Triacontanol Reverses Abscisic Acid Effects on Stomatal Regulation in Solanum lycopersicum L. under Drought Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Application of Treatments in a Greenhouse Tomato Crop
2.3. Drought Stress Conditions
2.4. Measurement of Chlorophyll Content
2.5. Measurement of the Operating Efficiency of PSII Photochemistry at Different Times of the Day
2.6. Measurement of Stomatal Conductance at Different Times of the Day
2.7. Morphometric Characterisation of Stomata
2.8. Morphometric Characterisation of Chloroplasts
2.9. TRIA Effect on ABA in Epidermal Strips
2.10. Statistical Analysis
3. Results
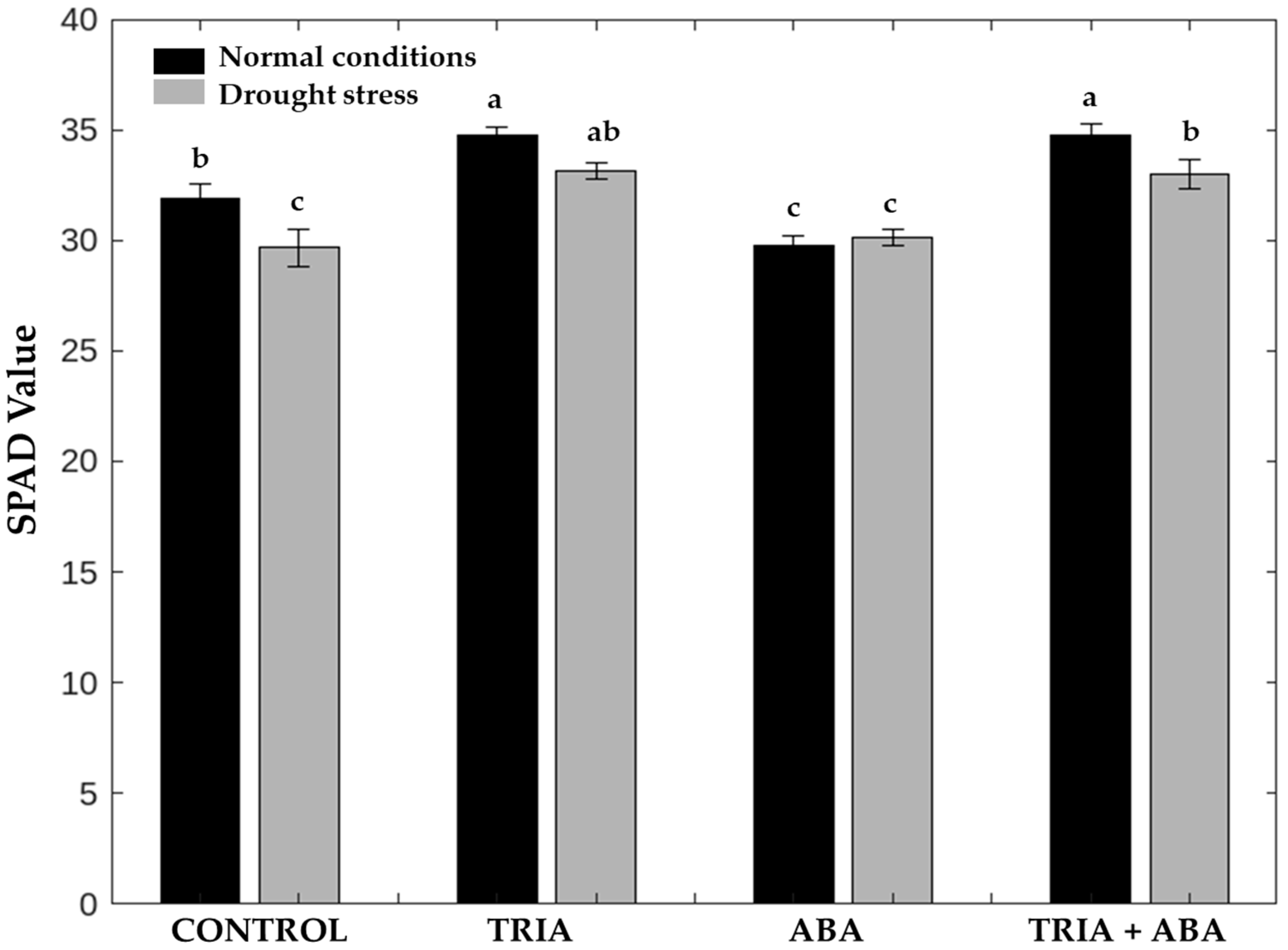
3.1. Chlorophyll Content
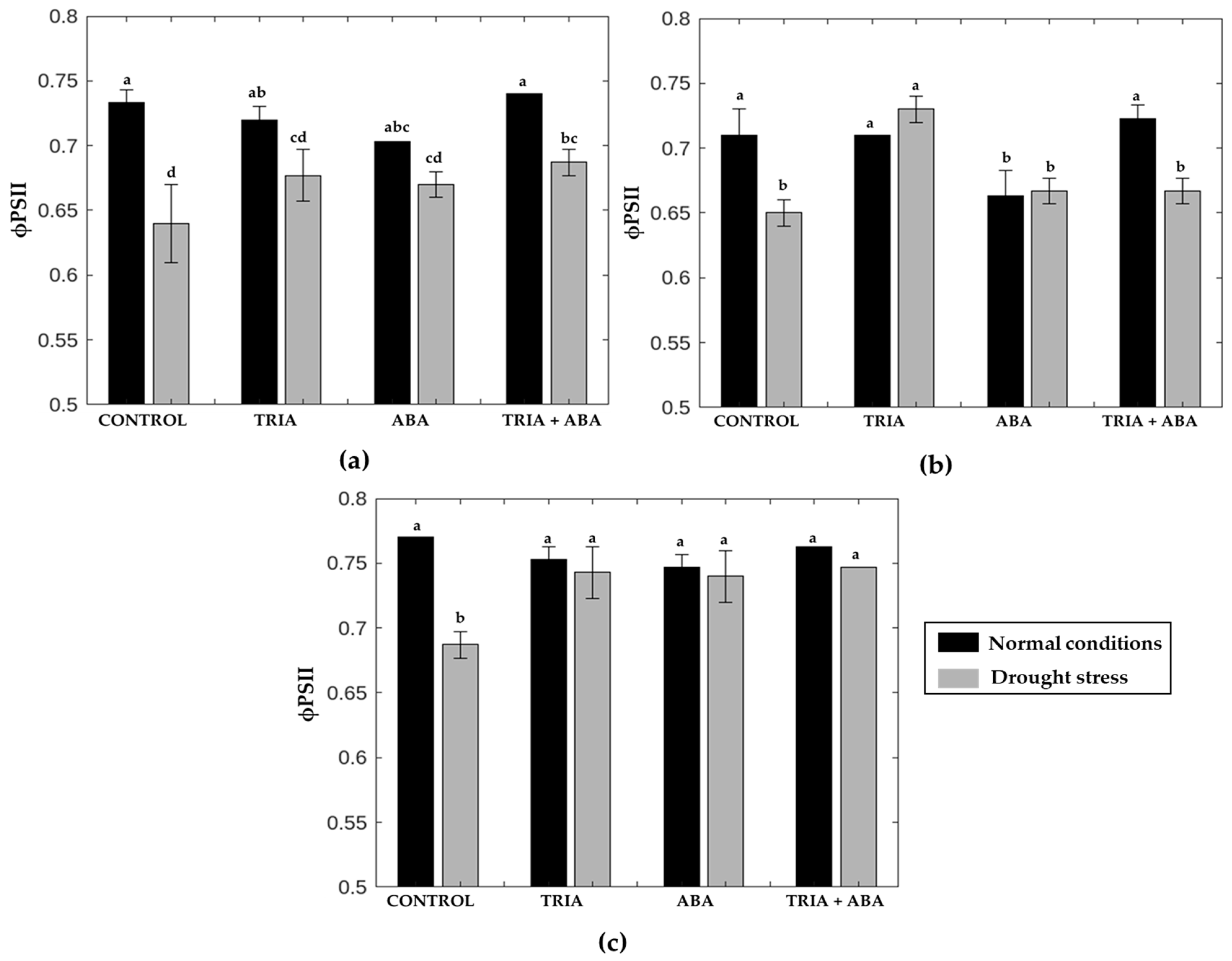
3.2. The Operating Efficiency of PSII Photochemistry, Fq′/Fm′
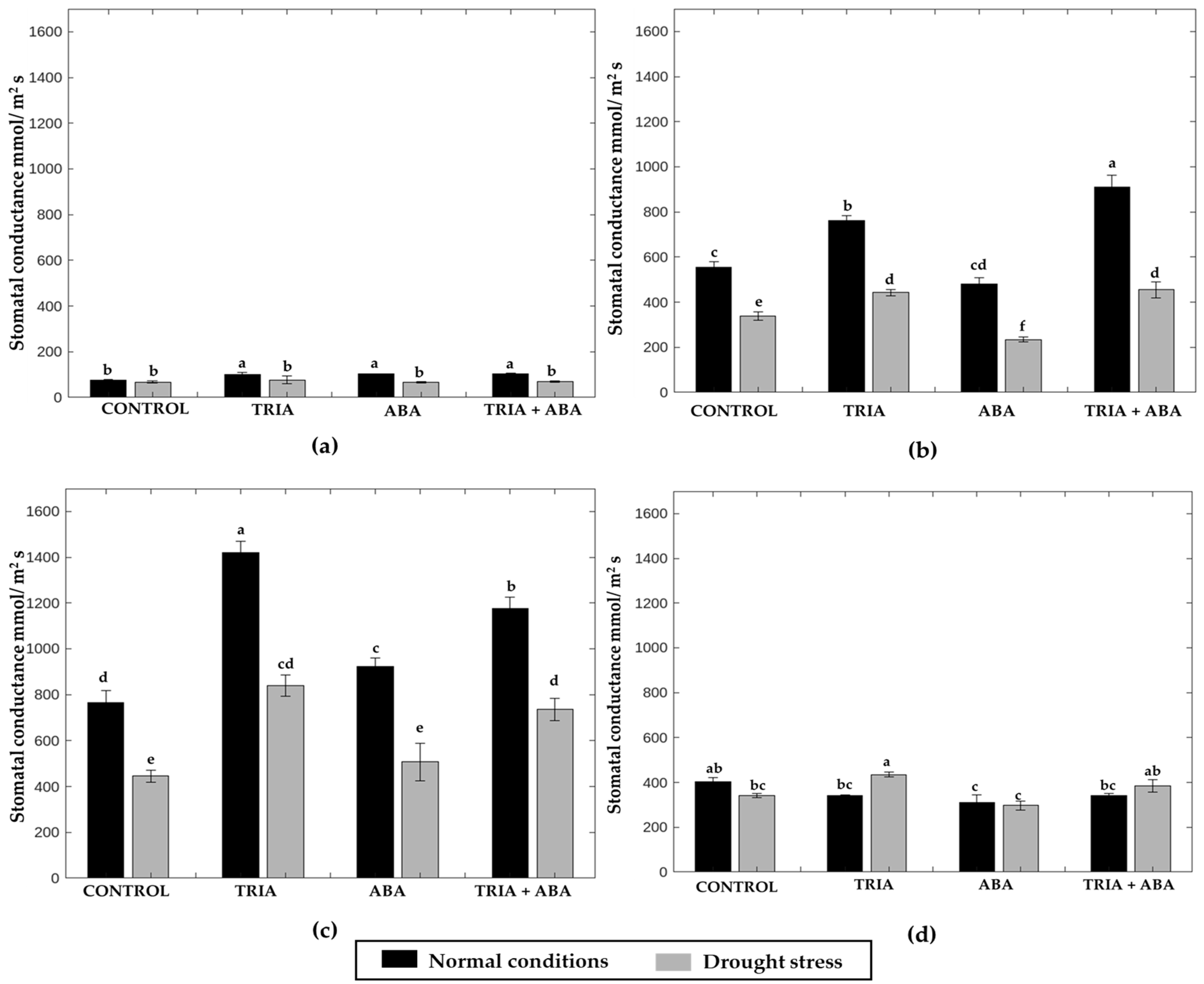
3.3. Stomatal Conductance
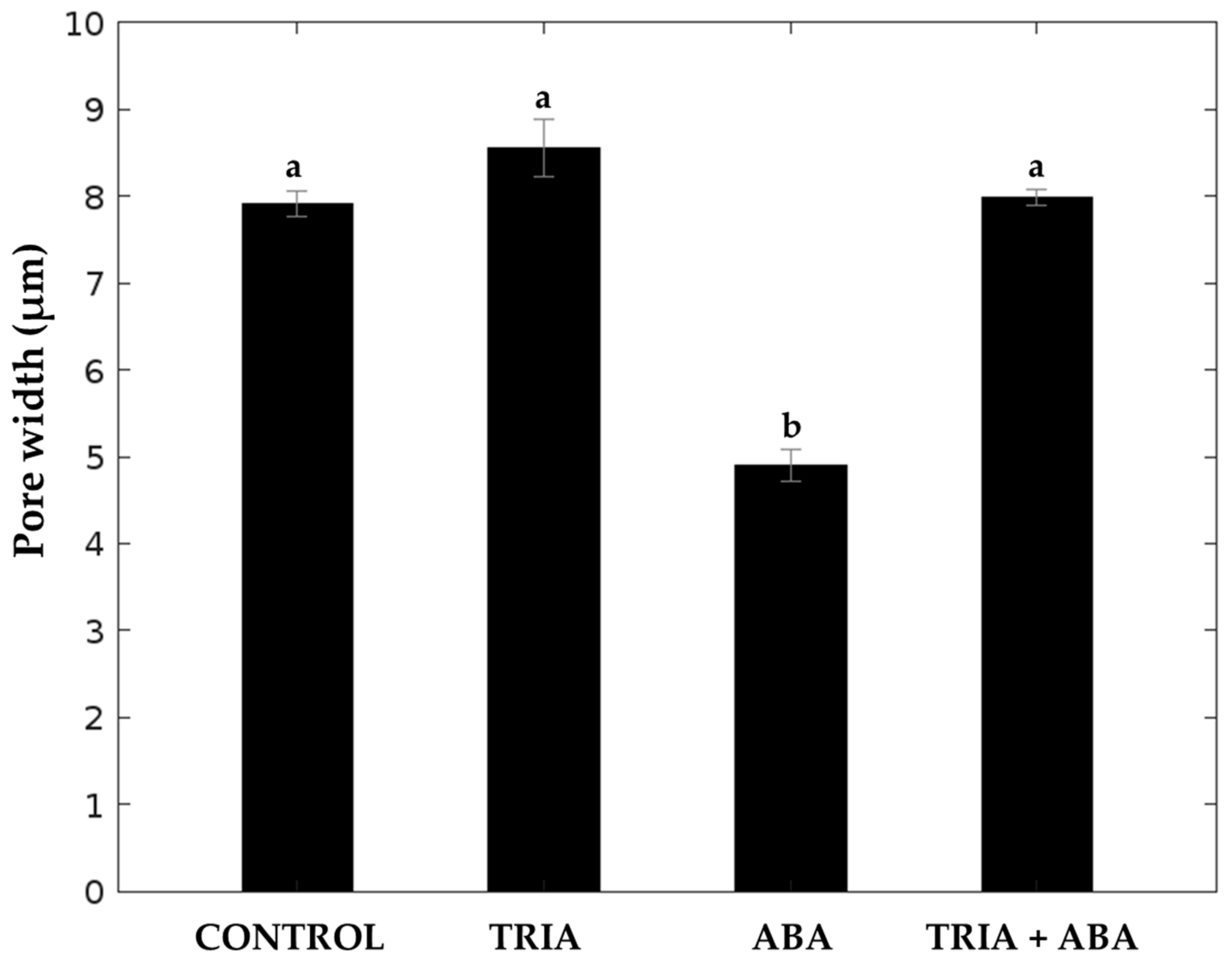
3.4. Stomata Morphometric Characteristics
3.5. Chloroplast Morphometric Characteristics
3.6. TRIA’s Effect on ABA in Epidermal Strips
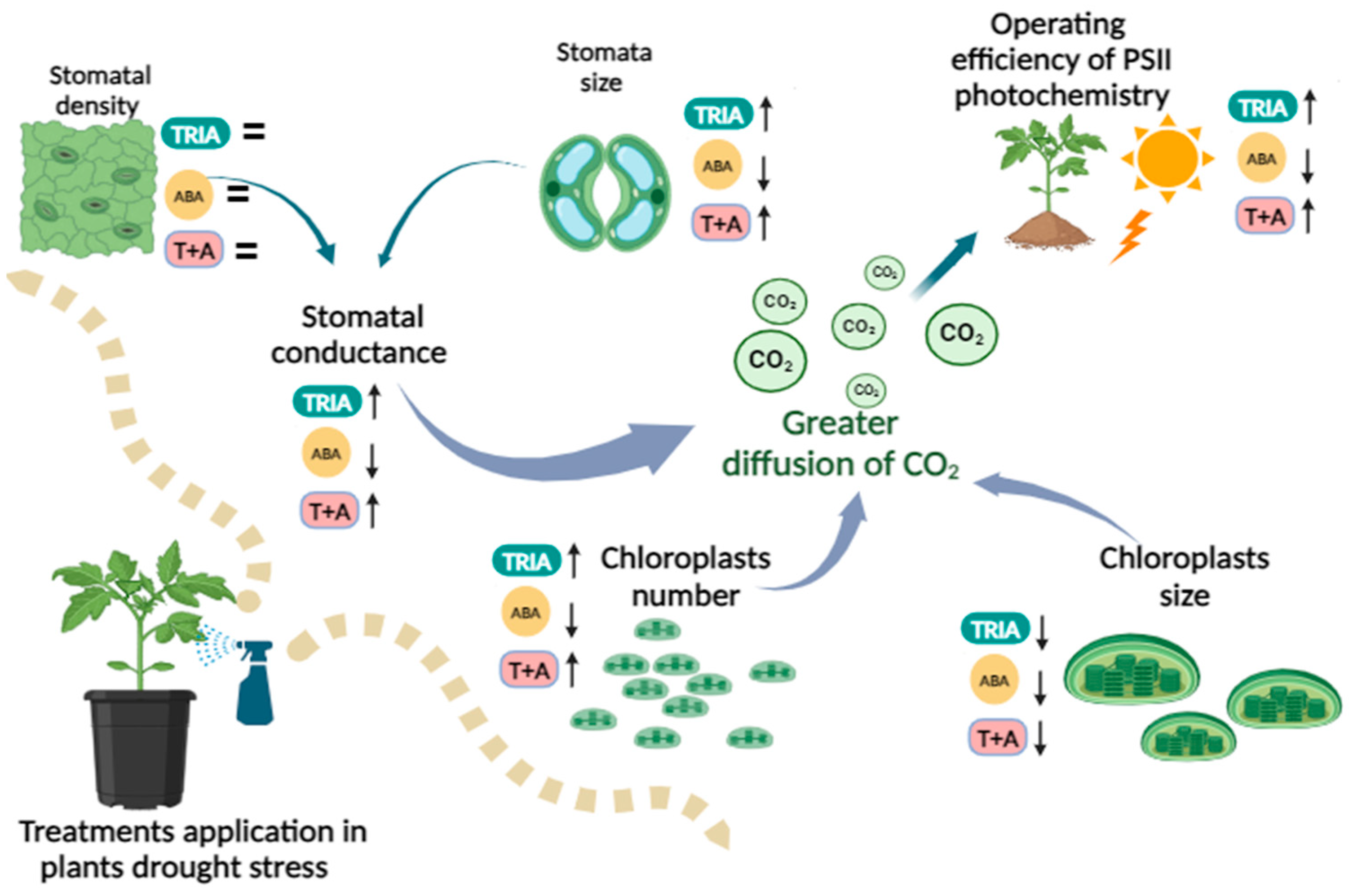
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Moazzam-Jazi, M.; Ghasemi, S.; Seyedi, S.M.; Niknam, V. COP1 plays a prominent role in drought stress tolerance in Arabidopsis and Pea. Plant Physiol. Biochem. 2018, 130, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Gantait, S.; Azizi, P.; Mazumdar, P. Drought tolerance improvement in Solanum lycopersicum: An insight into “OMICS” approaches and genome editing. 3 Biotech 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef]
- Jensen, N.B.; Ottosen, C.O.; Zhou, R. Exogenous melatonin alters stomatal regulation in tomato seedlings subjected to combined heat and drought stress through mechanisms distinct from ABA signaling. Plants 2023, 12, 1156. [Google Scholar] [CrossRef]
- Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S.; Guerriero, G.; Quinet, M. Comparison of Drought and Heat Resistance Strategies among Six Populations of Solanum chilense and Two Cultivars of Solanum lycopersicum. Plants 2021, 10, 1720. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Benešová, M.; Hola, D.; Fischer, L.; Jedelský, P.L.; Hnilička, F.; Wilhelmová, N.; Rothová, O.; Kočová, M.; Procházková, D.; Honnerová, J.; et al. The physiology and proteomics of drought tolerance in maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS ONE 2012, 7, e38017. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef] [PubMed]
- Ristic, Z.; Cass, D.D. Chloroplast structure after water shortage and high temperature in two lines of Zea mays L. that differ in drought resistance. Bot. Gaz. 1991, 152, 186–194. [Google Scholar] [CrossRef]
- Głowacka, K.; Kromdijk, J.; Salesse-Smith, C.E.; Smith, C.; Driever, S.M.; Long, S.P. Is chloroplast size optimal for photosynthetic efficiency? New Phytol. 2023, 239, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of Sugarcane. Sugar. Technol. 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Ries, S.K.; Wert, V.; Sweeley, C.C.; Leavitt, R.A. Triacontanol: A new naturally occurring plant growth regulator. Science 1977, 195, 1339–1341. [Google Scholar] [CrossRef]
- Pang, Q.; Chen, X.; Lv, J.; Li, T.; Fang, J.; Jia, H. Triacontanol promotes the fruit development and retards fruit senescence in strawberry: A transcriptome analysis. Plants 2020, 9, 488. [Google Scholar] [CrossRef]
- Lu, X.; Fang, M.; Dai, Y.; Yang, Y.; Fan, A.; Xu, J.; Qin, Z.; Lu, Y.; Zhao, D.; Chen, X.; et al. Quantification of triacontanol and its PEGylated prodrug in rat plasma by GC-MS/MS: Application to a pre-clinical pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1089, 8–15. [Google Scholar] [CrossRef]
