The Potential of Sitka Spruce Bark as an Alternative to Peat Casing for Mushroom (Agaricus bisporus) Production
Abstract
1. Introduction
2. Materials and Methods
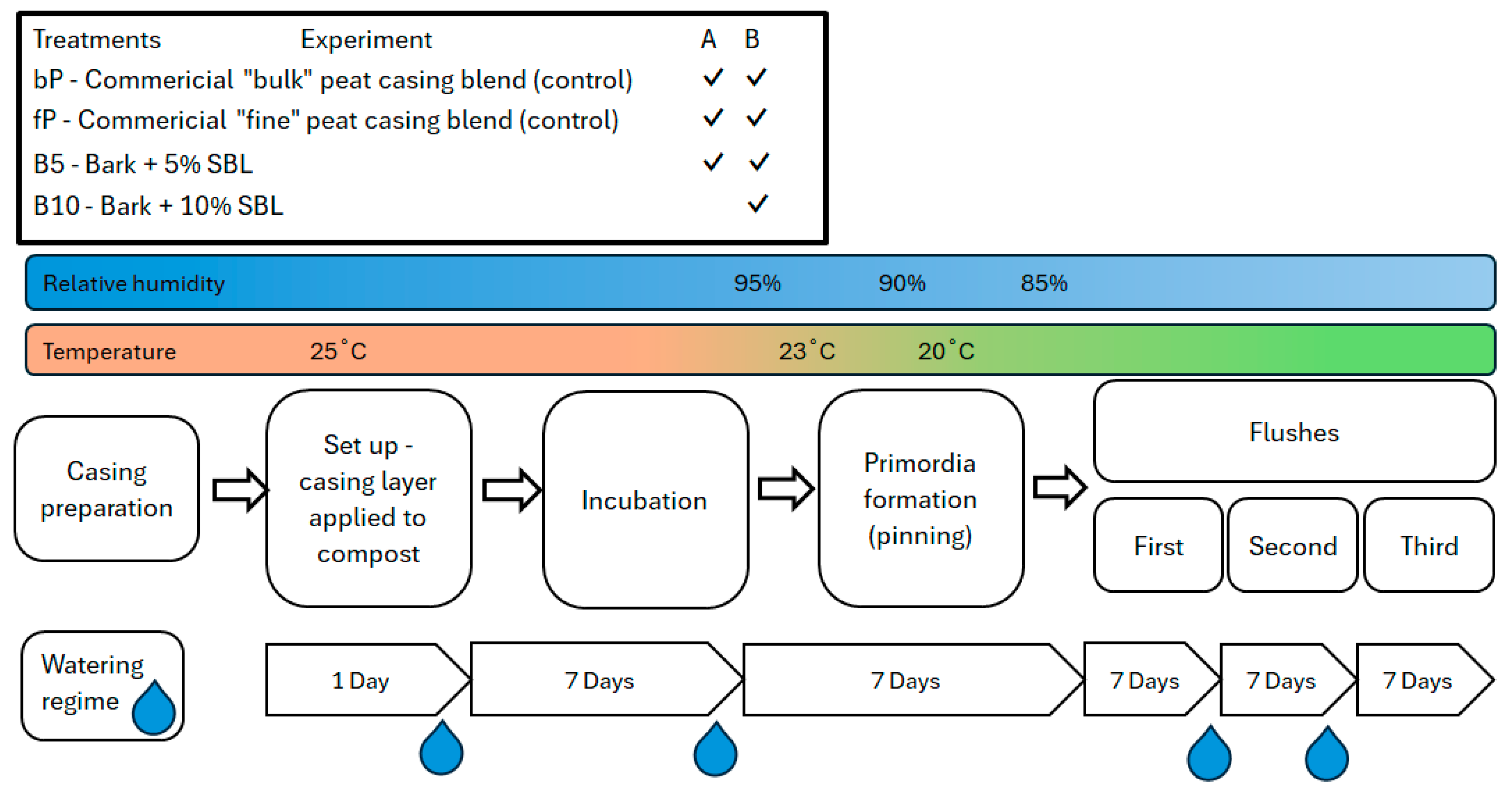
2.1. Casing Treatments
2.2. Mushroom Cropping Details
