Abstract
Pea sprouts, considered a nutritious and environmentally sustainable vegetable with significant cultivation prospects and market potential, face growth challenges due to salt stress. However, the underlying mechanisms associated with this stress have not been fully elucidated. To address this knowledge gap, we conducted a hydroponic study applying various concentrations of NaCl salt stress to pea sprouts. Systematic analysis was performed on key parameters including germination, plant height, biomass, and enzyme activity of pea sprouts under salt treatment. Our aim was to unravel the underlying mechanisms associated with the impact of salt stress on the growth of pea sprouts. Results revealed that salt treatment significantly inhibited the germination process of pea sprouts’ seeds, leading to a notable decrease in plant height and sprout yield. Salt stress induced an increase in MDA content, a decrease in chlorophyll content, and elevated relative conductivity. However, a low concentration of salt treatment enhanced SOD activity, suggesting the activation of oxidative stress resistance mechanisms in pea sprouts. Moreover, salt treatment exhibited an inhibitory effect on soluble protein content while promoting soluble sugar content in pea sprouts. Additionally, low-concentration salt treatment increased the crude fiber content of pea sprouts, while high-concentration salt treatment inhibited it. In summary, this study indicates that salt stress could cause physiological damage to pea sprouts, but pea sprouts may employ metabolic strategies to adapt to the low concentration of salt stress. These findings contribute to a deeper understanding of the physiological responses of pea sprouts to salt stress and provide valuable insights for its implementation of salt-tolerant cultivation.
1. Introduction
Sprouting vegetables, commonly referred to as “sprouts”, encompass a variety of beans, cereals, and vegetables, where their young stems, sprouts, and hypocotyls serve as the primary edible components [1]. These vegetables not only possess abundant nutrients but also offer a crisp texture [1]. In contrast to traditional soil cultivation methods, sprout production predominantly relies on hydroponic technology, which represents an innovative approach to cultivation. During the germination process, seeds primarily rely on their internal nutrient reserves, making sprouts an environmentally friendly and verdant vegetable variant [2]. Sprout cultivation is characterized by its simplicity, ease of implementation, minimal environmental requirements, and manual control feasibility. It remains unrestricted by geographical or climatic conditions and can be cultivated in a three-dimensional manner within a controlled temperature environment. Moreover, the production equipment is relatively straightforward, requiring minimal capital investment, thereby facilitating year-round cultivation [3]. Additionally, sprouts are enriched with diverse vitamins and offer various health benefits [3]. Sprouts are low in calories, making them suitable for individuals seeking weight loss [4]. Moreover, soybean sprouts can alleviate fatigue by reducing lactic acid accumulation in the human body [5]. In recent years, pea sprouts have gained popularity in the vegetable market due to their sweet and tender taste, lush appearance, and rich nutritional composition, thereby exhibiting promising market prospects [6]. As a green, harmless, and nutritious vegetable variant, pea sprouts possess immense cultivation prospects and market potential. Conducting comprehensive research on their production technology and physiological characteristics will contribute to the sustainable development of the sprout industry. However, salt stress represents a significant hindrance to pea sprouts’ growth [7].
As a critical environmental factor, salt stress exerts a substantial influence on every stage of crop growth and development [8]. Among these stages, seed germination holds exceptional importance as it marks the initiation of the crop’s life cycle and demonstrates its susceptibility and tolerance to external environmental stresses. The ability of seeds to absorb water, swell, and germinate smoothly directly impacts the emergence rate and subsequent growth conditions, thereby serving as a vital indicator for evaluating crop salt tolerance [9]. Under salt stress conditions, changes occur in the osmotic potential of seeds, affecting the processes of water absorption and swelling. Specifically, excessive concentrations of salt ions disrupt the water potential and ion distribution equilibrium within the seeds. This disturbance of cellular homeostasis not only damages seed cell membranes and molecular structures but also impairs the activity of various enzymes [10]. Additionally, salt stress can inhibit cell division and expansion processes, ultimately resulting in a reduced seed germination rate. In severe cases, it may impede plant growth and lead to plant mortality [11].
Beyond its impact on seed germination, salt stress exerts a direct influence on the overall growth and development of crops, manifesting in hindered plant tissue growth and organ differentiation, leading to accelerated plant development processes and shortened growth cycles [12,13]. Moreover, salt stress induces a reduction in leaf number and area, thickening of the leaf epidermis, and the formation of a waxy surface. Notably, comprehensive research has been conducted on the growth, physiological, and biochemical characteristics of hornbeam seedlings under NaCl stress. These investigations have revealed that prolonged exposure to salt stress progressively leads to salt-induced symptoms in seedlings, including leaf scorching, yellowing, and curling. Ultimately, the stems desiccate, darken, and result in plant mortality [14]. Salt stress, as an abiotic stress factor, profoundly disrupts ion balance in plants, leading to osmotic stress and ion toxicity [15]. This stress not only hampers plant metabolic processes but also triggers a substantial accumulation of reactive oxygen species (ROS) within the plant, thereby impeding growth, development, and reducing yield and quality to a significant extent [16]. To maintain ROS homeostasis and prevent oxidative damage, plants activate their antioxidant defense systems, including upregulation of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) [9,17]. The impact of salt stress is pervasive, affecting various aspects of plant life processes, including crucial factors such as growth, development, photosynthesis, and lipid and energy metabolism [18]. Consequently, salt stress poses a substantial challenge to the sustainable advancement of contemporary agriculture, resulting in significant economic losses [19]. However, the short-term physiological and biochemical effects of salt stress on pea sprouts are still unclear.