- Verma, T.; Bhardwaj, S.; Singh, J.; Kapoor, D.; Prasad, R. Triacontanol as a versatile plant growth regulator in overcoming negative effects of salt stress. J. Agric. Res. 2022, 10, 100351. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Singh, M.; Ram, M. Stimulation of crop productivity, photosynthesis and artemisinin production in Artemisia annua L. by triacontanol and gibberellic acid application. J. Plant Interact. 2010, 5, 273–281. [Google Scholar] [CrossRef]
- Ramos-Zambrano, E.; Juárez-Yáñez, T.E.; Tapia-Maruri, D.; Camacho-Díaz, B.H.; Jiménez-Aparicio, A.R.; Martínez-Ayala, A.L. Effects of triacontanol and light on stomatal and photochemical responses in Solanum lycopersicum L. J. Plant Growth Regul. 2021, 40, 2208–2220. [Google Scholar] [CrossRef]
- Manai, M.; Fiorillo, A.; Matuozzo, M.; Li, M.; D’Ambrosio, C.; Franco, L.; Scaloni, A.; Fogliano, V.; Camoni, L.; Marra, M. Phenotypical and biochemical characterization of tomato plants treated with triacontanol. Sci. Rep. 2024, 14, 12096. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, A.; Grzesiak, M.T.; Hura, T. Exogenous application of growth stimulators improves the condition of maize exposed to soil drought. Acta Physiol. Plant. 2021, 43, 62. [Google Scholar] [CrossRef]
- Islam, S.; Zaid, A.; Mohammad, F. Role of Triacontanol in counteracting the III effects of salinity in plants: A review. J. Plant Growth Regul. 2021, 40, 1–10. [Google Scholar] [CrossRef]
- Faiz, H.; Khan, O.; Ali, I.; Hussain, T.; Haider, S.T.; Siddique, T.; Liaquat, M.; Noor, A.; Khan, R.W.; Ashraf, S.; et al. Foliar application of triacontanol ameliorates heat stress through regulation of the antioxidant defense system and improves yield of eggplant. Braz. J. Biol. 2022, 84, e253696. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ismail, S.A.; Ibrahim, N.M.; Shehata, W.F.; Alkhateeb, A.A.; Ghazzawy, H.S.; El-Mogy, M.M.; Sayed, E.G. Unravelling the Effect of Triacontanol in Combating Drought Stress by Improving Growth, Productivity, and Physiological Performance in Strawberry Plants. Plants 2022, 11, 1913. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Abdulmajeed, A.M.; Hassan, H. Biochemical and molecular effects induced by triacontanol in acquired tolerance of rice to drought stress. Genes 2021, 12, 1119. [Google Scholar] [CrossRef]
- Shukla, A.; Farooqi, A.A.H.; Shukla, Y.N.; Sharma, S. Effect of triacontanol and chlormequat on growth, plant hormones and artemisinin yield in Artemisia annua L. Plant Growth Regul. 1992, 11, 165–171. [Google Scholar] [CrossRef]
- Waqas, M.; Shahzad, R.; Khan, A.L.; Asaf, S.; Kim, Y.H.; Kang, S.M.; Bilal, S.; Hamayun, M.; Lee, I.J. Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef]
- Flores, J.; Ojeda-Bustamante, W.; López, I.; Rojano, A.; Salazar, I. Requerimientos de riego para tomate de invernadero. Terra Latinoam. 2007, 25, 127–134. [Google Scholar]
- Maai, E.; Nishimura, K.; Takisawa, R.; Nakazaki, T. Light stress-induced chloroplast movement and midday depression of photosynthesis in sorghum leaves. Plant Prod. Sci. 2020, 23, 172–181. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, B. Using clear nail polish to make Arabidopsis epidermal impressions for measuring the change of stomatal aperture size in immune response. Methods Mol. Biol. 2017, 1578, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Li, J.; Tseng, T.S.; Schroeder, J.I.; Ehrhardt, D.W.; Briggs, W.R. COP1 jointly modulates cytoskeletal processes and electrophysiological responses required for stomatal closure. Mol. Plant 2014, 7, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ollion, J.; Cochennec, J.; Loll, F.; Escudé, C.; Boudier, T. TANGO: A generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 2013, 29, 1840–1841. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Ding, L.; Shen, Q.; Peng, S.; Guo, S. Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS ONE 2013, 8, e62036. [Google Scholar] [CrossRef]
- Naeem, M.; Khan, M.M.A.; Siddiqui, M.H. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.). Sci. Hortic. 2009, 121, 389–396. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Ali, Q.; Alam, M.W.; Haider, M.S.; Mehboob, W. Triacontanol modulates salt stress tolerance in cucumber by altering the physiological and biochemical status of plant cells. Sci. Rep. 2021, 11, 24504. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Xiong, D.; Huang, J.; Peng, S.; Li, Y. A few enlarged chloroplasts are less efficient in photosynthesis than a large population of small chloroplasts in Arabidopsis thaliana. Sci. Rep. 2017, 7, 5782. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Angelov, M.N. Photosynthesis response to triacontanol correlates with increased dynamics of mesophyll protoplast and chloroplast membranes. Plant Growth Regul. 1977, 21, 145–152. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Verma, A.; Malik, C.P.; Gupta, V.K.; Bajaj, B.K. Effects of in vitro triacontanol on growth, antioxidant enzymes, and photosynthetic characteristics in Arachis hypogaea L. Braz. J. Plant Physiol. 2011, 23, 271–277. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Alam, M.W.; Ali, Q.; Ayyub, C.M.; Haider, M.S.; Ashraf, M.I.; Mahboob, W. Triacontanol regulates morphological traits and enzymatic activities of salinity affected hot pepper plants. Sci. Rep. 2022, 12, 3736. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001, 125, 935–942. [Google Scholar] [CrossRef]
