Abstract
Begonia rex cv. DS-EYWA is an important plant for indoor and outdoor cultivation, and cv. DS-EYWA is a rare unique cultivar with curly, colorful leaves. Due to their importance, applying plant tissue culture techniques for mass and healthy production in a short period of time without seasonal limitation is of immense economic value. Applying several concentrations of silver nitrate (AgNO3) in combination with varied concentrations of cytokinins including 6-benzylaminopurine (BAP), thidiazuron (TDZ) (0, 0.5, 1, 1.5 mgL−1), and 1-naphthaleneacetic acid (NAA) auxin (0, 0.5, 1 mgL−1) via focusing on transverse thin cell layer (tTCL) petiole explants for high-scale production was used to establish an efficient in vitro propagation protocol. Our results showed that even low concentrations (25 mgL−1) can control internal bacterial infection and increase shoot direct regeneration efficiency. A combination of 1.5 mgL−1 BAP, 0.5 mgL−1 NAA, and 25 mgL−1 AgNO3 was the best treatment to increase the number of direct regenerated shoots, and a lower concentration of BAP (0.5 mgL−1) can be suggested for shoot elongation. Elongated shoots were successfully rooted in MS basal medium and acclimatized in a 1:1 peat moss/perlite sterilized pot mixture.
1. Introduction
Begonia rex cv. DS-EYWA is a perennial herbaceous ornamental pot plant with novel colorful leaves that belongs to the large Begoniaceae family and was produced by a Ukrainian begonia breeder for the first time [1]. The popularity of begonia species, especially B. rex cultivars, is not a secret to anyone. Many flower lovers prefer various cultivars of B. rex pot plants due to their colorful patterned leaves, which leads to ever-increasing commercial demands [2,3]. Due to their colorful patterned leaves, depending on the climatic conditions and their sensitivity, they can be good candidates for various decorative purposes, both indoors and outdoors, which enhances their popularity among plant lovers. Due to their esthetic value and high commercial demands in flower industries, over 200 species including B. rex have been commercialized [4]. As a consequence of high commercial demands, expanding the production rate seems to be essential.
Begonias can easily be propagated by conventional methods such as seeds, leaf/shoot cuttings, and splitting [5]. Because of the low propagation coefficient with the possibility of transferring different kinds of diseases and pathogens including bacterial and fungal contaminations, using plant tissue culture techniques with the advantages of achieving season-free, mass-scale, and healthy production in a limited period of time has long been suggested as a viable approach [2,6,7,8]. On the other hand, recent years have seen a new focus on flower color variations, the production of disease/pathogen-tolerant varieties, and the introduction of lines with resistance/tolerance to environmental stress conditions [8]. For quite some time, interspecific crossings and mutation inductions have been carried out to develop breeding programs in begonia. However, a lack of documented genomic data and the long propagation cycles of conventional breeding techniques have limited the success in attaining viable variations in begonia species. Genetic engineering programs can be good candidates for resolving different breeding goals in begonia species [9].
Successful genetic engineering programs require establishing several biotechnological steps, particularly direct/indirect organogenesis and somatic embryogenesis procedures [9,10]. Among various methods of in vitro plant production, thin cell layering (TCL) culture of tiny explants via either transversal or longitudinal positions has been promoted in recent decades. Currently, TCL techniques are successfully used for the in vitro propagation of various horticultural plants including ornamentals, medicinals, and vegetables [11]. The quick sampling of appropriate explants from different plant parts and the low number of required mother plants in comparison with other plant tissue culture techniques are the main advantages of the technique’s popularity among tissue culture researchers [12,13,14]. Moreover, the small size and large surface area of TCL explants, and their close exposure to the ingredients of culture medium including Plant Growth Regulators (PGRs) enhance the faster diffusion of nutrients and reduce the potential for in vitro tensions, inversely increasing the potential for direct/indirect organogenesis or embryogenesis rates [15,16].
The successful in vitro propagation of begonia species via leaf and petiole explants, which was mainly based on shoot direct/indirect regeneration or somatic embryogenesis, was reported recently [2,15]. The TCL culture plays an essential role in the shoot regeneration of various horticultural crops, including begonia species; however, there are limited reported data for the TCL culture of begonia, and what little data are available are mainly related to b. tuberous [5,17]. Additionally, the quality and quantity of in vitro regenerated plantlets is in part determined by the presence of effective growth adjuvants in the culture medium, the type of TCL explants (transverse thin cell layering (tTCL) or longitudinal thin cell layering (lTCL)), and the endogenous amount of PGRs presents in the TCL explants, from either in vitro or ex vitro sources [5].
