Micropropagation of Rare Endemic Species Allium microdictyon Prokh. Threatened in Kazakhstani Altai
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Establishment of In Vitro Culture
2.3. Protocol for In Vitro Micropropagation of A. microdictyon
2.4. Adaptation to Ex Vitro Conditions
2.5. Statistical Analyses
3. Results
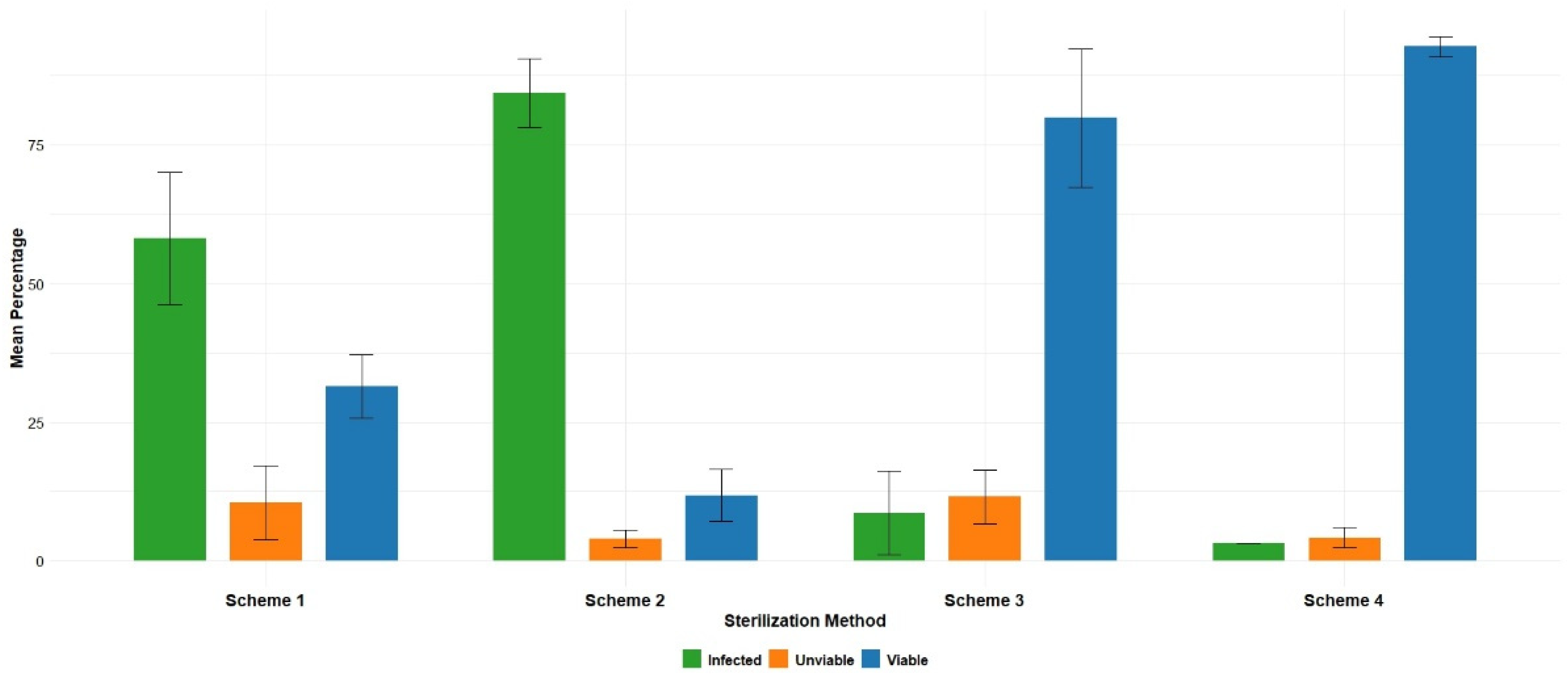
3.1. Establishment of In Vitro Culture of A. microdictyon
3.2. In Vitro Micropropagation of A. microdictyon
3.3. Ex Vitro Adaptation of A. microdictyon
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maryam, A.; Tariq, R.; Chuadhary, S.; Azmat, R.; Javed, S.; Khanam, S.J. A review: Role of tissue culture (in vitro) techniques in the conservation of rare and endangered species. Pac. J. Life Sci. 2014, 2, 93–103. [Google Scholar]
- Khapilina, O.; Raiser, O.; Danilova, A.; Shevtsov, V.; Turzhanova, A.; Kalendar, R. DNA profiling and assessment of genetic diversity of relict species Allium altaicum Pall. on the territory of Altai. PeerJ 2021, 9, e10674. [Google Scholar] [CrossRef]
- Khapilina, O.; Turzhanova, A.; Danilova, A.; Tumenbayeva, A.; Shevtsov, V.; Kotukhov, Y.; Kalendar, R. Primer Binding Site (PBS) Profiling of Genetic Diversity of Natural Populations of Endemic Species Allium ledebourianum Schult. BioTech 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Allium microdictyon Prokh. In GBIF Backbone Taxonomy; Checklist Dataset; GBIF Secretariat: Copenhagen, Denmark, 2023. [CrossRef]
- Fomina, T.I.; Kukushkina, T.A. Content of biologically active substances in the aboveground part of some onion spe-cies (Allium L.). Chem. Plant Raw Mater. 2019, 3, 177–184. [Google Scholar] [CrossRef]
