Drought Stress Effects and Ways for Improving Drought Tolerance in Impatiens walleriana Hook.f.—A Review
Abstract
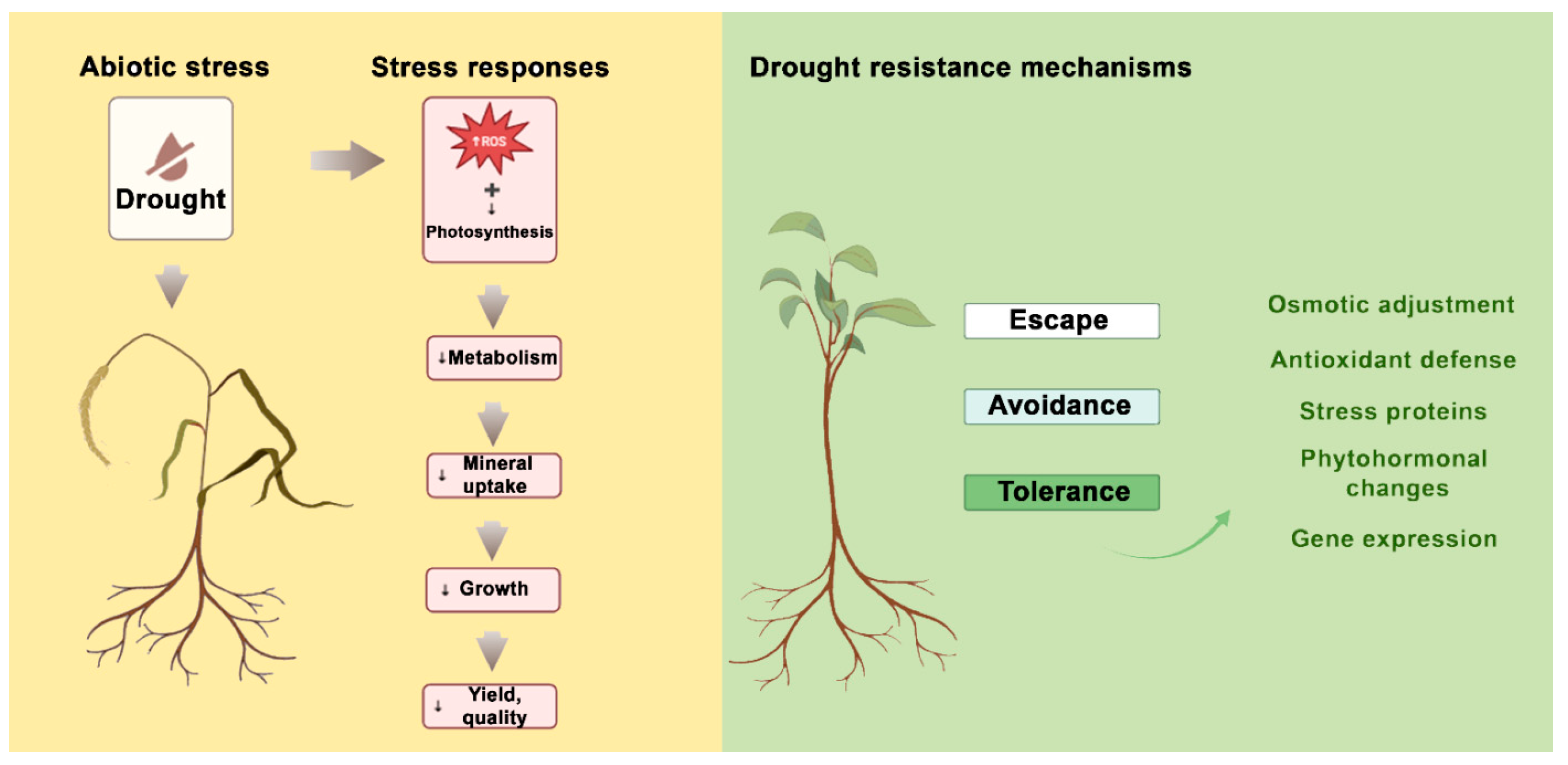
1. General Introduction
2. Impatiens walleriana—Species Characteristics
3. Morphological Changes in Drought-Stressed I. walleriana
4. Physiological Changes in Drought-Stressed I. walleriana
4.1. Photosyntesis, Photosyntetic Pigments and Osmotic Adjustment
4.2. Changes in Endogenous Abscisic Acid
4.3. Changes in Stress-Related Proteins
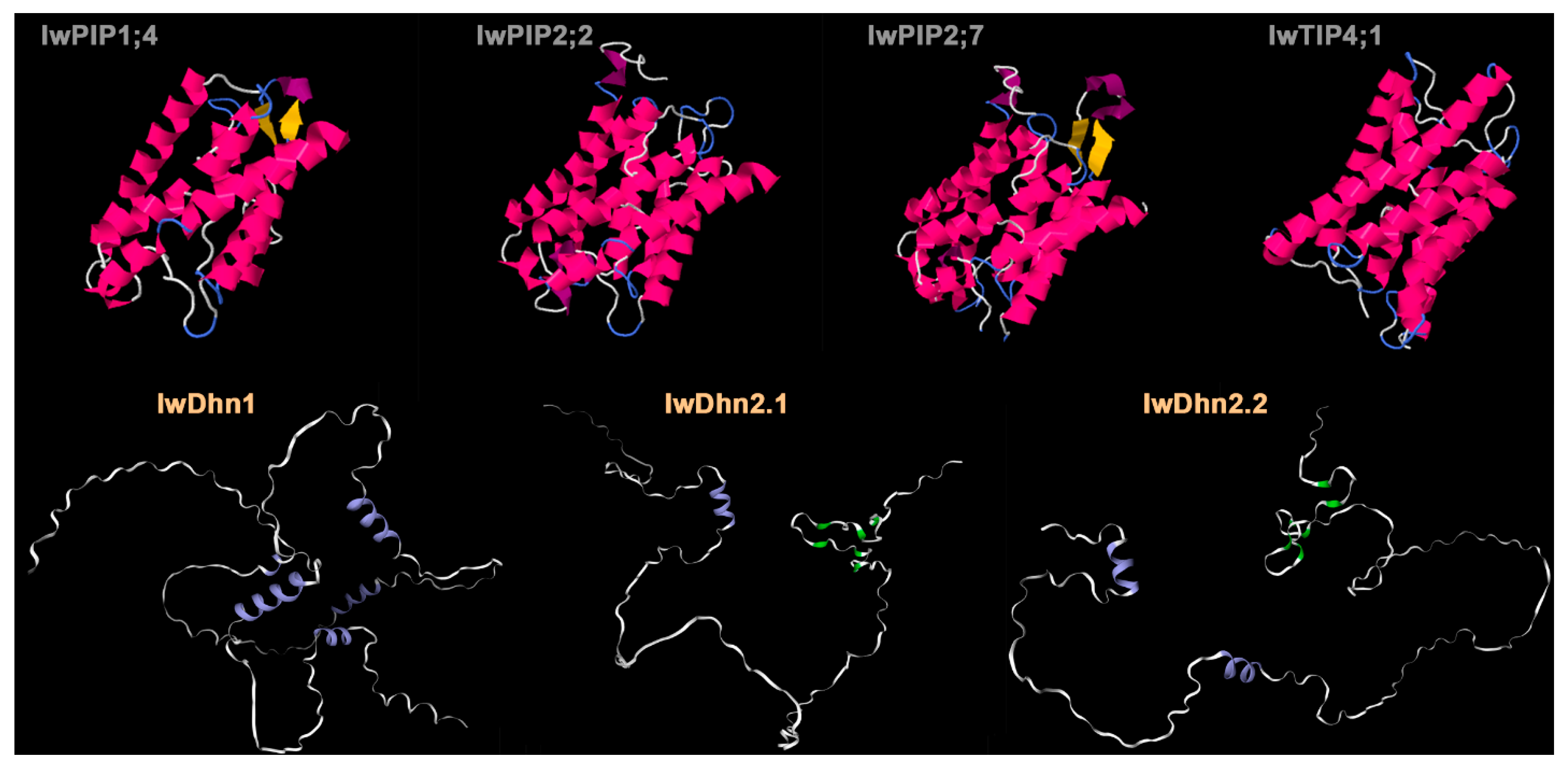
4.3.1. Aquaporins
4.3.2. Dehydrins
5. Biochemical Changes in Drought-Stressed I. walleriana
Reactive Oxygen Species and Antioxidant Defense
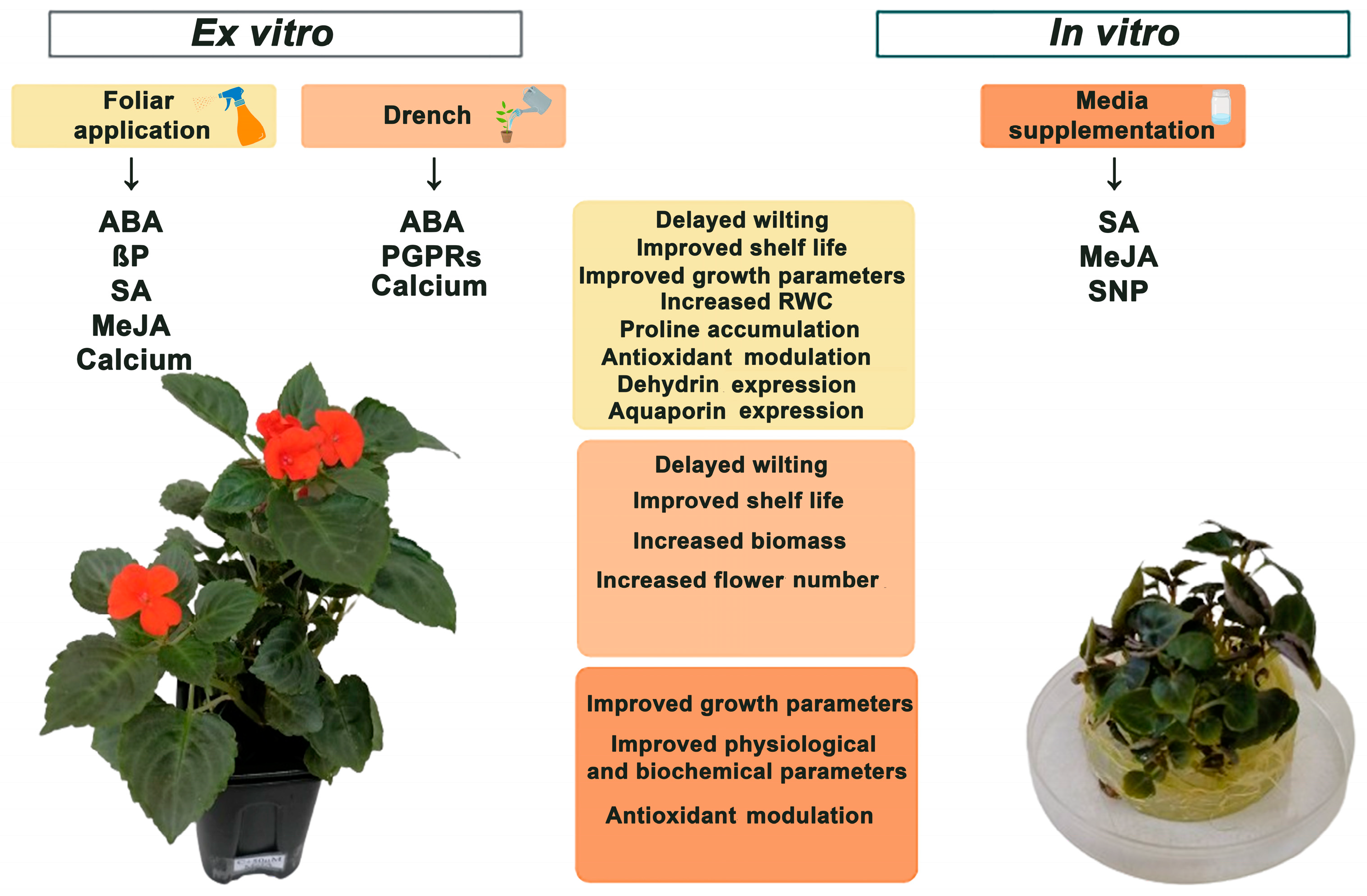
6. Mitigation Strategies for Drought Stress
The PGRs and PGPB Application for Drought-Tolerance Improvement in I. walleriana
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Rasool, F.; Habib, R.; Javed, T.; Sardar, K.; Ayub, M.M.; Ayub, M.A.; Rasool, A. A review of morphological, physiological and biochemical responses of plants under drought stress conditions. Imp. J. Interdiscip. Res. 2016, 2, 1600–1606. [Google Scholar]
- Novák, V. Physiological drought—How to quantify it? In Bioclimatology and Natural Hazards. Springer: Dordrecht, The Netherlands, 2009; pp. 89–95. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Heschel, M.S.; Riginos, C. Mechanisms of selection for drought stress tolerance and avoidance in Impatiens capensis (Balsaminaceae). Am. J. Bot. 2005, 92, 37–44. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Li, H.; Mo, Y.; Cui, Q.; Yang, X.; Guo, Y.; Wei, C.; Yang, J.; Zhang, Y.; Ma, J.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and-susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Mithra, S.V.A.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-induced biochemical and molecular manifestations to improve drought tolerance in rice (Oryza sativa L.) through seed-priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef]
- Nataraja, K.N.; Dhanyalakshmi, K.H.; Govind, G.; Oelmüller, R. Activation of drought tolerant traits in crops: Endophytes as elicitors. Plant Signal. Behav. 2022, 17, 2120300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dong, K.; Ge, P.; Bian, Y.; Dong, L.; Deng, X.; Li, X.; Yan, Y. Identification of leaf proteins differentially accumulated between wheat cultivars distinct in their levels of drought tolerance. PLoS ONE 2015, 10, e0125302. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E.S.; Sadak, M.S.; El-Lethy, S.R. Research article comparison between the physiological role of carrot root extract and β-carotene in inducing Helianthus annuus L. Drought Tolerance. Asian J. Biol. Sci. 2019, 12, 231–241. [Google Scholar] [CrossRef]
- Janssens, S.B.; Knox, E.B.; Huysmans, S.; Smets, E.F.; Merckx, V.S. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: Result of a global climate change. Mol. Phylogenet. Evol. 2009, 52, 806–824. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E. Balsaminaceae. In Flowering Plants·Dicotyledons: Celastrales, Oxalidales, Rosales, Cornales, Ericales; Springer: Berlin/Heidelberg, Germany, 2004; pp. 20–25. [Google Scholar] [CrossRef]
- Grey-Wilson, C. Hydrocera triflora, its floral morphology and relationship with Impatiens: Studies in Balsaminaceae: V. Kew Bulletin 1980, 35, 213–219. [Google Scholar] [CrossRef]
- Subotić, A.; Jevremović, S.; Cingel, A.; Milošević, S. Effect of urea—Type citokinins on axillary shoots regeneration of Impatiens walleriana L. Biotechnol. Biotechnol. Equip. 2008, 22, 817–819. [Google Scholar] [CrossRef]
- Milošević, S.; Simonović, A.; Cingel, A.; Jevremović, S.; Todorović, S.; Filipović, B.; Subotić, A. Response of antioxidative enzymes to long-term Tomato spotted wilt virus infection and virus elimination by meristem-tip culture in two Impatiens species. Physiol. Mol. Plant Pathol. 2012, 79, 79–88. [Google Scholar] [CrossRef]
- Milošević, S.; Subotić, A.; Cingel, A.; Jevremović, S.; Stanković, I.; Bulajić, A.; Krstić, B. Virus elimination from ornamental plants with the use of in vitro culture techniques. Pestic. Phytomed. 2012, 27, 203–211. [Google Scholar] [CrossRef]
- Milošević, S.; Simonović, A.; Cingel, A.; Nikolić, D.; Ninković, S.; Subotić, A. Introduction of dsRNA-specific ribonuclease pac1 into Impatiens walleriana provides resistance to Tomato spotted wilt virus. Sci. Hortic. 2013, 164, 499–506. [Google Scholar] [CrossRef]