Silver compounds such as silver nitrate (AgNO3), or silver nanoparticles (AgNPs), recognized as in vitro growth adjuvants, have significant value in tissue culture experiments to enhance in vitro growth rate and development. On the other hand, the positive effects of silver compounds on the explant sterilization stage or the elimination of in vitro contaminations by use in the culture medium have been reported [18,19]. Hence, depending on the plant species, applying silver compounds may have positive effects on the sterilization and regeneration of plant in vitro explants. The main objective of this study is to investigate the effects of different levels of AgNO3 in combination with various PGRs on the direct organogenesis potential of B. rex cv. DS-EYWA via tTCL ex vitro petiole explants for the first time.
2. Materials and Methods
2.1. Plant Materials
Petioles of B. rex cv. DS-EYWA (1 × 1) were used as explants for direct organogenesis experiments [20]. They were selected from one-year-old mother plants which were cultivated in a cocopeat pot mixture. After being washed with running tap water for 15 min, they were soaked in 1% solution of sodium hypochlorite and 0.5% Tween 20 for 10 min. Finally, sterilization was concluded by rinsing 3 times with autoclaved double distilled water with the intervals of 5, 10, and 15 min. After sterilization, petioles were transferred to autoclaved filter paper to dry out excess water before cultivation.
2.2. Establishment of Transverse Thin Cell Layer Explants (tTCLs) for Controlling In Vitro Contaminations
Murashige and Skoog (MS) [21] semi-solid medium containing 30 gL−1 sucrose and 5 gL−1 agar was used as the basal culture medium for all establishment, organogenesis, proliferation, and rooting stages. Then, 1 mm petiole and leaf explants were selected as tTCL explants for the establishment stage. In order to control possible in vitro contaminations, select healthy explants, and evaluate silver nitrate’s impact on inhibiting internal contaminations, all tTCL explants were transferred to establishment medium consisting of MS basal medium without any PGRs in combination with different concentrations of silver nitrate (AgNO3) (25, 50, 75, 100 mgL−1) and control explants on the medium without AgNO3. After four weeks, the percentage of infected explants was measured (Table 1). The pH of all prepared media was adjusted to 5.8 before autoclaving at 121 °C, 2 bar for 20 min. All cultivated explants were incubated in a growth chamber at 23 ± 2 °C temperature and 16/8 h dark/light photoperiods (T-10 Fluorescent lamps (The Lamp Company Co. Ltd, London, UK) [at 37.5 µmol m−2 s−1 photosynthetic photon flux (PPF)]).

Table 1.
Designed treatments and investigated characteristics in establishment stage.
2.3. Direct Organogenesis of Transverse Thin Cell Layer Explants (tTCLs) and Culture Conditions
Fresh non-infected explants, selected in the establishment stage, were transferred to organogenesis medium containing MS basal medium in combination with different types and concentrations of PGRs including 6-benzylaminopurine (BAP) (0, 0.5, 1, 1.5 mgL−1), thidiazuron (TDZ) (0, 0.5, 1, 1.5 mgL−1), and 1-naphthaleneacetic acid (NAA) (0, 0.5, 1 mgL−1). Stable 25 mgL−1 AgNO3 was used in all designed regeneration treatments (Table 2). The explants were subcultured every 6 weeks to the fresh organogenesis medium. Regenerated shoots were subcultured in MS medium containing 0.5 mgL−1 BAP without any auxins for elongation, and desirable elongated shoots with 3–4 leaves were transferred to MS basal medium devoid of PGRs for rooting. Rooted plantlets were acclimatized in tissue culture room with 25 ± 1 °C and 80% relative humidity for one month. After one month, they were transferred to a greenhouse with 25 ± 1 °C and 60% relative humidity. Transparent plastic bags were used to supply the required relative humidity in the first acclimatization stage. The culture conditions, medium preparation, and growth chamber environmental parameters were similar to those of the establishment stage.

Table 2.
Designed treatments and investigated characteristics in organogenesis stage.
2.4. Experimental Design and Statistical Analysis
Establishment stage tests were carried out in Completely Randomized Design (CRD) with 5 treatments and 3 replicates for each treatment (each replication included 3 explants). The same experimental design with 21 treatments and 3 replicates for each treatment was used for organogenesis tests. Three explants were considered for each replicate in all experiments. All culture medium contents and PGRs were prepared from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and Duchefa (Duchefa-Biochemie, Haarlem, The Netherlands), respectively. The percentage of infected explants was measured in the establishment stage. Regenerated shoot number, regenerated shoot length (cm), and number of new leaves per shoot were recorded after organogenesis stage. Means were separated by one-way analysis of variance (ANOVA), and significant differences between treatments were assessed with Duncan’s multiple range test at p ≤ 0.05 using SPSS v. 28.0.