2.3. Experiments
2.4. Casing Water Content
2.5. pH
2.6. Mushroom Colour Assessment
2.7. Experimental Design and Statistical Analysis
3. Results
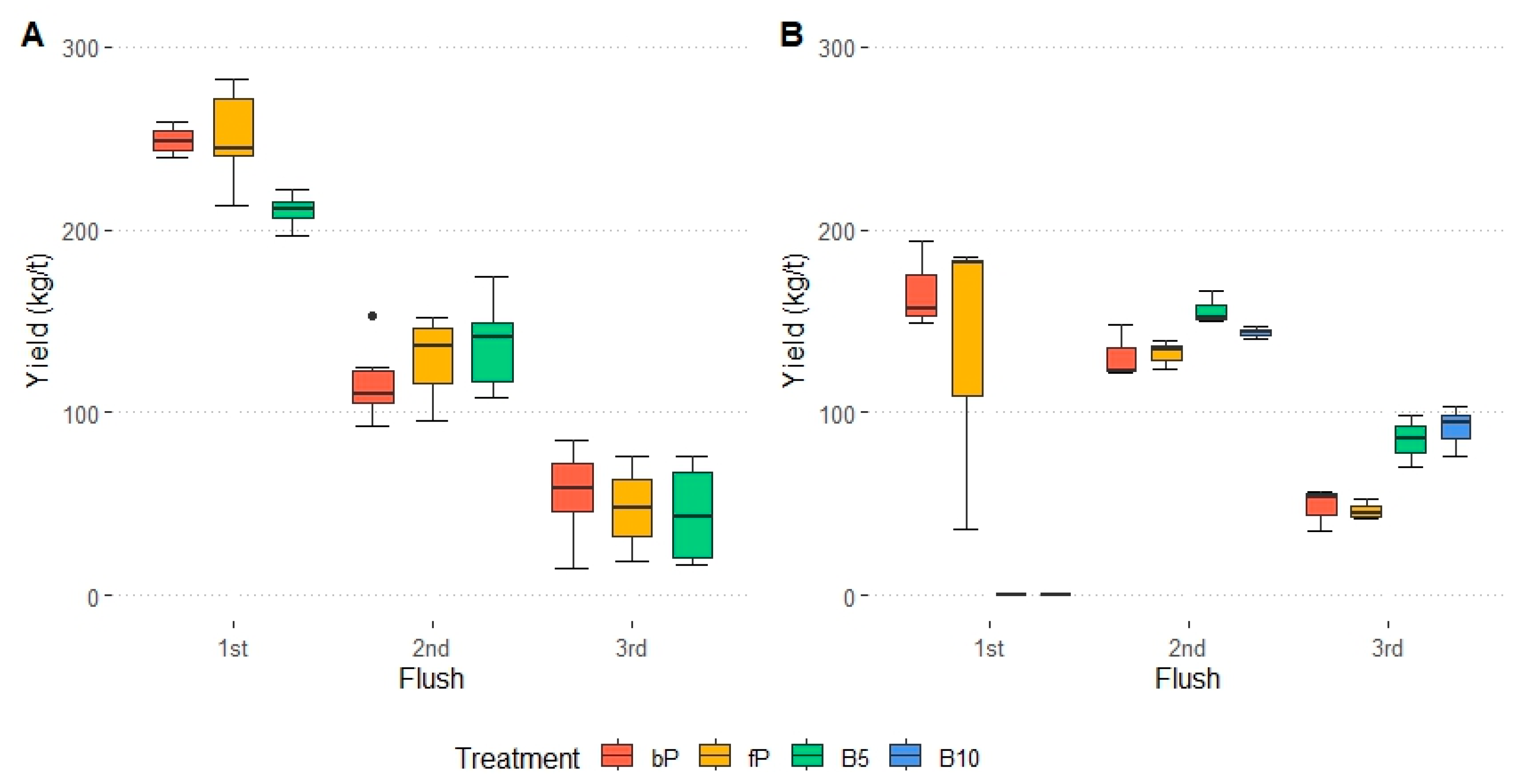
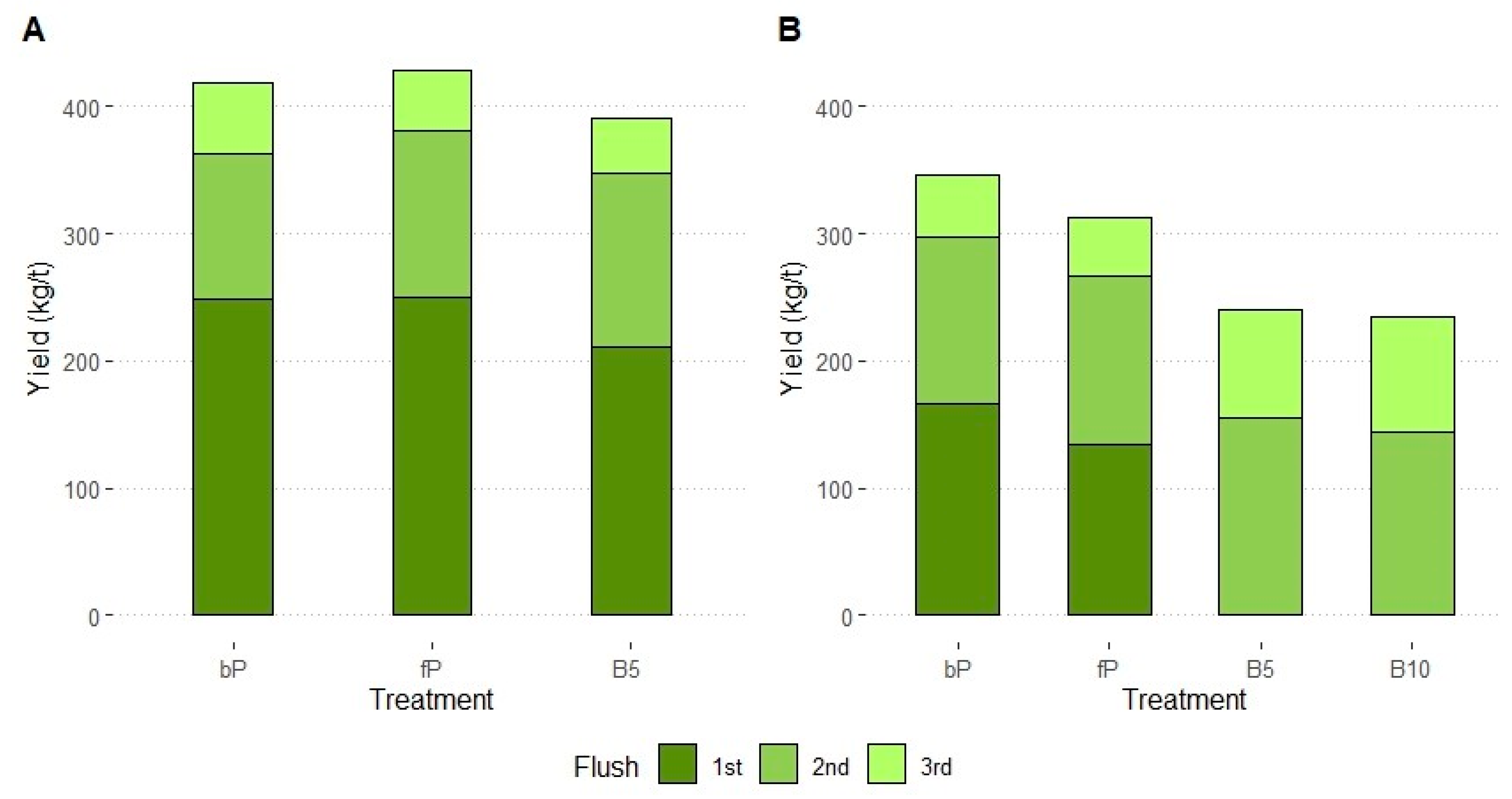
3.1. Yield
