Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl.
Abstract
1. Introduction
2. Material and Method
2.1. Plant Materials
2.2. Metabolomics Analysis
2.3. The Identification and Quantification of Metabolites
2.4. RNA Extraction and Transcriptome
2.5. Transcriptome Assembly, Annotation, and Differential Expression Analysis of the Genes
2.6. Validation of Transcriptome Data
2.7. Construction of Gene Silencing, Overexpression, and Subcellular Localization Vectors
2.8. Agrobacterium-Mediated Transient Infection
2.9. Changes in Aromatic Compound Content after Transient Infection of RfFDPS
2.10. Statistical Analysis
3. Results
3.1. Analysis of Terpenoids in Rhododendron Species with Different Aroma Types
3.2. Analysis of Terpenoids in Petals of Fragrant YJ at Different Development Stages
3.3. Differential Accumulation of Terpenoids
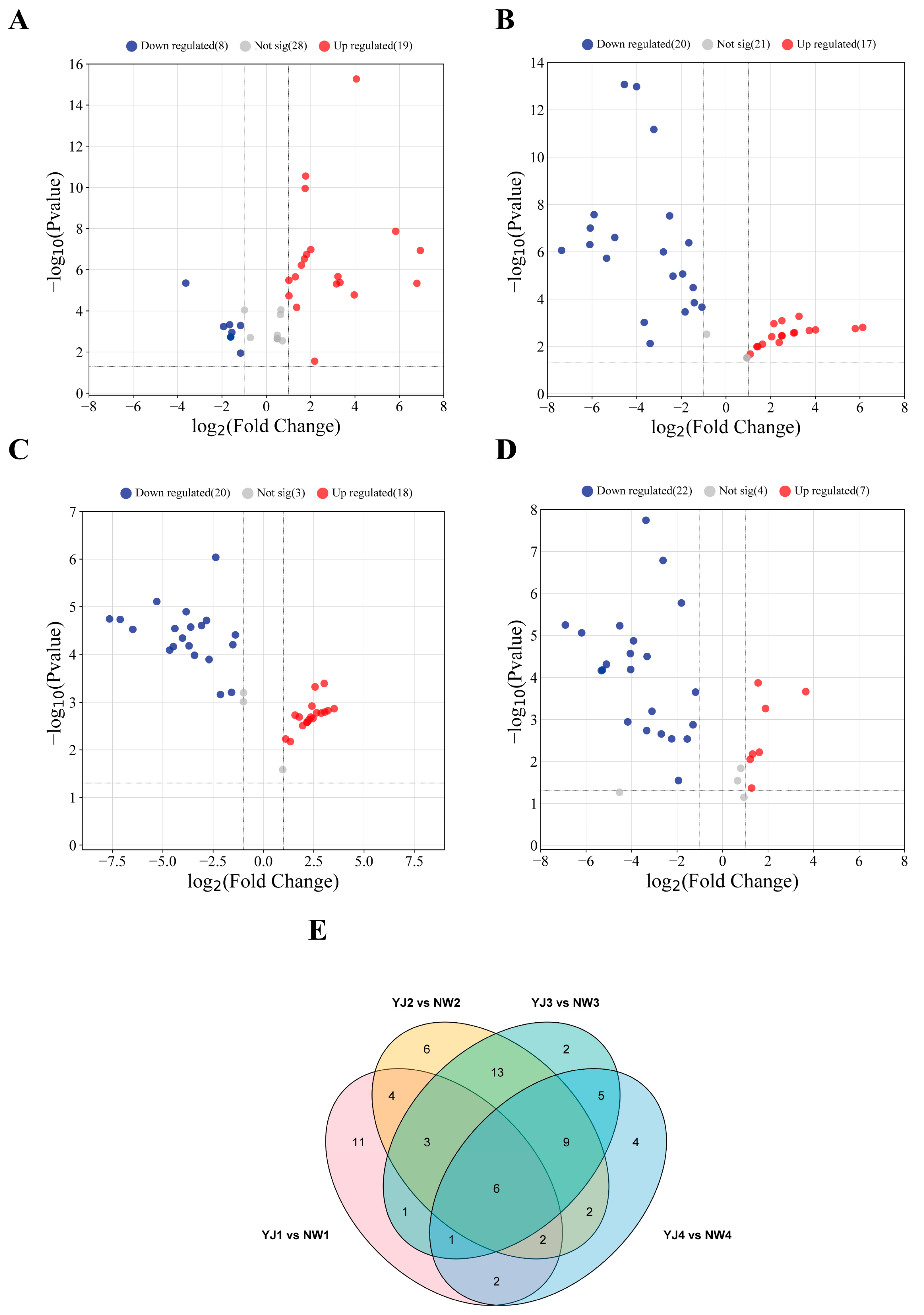
3.4. Transcriptome Data Analysis of Different Aroma Types of Rhododendrons
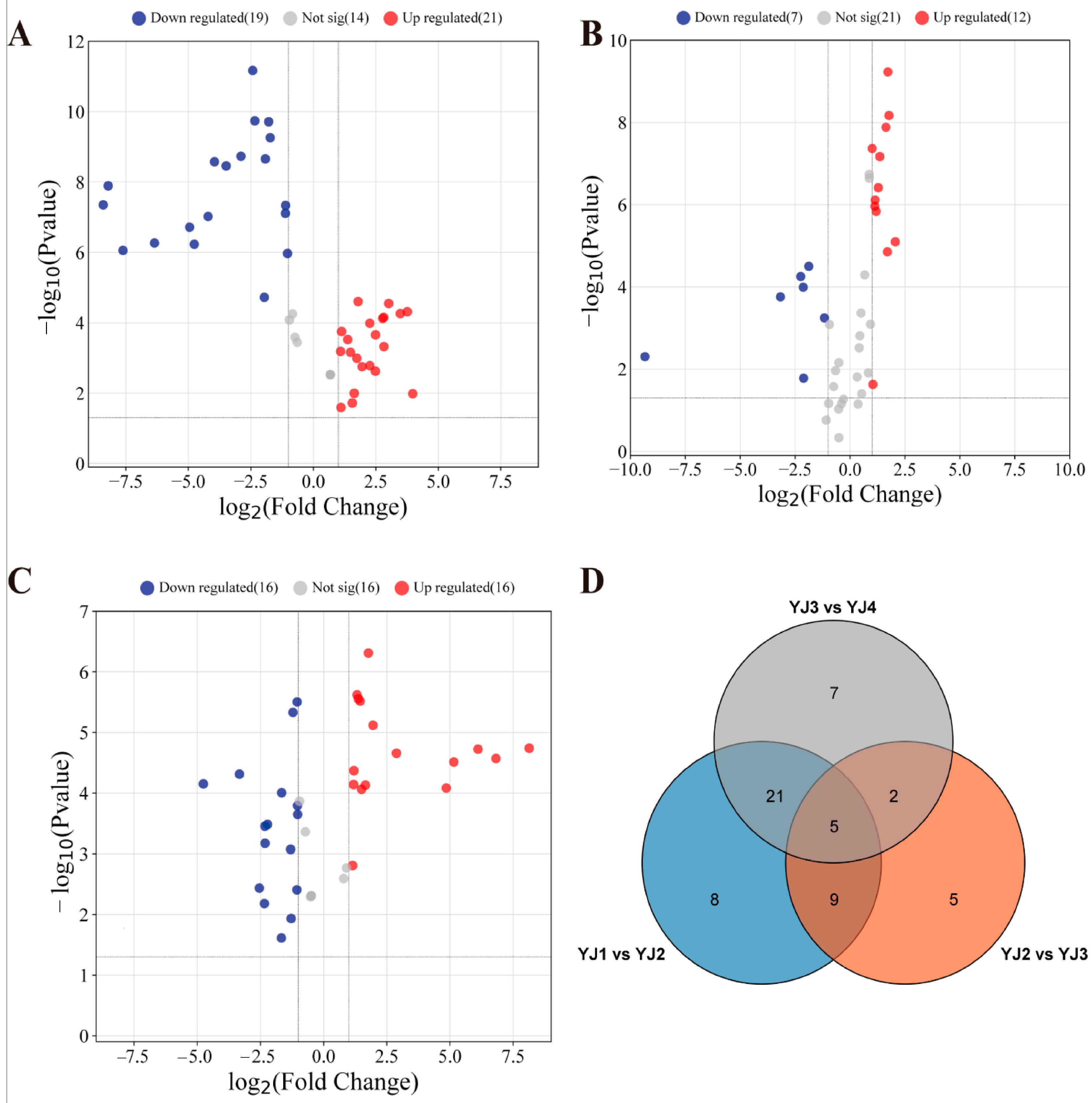
3.5. Transcriptome Data Analysis of YJ with Fragrance at Different Developmental Stages
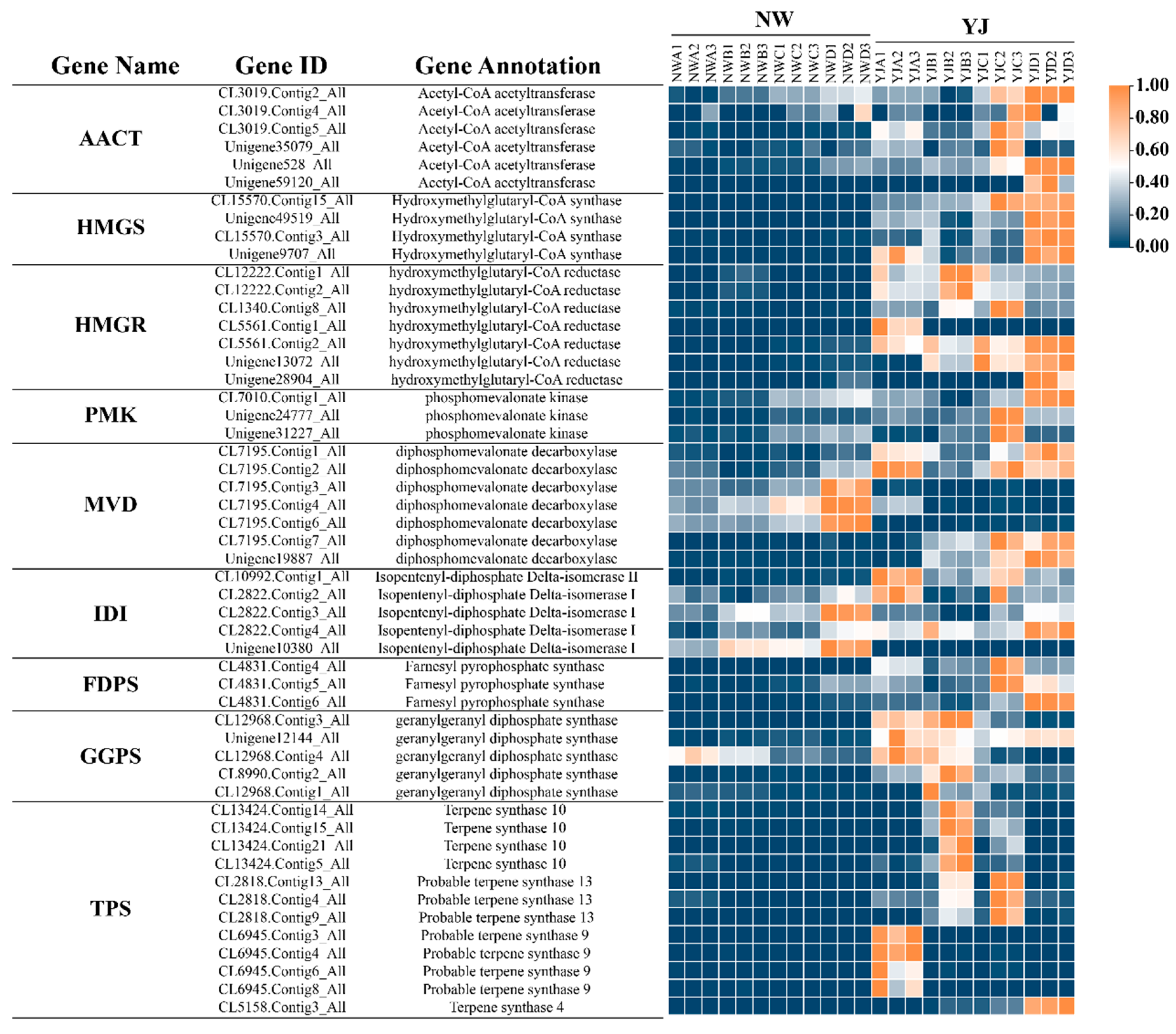
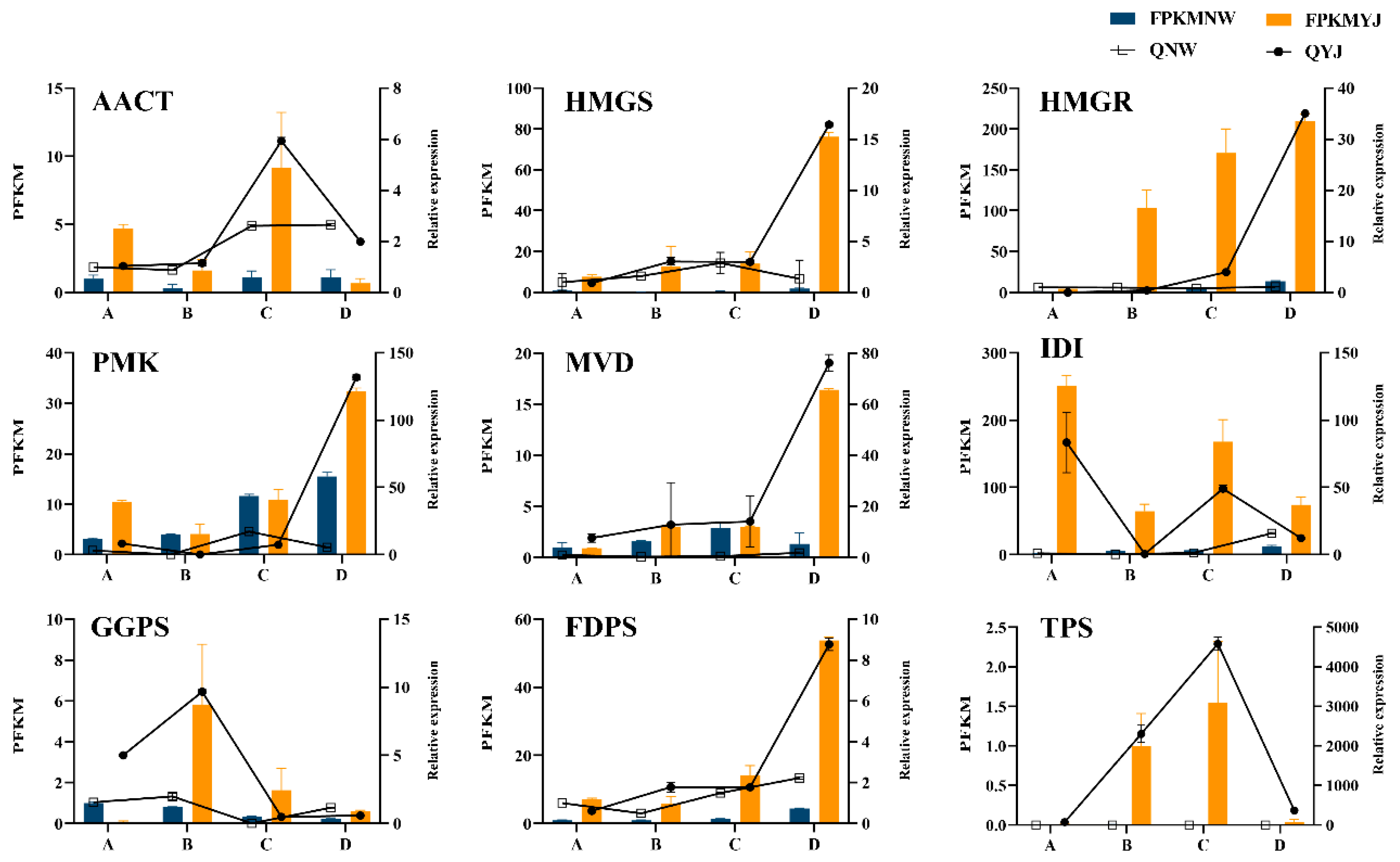
3.6. Screening DEGs in Terpenoid Synthesis Pathway
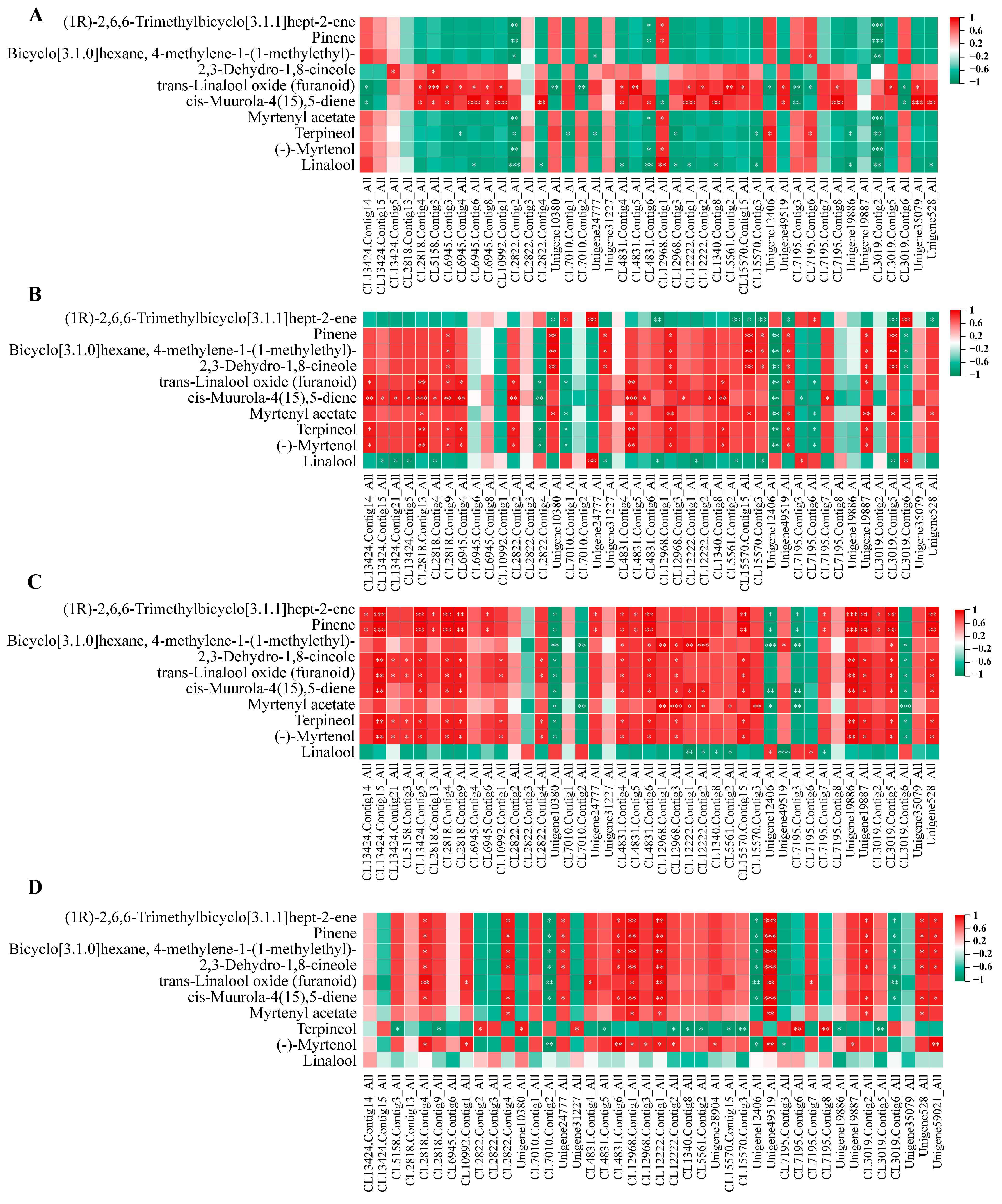
3.7. Joint Analysis of Transcriptome and Metabolome
3.8. Transcriptome and Metabolome Data Validation
3.9. Changes in Relative Content of Major Volatile Compounds following Transient Overexpression and Gene Silencing of RfFDPS
4. Discussion
4.1. Metabolomic Data Analysis of YJ, an Aromatic Rhododendron
4.2. Key Genes for Terpeneoid Synthesis in YJ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.H.; Yao, G.; Neilsen, J.; Liu, D.T.; Zhang, L.; Ma, Y.P. Rhododendron kuomeianum (Ericaceae), a new species from northeastern Yunnan (China), based on morphological and genomic data. Plant Divers. 2021, 43, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hakeem Said, I.; Grimbs, A.; Thielen, N.; Lansing, L.; Schepker, H.; Kuhnert, N. Determination of hydroxycinnamic acids present in Rhododendron species. Phytochemistry 2017, 144, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Qin, Y.; Jia, Y.; Xie, X.; Li, D.; Jiang, B.; Wang, Q.; Feng, S.; Wu, Y. Transcriptomic and metabolomic data reveal key genes that are involved in the phenylpropanoid pathway and regulate the floral fragrance of Rhododendron fortunei. BMC Plant Biol. 2023, 23, 8. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Wu, X.; Wen, X.; Li, D.; Zhou, H.; Li, Z.; Liu, B.; Wei, J.; Chen, F.; et al. High-quality evergreen azalea genome reveals tandem duplication-facilitated low-altitude adaptability and floral scent evolution. Plant Biotechnol. J. 2021, 19, 2544–2560. [Google Scholar] [CrossRef]