3. Results
3.1. The Effect of Silver Nitrate on In Vitro Bacterial Infection in tTCL Petiole Begonia Explants
Here, 1 mm thin transverse segments were surface sterilized with varying concentrations of AgNO3 and kept on MS basal medium without any PGRs. Our statistical analysis showed that applying silver nitrate to the culture medium significantly decreased the incidence of internal bacterial infection F (4, 15) = 48.975, p ≤ 0.01. As shown in Figure 1, in the control group, an incidence of 77.77% bacterial infection was observed, whereas this dropped significantly after the addition of even small amounts of silver nitrate (25 mgL−1). Crucially, however, using higher concentrations of silver nitrate induced tissue necrosis that resulted in explant death, negating any benefit of a further decrease in infections.
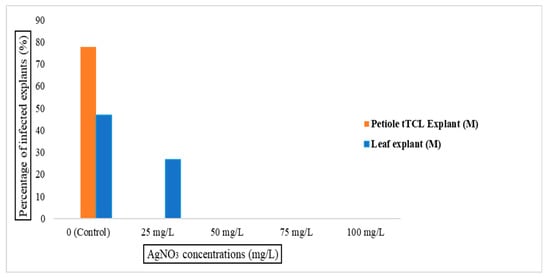
Figure 1.
Effect of different concentrations of silver nitrate (AgNO3) on in vitro bacterial infection of B. rex cv. DS-EYWA via direct organogenesis of two different explants including tTCL petiole explant and leaf explant.
3.2. The Combined Impacts of PGRs and AgNO3 on Direct Organogenesis of tTCL Petiole Begonia Explants
tTCL explants were regenerated using 25 mgL−1 AgNO3 and varying concentrations of BAP, TDZ, and NAA. ANOWA analysis showed that 1.5 mgL−1 BAP in combination with 0.5 mgL−1 NAA produced the highest number of regenerated shoots (M = 35.66, SD = 0.57), and the lowest was found in control treatments without any PGRs (M = 0.00, SD = 0.00) F (18, 57) = 642.101, p ≤ 0.01. The results clearly showed that direct organogenesis occurred in all treatments containing BAP and TDZ in combination with or without NAA, but the regeneration efficiency and the number of regenerated shoots varied. BAP performed better than TDZ for direct organogenesis from tTCL begonia petiole explants (Figure 2A). In Figure 3(A1–A6), different stages of in vitro direct organogenesis from mother plant selection, tape of explant, direct regeneration after two and four weeks in BAP treatment, and the elongation stage before acclimatization are displayed in detail.
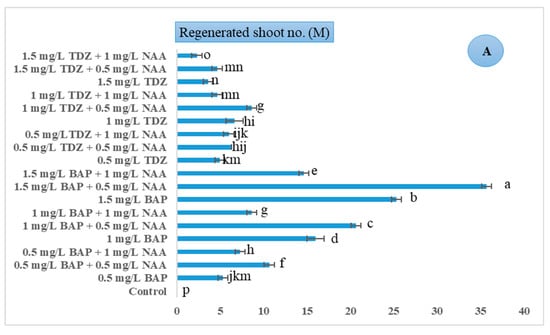
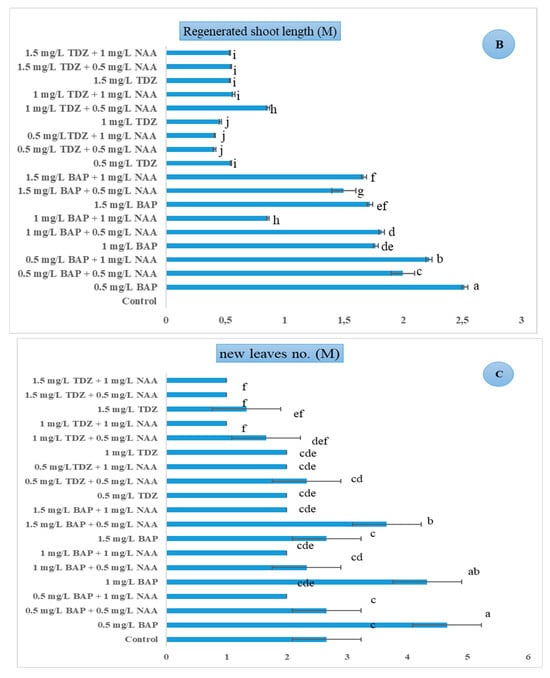
Figure 2.
Effect of different concentrations of PGRs (mg/L) on in vitro morphological traits of B. rex cv. DS-EYWA via direct organogenesis of tTCL petiole explant: (A) Regenerated shoot number (M), (B) regenerated shoot length (M) (cm), (C) new leaf number per regenerated explant (M), p ≤ 0.05. The same lowercase letters indicate no significant differences between treatments by the Duncan test at a 5% probability (Mean ± Standard Deviation).