- Milošević, S.; Cingel, A.; Subotić, A. Agrobacterium-mediated transformation of ornamental species: A review. Genetika 2015, 47, 1149–1164. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Saglam, A.; Kadioglu, A.; Demiralay, M.; Terzi, R. Leaf rolling reduces photosynthetic loss in maize under severe drought. Acta Bot. Croat. 2014, 73, 315–323. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Augé, R.M.; Stodola, A.J.; Moore, J.L.; Klingeman, W.E.; Duan, X. Comparative dehydration tolerance of foliage of several ornamental crops. Sci. Hortic. 2003, 98, 511–516. [Google Scholar] [CrossRef]
- Burnett, S.; van Iersel, M.; Thomas, P. PEG-8000 alters morphology and nutrient concentration of hydroponic impatiens. HortScience 2005, 40, 1768–1772. [Google Scholar] [CrossRef]
- Henson, D.Y.; Newman, S.E.; Hartley, D.E. Performance of selected herbaceous annual ornamentals grown at decreasing levels of irrigation. HortScience 2006, 41, 1481–1486. [Google Scholar] [CrossRef]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Blanusa, T.; Vysini, E.; Cameron, R.W. Growth and flowering of Petunia and Impatiens: Effects of competition and reduced water content within a container. HortScience 2009, 44, 1302–1307. [Google Scholar] [CrossRef]
- Andersson, N.E. The influence of water stress and air velocity on growth of Impatiens walleriana and Petunia × hybrid. Sci. Hortic. 2011, 128, 146–151. [Google Scholar] [CrossRef]
- Antonić, D.; Milošević, S.; Cingel, A.; Lojić, M.; Trifunović-Momčilov, M.; Petrić, M.; Subotić, A.; Simonović, A. Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Đurić, M.; Subotić, A.; Trifunović-Momčilov, M.; Milošević, S. Improvement of water deficit stress tolerance of Impatiens walleriana shoots grown in vitro by methyl jasmonate. Plant Cell Tissue Organ Cult. 2023, 154, 351–365. [Google Scholar] [CrossRef]
- Antonić, D.D.; Subotić, A.R.; Dragićević, M.B.; Pantelić, D.; Milošević, S.M.; Simonović, A.D.; Momčilović, I. Effects of exogenous salicylic acid on drought response and characterization of dehydrins in Impatiens walleriana. Plants 2020, 9, 1589. [Google Scholar] [CrossRef] [PubMed]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Cingel, A.; Vujičić, M.; Milošević, S. Morpho-physiological and molecular evaluation of drought and recovery in Impatiens walleriana grown ex vitro. Plants 2020, 9, 1559. [Google Scholar] [CrossRef]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Foliar application of methyl jasmonate affects impatiens walleriana growth and leaf physiology under drought stress. Plant Signal. Behav. 2023, 18, 2219936. [Google Scholar] [CrossRef]
- Safari, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2021, 19, 969–984. [Google Scholar] [CrossRef]
- Descamps, C.; Boubnan, N.; Jacquemart, A.L.; Quinet, M. Growing and flowering in a changing climate: Effects of higher temperatures and drought stress on the bee-pollinated species Impatiens glandulifera royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef]
- Shintiavira, H.; Purba, A.E.; Kartikaningrum, S.; Koseki, A. Identifying Drought-Tolerant Impatiens Genotypes by Using Water Stress Treatment. Caraka Tani J. Sustain. Agric. 2023, 38, 40–52. [Google Scholar] [CrossRef]
- Todd, G.W.; Richardson, P.E.; Sengupta, S.P. Leaf and stem anatomical anomalies in a drought-susceptible species, Impatiens balsamina, under conditions of drought stress. Bot. Gaz. 1974, 135, 121–126. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 23, 407–418. [Google Scholar]
- Ansari, W.A.; Atri, N.; Pandey, M.; Singh, A.K.; Singh, B.; Pandey, S. Influence of drought stress on morphological, physiological and biochemical attributes of plants: A review. Biosci. Biotechnol. Res. Asia 2019, 16, 697–709. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar]
- Zaefyzadeh, M.; Quliyev, R.A.; BABAYEVA, S.; Abbasov, M.A. The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar] [CrossRef]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A.; Darvishzadeh, R.; Samadi, A. Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J. Med. Plants Res. 2011, 5, 2483–2488. [Google Scholar]