- Raguso, R.A. Floral scent in a whole-plant context: Moving beyond pollinator attraction. Funct. Ecol. 2009, 23, 837–840. [Google Scholar] [CrossRef]
- Prieto-Benitez, S.; Dotterl, S.; Gimenez-Benavides, L. Diel Variation in Flower Scent Reveals Poor Consistency of Diurnal and Nocturnal Pollination Syndromes in Sileneae. J. Chem. Ecol. 2015, 41, 1095–1104. [Google Scholar] [CrossRef]
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent Advances in the Emission and Functions of Plant Vegetative Volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-beta-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Abdollahi, A.; Ghanbariasad, A.; Osanloo, M. Antibacterial and leishmanicidal activities of Syzygium aromaticum essential oil versus its major ingredient, eugenol. Flavour Fragr. J. 2020, 35, 534–540. [Google Scholar] [CrossRef]
- Ramya, M.; Jang, S.; An, H.R.; Lee, S.Y.; Park, P.M.; Park, P.H. Volatile Organic Compounds from Orchids: From Synthesis and Function to Gene Regulation. Int. J. Mol. Sci. 2020, 21, 1160. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Matteo, C.; Valentina, S. The Contribution of Volatile Organic Compounds (VOCs) Emitted by Petals and Pollen to the Scent of Garden Roses. Horticulturae 2022, 8, 1049. [Google Scholar] [CrossRef]
- Shanshan, L.; Ling, Z.; Miao, S.; Mengwen, L.; Yong, Y.; Wenzhong, X.; Liangsheng, W. Biogenesis of flavor-related linalool is diverged and genetically conserved in tree peony (Paeonia × suffruticosa). Hortic. Res. 2023, 10, uhac253. [Google Scholar]
- Jiang, F.; Liu, D.; Dai, J.; Yang, T.; Zhang, J.; Che, D.; Fan, J. Cloning and Functional Characterization of 2-C-methyl-D-erythritol-4-phosphate cytidylyltransferase (LiMCT) Gene in Oriental Lily (Lilium ‘Sorbonne’). Mol. Biotechnol. 2024, 66, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, W.; Liu, S.; Wu, J.; Leng, P.; Hu, Z. LiNAC100 contributes to linalool biosynthesis by directly regulating LiLiS in Lilium ‘Siberia’. Planta 2024, 259, 73. [Google Scholar] [CrossRef] [PubMed]