Figure 3.
Various in vitro stages of direct organogenesis in B. rex cv. DS-EYWA: (A1). Mother plant for explant isolation, (A2) shoot regeneration from tTCL petiole explant (two weeks after cultivation), (A3) shoot regeneration from tTCL petiole explant (four weeks after cultivation), (A4) regenerated shoot in elongation stage, (A5) proliferation stage of elongated shoots, (A6) acclimatization stage of rooted shoots.
3.3. Enhancing the Length of Regenerated Shoots Can Be in Parallel with New Leaf Production in the Same Treatment
After shoot regeneration, desirable shoots were progressed to the elongation stage. The length of regenerated shoots was evaluated as the indicator of the elongation stage. Figure 2B shows the longest in vitro regenerated shoots were observed in MS medium containing 0.5 mgL−1 BAP without any auxin (M = 2.52 cm, STD = 0.025), F (18, 57) = 1321.292, p ≤ 0.01. Furthermore, the highest number of new leaves was produced in developed shoots in MS medium containing 0.5–1 mgL−1 BAP (M = 4.66–4.33, STD = 0.57–0.57), F (18, 57) = 18, p ≤ 0.01 (Figure 2C). Results showed that the direct regeneration of tTCL petiole explants of B. rex cv. DS-EYWA was associated with the presence of both auxin and cytokinin in the culture medium with different ratios (Figure 4a–c). After the selection of suitable regenerated shoots with desirable heights, they were transferred to the rooting stage. MS basal medium without any PGRs was used for the rooting stage, and regenerated plantlets produced roots successfully during 6–8 weeks. Rooted plantlets were acclimatized in tissue culture room with 25 ± 1 °C and 80% relative humidity for one month. Transparent plastic bags were used to supply the required relative humidity in the first acclimatization stage. After one month, they were transferred to the greenhouse with 25 ± 1 °C and 60% relative humidity. Thus, 98% of plantlets were acclimatized successfully.
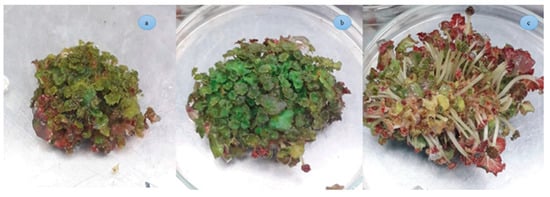
Figure 4.
Direct adventitious bud regeneration of B. rex cv. DS-EYWA via tTCL petiole explant: (a) Two weeks after subculturing in regeneration medium, (b) four weeks after subculturing in regeneration medium (upper side), (c) four weeks after subculturing in regeneration medium (bottom side).
4. Discussion
In 1700, silver nitrate, AgNO3, was used for the treatment of microbial human diseases. The toxicity of silver ions (Ag+) and their compounds towards microbes is characterized by their stronger antibacterial activity, broad antibacterial spectrum, and higher stability. Currently, silver nitrate as an antimicrobial agent can be used in plant tissue culture [22]. Our results showed that using silver nitrate in medium after surface sterilization was effective in decreasing bacterial infection in begonia tTCL petiole explants. Controlling in vitro contaminations, especially bacterial ones, is a time-consuming and limiting stage for plant in vitro culture techniques. Using antimicrobial agents such as silver compounds during the sterilization procedure or in the culture medium after sterilization are suggested methods for limiting bacterial growth. Moreover, using silver nitrate after sterilization in the culture medium can increase the antibacterial effect of silver compounds due to the cut surface of explants after sterilization [23,24]. The toxicity of silver ions (Ag+) and their compounds towards microbes is characterized by their stronger antibacterial activity, broad antibacterial spectrum and higher stability [25,26]. Internal bacterial infections (from different genera) are considered as limiting factors for the in vitro culture of many plants, which mainly relates to the mother plant’s culture conditions [27]. Silver compounds, especially silver nanoparticles, are toxic to bacteria at low concentrations, and when incorporated in modified MS medium of culturing plants, they have good potential for the removal of bacterial contaminants in plant tissue culture procedures [28].
Moreover, previous research on Brassica juncea showed that applying silver nitrate in in vitro conditions can induce an initial oxidative milieu followed by a less-oxidized cellular environment. Furthermore, it can upregulate cytokinin receptors and negative regulators of auxin biosynthesis genes [29]. Silver components can regulate protein accumulation and the expression of some genes involved in cellular metabolism. On the other hand, controlling internal bacterial contaminations is one of the positive impacts of silver components, which is mentioned in several research studies related to plant species such as Valeriana officinalis, Araucaria excelsa, and Brassica juncea without inducing any mutations [22,30,31]. The uptake, accumulation, and toxicity of silver components are highly dependent on their concentrations. Therefore, depending on their concentrations, positive or negative responses of silver compounds can be different, and this is obvious in the morphological traits of in vitro plants [31] (Figure 5).