- Fomina, T.I.; Kukushkina, T.A. Bioactive compounds in the aboveground part of hemiephemeroid onions (Allium L.). Proc. Appl. Bot. Genet. Breed. 2020, 181, 37–43. [Google Scholar] [CrossRef]
- Olennikov, D. Flavonol glycosides from leaves of Allium microdictyon. Chem. Nat. Compd. 2020, 56, 1035–1039. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.-H. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef]
- Soldatenko, A.V.; Ivanova, M.I.; Bukharov, A.F.; Kashleva, A.I.; Seredin, T.M. Prospects for introducing into the culture wild species of the genus Allium L. food direction. Veg. Crops Russ. 2021, 1, 20–32. (In Russian) [Google Scholar] [CrossRef]
- Fomina, T.; Kukushkina, T. Edible onion flowers (Allium L.) as a source of biologically active substances. Khimiya Rastit. Syr’ya 2021, 4, 291–297. [Google Scholar] [CrossRef]
- Baitulin, I.O. Baitulin Idiscussion of economically valuable rare plant species. Proc. Natl. Acad. Sci. Repub. Kazakhstan 2008, 1, 3–8. (In Russian) [Google Scholar]
- Kotukhov, Y.A.; Danilova, A.B.; Anufrieva, O.A. The Current State of Populations of Rare and Endangered Plants of Eastern Kazakhstan; Tethys: Almaty, Kazakhstan, 2006; Book 1; pp. 60–77. ISBN 9965-9822-2-8. [Google Scholar]
- Tukhvatullina, L.A.; Zhigunov, O.Y. Shadow bows during introduction in the South Ural Botanical Garden-Institute. Bull. State Nikitsk. Bot. Gard. 2019, 130, 73–78. (In Russian) [Google Scholar] [CrossRef]
- Burenin, V.; Shumilina, V. Distant hybridization of plants of Allium L. Veg. Crops Russ. 2016, 1, 10–13. [Google Scholar] [CrossRef][Green Version]
- Red Data Book of Kazakh SSR, Rare and Vulnerable Species of Animals and Plants; Nauka KazSSR: Alma-Ata, Kazakhstan, 2014.