- Jiawei, L.; Hongmin, H.; Huimin, S.; Qingyin, T.; Wenjie, D.; Xiulian, Y.; Lianggui, W.; Yuanzheng, Y. Insights into the Cytochrome P450 Monooxygenase Superfamily in Osmanthus fragrans and the Role of OfCYP142 in Linalool Synthesis. Int. J. Mol. Sci. 2022, 23, 12150. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Zhihui, D.; Xiyu, Y.; Lanlan, W.; Kuaifei, X.; Zhilin, C. An Integrated Analysis of Metabolomics and Transcriptomics Reveals Significant Differences in Floral Scents and Related Gene Expression between Two Varieties of Dendrobium loddigesii. Appl. Sci. 2022, 12, 1262. [Google Scholar] [CrossRef]
- Wang, X.; Song, Z.; Ti, Y.; Ma, K.; Li, Q. Comparative transcriptome analysis linked to key volatiles reveals molecular mechanisms of aroma compound biosynthesis in Prunus mume. BMC Plant Biol. 2022, 22, 395. [Google Scholar]
- Wang, D.; Liu, G.; Yang, J.; Shi, G.; Niu, Z.; Liu, H.; Xu, N.; Wang, L. Integrated metabolomics and transcriptomics reveal molecular mechanisms of corolla coloration in Rhododendron dauricum L. Plant Physiol. Biochem. 2024, 207, 108438. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, Y.; Fang, L.; Liu, Y.; Zhu, X.; Dong, H.; Wang, S. Metabolite analysis reveals flavonoids accumulation during flower development in Rhododendron pulchrum sweet (Ericaceae). PeerJ 2024, 12, e17325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, Y.; Sun, D.; Li, Y.; Li, H.; Chen, L. Chromene meroterpenoids from Rhododendron dauricum L. and their anti-inflammatory effects. Phytochemistry 2024, 225, 114200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, L.; Feng, Y.; Zhang, H.; Gao, B.; Ma, X.; Sun, Y.; Abudurexiti, A.; Yao, G. Discovery of highly functionalized grayanane diterpenoids from the flowers of Rhododendron molle as potent analgesics. Bioorganic Chem. 2024, 142, 106928. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, L.; Feng, Y.; Zhang, H.; Ma, X.; Gao, B.; Sun, Y.; Abudurexiti, A.; Yao, G. Structurally diverse analgesic diterpenoids from the flowers of Rhododendron molle. Fitoterapia 2024, 172, 105770. [Google Scholar] [CrossRef]
- Huang, H.; Kuo, Y.W.; Chuang, Y.C.; Yang, Y.P.; Huang, L.M.; Jeng, M.F.; Chen, W.H.; Chen, H.H. Terpene Synthase-b and Terpene Synthase-e/f Genes Produce Monoterpenes for Phalaenopsis bellina Floral Scent. Front. Plant Sci. 2021, 12, 700958. [Google Scholar] [CrossRef]
