Abstract
A survey of plant parasitic nematodes was carried out in 650 garlic fields in Korea from 2020 to 2022. Migratory endoparasite nematodes (Ditylenchus sp.) were recovered from 6% of the garlic samples, with an average density of 494 individuals per garlic bulb. The morphological characteristics of males and females from the 2022 survey were very similar to D. destructor, but D. dipsaci was not found. The Korean population traits have a lateral field containing six incisures, and the posterior esophagus part overlaps the intestine dorsally. PCR and DNA sequencing were performed for the D2/D3 region of the ribosomal DNA 28S and the ITS region, and the phylogenetic analysis strongly supports the monophyly of D. destructor. This is the first report of D. destructor parasitizing garlic in the Republic of Korea. In Korea, due to changes in agricultural or environmental conditions, the most damaging potential PPNs changed from D. dipsaci to D. destructor in garlic cultivation.
1. Introduction
Garlic (Allium sativum L.) is a spice crop with a major component, allicin, belonging to the genus Allium L. in the Liliaceae Juss. family. In Korea, annual garlic consumption per capita was the second highest in the world, with 7.6 kg in 2018 [1]. The domestic garlic cultivation area was 22,362 ha, with a production of 272,759 tons and yield of 1220 kg per 10 acres. In 2022, the main garlic-producing regions were Gyeongnam (28.8%), Gyeongbuk (22.8%), and Jeonnam (15.7%) [2].
Plant-parasitic nematodes (PPNs) are described in over 4100 species and cause agricultural losses estimated at USD 173 billion annually [3]. Among the PPNs, the genus Ditylenchus Filipjev, 1936, encompasses more than 80 species, though only a few are plant parasites [4,5]. These nematodes have over 500 known host species, including garlic, onion (A. cepa), lily (Lilium longiflorum, L. regale), potato (Solanum tuberosum), daffodil (Narcissus tazetta), and corn (Zea mays), and they are detected worldwide [6,7,8]. In garlic plants, Ditylenchus dipsaci (Kühn, 1857) Filipjev, 1936, and Ditylenchus destructor Thorne, 1945, are well-known parasitic nematodes and are listed as internationally quarantined species. The symptoms of damage vary depending on the host plant, but in garlic, these nematodes invade between the peel and the bulb, damaging the tissue. This damage weakens bulb development and promotes decay and death [9,10]. Additionally, the growth of the above-ground parts is stunted, causing chlorosis. Due to the nematodes’ habit of parasitizing the stem and bulb, the damage persists even after harvest, leading to a rapid reduction in storage capacity [11].
The garlic grown in Korea is divided into the northern ecotype and the southern ecotype. The northern ecotype cultivars are grown mainly in the central and northern regions of Korea, where winters are cold, while the southern ecotype cultivars are mainly grown in the southern part of the country, where winters are mild [12]. The southern ecotype cultivars include ‘Namdo’ garlic introduced from China, ‘Daeseo’ garlic from Spain, and ‘Jabong’ garlic from Indonesia. Because of global warming caused by climate change, the cultivation area of the southern ecotype cultivars has been expanding northward, along the West Sea coastline, since the early 1990s. Currently, 50–70% of the southern ecotype garlic is cultivated in Seosan and Taean, which were previously northern ecotype garlic cultivation areas [13].
In Korea, a previous study on garlic parasitic nematodes found that in 1986, D. dipsaci was detected in 81% of garlic fields, and when severely infected, the yield decreased by about 40% [14]. The infection rate of D. dipsaci in garlic fields was 10% in 1994 [15]. On the other hand, D. destructor has been identified as one of the most serious pathogens affecting garlic production in Japan, and the nematode has also been found Canada and the United States [16,17,18]. Despite many changes in the garlic cultivation system in Korea, such as recent garlic imports, global warming, and the expansion of southern ecotype cultivars cultivation, there has been no investigation of garlic nematodes for about 30 years. Therefore, a survey was conducted to investigate the occurrence and distribution of Ditylenchus species in garlic cultivation fields in Korea. During the investigation, nematodes similar to D. destructor were identified, and damage symptoms on garlic, as well as morphological and molecular characteristics were analyzed. This study presents the first report of potato rot nematodes, D. destructor, parasitizing garlic in Korea.
