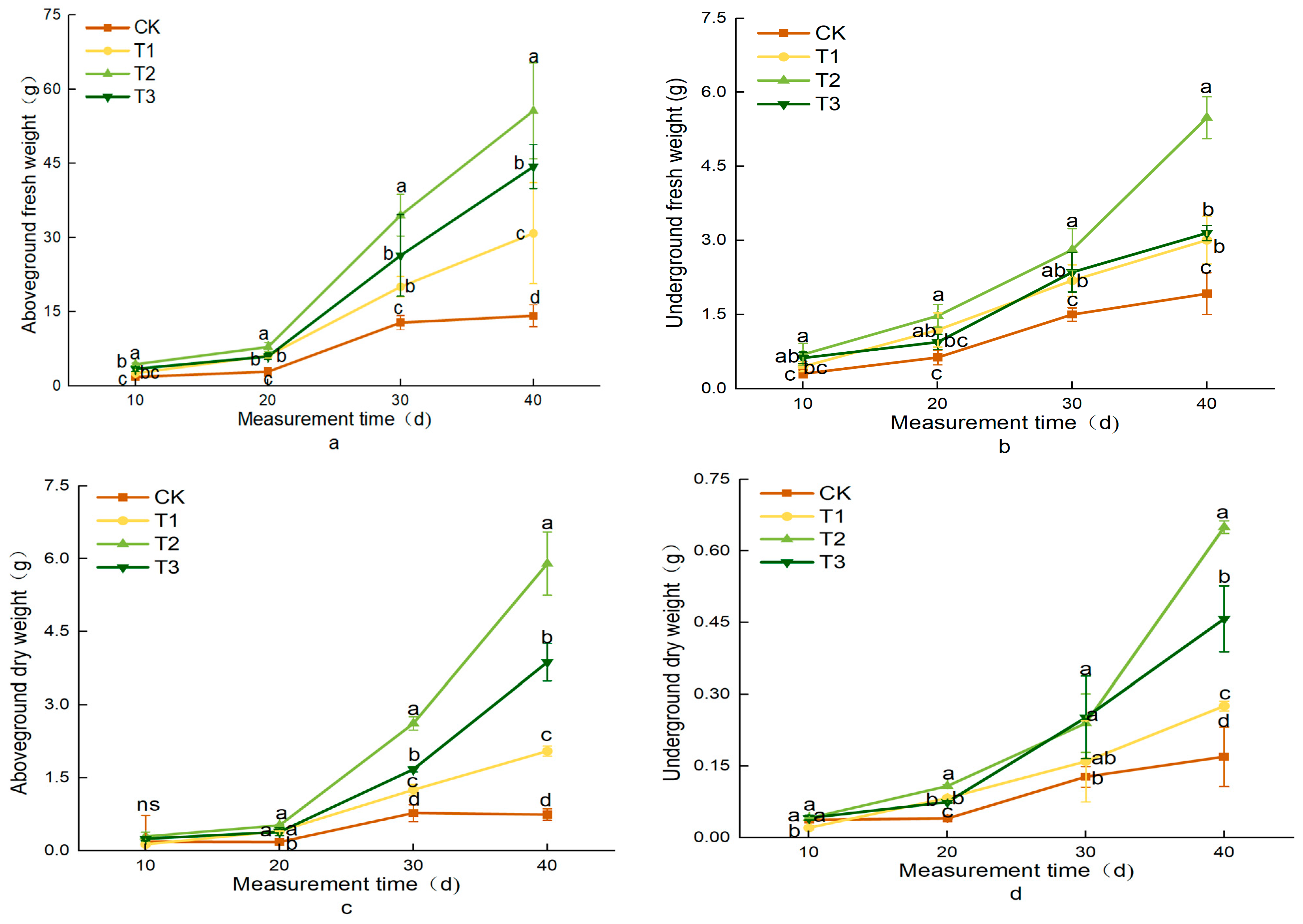
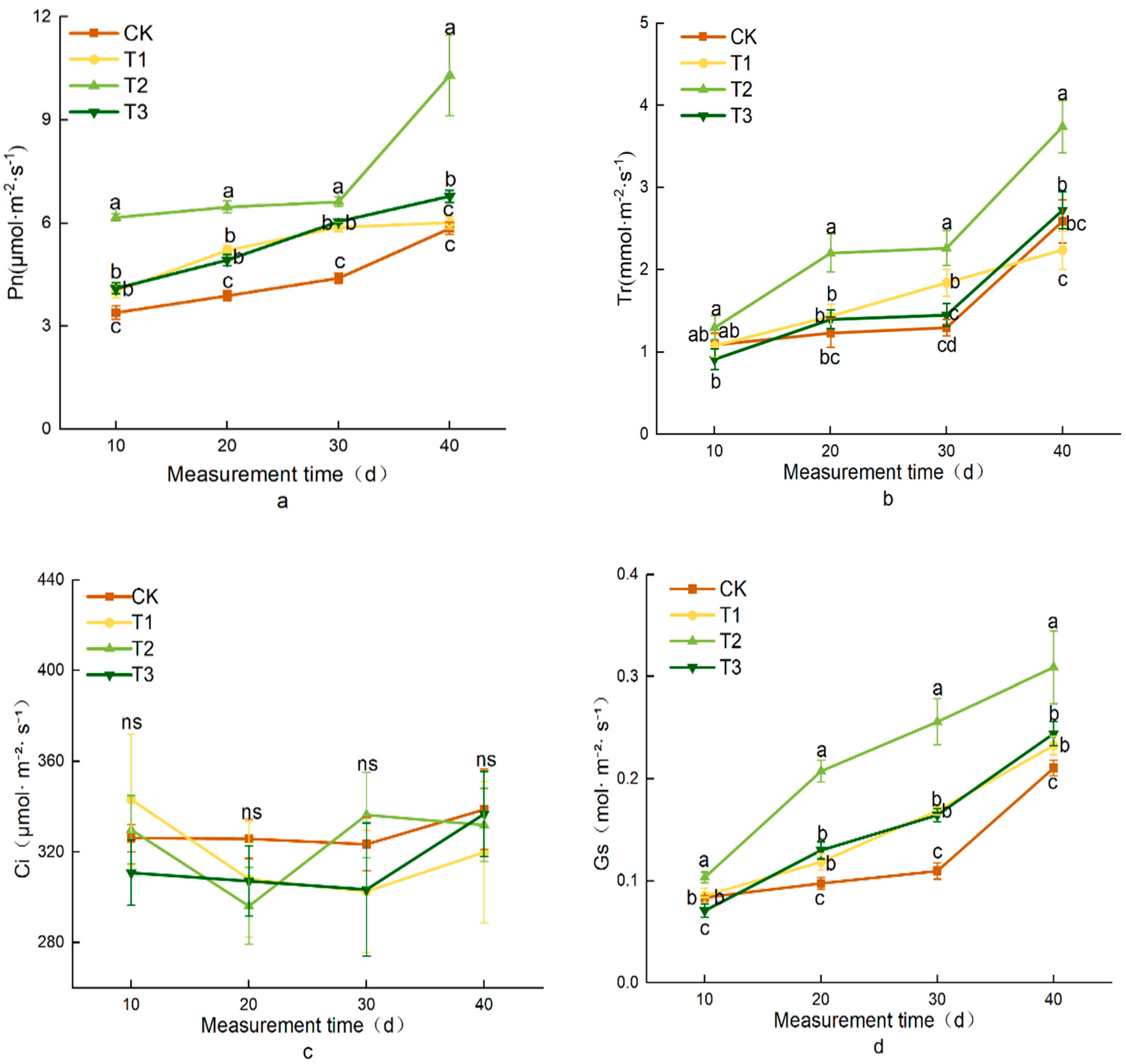
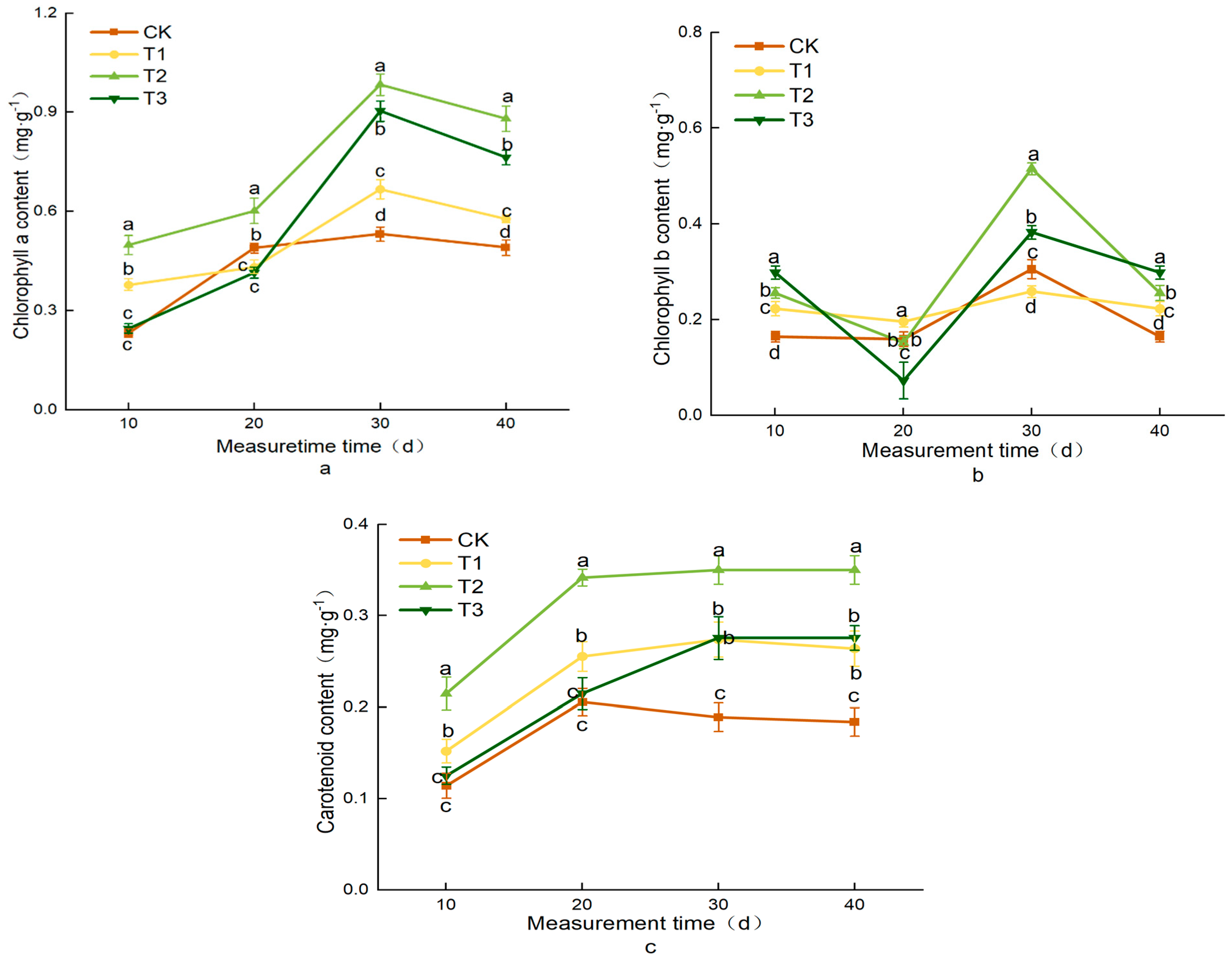
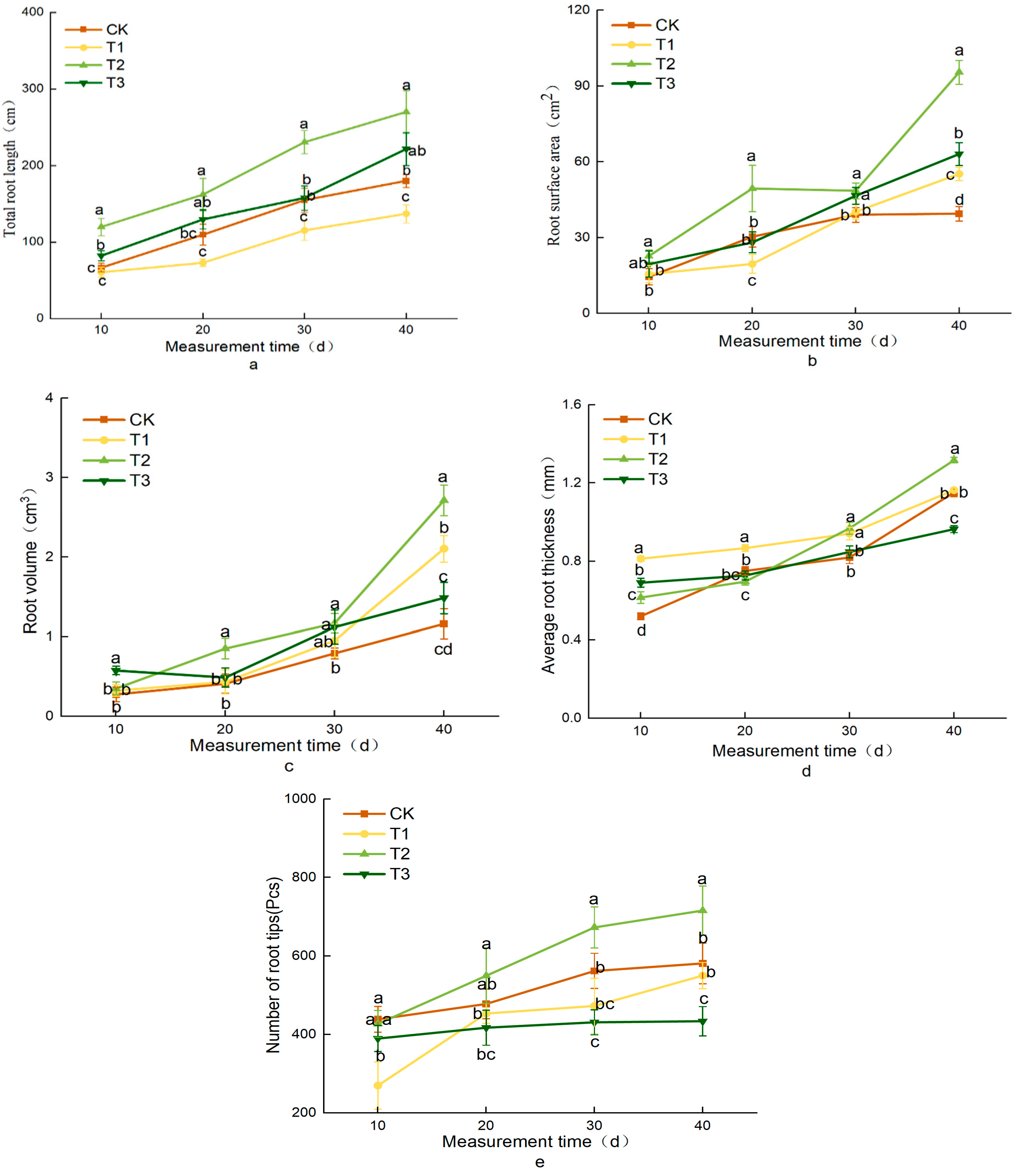
| Treatment | Aboveground Fresh Weight (g) | Underground Fresh Weight (g) | Aboveground Dry Weight (g) | Underground Dry Weight (g) |
|---|---|---|---|---|
| CK | 14.21 ± 2.23 d | 1.92 ± 0.42 c | 0.75 ± 0.12 d | 0.17 ± 0.06 d |
| T1 | 30.91 ± 10.22 c | 3.01 ± 0.38 b | 2.05 ± 0.10 c | 0.28 ± 0.01 c |