- Bobo-Pinilla, J.; Salmerón-Sánchez, E.; Mota, J.F.; Penas, J. Genetic conservation strategies of endemic plants from edaphic habitat islands: The case of Jacobaea auricula (Asteraceae). J. Nat. Conserv. 2021, 61, 126004. [Google Scholar] [CrossRef]
- Kulak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In vitro technology in plant conservation: Relevance to biocultural diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Sato, A. In vitro conservation of Mandevilla moricandiana (Apocynaceae): Short-term storage and encapsulation–dehydration of nodal segments. Vitr. Cell. Dev. Biol-Plant 2014, 50, 326–336. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. Vitr. Cell. Dev. Biol-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and conservation of plant biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Leung, D.W. Plant biotechnology helps quest for sustainability: With emphasis on climate change and endangered plants. In Forum on Public Policy: A Journal of the Oxford Round Table; Forum on Public Policy: Toronto, ON, Canada, 2018. [Google Scholar]
- Jafari, S.; Hassandokht, M.; Taheri, M. Optimizing proliferation and assessment of valak morpho-phenological traits; an endangered nutritious Allium endemic to Iran. 2021; preprint. Available online: https://www.researchsquare.com/article/rs-250915/v1 (accessed on 30 July 2024).
- Nasircilar, A.G. In vitro clonal propagation of endemic Allium junceum subs. tridentatum via immature and mature embryo culture method. Comptes Rendus L’academie Bulg. Sci. 2021, 74, 1259–1268. [Google Scholar]
- Kaur, N.; Kaur, N.; Saggoo, M. Conservation strategies for medicinal plants in the face of environmental challenges. In Environmental Challenges and Medicinal Plants: Sustainable Production Solutions under Adverse Conditions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 461–485. [Google Scholar]
- Unal, B.T.; Turker, H.; Ozturk, M. Ex-situ Conservation of Medicinal and Aromatic Plants Using In Vitro Techniques. In Plants as Medicine and Aromatics; CRC Press: Boca Raton, FL, USA, 2023; pp. 13–22. [Google Scholar]
- Radomir, A.-M.; Stan, R.; Florea, A.; Ciobotea, C.-M.; Bănuță, F.M.; Negru, M.; Neblea, M.A.; Sumedrea, D.I. Overview of the Success of In Vitro Culture for Ex Situ Conservation and Sustainable Utilization of Endemic and Subendemic Native Plants of Romania. Sustainability 2023, 15, 2581. [Google Scholar] [CrossRef]
- Gantait, S.; Mandal, N.; Das, P. An Overview on in vitro culture of genus allium. Am. J. Plant Physiol. 2010, 5, 325–337. [Google Scholar] [CrossRef]
- Eady, C.; Butler, R.; Suo, Y. Somatic embryogenesis and plant regeneration from immature embryo cultures of onion (Allium cepa L.). Plant Cell Rep. 1998, 18, 111–116. [Google Scholar] [CrossRef]
- Yan, M.-M.; Xu, C.; Kim, C.-H.; Um, Y.-C.; Bah, A.A.; Guo, D.-P. Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci. Hortic. 2009, 123, 124–128. [Google Scholar] [CrossRef]