- Dastborhan, S.; Ghassemi-Golezani, K. Influence of seed priming and water stress on selected physiological traits of borage. Folia Hortic. 2015, 27, 151–159. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Irani, S.; Majidi, M.M.; Mirlohi, A.; Zargar, M.; Karami, M. Assessment of drought tolerance in sainfoin: Physiological and drought tolerance indices. Agron. J. 2015, 107, 1771–1781. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Goodarzian Ghahfarokhi, M.; Mansurifar, S.; Taghizadeh-Mehrjardi, R.; Saeidi, M.; Jamshidi, A.M.; Ghasemi, E. Effects of drought stress and rewatering on antioxidant systems and relative water content in different growth stages of maize (Zea mays L.) hybrids. Arch. Agron. Soil. Sci. 2015, 61, 493–506. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 2016, 7, 182193. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.A.R.; Cardoso, M.N.; de Oliveira, A.C.A.; Machado, C.D.A.; Cardoso, B.T.; Muniz, A.D.S.; Ledo, A.D.S. Effects of In Vitro Drought Stress on Growth, Proline Accumulation and Antioxidant Defense in Sugarcane. 2018. Available online: https://www.ccsenet.org/journal/index.php/jas/article/view/73121 (accessed on 25 May 2024).
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular mechanisms for the reaction between •OH radicals and proline: Insights on the role as reactive oxygen species scavenger in plant stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Alterations in Physiological, Biochemical, and Molecular Responses of Impatiens walleriana to Drought by Methyl Jasmonate Foliar Application. Genes 2023, 14, 1072. [Google Scholar] [CrossRef]
- Quinet, M.; Descamps, C.; Coster, Q.; Lutts, S.; Jacquemart, A.L. Tolerance to water stress and shade in the invasive Impatiens parviflora. Int. J. Plant Sci. 2015, 176, 848–858. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Falara, V.; Pateraki, I.; López-Carbonell, M.; Cela, J.; Kanellis, A.K. Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. J. Plant Physiol. 2009, 166, 136–145. [Google Scholar] [CrossRef]
- Zeng, X.; Bai, L.; Wei, Z.; Yuan, H.; Wang, Y.; Xu, Q.; Tang, Y.; Nyima, T. Transcriptome analysis revealed the drought-responsive genes in Tibetan hulless barley. BMC Genom. 2016, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-R.; Wang, Y.-H.; Li, T.; Tan, G.-F.; Tao, J.-P.; Su, X.-J.; Xu, Z.-S.; Tian, Y.-S.; Xiong, A.-S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Molinari, M.D.C.; Fuganti-Pagliarini, R.; Marin, S.R.R.; Ferreira, L.C.; Barbosa, D.D.A.; Marcolino-Gomes, J.; Oliveira, M.C.N.D.; Mertz-Henning, L.M.; Kanamori, N.; Takasaki, H.; et al. Overexpression of AtNCED3 gene improved drought tolerance in soybean in greenhouse and field conditions. Genet. Mol. Biol. 2020, 43, e20190292. [Google Scholar] [CrossRef]
- Kim, J.; Malladi, A.; Van Iersel, M.W. Physiological and molecular responses to drought in Petunia: The importance of stress severity. J. Exp. Bot. 2012, 63, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Hong, Y.; Yao, J.; Ren, Z.; Shi, H.; Zhu, J.K. The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Mol. Plant 2018, 11, 1184–1197. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.D.C.; Carvajal, M. Mutual interactions between aquaporins and membrane components. Front. Plant Sci. 2016, 7, 1322. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 1–11. [Google Scholar] [CrossRef]
- Grondin, A.; Mauleon, R.; Vadez, V.; Henry, A. Root aquaporins contribute to whole plant water fluxes under drought stress in rice (Oryza sativa L.). Plant Cell Environ. 2016, 39, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Espinosa, J.; Carvajal, M. Genome-wide identification and biological relevance of broccoli aquaporins. Plant Genome 2022, 15, e20262. [Google Scholar] [CrossRef]
- Lopez-Zaplana, A.; Martinez-Garcia, N.; Carvajal, M.; Bárzana, G. Relationships between aquaporins gene expression and nutrient concentrations in melon plants (Cucumis melo L.) during typical abiotic stresses. Environ. Exp. Bot. 2022, 195, 104759. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M.J.A.P. Aquaporins in plants. Acta Physiol. 2006, 187, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Đurić, M.J.; Subotić, A.R.; Prokić, L.T.; Trifunović-Momčilov, M.M.; Cingel, A.D.; Dragićević, M.B.; Simonović, A.D.; Milošević, S.M. Molecular characterization and expression of four aquaporin genes in Impatiens walleriana during drought stress and recovery. Plants 2021, 10, 154. [Google Scholar] [CrossRef]