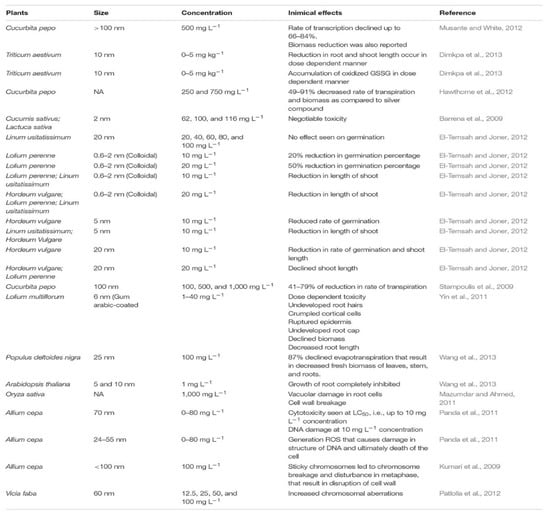
Figure 5.
The side effects of different concentrations of silver nitrate on different plant species [31,32,33,34,35,36,37,38,39,40,41,42,43].
The disruption of cell membranes, inhibition of enzymatic activity, and DNA binding are the three main strategies of silver ions for bacterial growth inhibition [44]. The combination of silver ion interactions with membrane proteins and lipids, ROS generation during induced oxidative stress conditions, and the inhibition of key cellular functions are major strategies of silver compounds for the disruption of cell membranes and the inhibition of internal bacterial growth. In addition to the limitation of bacterial growth, it is well recognized that silver components are effective for controlling ethylene accumulation in in vitro vessels [30]. Silver components such as silver thiosulfate, silver nitrate, and silver nanoparticles can reduce ethylene accumulation in plant tissue culture vessels by using various strategies including the following: 1. Ethylene binding: Silver ions can bind to ethylene to form a complex which inhibits ethylene interaction with ethylene receptors and triggers physiological responses, ultimately resulting in decreasing negative effects on plant tissues. 2. Inhibiting ethylene biosynthesis: Interference with enzymes from ethylene biosynthesis including ACC synthase and ACC oxidase results in a reduction in ethylene production in plant tissue culture vessels. 3. Antimicrobial properties: A reduction in microorganism growth, especially of bacterial agents, will lead to decreased ethylene biosynthesis in vitro [20,24,27]. It is important to set up balanced concentrations of silver components for ethylene inhibition or antimicrobial effects, because their high concentrations will induce toxicity for plant tissues.
A balanced combination of cytokinin and auxin is necessary for shoot direct regeneration in plant species [45]. Our results show that a combination of BAP and NAA performed better than the combination of TDZ and NAA. Moreover, among four concentrations of cytokinins, 1.5 mgL−1 was best for BAP and 1 mgL−1 for TDZ. On the other hand, among three concentrations of NAA in combination with both types of cytokinins, 0.5 mgL−1 responded better than others. In contrast, [2] reported equal concentrations of auxin and cytokinin for the direct adventitious bud formation of B. rex leaf explants. Without considering the concentration of PGRs, the direct regeneration of begonia explants (via tTCL petiole or leaf explants) will occur in the presence of both auxin and cytokinin, and the desirable concentrations are highly dependent on the genotype and explant type. Due to the importance of direct regeneration for genetic engineering programs, establishing an efficient protocol especially for new cultivars is necessary. Various in vitro factors including PGRs; growth adjuvants; and in vitro environmental factors such as temperature, humidity, and light conditions can directly influence regeneration efficiency.
Cytokinins are one of several in vitro ingredients that have a determinative effect on direct regeneration efficiency. Our results showed that BAP in combination with low concentrations of NAA can induce cell division and in vitro growth and ultimately enhance the regeneration rate more than the combination of TDZ and NAA. It is noticeable that cytokinins can potentially induce cell division by increasing protein synthesis and enzymatic activation and the elimination of apical dominance, but depending on the plant species and the in vitro explant type, results can be varied [46].