- Pazouki, L.; Niinemets, U. Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance. Front. Plant Sci. 2016, 7, 1019. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Degenhardt, J.; Kollner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Magnard, J.-L.; Roccia, A.; Caissard, J.-C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia × hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Conart, C.; Bomzan, D.P.; Huang, X.Q.; Bassard, J.E.; Paramita, S.N.; Saint-Marcoux, D.; Rius-Bony, A.; Hivert, G.; Anchisi, A.; Schaller, H.; et al. A cytosolic bifunctional geranyl/farnesyl diphosphate synthase provides MVA-derived GPP for geraniol biosynthesis in rose flowers. Proc. Natl. Acad. Sci. USA 2023, 120, e2221440120. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Su, W.; Hussain, M.A.; Mehmood, S.S.; Zhang, X.; Cheng, Y.; Zou, X.; Lv, Y. Integrated Analysis of Metabolome and Transcriptome Reveals Insights for Cold Tolerance in Rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 721681. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, Z.; Wang, Y.; Ding, J.; Zheng, Y.; Tang, H.; Yang, L. Transcriptome and Metabolome Analysis Revealed the Freezing Resistance Mechanism in 60-Year-Old Overwintering Camellia sinensis. Biology 2021, 10, 996. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, R.; Zhang, W.; Wei, G.; Ma, X.; Zheng, J.; Kollner, T.G.; Chen, F. Composition and Biosynthesis of Scent Compounds from Sterile Flowers of an Ornamental Plant Clematis florida cv. ‘Kaiser’. Molecules 2020, 25, 1711. [Google Scholar] [CrossRef]
- Basir, S.; Akbar, M.A.; Talip, N.; Baharum, S.N.; Bunawan, H. An Integrative Volatile Terpenoid Profiling and Transcriptomics Analysis in Hoya cagayanensis, Hoya lacunosa and Hoya coriacea (Apocynaceae, Marsdenieae). Horticulturae 2022, 8, 224. [Google Scholar] [CrossRef]
- Liu, Z.-q.; Gao, Q.; Li, H.; Jiang, M.-f.; Chen, R. Expression analysis of key genes in terpenoid biosynthesis of Ginkgo biloba under different growth years based on metabolomics and transcriptome. Chin. Tradit. Herb. Drugs 2022, 53, 1138–1147. [Google Scholar]
- Lee, D.K.; Ahn, S.; Cho, H.Y.; Yun, H.Y.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Metabolic response induced by parasitic plant-fungus interactions hinder amino sugar and nucleotide sugar metabolism in the host. Sci. Rep. 2016, 6, 37434. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Z.; Chai, P.; Chen, S.; Ma, H. Transcriptome analysis of San Pedro-type fig (Ficus carica L.) parthenocarpic breba and non-parthenocarpic main crop reveals divergent phytohormone-related gene expression. Tree Genet. Genomes 2017, 13, 83. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, G.; Xi, J.; Ji, X.; Li, M.-Z.; Xie, D.-Y. Non-plastidial expression of a synthetic insect geranyl pyrophosphate synthase effectively increases tobacco plant biomass. J. Plant Physiol. 2018, 221, 144–155. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Thabet, I.; Guirimand, G.; Guihur, A.; Lanoue, A.; Courdavault, V.; Papon, N.; Bouzid, S.; Giglioli-Guivarc’h, N.; Simkin, A.J.; Clastre, M. Characterization and subcellular localization of geranylgeranyl diphosphate synthase from Catharanthus roseus. Mol. Biol. Rep. 2012, 39, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Su, S.; Guo, K.; Wang, L.; Tang, Z.; Huo, J.; Song, H. Characterization of key aroma-active compounds in blue honeysuckle (Lonicera caerulea L.) berries by sensory-directed analysis. Food Chem. 2023, 429, 136821. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Li, Y.; Si, D.; Yan, S.; Liu, J.; Si, J.; Zhang, X. Identification, quantitative and bioactivity analyses of aroma and alcohol-soluble components in flowers of Gardenia jasminoides and its variety during different drying processes. Food Chem. 2023, 420, 135846. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Z.; Zhu, Z.; Sun, J.; Guo, W.; Zhang, J.; Ding, P.; Liu, M.; Gao, Z. Identification of floral aroma components and molecular regulation mechanism of floral aroma formation in Phalaenopsis. J. Sci. Food Agric. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Shang, J.; Feng, D.; Liu, H.; Niu, L.; Li, R.; Li, Y.; Chen, M.; Li, A.; Liu, Z.; He, Y.; et al. Evolution of the biosynthetic pathways of terpene scent compounds in roses. Curr. Biol. CB 2024, 34, 3550–3563.e3558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Y.; Huang, H.; Wu, D.; Chen, X.; Li, J.; Zheng, H.; Zhan, R.; Chen, L. Functional analysis of Pogostemon cablin farnesyl pyrophosphate synthase gene and its binding transcription factor PcWRKY44 in regulating biosynthesis of patchouli alcohol. Front. Plant Sci. 2022, 13, 946629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Bao, H.; Ren, S.; Cui, J.; Jin, B. Genome-Wide Identification and Characterization of Long Non-Coding RNAs Associated with Floral Scent Formation in Jasmine (Jasminum sambac). Biomolecules 2023, 14, 45. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.