- Hassan, N.M.; El-Bastawisy, Z.M.; El-Sayed, A.K.; Ebeed, H.T.; Alla, M.M.N. Roles of dehydrin genes in wheat tolerance to drought stress. J. Adv. Res. 2015, 6, 179–188. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant dehydrins: Expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2022, 41, 519–533. [Google Scholar] [CrossRef]
- Yang, Z.; Sheng, J.; Lv, K.; Ren, L.; Zhang, D. Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Sci. 2019, 284, 143–160. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Wang, X.; Zhang, M.; Xi, Y.; Wang, A.; Zhu, J. Overexpression of Saussurea involucrata dehydrin gene SiDHN promotes cold and drought tolerance in transgenic tomato plants. PLoS ONE 2019, 14, e0225090. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-C.; Zhang, H.-F.; Pan, X.-X.; Chen, N.; Hu, H.-F.; Haq, S.U.; Khan, A.; Chen, R.-G. CaDHN3, a pepper (Capsicum annuum L.) dehydrin gene enhances the tolerance against salt and drought stresses by reducing ROS accumulation. Int. J. Mol. Sci. 2021, 22, 3205. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Role of dehydrins in plant stress response. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–196. [Google Scholar]
- Cuevas-Velazquez, C.L.; Rendón-Luna, D.F.; Covarrubias, A.A. Dissecting the cryoprotection mechanisms for dehydrins. Front. Plant Sci. 2014, 5, 108360. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative stress and antioxidant defense in plants under drought conditions. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Castro, S. Signaling role of ROS in modulating drought stress tolerance. In Drought Stress. Tolerance in Plants, Vol. 1: Physiology and Biochemistry; Springer: Cham, Switzerland, 2016; pp. 309–330. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Oxidative stress, protein carbonylation, proteolysis and antioxidative defense system as a model for depicting water deficit tolerance in Indica rice seedlings. Plant Growth Regul. 2013, 69, 149–165. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Jacques, S.; Huang, J.; Messens, J.; Van Breusegem, F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 2015, 66, 2923–2934. [Google Scholar] [CrossRef]
- AnAnjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Maurya, A.K. Oxidative stress in crop plants. In Agronomic Crops: Volume 3: Stress. Responses and Tolerance; Springer: Singapore, 2020; pp. 349–380. [Google Scholar] [CrossRef]
- Asthir, B. Protective mechanisms of heat tolerance in crop plants. J. Plant Interact. 2015, 10, 202–210. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, N.; Gupta, A.K. Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian J. Biochem. Biophys. 2011, 48, 47–53. [Google Scholar]
- Moharramnejad, S.A.J.J.A.D.; Sofalian, O.; Valizadeh, M.; Asghari, A.; Shiri, M.; Ashraf, M.U.H.A.M.M.A.D. Response of maize to field drought stress: Oxidative defense system, osmolytes’ accumulation and photosynthetic pigments. Pak. J. Bot. 2019, 51, 799–807. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. In Seminars in Dell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 80, pp. 3–12. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Parvin, K.; Nahar, K.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Hasanuzzaman, M. Plant phenolic compounds for abiotic stress tolerance. In Managing Plant Production under Changing Environment; Springer: Singapore, 2022; pp. 193–237. [Google Scholar]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as plant protective companion against abiotic stress. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; Volume 1, pp. 277–308. [Google Scholar]
- Matijević, A.; Šakonjić, A.; Murtić, S. Antioxidant response of Impatiens walleriana L. to drought. Acta Agric. Slov. 2022, 118, 1–7. [Google Scholar] [CrossRef]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: A review. J. Adv. Res. Biotechnol. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Agudelo-Morales, C.E.; Lerma, T.A.; Martínez, J.M.; Palencia, M.; Combatt, E.M. Phytohormones and Plant Growth Regulators—A Review. J. Sci. Technol. Appl. 2021, 10, 27–65. [Google Scholar] [CrossRef]
- LeLeontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Jones, M.L. Isolation of rhizosphere bacteria that improve quality and water stress tolerance in greenhouse ornamentals. Front. Plant Sci. 2020, 11, 549301. [Google Scholar] [CrossRef] [PubMed]