- Farhadi, N.; Panahandeh, J.; Azar, A.M.; Salte, S.A. Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sci. Hortic. 2017, 218, 80–86. [Google Scholar] [CrossRef]
- Zheng, S.-J.; Henken, B.; Krens, F.A.; Kik, C. The development of an efficient cultivar-independent plant regeneration system from callus derived from both apical and non-apical root segments of garlic (Allium sativum L.). Vitr. Cell. Dev. Biol-Plant 2003, 39, 288–292. [Google Scholar] [CrossRef]
- Tubić, L.; Anačkov, G.; Milojević, J.; Ghalawenji, N.; Mitić, N.; Igić, R.; Zdravković-Korać, S. High variability in the tissue culture response of root-tips of Allium ascalonicum individuals and optimization of the regeneration procedure. Plant Cell Tissue Organ Cult. PCTOC 2014, 118, 101–110. [Google Scholar] [CrossRef]
- Karakan, F.Y. The effects of GA3 treatments and nutrient media on in vitro seed germination of Allium tuncelianum (Kollman), Özhatay, Matthew, Şiraneci. Curr. Perspect. Med. Aromat. Plants 2021, 4, 103–107. [Google Scholar]
- Kataeva, N.V.; Butenko, R.G. Clonal Micropropagation of Apple Trees. Acta Hortic. 1987, 212, 585–588. [Google Scholar] [CrossRef]
- Buiteveld, J.; Van der Valk, P.; Jansen, J.; Creemers-Molenaar, J.; Colijn-Hooymans, C. Callus induction and plant regeneration from explants of commercial cultivars of leek (Allium ampeloprasum var. porrum L.). Plant Cell Rep. 1993, 12, 431–434. [Google Scholar] [CrossRef]
- Luciani, G.F.; Mary, A.K.; Pellegrini, C.; Curvetto, N.R. Effects of explants and growth regulators in garlic callus formation and plant regeneration. Plant Cell Tissue Organ Cult. 2006, 87, 139–143. [Google Scholar] [CrossRef]
- Zhuravlev, Y.N.; Omelko, A.M. Plant morphogenesis in vitro1. Russ. J. Plant Physiol. 2008, 55, 579–596. [Google Scholar] [CrossRef]
- Muraseva, D.S.; Novikova, T.I. In vitro culture initiation using immature seeds of a rare species Fritillaria meleagroides Patrin ex Schult. et Schult. fil. (Liliaceae). Vestn. Tomsk. Gos. Universiteta. Biol. 2018, 44, 172–187. [Google Scholar] [CrossRef]
- Nikolaeva, M.; Razumova, M.; Gladkova, V. Handbook on Germination of Resting Seeds; Science: Leningrad, Russian, 1985. [Google Scholar]
- Savchenko, O.M.M.E.L.; Kozlovskaya, L.N. Inhibitor of growth regulators on seed germination of victorious onion (allium victorialis L.) and bear onion (Allium ursinum L.) Izv. Timiryazev Agric. Acad. 2010, 6, 61–66. [Google Scholar]
- Seo, H.; Choi, B.; Moon, Y.; Kim, S.; Park, K.; Kwon, S. Effect of light conditions and wet cold treatments on seed germination in several wild vegetables. J. Agric. Life Environ. Sci. 2018, 30, 64–72. [Google Scholar]
- Fomina, T.I. Biology of seed germination of some types of onions (Allium L.). Tauride Bull. Agrar. Sci. 2021, 3, 180–190. [Google Scholar]
- Yazdanian, E.; Golkar, P.; Vahabi, M.R.; Taghizadeh, M. Elicitation effects on some secondary metabolites and antioxidant activity in callus cultures of Allium jesdianum Boiss. & Buhse.: Methyl jasmonate and putrescine. Appl. Biochem. Biotechnol. 2022, 194, 601–619. [Google Scholar]