Existing research highlights that salt stress can damage the structure and function of cell membranes, leading to intracellular metabolic disorders and subsequent reduction in seed viability or even loss of germination ability [20,21]. Further investigations have revealed that high-concentration NaCl stress poses difficulties for water absorption by plant seed sprouts (embryos), which significantly contributes to the notable decline in seed germination rate and potential [22]. Plants employ a complex network of physiological and biochemical mechanisms to maintain ROS homeostasis, osmotic balance, and ion homeostasis under salt stress conditions. The coordinated action of these responses allows plants to mitigate the damaging effects of salinity and adapt to saline environments. However, a lack of comprehensive research exists regarding the specific effects of salt stress on pea sprouts’ growth and development. Therefore, it holds significant importance to delve deeper into the underlying mechanisms that influence the impact of salt stress on pea sprouts’ growth and development.
To comprehensively investigate the physiological and biochemical response mechanisms of pea sprouts under varying concentrations of salt treatments, addressing the challenges posed by salt stress during the cultivation process and enhancing the yield and quality of pea sprouts, this study employed different concentrations of NaCl solutions in the cultivation of pea sprouts and measured the related physiological and biochemical characteristics. The primary objectives of this study were as follows: (1) to elucidate the effects of salt stress on the germination, growth, and development of pea sprouts; and (2) to unravel the physiological and biochemical mechanisms underlying the impact of salt stress on pea sprouts. By accomplishing these objectives, this study aims to illustrate the salt tolerance mechanism of pea sprouts and facilitate the optimal utilization of pea sprouts in production practices.
2. Materials and Methods
2.1. Salt Stress Effect on Pea Sprouts’ Seed Germination
Pea sprouts seeds were selected as the experimental material for this study. The seeds were uniformly placed on a germination bed, with each batch consisting of 50 seeds. To investigate the impact of salt stress on seed germination, three NaCl solution treatment groups were established, each with different concentrations: 100 mmol/L, 200 mmol/L, and 300 mmol/L. Each treatment group was replicated three times, and a control group treated with distilled water was included. After immersing the seeds in the respective solutions for 19 h, the solutions were drained, and the seeds were kept moist. Germination was then carried out under low-light conditions at room temperature, and the number of germinated seeds was recorded for each treatment group throughout the germination process.
2.2. Determination of Germination Index for Pea Sprouts Seeds
After soaking the seeds for 10 h, the number of germinated seeds was assessed every 8 h until the 72nd hour. The germination potential at the 40th hour and the germination rate at the 64th hour were considered important indicators for evaluating seed germination ability. The following formulas were used for calculation:
Germination potential (%) = (number of seeds germinated at the 40th hour/number of seeds tested) × 100%
Germination rate (%) = (number of germinated seeds/number of tested seeds) × 100%
Furthermore, the germination performance of the seeds was comprehensively evaluated by calculating the germination index (GI) using the formula: GI = ∑ (Gt/Dt), where Gt represents the number of germinated seeds at hour t, and Dt represents the corresponding number of hours.
2.3. Effects of Salt Stress on the Growth and Development of Pea Seedlings
Plump and intact pea sprouts seeds weighing 80 g were selected as the experimental material. After washing the seeds three times with distilled water, they were evenly distributed in breeding trays, with five trays assigned to each treatment group. Following a 10 h soaking period in distilled water, the seeds were cultured under low-light and room temperature conditions (25 °C). When the sprouts reached approximately 3 cm in length, they were treated with NaCl solutions of 100 mmol/L, 200 mmol/L, and 300 mmol/L. On days 0, 1, 2, 3, and 4 after treatment, samples were collected to measure various growth and development indicators, including plant height, fresh weight, chlorophyll content, soluble protein content, soluble sugar content, electrical conductivity, malondialdehyde (MDA) content, crude fiber content, and enzyme activities such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). Three replicate measurements were conducted for each treatment group to ensure data accuracy and reliability.
2.4. Determination of Morphological and Physiological Indicators of Pea Sprouts
In this study, seedling height was measured using standard protocols. Chlorophyll content was determined through the widely utilized ethanol extraction method, as established in previous studies [23]. Conductivity measurements were conducted using a conductivity meter to ensure result accuracy [24]. Malondialdehyde (MDA) content was assessed using the thiobarbituric acid (TBA) method, a reliable and commonly employed technique [23]. Superoxide dismutase (SOD) activity was measured using the nitroblue tetrazolium (NBT) method, known for its high sensitivity and accuracy in determining enzyme activity [25]. Peroxidase (POD) activity was determined through the guaiacol method, widely employed in enzyme activity studies [26]. Catalase (CAT) activity was assessed using the hydrogen peroxide method, known for its stability and repeatability in measuring CAT activity [26]. Soluble protein content was measured using the Coomassie Brilliant Blue G-250 staining method, which offers high specificity and sensitivity in protein quantification [27]. Soluble sugar content was determined using the anthrone colorimetric method, a classic and reliable approach [27]. The determination of crude fiber content was performed using the gravimetric method, widely employed in plant fiber content analysis [28].