Various researchers have reported the direct/indirect regeneration of begonia species by using leaf/petiole explants [7,47]. Depending on the genotype, explant type, and PGR types and concentrations, the regeneration capability will vary. Our results show that the presence of phytohormones is necessary for direct regeneration, but the type and concentration of the PGRs in combination with tTCL petiole explants can distinguish the final results. The results also show that no direct/indirect regenerated shoots were obtained in control treatments without PGRs, but divergent regeneration ratios were obtained in treatments including PGRs. Generally integrating equal ratios of auxins and cytokinins can induce callogenesis in begonia explants [48]. Ref. [2] delineated equally high concentrations of IBA and BA for the indirect regeneration of B. rex from leaf explants. Along with the existence of auxins and cytokinins, their balanced ratio can differentiate direct adventitious buds from begonia explants. Based on the genotype and endogenous proportions of PGRs, equal ratios of cytokinins and auxins or higher ratios of cytokinins than auxins are vital for the direct organogenesis of horticultural crops including begonias [5,47,49]. In our tests, high NAA-to-BAP/TDZ ratios were very effective for the direct adventitious bud regeneration of B. rex cv. DS-EYWA from tTCL petiole explants (Figure 4).
On the other hand, using tTCL explants for plant tissue culture studies opens up new avenues for the efficient in vitro production of plant species in a short period of time. Using a low number of mother plants to prepare tTCL explants is one of the most notable advantages of TCL techniques for plant tissue culture studies [14,15]. According to our results, applying low concentrations of AgNO3 (25 mgL−1) in combination with higher concentrations of BAP (1.5 mgL−1) than NAA (0.5 mgL−1) could significantly increase direct regeneration efficiency. Moreover, improvement in shoot qualities and the enhancement of leaf numbers per regenerated shoot correlated strongly with the presence of silver nitrate. The abilities of silver compounds to enhance endogenous polyamine synthesis and inhibit ethylene synthesis and accumulation can positively affect organogenesis efficiency, which has been observed in previous studies [20,24,50,51].
5. Conclusions
In this study, the multifunctional purposes of silver nitrate for the efficient in vitro propagation of B. rex cv. DS-EYWA have been investigated. Owing to the inhibition of internal bacterial infection that is considered as one of the limiting factors for the in vitro propagation of ornamental plants including begonias, direct shoot organogenesis from ex vitro internode tTCL explants was significantly enhanced on MS medium containing 1.5 mgL−1 BAP, 0.5 mgL−1 NAA, and 25 mgL−1 AgNO3. In addition, to our knowledge, this was the first use of internode tTCL explants of B. rex cv. DS-EYWA for direct organogenesis. Furthermore, the addition of silver nitrate on the culture medium had positive effects on morphological traits including shoot organogenesis, shoot length, and the number of green leaves per regenerated shoot. The present study represents a significant improvement in the efficient micropropagation of B. rex cv. DS-EYWA via TCL culture in a short period of time.
Author Contributions
M.D. conceived the study, designed the experiments, supervised the research, and wrote the manuscript. D.N.D. performed the experiments and data collection. H.M. analyzed the data and prepared related figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Aragol Baft Negin Tabarestan Co., Mazandaran, Sari, Iran (Grant No. 1541).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Christopher Schlosser for critical reading of the manuscript. The authors gratefully acknowledge TU Wien for the Open Access Funding Programme of TU Wien Bibliothek.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://www.etsy.com/at/listing/1105190163/begonia-rex-ds-eywa-ukrainian-variety?show_sold_out_detail=1&ref=nla_listing_details (accessed on 20 April 2023).
- Hosseinabadi, S.; Khaleghi, A.; Akramian, M.; Khadivi, A. A highly efficient plant regeneration of Begonia rex Putz. by direct organogenesis of leaf explants. J. Hortic. Sci. Biotechnol. 2022, 97, 496–502. [Google Scholar] [CrossRef]
- Zarei, S.; Ehsanpour, A.A. Ethylene inhibition with silver nitrate (AgNO3) and pyrazinamide (PZA) ameliorates in vitro salt tolerance of tomato (Lycopersicon esculentum L.) plantlets. Plant Cell Tiss. Organ. Cult. 2023, 154, 239–247. [Google Scholar] [CrossRef]