- Żabicka, J.; Żabicki, P.; Słomka, A.; Sliwinska, E.; Jędrzejczyk-Korycińska, M.; Nowak, T.; Migdałek, G.; Kwiatkowska, M.; Kuta, E. Re-introduction of an extinct population of Pulsatilla patens using different propagation techniques. Sci. Rep. 2022, 12, 14321. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Gemedjieva, N.; da Silva, J.A.T.; Ryabushkina, N. Representation of endemics in floristic subprovinces of Kazakhstan. Asian Australas. J. Plant Sci. Biotechnol. 2010, 4, 56–63. [Google Scholar]
- Foggi, B.; Viciani, D.; Baldini, R.M.; Carta, A.; Guidi, T. Conservation assessment of the endemic plants of the Tuscan Archipelago, Italy. Oryx 2015, 49, 118–126. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K. Recent development in micropropagation techniques for rare plant species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Mežaka, I.; Kļaviņa, D.; Kaļāne, L.; Kronberga, A. Large-Scale In Vitro Propagation and Ex Vitro Adaptation of the Endangered Medicinal Plant Eryngium maritimum L. Horticulturae 2023, 9, 271. [Google Scholar] [CrossRef]
- Diaz-Martin, Z.; Fant, J.; Havens, K.; Cinea, W.; Lima, J.M.T.; Griffith, M.P. Current management practices do not adequately safeguard endangered plant species in conservation collections. Biol. Conserv. 2023, 280, 109955. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; Xie, H.; Feng, X.; Wang, Y.; Xu, P. Overhauling the effect of surface sterilization on analysis of endophytes in tea plants. Front. Plant Sci. 2022, 13, 849658. [Google Scholar] [CrossRef]
- Sahu, P.K.; Tilgam, J.; Mishra, S.; Hamid, S.; Gupta, A.K.J.; Verma, S.K.; Kharwar, R.N. Surface sterilization for isolation of endophytes: Ensuring what (not) to grow. J. Basic Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef]
- Si, Y.; Haxim, Y.; Wang, L. Optimum sterilization method for in vitro cultivation of dimorphic seeds of the succulent halophyte Suaeda aralocaspica. Horticulturae 2022, 8, 289. [Google Scholar] [CrossRef]
- Khan, M.M.; Iqbal, M.J.; Abbas, M.; Raza, H.; Waseem, R.; Ali, A. Loss of vigour and viability in aged onion (Allium cepa L.) seeds. Int. J. Agric. Biol 2004, 6, 701–711. [Google Scholar]
- Kamenetsky, R.; Gębura, J.; Winiarczyk, K. Germination strategy of Allium victorialis, a wild edible plant with high commercial potential. Botany 2017, 95, 195–202. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Nonogaki, H. Seed Germination; AccessScience, McGraw Hill: Chicago, IL, USA, 2020. [Google Scholar] [CrossRef]
- Hedden, P. Gibberellin; AccessScience, McGraw Hill: Chicago, IL, USA, 2021. [Google Scholar] [CrossRef]
- Macleod, A.M.; Palmer, G.H. Interaction of indolyl acetic acid and gibberellic acid in the synthesis of α-amylase by barley aleurone. New Phytol. 1969, 68, 295–304. [Google Scholar] [CrossRef]