2.5. Statistical Analysis
Data analysis and statistical procedures in this study were carried out using WPS 2019 and SPSS 26.0 software, ensuring the accuracy and reliability of the data. All results were presented as arithmetic means with standard deviations. Analysis of variance followed by post-hoc Fisher Least Significant Difference test (FLSD) was used to compare treatment means. Values with the same letter in the same treatment are not significantly different (p < 0.05). Additionally, Origin 2021 software was utilized for graphical representation, facilitating the visual depiction of data differences.
3. Results
3.1. Effects of Different Concentrations of Salt Treatment on Pea Sprouts’ Seed Germination
The germination rates of pea sprouts seeds under different salt treatments were illustrated in Figure 1a. The germination rate of seeds subjected to the 100 mmol/L salt treatment did not show a significant difference compared to the control (CK). However, as the salt concentration increased, the germination rate exhibited a gradual decline. Specifically, compared to the CK, the germination rates of seeds treated with 200 mmol/L and 300 mmol/L salts decreased by 38.8% and 59.2%, respectively. These findings demonstrate the inhibitory effect of salt treatment on the germination rate of pea sprouts seeds, which becomes more pronounced with higher salt concentrations.
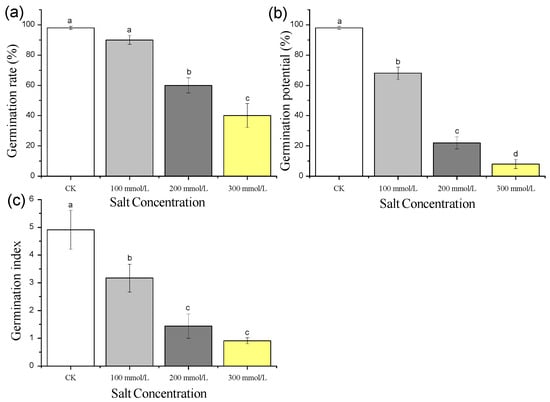
Figure 1.
Effect of different concentration of salt treatment on germination rate (a), germination potential (b) and germination index (c) of pea sprouts. Values with the same letter were not significantly different within different treatments (FLSD, p < 0.05).
The germination potential of pea sprouts seeds displayed a gradual decrease with increasing salt concentration. The germination potential of seeds under 100 mmol/L, 200 mmol/L, and 300 mmol/L salt treatments decreased by 30.6%, 77.6%, and 91.8%, respectively, compared to the CK, as shown in Figure 1b.
The germination index of pea sprouts seeds exhibited a gradual decline as the salt concentration increased. Compared to the CK, the germination index under 100 mmol/L, 200 mmol/L, and 300 mmol/L salt treatments decreased by 35.4%, 70.7%, and 81.5%, respectively (Figure 1c).
3.2. Effects of Different Concentrations of Salt Treatment on Morphological and Physiological Indicators of Pea Sprouts
3.2.1. Effects of Salt Treatment Concentrations on Plant Height and Yield of Pea Sprouts
The plant height of pea sprouts gradually decreased with increasing salt concentration, as evident in Figure 2. The inhibitory effect on plant height became more pronounced with prolonged treatment time. These results indicate that different salt concentrations exert an inhibitory effect on the plant height of pea sprouts, with the degree of inhibition positively correlated with the salt concentration.
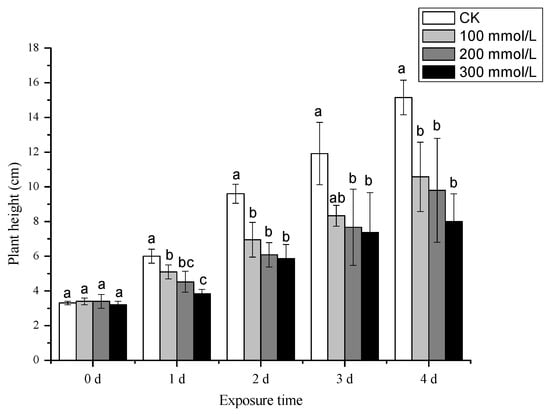
Figure 2.
Effects of different concentration of salt treatment on plant height of pea sprouts (cm). Values with the same letter were not significantly different within different treatments (p < 0.05).
The yield of pea sprouts exhibited a significant decrease under salt treatments compared to the CK (Figure 3). The yield reductions under 100 mmol/L, 200 mmol/L, and 300 mmol/L salt treatments were 46.8%, 59.3%, and 65.6%, respectively. These findings highlight the inhibitory effect of different salt concentrations on the yield of pea sprouts, with a stronger impact observed at higher salt concentrations.