- Kazemi, D.; Dehestani-Ardakani, M.; Hatami, M.; Ghorbanpour, M. Research on the Differences in Phenotypic and Photosynthetic Biophysical Parameters of Begonias (Begonia rex) Cultivars Under Various Light Spectral Compositions. J. Plant Growth Regul. 2023, 20, 106–121. [Google Scholar] [CrossRef]
- Nhut, D.T.; Hai, N.T.; Phan, M.X. A Highly Efficient Protocol for Micropropagation of Begonia tuberous. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S., Ochatt, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589. [Google Scholar] [CrossRef]
- Guo, B.; Xiong, Y.; Ren, H.; Wu, T.; SILVA, J.A.; Zeng, S.; Ma, G. Shoot organogenesis and plant regeneration in Begonia coptidifolia. Turk. J. Agric. For. 2022, 45, 381–388. [Google Scholar] [CrossRef]
- Hosseini, F.; Moshtaghi, N.; Sharifi, A.; Bagheri, A.; Marashi, H.; Keykha Akhar, F. Effect of Kind and Plant Growth Regulator Composition on Micropropagation of Three Begonia Species. J. Plant Prod. 2021, 44, 25–36. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Song, S.; Wang, C.; Sun, H. Highly effective organogenesis and somatic embryogenesis of Clivia. Sci. Hortic. 2022, 306, 111443. [Google Scholar] [CrossRef]
- Ho, T.; Pak, H.; Ri, S.; Kim, K.; Mun, N. Improvement of Water Stress Tolerance of Tuberous Begonia (Begonia × tuberhybrida) by OsmiR393a Gene Transformation. J. Plant Sci. 2021, 9, 257–265. [Google Scholar] [CrossRef]
- Hirutani, S.; Shimomae, K.; Yaguchi, A.; Chin, D.P.; Mii, M.; Igawa, T. Efficient plant regeneration and Agrobacterium-mediated transformation of Begonia semperflorens-cultorum. Plant Cell Tissue Organ Cult. 2020, 142, 435–440. [Google Scholar] [CrossRef]
- Iqbal, A.; Khan, R.S.; Khan, M.A.; Gul, K.; Aizaz, M.; Usman, M.; Arif, M. Efficient Regeneration in Sugarcane Using Thin Cell Layer (TCL) Culture System. Sugar Tech. 2023, 25, 168–176. [Google Scholar] [CrossRef]
- Da Silva, J.A.T. Thin cell layer technology in ornamental plant micropropagation and biotechnology. Afr. J. Biotechnol. 2003, 2, 683–691. [Google Scholar] [CrossRef]
- Da Silva, J.A.T. Thin Cell Layers: Power-Tool for Organogenesis of Floricultural Crops. In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S., Ochatt, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 589. [Google Scholar] [CrossRef]
- Sowmya, M.; Jinu, U.; Sarathikannan, D.; Geetha, N.; Girija, S.; Venkatachalam, P. Effect of silver nitrate and growth regulators on direct shoot organogenesis and in vitro flowering from internodal segment explants of Alternanthera sessilis L. Biocatal. Agric. Biotechnol. 2020, 30, 101855. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Plant Thin Cell Layers: A 40-Year Celebration. J. Plant Growth Regul. 2013, 32, 922–943. [Google Scholar] [CrossRef]
- Aswathi, N.V.; Thomas, T.D. Transverse thin cell layer (tTCL) technology: A promising tool for micropropagation of Centratherum punctatum Cass. Vitr. Cell Dev. Biol. Plant 2023, 18, 340–353. [Google Scholar] [CrossRef]
- Tung, H.T.; Van, H.T.; Bao, H.G.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Nhut, D.T. Silver nanoparticles enhanced efficiency of explant surface disinfection and somatic embryogenesis in Begonia tuberous via thin cell layer culture. Vietnam. J. Biotechnol. 2021, 19, 337–347. [Google Scholar] [CrossRef]
- Cuong, D.M.; Mai, N.T.; Tung, H.T.; Khai, H.D.; Luan, V.Q.; Phong, T.H.; Van The Vinh, B.; Phuong, H.T.; Van Binh, N.; Tan Nhut, D. Positive effect of silver nanoparticles in micropropagation of Limonium sinuatum (L.) Mill. ‘White’. Plant Cell Tissue Organ Cult. 2023, 22, 417–432. [Google Scholar] [CrossRef]
- Manokari, M.; Raj, M.C.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Shekhawat, M.S. Silver nanoparticles improved morphogenesis, biochemical profile and micro-morphology of Gaillardia pulchella Foug cv. ‘Torch Yellow’. Plant Cell Tiss Organ Cult. 2023, 6, 433–445. [Google Scholar] [CrossRef]
- Tung, H.T.; Hieu, T.; Phong, T.H.; Khai, H.D.; Hanh, N.T.; Van, K.T.; Nhut, D.T. The Application of Thin Cell Layer Culture Technique in Plant Regeneration and Micropropagation: Latest Achievements. In Plant Tissue Culture: New Techniques and Application in Horticultural Species of Tropical Region; Nhut, D.T., Tung, H.T., Yeung, E.C.T., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Nasir, S.M.; Abdulhussein, M.A.A. Effects of AgNO3 in combination with some plant growth regulators on micropropagation of strawberry (Fragaria ananassa Duch). Kufa J. Agric. Sci. 2022, 14, 33–40. [Google Scholar] [CrossRef]