- Popržen, T.; Nikolić, I.; Krstić-Milošević, D.; Uzelac, B.; Trifunović-Momčilov, M.; Marković, M.; Radulović, O. Characterization of the IAA-Producing and-Degrading Pseudomonas Strains Regulating Growth of the Common Duckweed (Lemna minor L.). Int. J. Mol. Sci. 2023, 24, 17207. [Google Scholar] [CrossRef] [PubMed]
- Popržen, T.; Jevremović, S.; Milošević, S.; Đurić, M.; Uzelac, B.; Stanković, S.; Radulović, O. Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid. Horticulturae 2024, 10, 562. [Google Scholar] [CrossRef]
- Du, Y.L.; Wang, Z.Y.; Fan, J.W.; Turner, N.C.; He, J.; Wang, T.; Li, F.M. Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct. Plant Biol. 2013, 40, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Oliveira, H.; Costa, A.; Santos, C. Improving elms performance under drought stress: The pretreatment with abscisic acid. Environ. Exp. Bot. 2014, 100, 64–73. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Mischke, S.; Meinhardt, L.W.; Zhang, D.; Zhu, X.; Li, X.; Fang, W. Exogenous abscisic acid significantly affects proteome in tea plant (Camellia sinensis) exposed to drought stress. Hortic. Res. 2014, 1, 14029. [Google Scholar] [CrossRef]
- Abdelaal, K.A. Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 2015, 6, 1771–1788. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by abscisic acid, salicylic acid and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef]
- Mohammadi, M.H.S.; Etemadi, N.; Arab, M.M.; Aalifar, M.; Arab, M.; Pessarakli, M. Molecular and physiological responses of Iranian Perennial ryegrass as affected by Trinexapac ethyl, Paclobutrazol and Abscisic acid under drought stress. Plant Physiol. Biochem. 2017, 111, 129–143. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, Z. Abscisic acid and glycine betaine mediated tolerance mechanisms under drought stress and recovery in Axonopus compressus: A new insight. Sci. Rep. 2020, 10, 6942. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef] [PubMed]
- AAwan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2021, 172, 809–819. [Google Scholar] [CrossRef]
- Alam, M.M.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2013, 7, 1053. [Google Scholar]
- Abbaspour, J.; Ehsanpour, A. The impact of salicylic acid on some physiological responses of Artemisia aucheri Boiss. under in vitro drought stress. Acta Agric. Slov. 2016, 107, 287–298. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crops Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Bilasvar, H.M.; Nassab, A.D.M. Improving rapeseed (Brassica napus L.) plant performance by exogenous salicylic acid and putrescine under gradual water deficit. Acta Physiol. Plant. 2019, 41, 1–8. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Alam, M.; Hayat, K.; Ullah, I.; Sajid, M.; Ahmad, M.; Basit, A.; Ahmad, I.; Muhammad, A.; Akbar, S.; Hussain, Z. Improving okra (Abelmoschus esculentus L.) growth and yield by mitigating drought through exogenous application of salicylic acid. FRES Environ. Bulle 2020, 29, 529–535. [Google Scholar]
- Hosain, M.T.; Kamrunnahar, M.; Rahman, M.; Hossain, M.; Munshi, M.; Rahman, S. Drought stress response of rice yield (Oryza sativa L.) and role of exogenous salicylic acid. Int. J. Biosci. 2020, 16, 222–230. [Google Scholar]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Tung, S.A.; Samad, R.A.; Wang, L.; Khan, I.; Rehman, N.U.; Shah, A.N.; Shahzad, B. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Fugate, K.K.; Lafta, A.M.; Eide, J.D.; Li, G.; Lulai, E.C.; Olson, L.L.; Deckard, E.L.; Khan, M.F.R.; Finger, F.L. Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J. Agron. Crop. Sci. 2018, 204, 566–576. [Google Scholar] [CrossRef]
- Karamian, R.; Ghasemlou, F.; Amiri, H. Physiological evaluation of drought stress tolerance and recovery in Verbascum sinuatum plants treated with methyl jasmonate, salicylic acid and titanium dioxide nanoparticles. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 154, 277–287. [Google Scholar] [CrossRef]
- Yosefi, A.; Mozafari, A.A.; Javadi, T. Jasmonic acid improved in vitro strawberry (Fragaria × ananassa Duch.) resistance to PEG-induced water stress. Plant Cell Tissue Organ Cult. 2020, 142, 549–558. [Google Scholar] [CrossRef]