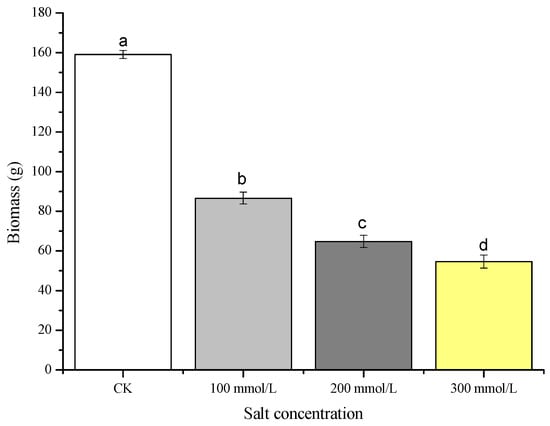
Figure 3.
Effects of different concentration of salt treatment on the biomass of pea sprouts. Values with the same letter were not significantly different within different treatments (p < 0.05).
3.2.2. Effects of Salt Treatment Concentrations on Chlorophyll Content of Pea Sprouts
Throughout the treatment period, the chlorophyll content increased with the time. The maximum value was observed on the 4th day after treatment. Compared to the CK, the chlorophyll content of pea sprouts decreased under various salt stress treatments, as shown in Figure 4. These results indicate that salt with different concentrations inhibit chlorophyll synthesis in pea sprouts.
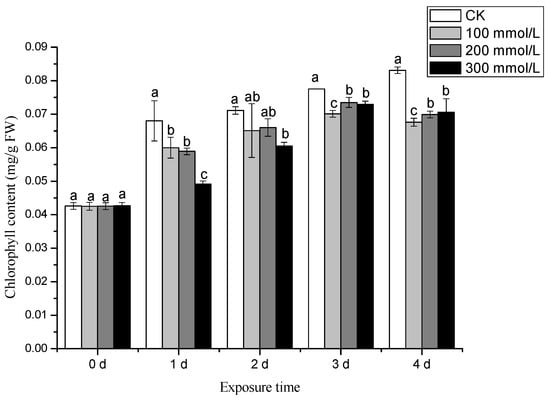
Figure 4.
Effects of different concentration of salt treatment on chlorophyll content of pea sprouts. Values with the same letter were not significantly different within different treatments (p < 0.05).
3.2.3. Effects of Different Salt Treatment Concentrations on the Relative Conductivity and MDA Content of Pea Sprouts
The impact of salt stress on the relative conductivity of pea sprouts at different stages is depicted in Figure 5. Compared with CK, salt stress significantly increased the relative conductivity of pea sprouts except day 0. Under different treatment conditions, the highest relative conductivity was obtained under the salt concentration of 300 mmol/L. Under salt stress conditions, the plasma membrane of pea sprouts may experience damage, resulting in the leakage of cytoplasmic substances and subsequently increasing relative conductivity while reducing resistance.
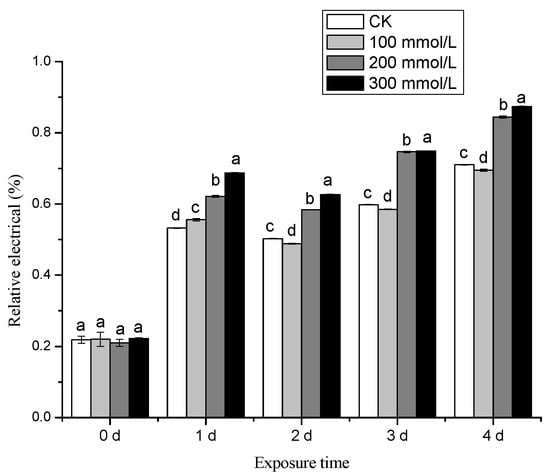
Figure 5.
Effects of different concentration of salt treatment on relative electrical conductivity of pea sprouts. Values with the same letter were not significantly different within different treatments (p < 0.05).
The MDA content of pea sprouts exhibited a trend under different salt concentration treatments: CK < 300 mmol/L < 200 mmol/L < 100 mmol/L. This observation indicates that salt stress can induce an elevation in MDA content in plants (Figure 6a).
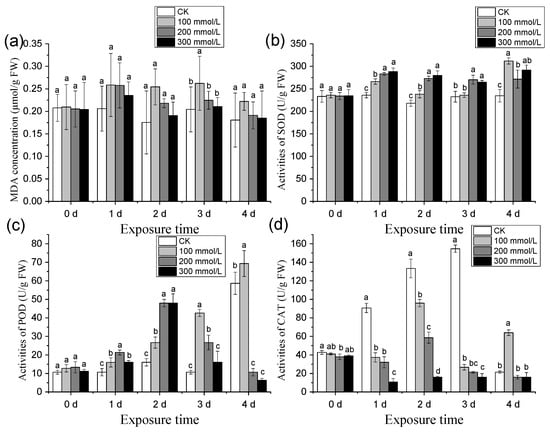
Figure 6.
Effects of different concentration of salt treatment on the activities of MDA (a), SOD (b), POD (c), and CAT (d) of pea sprouts. Values with the same letter were not significantly different within different treatments (p < 0.05).