3.2. Moisture
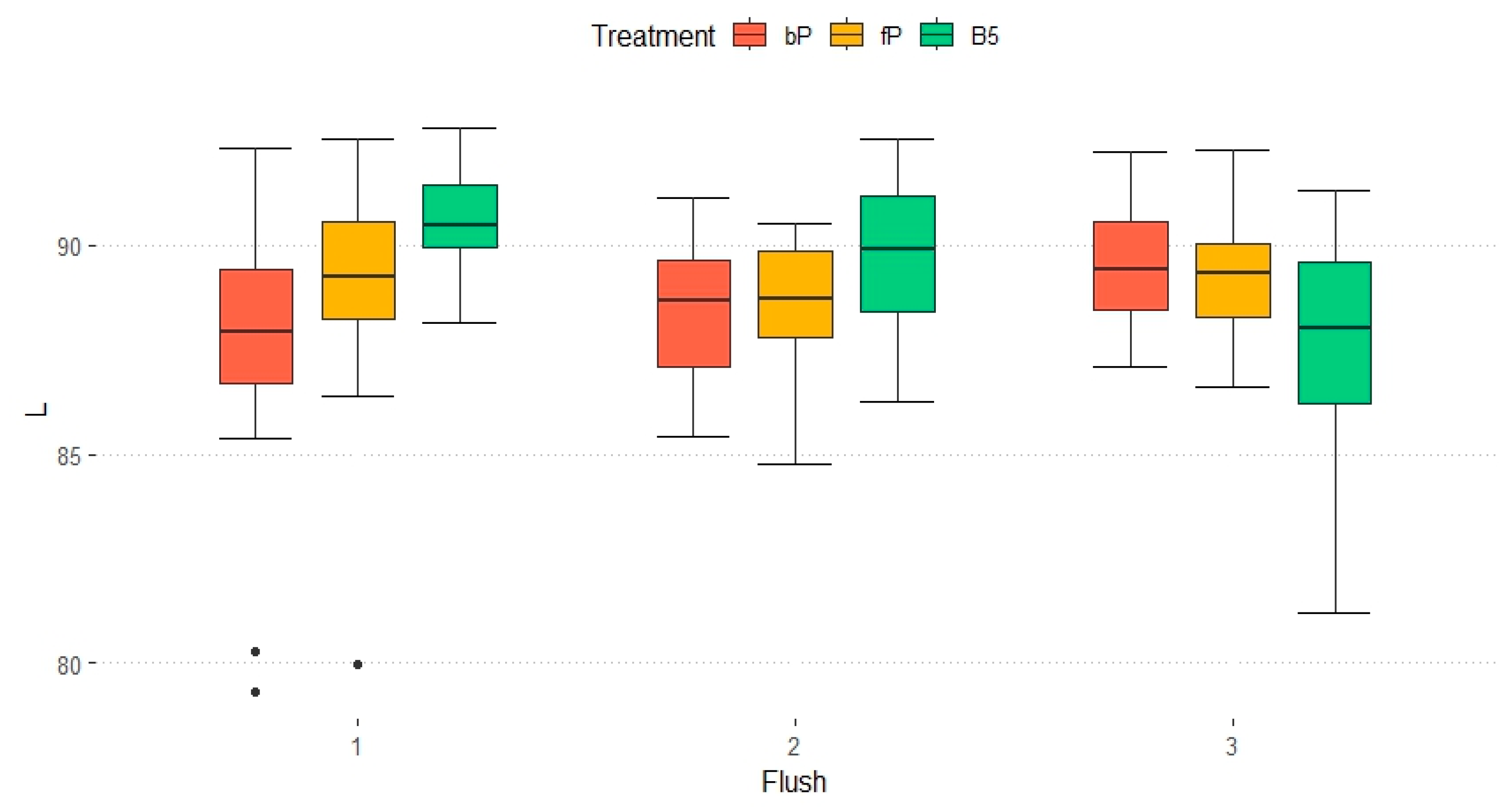
3.3. Mushroom Colour (Experiment A Only)
3.4. Additional Observations
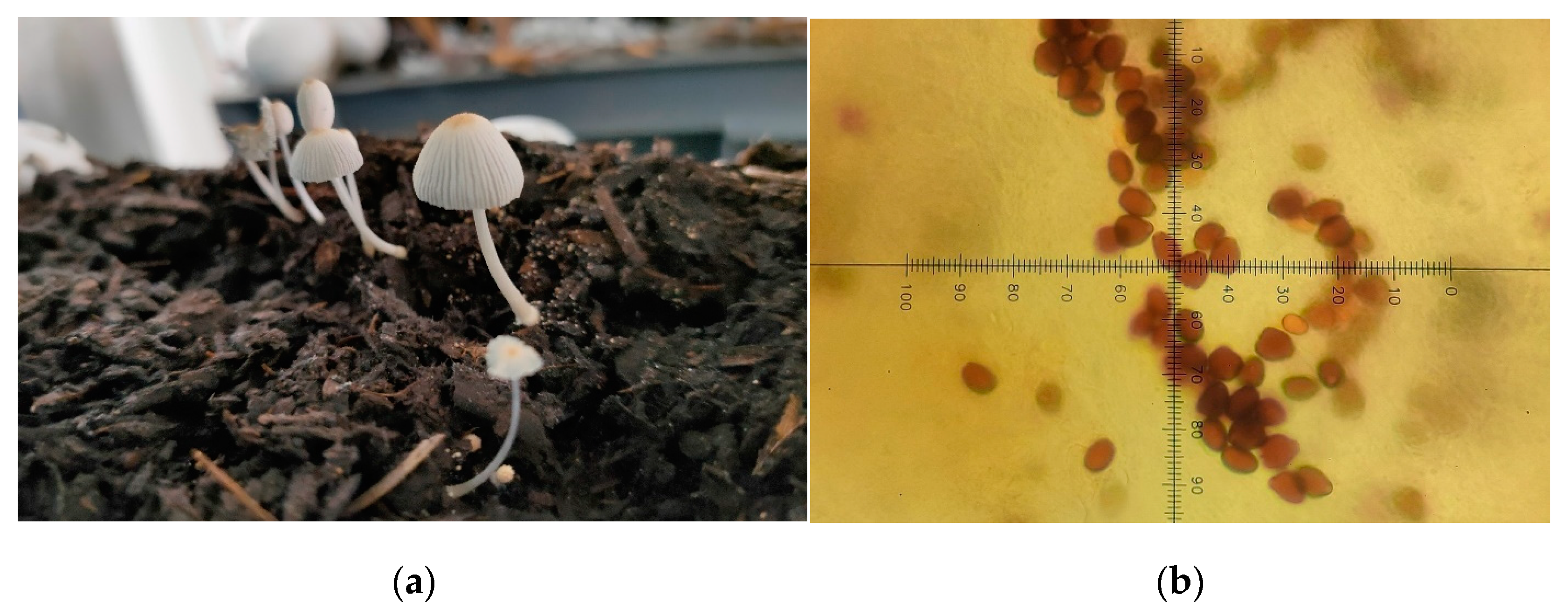
3.4.1. Contamination
3.4.2. Consistency of Bark
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 September 2024).
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V. Status and trends in world mushroom production-III-World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2021, 29, 75. [Google Scholar] [CrossRef]
- Bord Bia, Export Performance and Prospects Report 2023–2024. Available online: https://www.bordbia.ie/globalassets/bordbia.ie/industry/performance-and-prospects/bord-bia-exports-performance-and-prospects-report-2023---2024.pdf (accessed on 17 April 2024).