- Gull, I.; Noreen, A.; Aslam, M.S.; Athar, M.A. Comparative effect of different pyhtohormones on the micropropagation of Allium sativum. Pak. J. Biochem. Mol. Biol 2014, 47, 121–124. [Google Scholar]
- Rantau, D.E.; Wulandari, D.R.; Maharijaya, A. Growth response of shallot (Allium ascalonicum L.) seedlings cultured on MS solid and liquid medium supplemented with BAP, Thiamine and Adenine Sulphate. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012035. [Google Scholar] [CrossRef]
- Niranjan Sannatammappa Konchigeri, M.S.; Rajesh, S.; Karthikeyan, M.; Gnanam, R. In vitro shoot organogenesis in therapeutically important spice crop, Kodaikanal hill garlic (Allium sativum L.). Pharma Innov. J. 2022, 11, 465–467. [Google Scholar]
- Lee, C.H.; Jeong, M.J. In vitro culture and shoot proliferation of wild garlic (Allium ochotense Prokh.). J. Agric. Life Sci. 2018, 52, 73–80. [Google Scholar] [CrossRef]
- Marković, M.; Trifunović-Momčilov, M.; Radulović, O.; Paunović, D.M.; Antonić Reljin, D.D.; Uzelac, B.; Subotić, A. The Effects of Different Auxin–Cytokinin Combinations on Morphogenesis of Fritillaria meleagris Using Bulb Scale Sections In Vitro. Horticulturae 2023, 9, 910. [Google Scholar] [CrossRef]
- Zare, M.; Rabiei, M.; Mohammadi, S. The effect of plant growth regulators, basal medium and light on micropropagation of Allium jesdianum Boiss. & Buhse. Res. Sq. 2023; preprint. [Google Scholar]
- Azeri, F.N.; Öztürk, G. Microbulb and plantlet formation of a native bulbous flower, Lilium monodelphum M. Bieb, var. Armenum, through tissue culture propagation. Biotechnol. Rep. 2021, 32, e00665. [Google Scholar] [CrossRef] [PubMed]
- Yasemin, S.; Beruto, M. A Review on Flower Bulb Micropropagation: Challenges and Opportunities. Horticulturae 2024, 10, 284. [Google Scholar] [CrossRef]
- Jeong, M.J.; Yong, S.H. Rapid micropropagation of wild garlic (Allium victorialis var. platyphyllum) by the scooping method. J. Plant Biotechnol. 2022, 49, 213–221. [Google Scholar] [CrossRef]


| Scheme No. | Sterilizing Agent (%) and Exposure Time | ||||
|---|---|---|---|---|---|
| EtOH | HgCl2 | H2O2 | KMnO4 | NaClO | |
| 1 | 70% 30″ | 3% 20′ | |||
| 2 | 70% 30″ | 2% 20′ | |||
| 3 | 70% 30″ | 20% 20′ | |||
| 4 | 70% 30″ | 0.01% 20′ | |||
| Seeds | Seed Quantity, pcs. | Number of Germinated Seeds, pcs | Germination, % | |||
|---|---|---|---|---|---|---|
| 7th Day | 14th Day | 21th Day | 30th Day | |||
| ½ MC +10 mg L−1 GA3+ 0.1 mg L−1 IAA | ||||||
| Two years of storage | 300 | 0 | 0 | 1 | 4 | 1.3 |
| One year of storage | 300 | 0 | 0 | 11 | 17 | 5.7 |
| Freshly harvested | 300 | 25 | 81 | 175 | 278 | 92.7 |
| ½ MS without hormones | ||||||
| Two years of storage | 96 | 0 | 0 | 0 | 0 | 0 |
| One year of storage | 92 | 0 | 0 | 0 | 0 | 0 |
| Freshly harvested | 95 | 0 | 0 | 3 | 17 | 17.9 |
| Medium | The Concentration of Growth Regulators, mg L−1 | Number of Adventitious Shoots per Explant, pcs. | Length of Adventitious Shoots, cm | |
|---|---|---|---|---|
| BAP | ZEA | |||
| MS | 0 | 0 | 1.0 ± 0 l | 1.5 ± 0.6 |