- Bradford, K.J.; Sharkey, T.D.; Farquhar, G.D. Gas Exchange, Stomatal behavior, and deltaC values of the flacca tomato mutant in relation to abscisic acid. Plant Physiol. 1983, 72, 245–250. [Google Scholar] [CrossRef]
- Quarrie, S.A.; Jones, H.G. Effects of abscisic acid and water stress on development and morphology of wheat. J. Exp. Bot. 1977, 28, 192–203. [Google Scholar] [CrossRef]
- Lesniak, A.P.; Haug, A.; Ries, S.K. Stimulation of ATPase activity in barley (Hordeum vulgare) root plasma membranes after treatment of intact tissues and cell free extracts with triacontanol. Physiol. Plant. 1986, 68, 20–26. [Google Scholar] [CrossRef]
- Morré, D.J.; Selldén, G.; Zhu, X.Z.; Brightman, A. Triacontanol stimulates NADH oxidase of soybean hypocotyl plasma membrane. Plant Sci. 1991, 79, 31–36. [Google Scholar] [CrossRef]
- Irmak, S.; Dunford, N.T.; Milligan, J. Policosanol contents of beeswax, sugar cane and wheat extracts. Food Chem. 2006, 95, 312–318. [Google Scholar] [CrossRef]
- Lee, J.H.; Jia, Y.; Thach, T.T.; Han, Y.; Kim, B.; Wu, C.; Kim, Y.; Seo, W.D.; Lee, S.J. Hexacosanol reduces plasma and hepatic cholesterol by activation of AMP-activated protein kinase and suppression of sterol regulatory element-binding protein-2 in HepG2 and C57BL/6J mice. Nutr. Res. 2017, 1, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Carianopol, C.S.; Chan, A.L.; Dong, S.; Provart, N.J.; Lumba, S.; Gazzarrini, S. An abscisic acid-responsive protein interaction network for sucrose non-fermenting related kinase1 in abiotic stress response. Commun. Biol. 2020, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Hua, W.; Li, J.; Qiao, Y.; Yao, L.; Hao, W.; Li, R.; Fan, M.; De Jaeger, G.; Yang, W.; et al. TOR promotes guard cell starch degradation by regulating the activity of β-AMYLASE1 in Arabidopsis. Plant Cell 2022, 34, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, N.; Ramos-Zambrano, E.; Osorio-Ruiz, A.; Martínez-Ayala, A.L. Main mechanisms of action of policosanol in animal and plant cells. Int. J. Pharm. Res. Allied Sci. 2021, 10, 10–20. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, H.; Chen, R.; Zhu, L.; Du, B.; Weng, Q.; He, G. Isolation and characterization of triacontanol-regulated genes in rice (Oryza sativa L.): Possible role of triacontanol as a plant growth stimulator. Plant Cell Physiol. 2002, 43, 869–876. [Google Scholar] [CrossRef]
- Hussain, H.; Cheng, Y.; Wang, Y.; Yuan, Y.; Adnan; Li, Y.; Tian, H.; Hussain, S.; Chen, S.; Lin, R.; et al. ASR1 and ASR2, Two Closely Related ABA-Induced Serine-Rich Transcription Repressors, Function Redundantly to Regulate ABA Responses in Arabidopsis. Plants 2023, 12, 852. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.J.; Shin, S.Y.; Kim, Y.S.; Yoon, H.S. ASR Enhances Environmental Stress Tolerance and Improves Grain Yield by Modulating Stomatal Closure in Rice. Front. Plant Sci. 2020, 10, 1752. [Google Scholar] [CrossRef]
- Ramanarayan, K.; Swamy, G.S. Triacontanol negatively modulates the jasmonic acid-stimulated proteinase inhibitors in tomato (Lycopersicon esculentum). J. Plant Physiol. 2004, 161, 489–492. [Google Scholar] [CrossRef]
- Rehman, S.; Aziz, E.; Akhtar, W.; Ilyas, M.; Mahmood, T. Structural and functional characteristics of plant proteinase inhibitor-II (PI-II) family. Biotechnol. Lett. 2017, 39, 647–666. [Google Scholar] [CrossRef]
- Moloi, S.J.; Ngara, R. The roles of plant proteases and protease inhibitors in drought response: A review. Front. Plant Sci. 2023, 14, 1165845. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, W.; Wang, Y.; Zhang, M.; Aihara, Y.; Kinoshita, T. Protease Inhibitor-Dependent Inhibition of Light-Induced Stomatal Opening. Front. Plant Sci. 2021, 12, 735328. [Google Scholar] [CrossRef] [PubMed]