- Lachance, D.; Lavoie, C. Vegetation of Sphagnum bogs in highly disturbed landscapes: Relative influence of abiotic and anthropogenic factors. Appl. Veg. Sci. 2004, 7, 183. [Google Scholar] [CrossRef]
- Tiemeyer, B.; Albiac Borraz, E.; Augustin, J.; Bechtold, M.; Beetz, S.; Beyer, C.; Drösler, M.; Ebli, M.; Eickenscheidt, T.; Fiedler, S.; et al. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Change Biol. 2016, 22, 4134–4149. [Google Scholar] [CrossRef]
- Government of Ireland, Department of the Environment, Climate and Communications. Climate Action Plan. Available online: https://www.gov.ie/pdf/?file=https://assets.gov.ie/224574/be2fecb2-2fb7-450e-9f5f-24204c9c9fbf.pdf#page=null (accessed on 17 April 2023).
- Hirschler, O.; Thrän, D. Peat Substitution in Horticulture: Interviews with German Growing Media Producers on the Transformation of the Resource Base. Horticulturae 2023, 9, 919. [Google Scholar] [CrossRef]
- Noble, R.; Grogan, H.; Corbett, E.; Seymour, G. The Future of Casing—Review of Casing Materials and Availability of Peat for Mushroom Cultivation. Aust. Mushroom Grow. Assoc. 2023. Available online: https://isms.biz/Web/Web/Library/AMGA%20Casing%20Review%20Report%202023.aspx?hkey=ed8c4010-014c-4d47-abc3-c8502276cea2 (accessed on 30 October 2024).
- Young, G.; Grogan, H.; Walsh, L.; Noble, R.; Tracy, S.; Schmidt, O. Peat alternative casing materials for the cultivation of Agaricus bisporus mushrooms—A systematic review. Clean. Circ. Bioecon. 2024, 9, 100100. [Google Scholar] [CrossRef]
- Ben-Hakoun, E.; Shechter, M.; Hayuth, Y. Economic evaluation of the environmental impact of shipping from the perspective of CO2 emissions. J. Shipp. Trade 2016, 1, 5. [Google Scholar] [CrossRef]
- Jägerbrand, A.; Brutemark, A.; Svedén, J.; Gren, I. A review on the environmental impacts of shipping on aquatic and nearshore ecosystems. Sci. Total Environ. 2019, 695, 133637. [Google Scholar] [CrossRef]
- Seaby, D. The influence on yield of mushrooms (Agaricus bisporus) on the casing layer pore space volume and ease of water uptake. Compos. Sci. Util. 1999, 7, 56–65. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; de Juan, J.A.; Zied, D.C. Modelling the effect of the physical and chemical characteristics of the materials used as casing layers on the production parameters of Agaricus bisporus. Arch. Microbiol. 2010, 192, 1023–1030. [Google Scholar] [CrossRef]
- Siyoum, N.A.; Surridge, K.; Korste, L. Bacterial profiling of casing materials for white button mushrooms (Agaricus bisporus) using denaturing gradient gel electrophoresis. S. Afr. J. Sci. 2010, 106, 49–54. [Google Scholar]
- Noble, R.; Fermor, T.R.; Lincoln, S.; Dobrovin-Pennington, A.; Evered, C.; Mead, A.; Li, R. Primordia initiation of mushroom (Agaricus bisporus) strains on axenic casing materials. Mycologia 2003, 95, 620–629. [Google Scholar] [CrossRef]
- Noble, R.; Dobrovin-Pennington, A.; Evered, C.; Mead, A. Properties of peat-based casing soils and their influence on the water relations and growth of the mushroom (Agaricus bisporus). Plant Soil 1999, 207, 1–13. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Jafarpour, M.; Golparvar, A.R. The effects of various casing materials on yield and quantitative indices of Agaricus subrufescens and Agaricus bisporus. J. Biodivers. Environ. Sci. 2015, 6, 60–67. [Google Scholar]
- Eicker, A.; Van Greuning, M. Economical alternatives for topogenous peat as casing material in the cultivation of Agaricus bisporus in South Africa. S. Afr. J. Plant Soil 1989, 6, 129–135. [Google Scholar] [CrossRef][Green Version]
- Ghasemi, K.; Emadi, M.; Bagheri, A.; Mohammadi, M. Casing Material and Thickness Effects on the Yield and Nutrient Concentration of Agaricus bisporus. Sarhad J. Agric. 2020, 36, 734–1009. [Google Scholar] [CrossRef]
- Polat, E.; Önel, Ö. An alternative new casing material in the production of Agaricus bisporus. Mediterr. Agric. Sci. 2021, 34, 261–266. [Google Scholar] [CrossRef]
- Department of Agriculture, Food and the Marine, Ireland’s National Forest Inventory 2022. Available online: https://assets.gov.ie/246991/0b1fafb5-9475-4955-bbbc-26bd9effb509.pdf (accessed on 30 September 2024).