2. Materials and Methods
2.1. Collection of Garlic and Soil Samples
From 2020 to 2022, garlic plants and soil samples were collected from 32 fields in Gangwon, 9 fields in Gyeonggi, 76 fields in Chungnam, 20 fields in Chungbuk, 274 fields in Gyeongnam, 91 fields in Gyeongbuk, 36 fields in Jeonnam, and 112 fields in Jeju, making a total of 650 garlic cultivation fields during the harvest season. Fields selected for sampling were based on a minimum size of 0.1 acres (Table 1). For each field, 10 entire garlic plants were collected randomly. Soil samples were collected from around the garlic roots to a depth of 15 cm after removing the top 5 cm of soil. At least 1 kg of soil around the garlic roots in each field was collected using a zig-zag pattern, placed in a plastic bag, and transported to the laboratory for further analysis.

Table 1.
Detection frequencies of the genus Ditylenchus in South Korea from 2020 to 2022.
2.2. Nematode Extraction
The nematode extraction from soil was performed as follows: 300 g of soil from a 1 kg sample was placed in a bucket and stirred by hand, breaking up the clumps of soil while adding about 2 L of tap water. The suspension was poured through sieves with pore sizes of 125 μm and 45 μm. The 125 μm sieve was discarded, and the back-washed sediments from the 45 μm sieve were collected. Nematode extraction was performed using the modified Baermann funnel method. The procedures performed on the garlic samples were as follows: The garlic peels were stripped from the garlic bulbs. The garlic peel was cut into pieces measuring 2–3 mm, placed in a 200 mL beaker, and filled with tap water.
After 24 h, the extracted nematodes from soil and garlic samples were isolated using a 25 μm sieve. The extracted nematodes were then placed in a counting dish, and the nematodes were genus identified and counted using a stereo microscope (SZX61, Olympus, Tokyo, Japan).
2.3. Morphological Analysis
Female and male of nematodes were killed and fixed using 80 °C FG 4:1 fixative. Nematode specimens were prepared by the Seinhorst method and left for at least 24 h [19]. For microscopic observation, specimens were mounted on Cobb slides and sealed with a paraffin ring and glycerin [20]. Nematodes were observed, measured, and photographed with the aid of a compound microscope (BX53, Olympus) equipped with a digital camera (DP73, Olympus). The following metrics were measured: total body length, body width, stylet length, tail length and width, vulva location of females (%), number of lateral lines, and spicule length of males.
2.4. Molecular Analysis
For molecular analyses, garlic was re-collected from the infested fields where D. destructor was detected among the nematodes of the genus Ditylenchus. From isolated D. destructor populations by region, thirty female nematodes were picked and moved to a 2.0 mL microcentrifuge tube. Total genomic DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA, USA). Two rDNA fragments, i.e., the 28S rRNA D2-D3 expansion segments and the ITS region, were amplified. The primers for the amplification of the 28S rRNA region were D2A(5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and D3B(5′-TCGGAAGGAACCAGCTACTA-3′) [21], while the primers for the amplification of the ITS region were 18S(5′-TTGATTACGTCCCTGCCCTTT-3′) and 26S(5′-TTTCACTCGCCGTTACTAACG-3′) [22]. PCR reaction was conducted with a thermal cycler (C-100, Bio-Rad, Hercules, CA, USA), and the program was as follows: after an initial 2 min denaturation at 94 °C, the amplification was carried out for 35 cycles (1 min at 94 °C, 30 s at 55 °C, and 1 min at 72 °C), with a final extension for 4 min at 72 °C for the amplification of 28S rRNA. After an initial 90 s denaturation at 96 °C, the amplification was carried out for 40 cycles (45 s at 96 °C, 30 s at 50 °C, and 4 min at 72 °C), with a final extension for 10 min at 72 °C for the ITS region. Amplified PCR products were visualized in the 1% agarose gel. The PCR product was subsequently purified with a PCR Purification Kit (Qiagen Inc.). The amplicons were cloned in pGEM-T Easy Vector System (Promega, Madison, WI, USA), and the resultant plasmid DNA was isolated with a Plasmid Midiprep System (Promega) and sequenced at Macrogen.
2.5. Phylogenetic Analysis
For phylogenetic analysis, the newly obtained and published sequences for each gene were aligned using the Mega X program with default parameters [23]. Sequence alignments were manually edited using BioEdit [24]. The alignment quality was examined visually and optimized manually by adjusting the ambiguous nucleotide positions. Models of base substitution were evaluated using MODELTEST3.7 combined with PAUP4.0 [25,26,27]. The Akaike-supported model, the base frequency, the proportion of invariable sites, and the gamma distribution shape parameters and substitution rates in the AIC were then used in phylogenetic analyses. Bayesian analysis was performed to confirm the tree topology for each gene separately using MrBayes 3.2.7, running the chain for 1 × 106 generations and setting the ‘burn-in’ at 2500 [28]. The MCMC (Markov Chain Monte Carlo) method was used within a Bayesian framework to estimate posterior probabilities of the phylogenetic trees [29] and generate a 50% majority-rule consensus tree. The posterior probabilities are given on appropriate clades. Trees were visualized using TreeView [30].