3.2.4. Effects of Different Salt Treatment Concentrations on SOD, POD, and CAT Activity of Pea Sprouts
Salt stress exerted varying degrees of influence on the SOD activity of pea sprouts (Figure 6b). On the 1st and 2nd days following salt stress treatment, SOD activity gradually increased. On the 3rd day, SOD activity was ranked as follows: 200 mmol/L > 300 mmol/L > 100 mmol/L. By the 4th day, the highest SOD activity was observed under the 100 mmol/L treatment, followed by 300 mmol/L, and finally 200 mmol/L. These results demonstrate that different concentrations of salt treatment can enhance the SOD activity of pea sprouts.
Compared to the control (CK), on the first day following salt stress treatment, POD activity was ranked as follows: 200 mmol/L > 300 mmol/L > 100 mmol/L > CK. On the second day, POD activity exhibited the pattern: 200 mmol/L = 300 mmol/L > 100 mmol/L > CK. By the third day, the POD activity of all treatments surpassed that of the control but displayed a gradual decline. By the fourth day, the order of POD activity was 100 mmol/L > CK > 200 mmol/L > 300 mmol/L (Figure 6c). These findings indicate that salt treatment with different concentrations promotes POD activity in pea sprouts from the 1st to the 3rd day following treatment. However, on the 4th day, high-concentration salt treatment exerted an inhibitory effect on POD activity.
During the initial three days following treatment, the CAT activity under each salt concentration was lower than that of the control, and a downward trend was observed as the salt concentration increased. However, on the fourth day after treatment, only the CAT activity under 100 mmol/L salt treatment exceeded that of the control (Figure 6d). These findings indicate that within the first three days after treatment, salt treatment at various concentrations inhibits the CAT activity of pea sprouts, with a more pronounced inhibitory effect observed at higher salt concentrations. Conversely, on the fourth day, high salt concentrations exhibited an inhibitory effect on CAT activity.
3.2.5. Short-Term Effects of Different Salt Treatment Concentrations on Soluble Protein, Soluble Sugar, and Crude Fiber Content of Pea Sprouts
Compared to the control (CK), the soluble protein content of pea sprouts exhibited a gradual decrease with increasing salt concentration. Specifically, salt treatments of 100 mmol/L, 200 mmol/L, and 300 mmol/L resulted in a reduction in soluble protein content by 4.6%, 6.6%, and 10.4%, respectively (Figure 7a). These findings demonstrate that salt treatment at different concentrations inhibits the soluble protein content of pea sprouts, with a more pronounced inhibitory effect observed at higher salt concentrations.
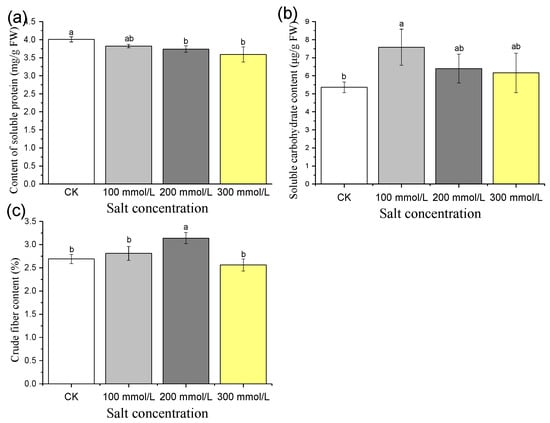
Figure 7.
Short-term effects of different concentration of salt treatment on the content of soluble protein (a), soluble carbohydrate (b), crude fiber (c) of pea sprouts. Values with the same letter were not significantly different within different treatments (p < 0.05).
Significantly, the soluble sugar content of sprouts treated with salt concentrations of 100 mmol/L, 200 mmol/L, and 300 mmol/L exceeded that of the control, exhibiting increases of 41.4%, 19.4%, and 14.9%, respectively (Figure 7b). These observations highlight the promoting effect of salt treatment at various concentrations on the soluble sugar content of pea sprouts.
Under salt stress of 100 mmol/L and 200 mmol/L, the crude fiber content of pea sprouts was higher than that of the control group, displaying increases of 4.5% and 16.7%, respectively (Figure 7c). However, when subjected to 300 mmol/L salt stress, the crude fiber content of pea sprouts was lower than that of the control, showing a decrease of 4.8% (Figure 7c). These results suggest that salt stress exhibits a promoting effect at low concentrations and an inhibitory effect at high concentrations on the crude fiber content of pea sprouts.
4. Discussion
In this study, we assessed the germination rate, germination potential, and germination index of pea sprouts seeds subjected to various salt concentrations. The results clearly demonstrated a downward trend in these indicators as salt concentration increased, with significant differences observed between different salt treatments (Figure 1). Additionally, we investigated the effects of different salt treatment concentrations on the plant height of pea sprouts. The findings revealed a decline in plant height as salt concentration increased, with a more pronounced downward trend observed at higher salt concentrations (Figure 2). This indicates a significant inhibitory effect of salt concentration on plant height growth. Moreover, salt stress substantially impacted the growth and development of pea sprouts, directly resulting in yield reduction (Figure 3). The experimental results from this study highlight the inhibitory effect of salt treatment on pea sprout yield, with a progressively more pronounced effect observed as salt concentration increases.