| MSPR1 | 0.1 | 0.1 | 2.0 ± 0.1 k | 1.2 ± 0.5 |
| MSPR2 | 0.1 | 0.5 | 2.5 ± 0.1 j | 2.6 ± 0.7 |
| MSPR3 | 0.1 | 1.0 | 12.2 ± 0.4 e | 2.5 ± 0.5 |
| MSPR4 | 0.1 | 1.5 | 13.7 ± 0.1 d | 2.2 ± 0.7 |
| MSPR5 | 0.1 | 2.0 | 10.3 ± 0.1 g | 3.6 ± 0.7 |
| MSPR6 | 0.5 | 0.1 | 11.5 ± 0.1 f | 1.0 ± 0.0 |
| MSPR7 | 0.5 | 0.5 | 14.8 ± 0.2 c | 4.5 ± 0.9 |
| MSPR8 | 0.5 | 1.0 | 16.1 ± 0.1 b | 3.4 ± 0.8 |
| MSPR9 | 0.5 | 1.5 | 18.4 ± 0.1 a | 9.4 ± 2.3 |
| MSPR10 | 0.5 | 2.0 | 18.2 ± 0.0 a | 6.8 ± 1.0 |
| MSPR11 | 1.0 | 0.1 | 13.7 ± 0.0 d | 2.5 ± 0.5 |
| MSPR12 | 1.0 | 0.5 | 11.3 ± 0.1 f | 1.6 ± 0.7 |
| MSPR13 | 1.0 | 1.0 | 7.7 ± 0.1 h | 5.6 ± 0.8 |
| MSPR14 | 1.0 | 1.5 | 2.6 ± 0.1 j | 9.5 ± 1.9 |
| MSPR15 | 1.0 | 2.0 | 3.8 ± 0.1 i | 4.8 ± 0.7 |
| F ratio | 75.9 | 1.0 | ||
| Sig. level | 0.000 *** | n.s. | ||
| Medium | Hormones | Number of Leaves, pcs. | Leaf Length, cm | Number of Roots, pcs. | Root Length, cm | ||||
|---|---|---|---|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 4 Weeks | 8 Weeks | 4 Weeks | 8 Weeks | 4 Weeks | 8 Weeks | ||
| MS | 1.1 ± 0.1 c | 1.5 ± 0.1 cd | 1.1 ± 0.2 | 1.1 ± 0.3 b. | 1.2 ± 0.2 bc | 1.3 ± 0.1 d | 0.7 ± 0.2 b | 0.7 ± 0.2 c | |
| MSB-IAA | 0.1 BAP 0.2 IAA | 1.2 ± 0.2 c | 1.2 ± 0.2 d | 1.3 ± 0.2 | 2.1 ± 0.2 a | 1.0 ± 0.1 c | 3.2 ± 0.1 b | 0.9 ± 0.1 b | 1.4 ± 0.1 b |
| MSB-IBA | 0.1 BAP 0.2 IBA | 2.5 ± 0.3 a | 7.2 ± 0.2 a | 1.0 ± 0.2 | 2.3 ± 0.1 a | 2.2 ± 0.2 a | 4.3 ± 0.2 a | 1.9 ± 0.2 a | 4.6 ± 0.2 a |
| MSB-NAA | 0.1BAP 0.2 NAA | 1.6 ± 0.3 bc | 1.9 ± 0.2 bc | 1.1 ± 0.2 | 2.0 ± 0.2 a | 1.5 ± 0.4 b | 2.4 ± 0.3 c | 0.7 ± 0.3 b | 1.3 ± 0.2 b |
| MSB-PAC | 0.1 BAP 0.2 PAC | 1.7 ± 0.2 b | 2.2 ± 0.1 b | 1.2 ± 0.3 | 1.9 ± 0.1 a | 2.0 ± 0.1 a | 2.6 ± 0.2 c | 0.8 ± 0.1 b | 1.1 ± 0.1 bc |
| F ratio | 13.3 | 331.9 | 1.1 | 17.9 | 19.1 | 56.4 | 48.4 | 92.1 | |
| Sig. level | 0.000 *** | 0.000 *** | n.s. | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagimanova, D.; Raiser, O.; Danilova, A.; Turzhanova, A.; Khapilina, O. Micropropagation of Rare Endemic Species Allium microdictyon Prokh. Threatened in Kazakhstani Altai. Horticulturae 2024, 10, 943. https://doi.org/10.3390/horticulturae10090943
Tagimanova D, Raiser O, Danilova A, Turzhanova A, Khapilina O. Micropropagation of Rare Endemic Species Allium microdictyon Prokh. Threatened in Kazakhstani Altai. Horticulturae. 2024; 10(9):943. https://doi.org/10.3390/horticulturae10090943
Chicago/Turabian StyleTagimanova, Damelya, Olesya Raiser, Alevtina Danilova, Ainur Turzhanova, and Oxana Khapilina. 2024. "Micropropagation of Rare Endemic Species Allium microdictyon Prokh. Threatened in Kazakhstani Altai" Horticulturae 10, no. 9: 943. https://doi.org/10.3390/horticulturae10090943
APA StyleTagimanova, D., Raiser, O., Danilova, A., Turzhanova, A., & Khapilina, O. (2024). Micropropagation of Rare Endemic Species Allium microdictyon Prokh. Threatened in Kazakhstani Altai. Horticulturae, 10(9), 943. https://doi.org/10.3390/horticulturae10090943