- Shirani Bidabadi, S.; Sharifi, P. Strigolactone and methyl jasmonate-induced antioxidant defense and the composition alterations of different active compounds in Dracocephalum kotschyi Boiss under drought stress. J. Plant Growth Regul. 2021, 40, 878–889. [Google Scholar] [CrossRef]
- Abeed, A.H.; Eissa, M.A.; Abdel-Wahab, D.A. Effect of exogenously applied jasmonic acid and kinetin on drought tolerance of wheat cultivars based on morpho-physiological evaluation. J. Soil. Sci. Plant Nutr. 2021, 21, 131–144. [Google Scholar] [CrossRef]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef]
- Meng, Y.; Liao, P.; Chen, Y.; Weng, W.; Chen, L.; Xu, F.; Hu, Q.; Xing, Z.; Wei, H.; Gao, H.; et al. Exogenous Application of Methyl Jasmonate Promotes Yield and Grain Quality of Rice under Terminal Drought Stress. Agronomy 2023, 13, 1903. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef]
- Gusain, Y.S.; Singh, U.S.; Sharma, A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr. J. Biotechnol. 2015, 14, 764–773. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Tiepo, A.N.; Hertel, M.F.; Rocha, S.S.; Calzavara, A.K.; De Oliveira, A.L.M.; Pimenta, J.A.; Oliveira, H.C.; Bianchini, E.; Stolf-Moreira, R. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol. Biochem. 2018, 130, 277–288. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil. 2020, 452, 423–440. [Google Scholar] [CrossRef]
- He, A.; Niu, S.; Yang, D.; Ren, W.; Zhao, L.; Sun, Y.; Meng, L.; Zhao, Q.; Paré, P.W.; Zhang, J. Two PGPR strains from the rhizosphere of Haloxylon ammodendron promoted growth and enhanced drought tolerance of ryegrass. Plant Physiol. Biochem. 2021, 161, 74–85. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2021, 41, 549–569. [Google Scholar] [CrossRef]
- Ferioun, M.; Srhiouar, N.; Tirry, N.; Belahcen, D.; Siang, T.C.; Louahlia, S.; El Ghachtouli, N. Optimized drought tolerance in barley (Hordeum vulgare L.) using plant growth-promoting rhizobacteria (PGPR). Biocatal. Agric. Biotechnol. 2023, 50, 102691. [Google Scholar] [CrossRef]
- Rigi, F.; Saberi, M.; Ebrahimi, M. Improved drought tolerance in Festuca ovina L. using plant growth promoting bacteria. J. Arid. Land. 2023, 15, 740–755. [Google Scholar] [CrossRef]
- Waterland, N.L.; Campbell, C.A.; Finer, J.J.; Jones, M.L. Abscisic acid application enhances drought stress tolerance in bedding plants. HortScience 2010, 45, 409–413. [Google Scholar] [CrossRef]
- Cochran, D.R.; Harkess, R.L.; Knight, P.R.; Tomaso-Peterson, M.; Blythe, E.K.; Gilliam, C.H. Evaluation of a Commercial Extract of Giant Knotweed on Drought Tolerance of Impatiens. HortScience 2014, 49, 1034–1040. [Google Scholar] [CrossRef]
- Park, S.; Mills, S.A.; Moon, Y.; Waterland, N.L. Evaluation of antitranspirants for enhancing temporary water stress tolerance in bedding plants. HortTechnology 2016, 26, 444–452. [Google Scholar] [CrossRef]
- Waterland, N.L.; Finer, J.J.; Jones, M.L. Benzyladenine and gibberellic acid application prevents abscisic acid-induced leaf chlorosis in pansy and viola. HortScience 2010, 45, 925–933. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Chapin, L.J.; Taylor, C.G.; Jones, M.L. Identification of Pseudomonas spp. that increase ornamental crop quality during abiotic stress. Front. Plant Sci. 2020, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Waterland, N.L. Evaluation of calcium application methods on delaying plant wilting under water deficit in bedding plants. Agronomy 2021, 11, 1383. [Google Scholar] [CrossRef]
- Đurić, M.; Trifunović-Momčilov, M.; Milošević, S.; Marković, M.; Radulović, O.; Subotić, A.; Uzelac, B. Does Sodium Nitroprusside Alleviate Water Deficit Stress in Impatiens walleriana Shoots Grown In Vitro? Agriculture 2023, 13, 1903. [Google Scholar] [CrossRef]


| Elicitor | Plant Species | Concentration/ Bacterial Strains | Way of Application | Effects | References |
|---|---|---|---|---|---|
| ABA | Triticum aestivum | 10 µM | Soil drenching | Increased ABA concentration, reduced stomatal conductance, decreased oxidative stress damages, increased antioxidant enzyme activities | [126] |
| Ulmus minor | 50 and 100 μM | Foliar application | Increased DW, reduced water loss, enhancement of antioxidant capacity | [127] | |
| Camellia sinensis | 50 mg L−1 | Foliar application | Leaf proteome changes | [128] | |