- Sgamma, T.; Thomas, B.; Muleo, R. Ethylene inhibitor silver nitrate enhances regeneration and genetic transformation of Prunus avium (L.) cv Stella. Plant Cell Tissue Organ Cult. 2015, 120, 79–88. [Google Scholar] [CrossRef]
- Abogarra, L.; Eisa, N.; EL-habbaa, G.; Darwesh, R.S.; EL-habbak, M. Superiority of Nano-Silver Nitrate and Nano-Chitosan in Controlling Bacterial Contamination and Promoting Growth of in vitro Date Palm Cultures. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 85–104. [Google Scholar] [CrossRef]
- Salman, H.D. Evaluation and comparison the antibacterial activity of silver nano particles (AgNPs) and silver nitrate (AgNO3) on some pathogenic bacteria. J. Glob. Pharma Technol 2017, 9, 238–248. [Google Scholar]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. Role of Silver Nitrate and Silver Nanoparticles on Tissue Culture Medium and Enhanced the Plant Growth and Development. In Plant Breeding towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer: Singapore, 2019; pp. 59–74. [Google Scholar] [CrossRef]
- Sibeko, L.; Johns, T.; Cordeiro, L.S. Traditional plant use during lactation and postpartum recovery: Infant development and maternal health roles. J. Ethnopharmacol. 2021, 279, 114377. [Google Scholar] [CrossRef] [PubMed]
- Paladi, R.K.; Rai, A.N.; Penna, S. Silver nitrate modulates organogenesis in Brassica juncea (L.) through differential antioxidant defense and hormonal gene expression. Sci. Hortic. 2017, 226, 261–267. [Google Scholar] [CrossRef]
- Sarmast, M.; Salehi, H.; Khosh-Khui, M. Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol. Hung. 2011, 62, 477–484. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef]
- Hawthorne, J.; Musante, C.; Sinha, S.K.; White, J.C. Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita pepo. Int. J. Phytoremed. 2012, 14, 429–442. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H.; Ahmed, G.U. Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int. J. ChemTech Res. 2011, 3, 1494–1500. [Google Scholar]
- Panda, K.K.; Achary, V.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the Ions: The Effects of Silver Nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Phong, T.H.; Hieu, T.; Tung, H.T.; Mai, N.T.; Khai, H.D.; Cuong, D.M.; Luan, V.Q.; Nam, N.B.; Nhut, D.T. Silver nanoparticles enhance the in vitro plant regeneration via thin cell layer culture system in purple passion fruit. Plant Cell Tissue Organ Cult. 2023, 155, 403–415. [Google Scholar] [CrossRef]
- Kaviani, B.; Hashemabadi, D.; Khodabakhsh, H.; Onsinejad, R.; Ansari, M.H.; Haghighat, N. Micropropagation of Begonia rex Putz. by 6-benzyladenine and α-naphthalene acetic acid. Int. J. Biosci. 2015, 6, 8–15. [Google Scholar] [CrossRef]
- Bao, H.G.; Tung, H.T.; Van, H.T.; Bien, L.T.; Khai, H.D.; Mai, N.T.; Luan, V.Q.; Cuong, D.M.; Nam, N.B.; Van the Vinh, B.; et al. Copper nanoparticles enhanced surface disinfection, induction and maturation of somatic embryos in tuberous begonias (Begonia × tuberhybrida Voss) cultured in vitro. Plant Cell Tissue Organ Cult. 2022, 151, 385–399. [Google Scholar] [CrossRef]
- Lai, I.L.; Lin, C.W.; Chen, T.Y.; Hu, W.H. Micropropagation shortens the time to blooming of Begonia montaniformis × Begonia ningmingensis var. bella F1 Progeny. HortScience 2018, 53, 1855–1861. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Induction of callogenesis, organogenesis, and embryogenesis in non-meristematic explants of bleeding heart and evaluation of chemical diversity of key metabolites from callus. Int. J. Mol. Sci. 2020, 21, 5826. [Google Scholar] [CrossRef] [PubMed]
- Davoudi Pahnekolayi, M.; Tehranifar, A.; Samiei, L.; Shoor, M. Optimizing culture medium ingredients and micrografting devices can promote in vitro micrografting of cut roses on different rootstocks. Plant Cell Tissue Organ Cult. 2019, 137, 265–274. [Google Scholar] [CrossRef]
- Nhut, D.T.; Khai, H.D.; Hung, N.V.; Vinh, N.Q.; Dung, D.M.; Tung, H.T.; Mai, N.T.N.; Luan, V.Q.; Cuong, D.M. Effect of explant age on phytochemicals and morphogenesis in begonia. Plant Cell Tissue Organ Cult. 2023, 155, 267–282. [Google Scholar] [CrossRef]
- Pawar, B.D.; Markad, N.R.; Wagh, R.S.; Neumann, M.; Kale, A.A.; Chimote, V.P. Proline and Silver Nitrate Promotes Multiple Shoot Induction from Mature Embryo and Shoot Tip Explants of Sorghum. Sugar Tech. 2023, 25, 1187–1195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).