| T2 | 55.67 ± 9.74 a | 5.49 ± 0.42 a | 5.90 ± 0.65 a | 0.65 ± 0.01 a |
| T3 | 44.39 ± 4.45 b | 3.15 ± 0.15 b | 3.88 ± 0.38 b | 0.46 ± 0.07 b |
| Treatment | Tr (mmol·m−2·s−1) | Pn (μmol·m−2·s−1) | Ci (μmol·m⁻2·s−1) | Gs (mol·m−2·s−1) |
|---|---|---|---|---|
| CK | 2.59 ± 0.26 bc | 5.84 ± 0.18 c | 338.81 ± 17.76 ns | 0.21 ± 0.01 c |
| T1 | 2.24 ± 0.24 c | 6.01 ± 0.18 c | 319.90 ± 31.20 ns | 0.23 ± 0.01 b |
| T2 | 3.74 ± 0.32 a | 10.30 ± 1.71 a | 331.91 ± 16.20 ns | 0.31 ± 0.04 a |
| T3 | 2.73 ± 0.23 b | 6.78 ± 0.18 b | 336.82 ± 18.73 ns | 0.24 ± 0.01 b |
| Treatment | Chlorophyll a (mg·g−1) | Chlorophyll b (mg·g−1) | Carotenoid content (mg·g−1) | Total Chlorophyll Content (mg·g−1) | Chlorophyll a/b |
|---|---|---|---|---|---|
| CK | 0.49 ± 0.02 d | 0.16 ± 0.01 d | 0.18 ± 0.02 c | 0.65 ± 0.02 d | 3.06 ± 0.05 b |
| T1 | 0.58 ± 0.01 c | 0.22 ± 0.01 c | 0.26 ± 0.02 b | 0.80 ± 0.02 c | 2.64 ± 0.01 c |
| T2 | 0.88 ± 0.04 a | 0.26 ± 0.01 b | 0.35 ± 0.02 a | 1.14 ± 0.05 a | 3.38 ± 0.05 a |
| T3 | 0.76 ± 0.02 b | 0.30 ± 0.01 a | 0.28 ± 0.01 b | 1.06 ± 0.03 b | 2.53 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Hu, W.; Chen, S.; Cui, H.; Qi, J.; Yu, Y.; Liu, S.; Wang, J. Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.). Horticulturae 2025, 11, 43. https://doi.org/10.3390/horticulturae11010043
Qiao J, Hu W, Chen S, Cui H, Qi J, Yu Y, Liu S, Wang J. Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.). Horticulturae. 2025; 11(1):43. https://doi.org/10.3390/horticulturae11010043
Chicago/Turabian StyleQiao, Jianlei, Wen Hu, Shanshan Chen, Hongbo Cui, Jiangtao Qi, Yue Yu, Shuang Liu, and Jianfeng Wang. 2025. "Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.)" Horticulturae 11, no. 1: 43. https://doi.org/10.3390/horticulturae11010043
APA StyleQiao, J., Hu, W., Chen, S., Cui, H., Qi, J., Yu, Y., Liu, S., & Wang, J. (2025). Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.). Horticulturae, 11(1), 43. https://doi.org/10.3390/horticulturae11010043