| Vicia faba | 0.1 mM | Foliar application | Improved morphological and anatomical characters, Chl concentrations, and yield | [129] | |
| Triticum aestivum | 10 μM | Supplemented in Hoagland solution | Increased shoot length, and shoot and root dry weights; decreased H2O2 and MDA, increased content of GSH and ASA | [130] | |
| Agrostis stolonifera | 5 μM | Foliar application | Lower electrolyte leakage, greater RWC, accumulation of organic acids | [131] | |
| Lolium perenne | 0.054 kg ai ha−1 | Foliar application | Enhanced RWC, decreased electrolyte leakage and H2O2, increased soluble sugar content and antioxidant enzyme activity | [132] | |
| Axonopus compressus | 100 μM | Foliar application | Reduced oxidative stress, increased pigment content, osmolyte accumulation | [133] | |
| Camellia sinensis | 50 mg L−1 | Foliar application | Metabolic changes, increased phenolic content | [134] | |
| Pennisetum glaucum | 100 μM | Supplemented in Hoagland solution | Improved Chl and RWC, increased activities of antioxidative enzymes | [135] | |
| SA | Brassica juncea | 50 μM | Foliar application | Increased RWC, Chl content, antioxidant enzyme activity, decreased H2O2 and lipid peroxidation level | [136] |
| Vicia faba | 1 mM | Foliar application | Improved morphological and anatomical characters, Chl concentrations, and yield | [129] | |
| Artemisia aucheri | 0.01 and 0.1 mM | In vitro | Improved DW and FW, Chl and carotenoid contents, increased soluble carbohydrates, increased biosynthesis of phenolic compounds | [137] | |
| Agrostis stolonifera | 10 μM | Foliar application | Lower electrolyte leakage, greater RWC, accumulation of amino acids and carbohydrates | [131] | |
| Carthamus tinctorius | 250 μM | Foliar application | Activation of non-enzymatic antioxidant defense system, increased proline content, decreased oxidative stress | [138] | |
| Brassica napus | 1 mM | Foliar application | Increased antioxidant enzyme activities, water content, membrane integrity and Chl, improvement in grain and oil yields | [139] | |
| Hordeum vulgare | 0.5 mM | Foliar application | Increased stem length, DW, Chl, RWC, activity of antioxidant enzymes, and grain yield; decreased lipid peroxidation, electrolyte leakage, O2·− and H2O2 | [140] | |
| Abelmoschus esculentus | 80, 160 and 240 mgL−l | Foliar application | SA at 240 mgL−1 improved the best all of the growth and yield attributes; had minimum days to flowering and picking, and maximum single-pod weight, average pod length, plant height, number of leaves per plant, number of pods per plant, stem diameter and yield | [141] | |
| Oryza sativa | 250, 500, 750 and 1000 µM m−2 | Foliar application | Improved grain yield and harvest index when 750 µM m−2 SA applied | [142] | |
| Triticum aestivum | 140 mg L− 1 | Foliar application | Increased grain yield, decreased MDA, H2O2 and O2•−, increased proline, soluble sugars and antioxidant enzyme activity | [143] | |
| Jasmonates | Triticum aestivum | 0.5 mM MeJA | Foliar application | Improved dry biomass, number of grains per spike, and grain weight and yield | [144] |
| Glycine max | 20 µM MeJA | Foliar application | Increased growth parameters, RWC, photosynthetic pigments, cell wall components, unsaturated fatty acids, and phenolic compounds | [145] | |
| Beta vulgaris | 0.01, 0.1, 1 or 10 μM MeJA | Foliar application | 1 and 10 μM MeJA reduced moderate- and severe-drought effects on RWC, photosynthesis rate, substomatal CO2 concentration and WUE, and altered drought-induced changes in proline accumulation | [146] | |
| Verbascum sinuatum | 200 µM MeJA | In vitro | MeJA negatively affected growth parameters and increased the content of MDA, H2O2, total saponin and activity of peroxidase and polyphenol oxidase | [147] | |
| Fragaria × ananassa | 0.01 and 0.05 mM JA | In vitro | Improved growth, RWC, and pigment content | [148] | |
| Dracocephalum kotschyi | 0.5 mM MeJA | Foliar application | Higher FW and DW, lower electrolyte leakage, MDA, H2O2, total phenol content, total antioxidant activity and antioxidant power assay | [149] | |
| Triticum sativum | 0.1 mM JA | Foliar application | Improved growth, restoration of shoot/root ratio, accumulation of osmolytes, regulated activity of antioxidant enzymes | [150] | |
| Pennisetum glaucum | 100 μM | Supplemented in Hoagland solution | Improved Chl and RWC, increased activities of antioxidative enzymes | [135] | |