- Houston Durrant, T.; Mauri, A.; de Rigo, D.; Caudullo, G. Picea sitchensis in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e0137a1+. Available online: https://www.researchgate.net/profile/Giovanni-Caudullo/publication/299470814_Picea_sitchensis_in_Europe_distribution_habitat_usage_and_threats/links/61ee7374dafcdb25fd4a06ab/Picea-sitchensis-in-Europe-distribution-habitat-usage-and-threats.pdf (accessed on 11 December 2024).
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; 2016; p. e012300+. Available online: https://boris.unibe.ch/80794/1/Picea_abies.pdf (accessed on 11 December 2024).
- Jarial, R.; Shandilya, T.R.; Jarial, K. Casing in mushroom beds—A review. Agric. Rev. 2005, 26, 261–271. [Google Scholar]
- Herman, K.C.; Bleichrodt, R. Go with the flow: Mechanisms driving water transport during vegetative growth and fruiting. Fungal Biol. Rev. 2022, 41, 10–23. [Google Scholar] [CrossRef]
- EN 13037:2012; Soil Improvers and Growing Media—Determination of pH. Available online: https://standards.iteh.ai/catalog/standards/sist/ab70113b-2e34-49a1-abcb-c07a7204f1f1/sist-en-13037-2012?srsltid=AfmBOor2HaMy8A_LxVM3AVm7FD3OIt_p4-xWxXQXpxnvPBlq539RTw8J (accessed on 12 January 2025).
- Zied, D.C.; Pardo-González, J.E.; Minhoni, M.T.A.; Pardo-Giménez, A. A reliable quality index for mushroom cultivation. J. Agric. Sci. 2011, 3, 50. [Google Scholar]
- Foulongne-Oriol, M.; Rodier ARousseau, T.; Savoie, J. Quantitative Trait Locus Mapping of Yield-Related Components and Oligogenic Control of the Cap Color of the Button Mushroom, Agaricus bisporus. Appl. Environ. Microbiol. 2012, 78, 2422–2434. [Google Scholar] [CrossRef]
- Pardo Giménez, A.; Pardo-González, J.E. Evaluation of casing materials made from spent mushroom substrate and coconut fibre pith for use in production of Agaricus bisporus (Lange) Imbach. Span. J. Agric. Res. 2008, 6, 683. [Google Scholar] [CrossRef]
- Dias, E.S.; Zied, D.C.; Rinker, D.L. Physiologic response of Agaricus subrufescens using different casing materials and practices applied in the cultivation of Agaricus bisporus. Fungal Biol. 2013, 117, 569–575. [Google Scholar] [CrossRef]
- Noble, R.; Dobrovin-Pennington, A. Partial substitution of peat in mushroom casing with fine particle coal tailings. Sci. Hortic. 2005, 104, 351–367. [Google Scholar] [CrossRef]
- Pardo, A.; de Juan, J.A.; Pardo, J.E. Production, characterization and evaluation of composted vine shoots as a casing soil additive for mushroom cultivation. Biol. Agric. Hortic. 2002, 19, 377–391. [Google Scholar] [CrossRef]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- Burton, K.; Noble, R. The influence of flush number, bruising and storage temperature on mushroom quality. Postharvest Biol. Technol. 1993, 3, 39–47. [Google Scholar] [CrossRef]
- Lin, X.; Sun, D. Research advances in browning of button mushroom (Agaricus bisporus): Affecting factors and controlling methods. Trends Food Sci. Technol. 2019, 90, 63–75. [Google Scholar] [CrossRef]