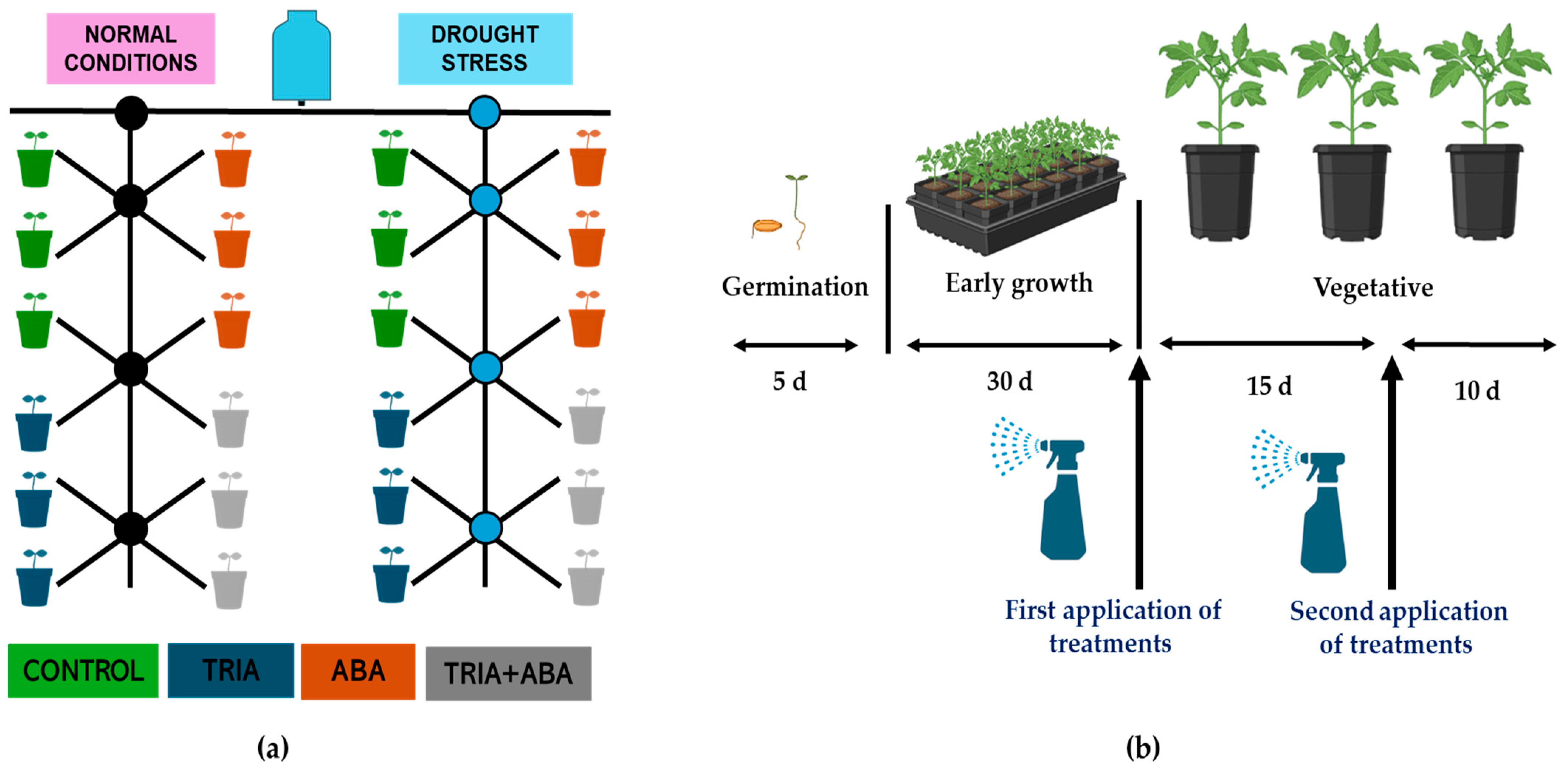



| Factor | Levels | Description | Reference |
|---|---|---|---|
| Treatment application | Control | Surfactant (0.1% (v/v) Tween 20 solution) | [20] |
| TRIA | 1 mg/L of TRIA dissolved in surfactant | ||
| ABA | 40 µM of ABA dissolved in surfactant | [11] | |
| TRIA + ABA | 1 mg/L of TRIA + 40 µM of ABA dissolved in surfactant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Díaz, M.A.; Ramos-Zambrano, E.; Juárez-Yáñez, T.E.; Perea-Flores, M.d.J.; Martínez-Ayala, A.L. Triacontanol Reverses Abscisic Acid Effects on Stomatal Regulation in Solanum lycopersicum L. under Drought Stress Conditions. Horticulturae 2024, 10, 985. https://doi.org/10.3390/horticulturae10090985
Bravo-Díaz MA, Ramos-Zambrano E, Juárez-Yáñez TE, Perea-Flores MdJ, Martínez-Ayala AL. Triacontanol Reverses Abscisic Acid Effects on Stomatal Regulation in Solanum lycopersicum L. under Drought Stress Conditions. Horticulturae. 2024; 10(9):985. https://doi.org/10.3390/horticulturae10090985
Chicago/Turabian StyleBravo-Díaz, María Asunción, Emilia Ramos-Zambrano, Tomás Ernesto Juárez-Yáñez, María de Jesús Perea-Flores, and Alma Leticia Martínez-Ayala. 2024. "Triacontanol Reverses Abscisic Acid Effects on Stomatal Regulation in Solanum lycopersicum L. under Drought Stress Conditions" Horticulturae 10, no. 9: 985. https://doi.org/10.3390/horticulturae10090985
APA StyleBravo-Díaz, M. A., Ramos-Zambrano, E., Juárez-Yáñez, T. E., Perea-Flores, M. d. J., & Martínez-Ayala, A. L. (2024). Triacontanol Reverses Abscisic Acid Effects on Stomatal Regulation in Solanum lycopersicum L. under Drought Stress Conditions. Horticulturae, 10(9), 985. https://doi.org/10.3390/horticulturae10090985