-H.; Amar, O.; Lastochkin, E.; Larkov, O.; Ravid, U.; et al. Enhanced Levels of the Aroma and Flavor Compound S-Linalool by Metabolic Engineering of the Terpenoid Pathway in Tomato Fruits. Plant Physiol. 2001, 127, 1256–1265. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, X.; Zhang, Q.; Guo, Z.; Hao, J.; Zhang, Z.; Sun, M.; Liu, Y. Determination of Volatile Organic Compounds and Endogenous Extracts and Study of Expression Patterns of TPS and BSMT in the Flowers of Seven Lilium Cultivars. Molecules 2023, 28, 7938. [Google Scholar] [CrossRef]
- Hendrickson, H.; Islam, M.; Wabo, G.F.; Mafu, S. Biochemical analysis of the TPS-a subfamily in Medicago truncatula. Front. Plant Sci. 2024, 15, 1349009. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Gao, M.; Wu, L.; Wang, Y. LcMYB106 suppresses monoterpene biosynthesis by negatively regulating LcTPS32 expression in Litsea cubeba. Tree Physiol. 2023, 43, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.J.; Green, S.A.; Chen, X.; Bailleul, E.J.; Matich, A.J.; Wang, M.Y.; Atkinson, R.G. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple. Plant Physiol. 2013, 161, 787–804. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and Accumulation of Monoterpene and the Key Terpene Synthase (TPS) Associated with Monoterpene Biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2015, 6, 1232. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Y.; Shi, X.; Yang, Y.; Chen, J.; Zhang, Q.; Sun, M. Overexpression of LiTPS2 from a cultivar of lily (Lilium ‘Siberia’) enhances the monoterpenoids content in tobacco flowers. Plant Physiol. Biochem. 2020, 151, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Wachtler, B.; Temp, U.; Krekling, T.; Seguin, A.; Gershenzon, J. A bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Picea abies. Plant Physiol. 2010, 152, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Rui-jie, L.; Hong, Z.; Shao-zhen, H.; Huan, Z.; Ning, Z.; Qing-chang, L. A geranylgeranyl pyrophosphate synthase gene, IbGGPS, increases carotenoid contents in transgenic sweetpotato. J. Integr. Agric. 2022, 21, 2538–2546. [Google Scholar]
- Ziang, L.; Xinwen, Z.; Kun, L.; Peiyun, W.; Chuanrong, L.; Xiuhua, S. Integrative analysis of transcriptomic and volatile compound profiles sheds new insights into the terpenoid biosynthesis in tree peony. Ind. Crops Prod. 2022, 188, 115672. [Google Scholar]