- Péneau, S.; Brockhoff, P.; Escher, F.; Nuessli, J. A comprehensive approach to evaluate the freshness of strawberries and carrots. Postharvest Biol. Technol. 2007, 45, 20–29. [Google Scholar] [CrossRef]
- Mohapatra, D.; Bira, Z.M.; Kerry, J.P.; Frías, J.M.; Rodrigues, F.A. Postharvest Hardness and Color Evolution of White Button Mushrooms (Agaricus bisporus). J. Food Sci. 2010, 75, E146–E152. [Google Scholar] [CrossRef]
- Shu, L.; Zeng, Z.; Dai, J.; Cheng, Y.; Lu, Y.; Chen, M.; Zeng, H. Morphological and metabolic changes in an aged strain of Agaricus bisporus As2796. Appl. Microbiol. Biotechnol. 2021, 105, 79978007. [Google Scholar] [CrossRef] [PubMed]
- Bechara, M.A. Alternative Mushroom Production System Using Non-Composted Grain-Based Substrates. Ph.D. Thesis, Pennsylvania State University, University Park, PA, USA, 2007. [Google Scholar]
- Pardo, A.; de Juan, J.A.; Pardo, J.E. Performance of composted vine shoots as a peat alternative in casing materials for mushroom cultivation. J. Appl. Hortic. 2003, 5, 11–15. [Google Scholar] [CrossRef]
- Van Jaarsveld, L.P.; Korsten, L. Chemical and physical properties of alternative casing media in commercial production of button mushrooms [Agaricus bisporus (Lange)]. Mushroom Sci. 2008, 17, 310–332. [Google Scholar]
- Duran, H.; Peksen, A.; Eren, E. Vermicompost, rose oil processing waste compost, and spent coconut fiber as casing material in button mushroom cultivation. Biomass Convers. Biorefinery 2023, 13, 4317–4329. [Google Scholar] [CrossRef]


| Experiment A | Experiment B | |
|---|---|---|
| bP | 7.48 | 7.83 |
| fP | 7.01 | 7.82 |
| B5 | 7.50 | 7.76 |
| B10 | - | 7.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, G.; Grogan, H.; Corbett, E.; McGuinness, B.W.; Gaffney, M.T.; Tracy, S.; Schmidt, O.; Walsh, L. The Potential of Sitka Spruce Bark as an Alternative to Peat Casing for Mushroom (Agaricus bisporus) Production. Horticulturae 2025, 11, 100. https://doi.org/10.3390/horticulturae11010100
Young G, Grogan H, Corbett E, McGuinness BW, Gaffney MT, Tracy S, Schmidt O, Walsh L. The Potential of Sitka Spruce Bark as an Alternative to Peat Casing for Mushroom (Agaricus bisporus) Production. Horticulturae. 2025; 11(1):100. https://doi.org/10.3390/horticulturae11010100
Chicago/Turabian StyleYoung, Gabrielle, Helen Grogan, Eoghan Corbett, Brian W. McGuinness, Michael T. Gaffney, Saoirse Tracy, Olaf Schmidt, and Lael Walsh. 2025. "The Potential of Sitka Spruce Bark as an Alternative to Peat Casing for Mushroom (Agaricus bisporus) Production" Horticulturae 11, no. 1: 100. https://doi.org/10.3390/horticulturae11010100
APA StyleYoung, G., Grogan, H., Corbett, E., McGuinness, B. W., Gaffney, M. T., Tracy, S., Schmidt, O., & Walsh, L. (2025). The Potential of Sitka Spruce Bark as an Alternative to Peat Casing for Mushroom (Agaricus bisporus) Production. Horticulturae, 11(1), 100. https://doi.org/10.3390/horticulturae11010100