| Brassica rapa | 10 µM JA | Foliar application | Improved photosynthetic rate, photosynthetic pigments, stomatal conductance, transpiration rate and antioxidant defence, increased osmolyte accumulation, and decreased membrane damage | [151] | |
| Oryza sativa | 100 μmol L−1 MeJA | Foliar application | Increased grain yield and quality | [152] | |
| PGPBs | Zea mays | Proteus penneri, Pseudomonas aeruginosa, and Alcaligenes faecalis | Seed priming | Improved plant biomass, root and shoot length, leaf area, RWC, protein and sugar content | [153] |
| Oryza sativa | Pseudomonas sp., Bacillus cereus, Arthrobacter nitroguajacolicus | Soil inoculation | Enhanced growth, higher proline content and antioxidant enzyme activities, lower MDA and H2O2 | [154] | |
| Triticum aestivum | Klebsiella sp., Enterobacter ludwigii, Flavobacterium sp. | Seed priming, soil inoculation | Transcriptomic changes, improved root length and number, shoot DW, root FW and DW, and physiological and biochemical parameters | [155] | |
| Setaria italica | Pseudomonas fluorescens, P. migulae, Enterobacter hormaechei | Seed priming, soil inoculation | Stimulated seed germination and seedling growth | [156] | |
| Trema micrantha, Cariniana estrellensis | Azospirillum brasilense, Bacillus sp., Azomonas sp., Azorhizophillus sp. | Seed priming, soil inoculation | Increased drought tolerance, growth parameters, and physiological and biochemical attributes | [157] | |
| Ziziphus jujuba | Pseudomonas, Bacillus, Serratia | Soil inoculation | Pseudomonas lini and Serratia plymuthica increased plant height, shoot and root dry matter, RWC, and antioxidant enzyme activities; decreased MDA and ABA | [158] | |
| Thymus, Sarcocornia, Mentha | Pseudomonas, Pantoea, Acinetobacter | Soil inoculation | in vitro PGP-associated traits, including phosphate solubilization, indole-3-acetic acid production, and 1-aminocyclopropane-1-carboxylate deaminase activity; increased tolerance to salinity and drought | [122] | |
| Petunia, Pelargonium | Pseudomonas, Arthrobacter, Herbaspirillium, | Soil inoculation | Increased biomass; increased number of flowers; mitigation of the reduction in photosynthetic parameters of water-stressed P. hybrida and Pelargonium × hortorum | [123] | |
| Haloxylon ammodendron | Bacillus sp., Pseudomonas sp. | Seed priming, soil inoculation | Improved root system and growth, RWC, photosynthetic capacity, antioxidant enzyme activities and regulated ABA content | [159] | |
| Triticum aestivum | Bacillus megaterium, B. licheniformis | Seed priming, soil inoculation | Increased germination index, promptness index, seedling vigor index, FW and DW, RWC, photosynthetic pigments, osmolytes, and activities of defense-related antioxidant enzymes | [160] | |
| Hordeum vulgare | four bacterial isolates (MFC1, MFE3, MFF2, and MFF5) | Soil inoculation | Increased shoot dry weight, RWC, Chl content, photosynthesis efficiency, and proline content; decreased MDA and H2O2 | [161] | |
| Festuca ovina | Azotobacter vinelandii, Pantoea agglomeran, Pseudomonas putida | Seed priming | Improved seed germination, plant growth, and nutrient uptake | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovančević, M.; Trifunović-Momčilov, M.; Radulović, O.; Milošević, S.; Subotić, A. Drought Stress Effects and Ways for Improving Drought Tolerance in Impatiens walleriana Hook.f.—A Review. Horticulturae 2024, 10, 903. https://doi.org/10.3390/horticulturae10090903
Milovančević M, Trifunović-Momčilov M, Radulović O, Milošević S, Subotić A. Drought Stress Effects and Ways for Improving Drought Tolerance in Impatiens walleriana Hook.f.—A Review. Horticulturae. 2024; 10(9):903. https://doi.org/10.3390/horticulturae10090903
Chicago/Turabian StyleMilovančević, Marija, Milana Trifunović-Momčilov, Olga Radulović, Snežana Milošević, and Angelina Subotić. 2024. "Drought Stress Effects and Ways for Improving Drought Tolerance in Impatiens walleriana Hook.f.—A Review" Horticulturae 10, no. 9: 903. https://doi.org/10.3390/horticulturae10090903
APA StyleMilovančević, M., Trifunović-Momčilov, M., Radulović, O., Milošević, S., & Subotić, A. (2024). Drought Stress Effects and Ways for Improving Drought Tolerance in Impatiens walleriana Hook.f.—A Review. Horticulturae, 10(9), 903. https://doi.org/10.3390/horticulturae10090903