In general, stress conditions lead to the accumulation of reactive oxygen species (ROS) in plants, causing oxidative damage to the organism [29]. Excessive ROS disrupts the intracellular balance of reactive oxygen metabolism, inducing oxidative stress in plant cells. Consequently, cell membrane structures are damaged, severely affecting biological membranes and macromolecules such as mitochondria, chloroplasts, and plasma membranes [16]. The test results of this study revealed that only the 100 mmol/L salt treatment from days 2 to 4 reduced the relative conductivity of pea sprouts. This can be attributed to the adaptation of pea sprouts to this concentration after two days of salt treatment exposure. However, at other time points, the relative conductivity of sprouts increased to varying degrees under salt treatment (Figure 7). Additionally, a significant reduction in chlorophyll content was observed, further confirming the detrimental impact of oxidative stress caused by ROS accumulation on plasma membranes and chloroplasts [30]. When excessive ROS accumulation in cells is not promptly cleared, lipid peroxidation of cell membranes occurs. Malondialdehyde (MDA) is a byproduct of cell membrane lipid peroxidation, and changes in its content reflect the extent of cell membrane damage [31]. In this experiment, plant MDA content increased following salt stress treatment, providing further evidence of the detrimental effect of salt stress on pea sprouts cell membranes.
SOD, POD, and CAT are critical enzymes within the cellular membrane system, playing a protective role. Their primary function involves facilitating the elimination of reactive oxygen species, thereby maintaining the balance of reactive oxygen species metabolism and preserving the integrity of the cell membrane [32]. The results of this experiment further illustrate that different salt treatments exert a stimulating effect on SOD activity in pea sprouts. Particularly during the initial two days following treatment, the stimulating effect became increasingly significant with higher salt concentrations. Likewise, various salt concentrations induced POD activity in pea sprouts from the first to the third day post treatment. However, on the fourth day after treatment, the 100 mmol/L salt treatment continued to stimulate POD activity, while the 200 mmol/L and 300 mmol/L salt treatments transitioned to an inhibitory effect. The stimulation of POD activity in the 100 mmol/L salt treatment can be attributed to the activation of defense mechanisms in response to moderate salt stress. Increased POD activity helps scavenge reactive oxygen species (ROS) generated under salt stress, thus mitigating oxidative damage and maintaining cellular homeostasis [33,34]. Conversely, the transition to an inhibitory effect of POD activity in the 200 mmol/L and 300 mmol/L salt treatments suggests a disruption or impairment of the antioxidant defense system. This inhibition may be due to excessive ROS production or perturbations in the enzymatic machinery [35,36]. Regarding CAT activity, the test results revealed that different salt treatments inhibit CAT activity in pea sprouts from the first to the third day post treatment, with a more pronounced inhibitory effect at higher salt concentrations. However, on the fourth day after treatment, the 100 mmol/L salt treatment shifted to a stimulating effect on CAT activity, while the 200 mmol/L and 300 mmol/L salt treatments persisted in exhibiting an inhibitory effect. The shift to a stimulating effect on catalase (CAT) activity in the 100 mmol/L salt treatment after the fourth day may indicate an adaptive response of pea sprouts to prolonged exposure to moderate salt stress. CAT plays a crucial role in scavenging hydrogen peroxide (H2O2) generated during oxidative stress [33,34]. The increase in CAT activity suggests an enhanced ability to counteract the accumulation of H2O2 and maintain redox homeostasis. On the other hand, the persistent inhibitory effect of CAT activity in the 200 mmol/L and 300 mmol/L salt treatments indicates a sustained disruption or impairment of the antioxidant defense system. This inhibition could be attributed to the overwhelming accumulation of reactive oxygen species (ROS) or the inhibition of CAT enzyme activity itself [35,36]. These higher salt concentrations may induce more severe oxidative stress, leading to a compromised antioxidant response. It is worth noting that SOD activity on the second day after treatment may have been influenced by prolonged stress, affecting the enzyme activation process. Similarly, POD and CAT may have been similarly affected on the fourth day after treatment. In this experiment, the activities of SOD and POD surpassed those of the control group (CK), which aligns with findings from other studies [37,38]. However, CAT activity in plants decreased with high salt stress concentrations. In summary, this experiment further elucidates the influence of salt stress on the antioxidant enzyme systems in plants by assessing the activities of SOD, POD, and CAT in pea sprouts subjected to different salt treatments. These results provide valuable insights for understanding the underlying physiological response mechanisms of pea sprouts under salt stress.