- Kalita, R.; Patar, L.; Shasany, A.K.; Modi, M.K.; Sen, P. Molecular cloning, characterization and expression analysis of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Centella asiatica L. Mol. Biol. Rep. 2015, 42, 1431–1439. [Google Scholar] [CrossRef]
- Afroz, S.; Warsi, Z.I.; Khatoon, K.; Sangwan, N.S.; Khan, F.; Rahman, L.U. Molecular cloning and characterization of Triterpenoid Biosynthetic Pathway Gene HMGS in Centella asiatica (Linn.). Mol. Biol. Rep. 2022, 49, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Ladevèze, V.; Marcireau, C.; Delourme, D.; Karst, F. General resistance to sterol biosynthesis inhibitors in Saccharomyces cerevisiae. Lipids 1993, 28, 907–912. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Huang, L.; Wang, G. The expression of AcIDI1 reveals diterpenoid alkaloids’ allocation strategies in the roots of Aconitum carmichaelii Debx. Gene 2024, 920, 148529. [Google Scholar] [CrossRef]
- Chai, J.; Wang, D.; Peng, Y.; Zhao, X.; Zhang, Q.; Li, P.; Fang, X.; Wang, M.; Cai, X. Molecular cloning, expression and immunolocalization analysis of diphosphomevalonate decarboxylase involved in terpenoid biosynthesis from Euphorbia helioscopia L. Biotechnol. Biotechnol. Equip. 2017, 31, 1106–1115. [Google Scholar] [CrossRef]
- Shixin, W.; Zengcai, L.; Xutong, W.; Tingting, S.; Li, Z. Cloning and characterization of a phosphomevalonate kinase gene from Sanghuangporus baumii. Biotechnol. Biotechnol. Equip. 2021, 35, 934–942. [Google Scholar]
- Redding-Johanson, A.M.; Batth, T.S.; Chan, R.; Krupa, R.; Szmidt, H.L.; Adams, P.D.; Keasling, J.D.; Lee, T.S.; Mukhopadhyay, A.; Petzold, C.J. Targeted proteomics for metabolic pathway optimization: Application to terpene production. Metab. Eng. 2011, 13, 194–203. [Google Scholar] [CrossRef]
- Woo, H.M.; Murray, G.W.; Batth, T.S.; Prasad, N.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Application of targeted proteomics and biological parts assembly in E. coli to optimize the biosynthesis of an anti-malarial drug precursor, amorpha-4,11-diene. Chem. Eng. Sci. 2013, 103, 21–28. [Google Scholar] [CrossRef]
- Ma, R.; Yang, P.; Jing, C.; Fu, B.; Teng, X.; Zhao, D.; Sun, L. Comparison of the metabolomic and proteomic profiles associated with triterpene and phytosterol accumulation between wild and cultivated ginseng. Plant Physiol. Biochem. 2023, 195, 288–299. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Qin, C.; Cao, H.; Kong, L.; Liu, T.; Jiang, S.; Ma, L.; Liu, X.; Ren, W.; et al. Cloning, Expression Characteristics of Farnesyl Pyrophosphate Synthase Gene from Platycodon grandiflorus and Functional Identification in Triterpenoid Synthesis. J. Agric. Food Chem. 2024, 72, 11429–11437. [Google Scholar] [CrossRef]