3. Results
3.1. Frequency of Occurrence of Ditylenchus sp. in Garlic from Korea
Ditylenchus sp. were detected in 39 samples (6.0% of the 650 samples) from garlic fields in five of eight provinces (Table 1). The greatest detection frequency of Ditylenchus sp. was found in the province of Gyeonggi (34%), which showed the lowest average density of individuals per bulb (373/bulb). The lowest infestation frequency was found in the province of Chungnam (2.6%), which showed the highest average density of individuals per bulb (673/bulb). The nematodes were not detected in Jeju, Chungbuk, and Gangwon. Furthermore, the infection rates were relatively evenly distributed, with 14% in the northern ecotype and 9% in the southern ecotype.
3.2. Morphological Characteristics of Ditylenchus destructor
The damage symptoms observed in garlic infected with D. destructor collected from Namhae, Gyeongnam, during storage included blackened garlic peels and clove rot, with 2440 individuals per garlic bulb (Figure 1A). The nematode was observed in the garlic peel after staining (Figure 1B). Morphological characteristics and morphometric studies were performed from the recovered females and males of the isolate D. destructor (Table 2).
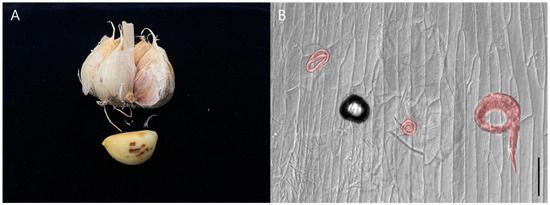
Figure 1.
Damage symptoms of garlic bulb (A) and the garlic peel infected by Ditylenchus destructor (B). Scale bars: (B) = 100 μm.

Table 2.
Comparative morphometrics and morphological traits of the reported populations of Ditylenchus destructor (females and males) infesting garlic, along with the original description.
The morphological characteristics of females were very similar to those of D. destructor, with an elongated and straight body (Figure 2A). The posterior esophagus part was overlapped dorsally with the intestine (Figure 2E), the tail tip was less sharp (Figure 2D) and had six lateral lines (Figure 2B). The post-uterine sac was long, extended to about 1/2 of the tail terminus (Figure 3). The males of D. destructor had a spicule that was ventrally curved, a gubernaculum was short, and a wide bursa that extended to near the middle of the tail (Figure 4).
3.3. Molecular Profiles and Phylogenetic Status of Ditylenchus destructor
The sequenced LSU D2-D3 segments and the ITS region are 779–780 bp and 1130–1033 bp, respectively. The newly obtained sequences of D. destructor populations collected from various geographical regions were submitted to the GenBank database under the accession numbers listed in Table 3.