Soluble protein is a crucial substance involved in various metabolic activities within plants, and changes in its content serve as an important indicator for evaluating the overall metabolic status of plants [39]. On the other hand, soluble sugar is an adaptive product synthesized by plants in response to different salt concentrations, and fluctuations in its content reflect the plant’s coping mechanisms under salt stress [7]. The findings of this study demonstrated a declining trend in soluble protein content as salt concentration gradually increases in pea sprouts. This observation further confirms the inhibitory impact of salt stress on protein metabolism in plants [40]. Simultaneously, we observed a pattern where soluble sugar content initially increased and subsequently decreased with the rise in salt concentration, with its content in all treatment groups surpassing that of the control group (CK). This indicates that under moderate salt stress, plants may enhance their stress tolerance by elevating the levels of soluble sugar. Furthermore, crude fiber content significantly influences the taste of pea sprouts and is substantially affected by salt treatment [41]. The test results revealed that salt treatments of 100 mmol/L and 200 mmol/L increased the crude fiber content of pea sprouts, while the 300 mmol/L salt treatment led to a reduction in content. Crude fiber refers to the indigestible portion of plant material, mainly composed of cellulose, hemicellulose, and lignin. It provides structural support to plants and plays a role in their overall physiology [42]. The fiber content of food is also important from a nutritional perspective, as it affects factors such as satiety, digestion, and gut health [43]. In response to the lower salt treatments (100 mmol/L and 200 mmol/L), pea sprouts may have increased their production of crude fiber as a protective mechanism. However, at higher salt concentrations (300 mmol/L), pea sprouts may experience more severe stress, leading to a reduction in crude fiber content. This reduction could be attributed to the detrimental effects of high salt levels on cell wall integrity and overall plant health. The compromised cellular function under extreme salt stress may result in reduced fiber production [44]. This finding provides novel insights into understanding the effects of salt stress on the quality of pea sprouts.
Overall, salt stress significantly impacts the physiological metabolism and quality of pea sprouts. Moderate salt treatment has the potential to improve the quality of pea sprouts by modulating enzyme activity and modifying the levels of biochemical indicators. However, excessively high salt concentrations may impede these benefits. Therefore, careful regulation of salt concentrations is crucial during the cultivation of pea sprouts, with the aim of reducing the effect of salt stress on the yield of pea sprouts. This investigation deepens our understanding of the physiological response and quality alteration mechanisms of pea sprouts under salt stress.
5. Conclusions
In conclusion, this study examined the effects of salt stress on pea sprouts. The findings revealed that salt stress hindered the germination of seeds, reduced the yield, and inhibited the growth of pea sprouts. In addition, it increased the activity of the antioxidant enzyme SOD, thereby mitigating oxidative damage. The activities of POD and CAT were influenced by both time and salt concentration, exhibiting complex patterns. Salt stress inhibited soluble protein content but promoted the accumulation of soluble sugars, particularly at moderate salt concentrations. Additionally, salt stress influenced the crude fiber content of pea sprouts. Pea sprouts could activate the activities of MDA, SOD, and POD in response to low concentrations of salt stress, but high concentrations of salt stress had a serious inhibitory effect on its physiological and biochemical characteristics. These results offer valuable insights into the physiological responses of pea sprouts to salt stress.
Author Contributions
Conceptualization, T.W.; methodology, L.Z. and X.S.; formal analysis, J.G.; investigation, J.G.; data curation, J.G.; writing—original draft preparation, J.G.; writing—review and editing, J.G.; funding acquisition, T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Projects of the Agriculture Department of Guangdong Province (Funding No. 2023-NPY-00-009) and the Guangdong Province Science and Technology Planning Project (No. KTP20210007).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors were grateful to Guangdong Ocean University and Guangdong Academy of Agricultural Sciences/Guangdong Key Laboratory for New Technology Research of Vegetables for supplying laboratory equipment in this experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Y.; Zhang, Z.; Fan, S.; Qi, Y.; Wang, F.; Wang, M.; Feng, M.; Liu, X.; Ren, H. Advance research on the pre-harvest sprouting trait in vegetable crop seeds. Int. J. Mol. Sci. 2023, 24, 17171. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. Optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Beatriz, M.; Oliveira, P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Novidiyanto; Asrullah, M.; Arsanti Lestari, L.; Helmyati, S.; Farmawati, A. Effect supplementation of mung bean sprouts (Phaseolus radiatus L.) and vitamin e in rats fed high fat diet. KnE Life Sci. 2019, 4, 36. [Google Scholar]