- Lin, P.; Yan, Z.F.; Li, C.T. Effects of Exogenous Elicitors on Triterpenoids Accumulation and Expression of Farnesyl Diphosphate Synthase Gene in Inonotus obliquus. Biotechnol. Bioprocess Eng. 2020, 25, 580–588. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.; Chao, N.; Ma, C.; Chen, Q.; Sun, J.; Wu, Y. Positive selection and functional divergence of farnesyl pyrophosphate synthase genes in plants. BMC Mol. Biol. 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, J.-L.; Liu, B.-Y.; Ye, H.-C.; Wang, H.; Li, Z.-Q.; Li, G.-F. Effects of Overexpression of the Endogenous Farnesyl Diphosphate Synthase on the Artemisinin Content in Artemisia annua L. J. Integr. Plant Biol. 2006, 48, 482–487. [Google Scholar] [CrossRef]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Andreas, F.; Hur-Song, C.; Tong, Z.; Xun, W.; Heifetz, P.B.; Wilhelm, G.; Markus, L. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Fan, Y.; Dong, J.; Gao, P.; Jiang, W.; Yang, T.; Che, D. RNA sequencing analysis reveals PgbHLH28 as the key regulator in response to methyl jasmonate-induced saponin accumulation in Platycodon grandiflorus. Hortic. Res. 2024, 11, uhae058. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Yang, G.; Li, D.; Zhang, D.; Chen, Z.; Yang, Z.; Yang, K.; Xie, X.; Wu, Y. Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl. Horticulturae 2024, 10, 959. https://doi.org/10.3390/horticulturae10090959
Qin Y, Yang G, Li D, Zhang D, Chen Z, Yang Z, Yang K, Xie X, Wu Y. Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl. Horticulturae. 2024; 10(9):959. https://doi.org/10.3390/horticulturae10090959
Chicago/Turabian StyleQin, Yi, Guoxia Yang, Dongbin Li, Danyidie Zhang, Zhihui Chen, Zhongyi Yang, Kaitai Yang, Xiaohong Xie, and Yueyan Wu. 2024. "Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl." Horticulturae 10, no. 9: 959. https://doi.org/10.3390/horticulturae10090959
APA StyleQin, Y., Yang, G., Li, D., Zhang, D., Chen, Z., Yang, Z., Yang, K., Xie, X., & Wu, Y. (2024). Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in the Synthesis of Terpenoids in Rhododendron fortunei Lindl. Horticulturae, 10(9), 959. https://doi.org/10.3390/horticulturae10090959