- Khan, M.A.H.; Baset Mia, M.A.; Quddus, M.A.; Sarker, K.K.; Rahman, M.; Skalicky, M.; Brestic, M.; Gaber, A.; Alsuhaibani, A.M.; Hossain, A. Salinity-induced physiological changes in pea (Pisum sativum L.): Germination rate, biomass accumulation, relative water content, seedling vigor and salt tolerance index. Plants 2022, 11, 3493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Alencar, N.L.M.; Enéas, G.-G. Comparison between the water and salt stress effects on plant growth and development. Responses Org. Water Stress 2013, 4, 67–94. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Poojan Yadav, S.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Kumar Singh, R.; Kumar Prasad, S. Impact of salt stress on growth, productivity and physicochemical properties of plants: A review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, N.; Saadaoui, W.; Mezghani, N.; Pavli, O.I.; Falleh, H.; Petropoulos, S.A. The effects of salt stress on germination, seedling growth and biochemical responses of tunisian squash (cucurbita maxima duchesne) germplasm. Plants 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Plessis, A. Abiotic stress experiments need a reality check to improve translation to the field. J. Exp. Bot. 2023, 74, 1741–1744. [Google Scholar] [CrossRef]
- Gul, Z.; Tang, Z.H.; Arif, M.; Ye, Z. An insight into abiotic stress and influx tolerance mechanisms in plants to cope in saline environments. Biology 2022, 11, 597. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Bioch. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Lu, Q.; Ge, G.T.; Sa, D.W.; Wang, Z.J.; Hou, M.L.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ 2021, 9, e11729. [Google Scholar] [CrossRef]
- Trușcă, M.; Gâdea, Ș.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the research challenges and perspectives in ecophysiology of plants affected by salinity stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Lei, Z.; Huang, Q.; Li, T.; Shen, F.; Cheng, Q. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 2020, 10, 10626. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Chen, Y.; Zhang, L.; Kudusi, K.; Song, J. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest china. Front. Ecol. Evol. 2022, 10, 1026095. [Google Scholar] [CrossRef]
- Guo, J.; Du, M.; Tian, H.; Wang, B. Exposure to high salinity during seed development markedly enhances seedling emergence and fitness of the progeny of the extreme halophyte suaeda salsa. Front. Plant Sci. 2020, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Ze Jaafar, H.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, J.; Wang, J. Protective enzyme activity regulation in cotton (Gossypium hirsutum L.) in response to scirpus planiculmis stress. Front. Plant Sci. 2022, 13, 1068419. [Google Scholar] [CrossRef]
- Leonowicz, G.; Trzebuniak, K.F.; Zimak-Piekarczyk, P.; Ślesak, I.; Mysliwa-Kurdziel, B. The activity of superoxide dismutases (sods) at the early stages of wheat deetiolation. PLoS ONE 2018, 13, e0194678. [Google Scholar] [CrossRef]
- Talaat, N.B.; Nesiem, M.R.A.; Gadalla, E.G.; Ali, S.F. Gibberellic acid and salicylic acid dual application improves date palm fruit growth by regulating the nutrient acquisition, amino acid profile, and phytohormone performance. J. Soil. Sci. Plant Nutr. 2023, 23, 6216–6231. [Google Scholar] [CrossRef]
- Jacomassi, L.M.; Pacola, M.; Momesso, L.; Viveiros, J.; Júnior, O.A.; Siqueira, G.F.d.; Campos, M.d.; Crusciol, C.A.C. Foliar application of amino acids and nutrients as a tool to mitigate water stress and stabilize sugarcane yield and bioenergy generation. Plants 2024, 13, 461. [Google Scholar] [CrossRef]
- Hu, P.; Wang, L.; Hu, Z.; Jiang, L.; Hu, H.; Rao, Z.; Wu, L.; Tang, Z. Effects of multi-bacteria solid-state fermented diets with different crude fiber levels on growth performance, nutrient digestibility, and microbial flora of finishing pigs. Animals 2021, 11, 3079. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Pehlivan, N.; Sun, L.; Jarrett, P.; Yang, X.; Mishra, N.; Chen, L.; Kadioglu, A.; Shen, G.; Zhang, H. Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic arabidopsis plants. Plant Cell Physiol. 2016, 57, 1069–1084. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced a. Tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ye, Z.H.; Zhang, H.J.; Miao, L.X. Broccoli plants over-expressing an erf transcription factor gene boerf1 facilitates both salt stress and sclerotinia stem rot resistance. J. Plant Growth Regul. 2019, 38, 1–13. [Google Scholar] [CrossRef]
- Rahnama, H.; Ebrahimzadeh, H. The effect of nacl on antioxidant enzyme activities in potato seedlings. Biol. Plant. 2005, 49, 93–97. [Google Scholar] [CrossRef]
- Akbari, M.; Katam, R.; Husain, R.; Farajpour, M.; Mazzuca, S.; Mahna, N. Sodium chloride induced stress responses of antioxidative activities in leaves and roots of pistachio rootstock. Biomolecules 2020, 10, 189. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of broussonetia papyrifera. South. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Polesskaya, O.G.; Kashirina, E.I.; Alekhina, N.D. Effect of salt stress on antioxidant system of plants as related to nitrogen nutrition. Russ. J. Plant Physl 2006, 53, 186–192. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium chloride (nacl)-induced physiological alteration and oxidative stress generation in Pisum sativum (L.): A toxicity assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef]
- López-Cristoffanini, C.; Bundó, M.; Serrat, X.; San Segundo, B.; López-Carbonell, M.; Nogués, S. A comprehensive study of the proteins involved in salinity stress response in roots and shoots of the fl478 genotype of rice (Oryza sativa L. Ssp. Indica). Crop J. 2021, 9, 1154–1168. [Google Scholar] [CrossRef]
- Ghezal, N.; Rinez, I.; Sbai, H.; Saad, I.; Farooq, M.; Rinez, A.; Zribi, I.; Haouala, R. Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. South. Afr. J. Bot. 2016, 105, 240–250. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant. Nutr. Ecol. Rumin. 2019, 44, 2552–2561. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).