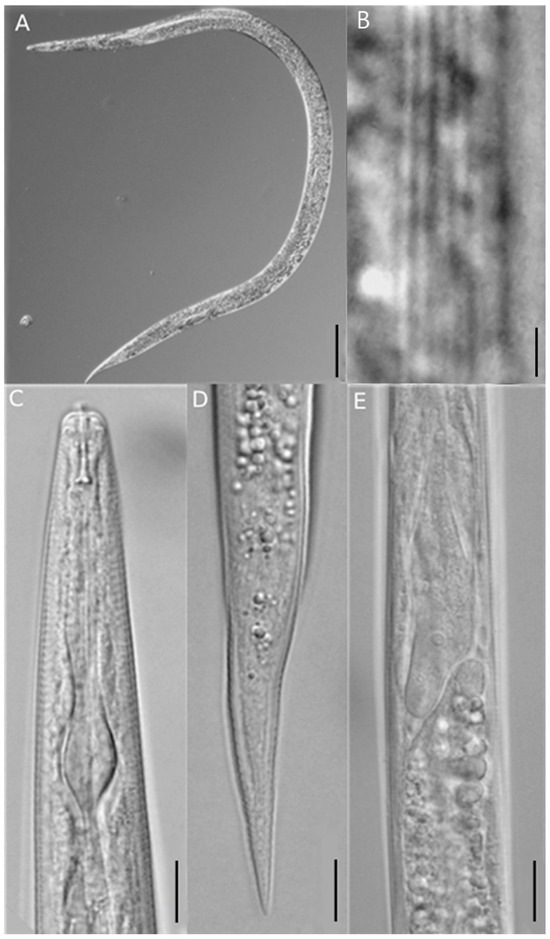
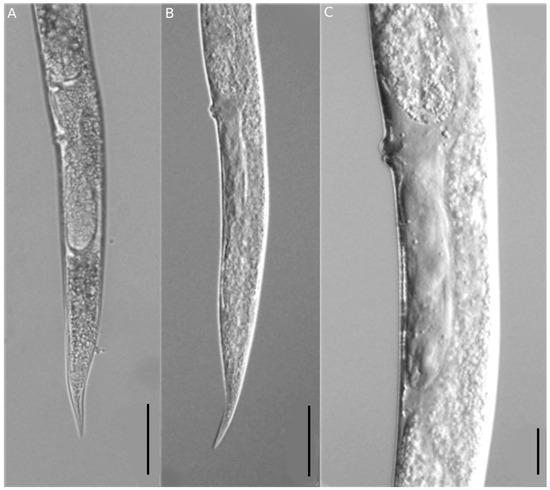
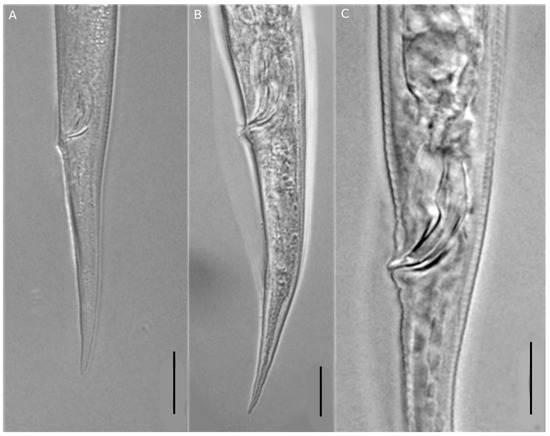

Figure 2.
Photographs of females of Ditylenchus destructor. Entire body (A); lateral line (B); anterior region (C); posterior region (D); posterior esophagus part (E). Scale bars: (A) = 100 μm, (B) = 2 μm, (C–E) = 10 μm.

Figure 3.
Photographs of vulva and tail region of Ditylenchus destructor. Tail of female (A,B); vulva region (C). Scale bars: (A,B) = 50 μm, (C) = 20 μm.

Figure 4.
Photographs of spicule and tail region of Ditylenchus destructor. Tail of female (A,B); spicule region (C). Scale bars: (A) = 20 μm, (B,C) = 10 μm.

Table 3.
List of host, geographical regions, isolates, and GenBank accession numbers of Ditylenchus destructor used.
A BLAST search of D. destructor on the LSU D2-D3 segments revealed high-scoring matches with some Ditylenchus species, with the closest match being D. destructor (GenBank accession number MT585824), which is the species isolated from the maize in Heilongjiang Province in China. The identities of these two sequences are 100.0% (780/780), with no insertions/deletions. However, compared with D. dipsaci (KT806478), D. destructor shows an 88.47% identity (330/373) and has five gaps (1%). A BLASTn search of D. destructor on the ITS region also revealed similarities with some Ditylenchus species. The highest match was D. destructor (MK979365), with 99% identity (1126/1133) and three insertions/deletions, which is the species isolated from the potato in Liaoning Province in China. Compared with D. dipsaci (KY348764), D. destructor shows an 84% identity (310/369) and has 10 gaps (2%).
The molecular phylogenetic relationships of the Korean populations are shown in Figure 5 and Figure 6. Figure 5 represents a phylogenetic tree based on the LSU D2-D3 segments. The average nucleotide composition is as follows: 22.65% A, 22.71% C, 27.26% G, and 27.38% T. Using Bursaphelenchus xylophilus as the outgroup taxon, the molecular phylogeny strongly supports the monophyly of Ditylenchus. Figure 6 represents a phylogenetic tree based on the ITS region. The average nucleotide composition is as follows: 23.59% A, 20.38% C, 26.09% G, and 29.94% T. In addition, using Bursaphelenchus xylophilus as the outgroup taxon, all these species grouped independently from other Ditylenchus species, and the molecular phylogeny supports the monophyly of D. destructor.
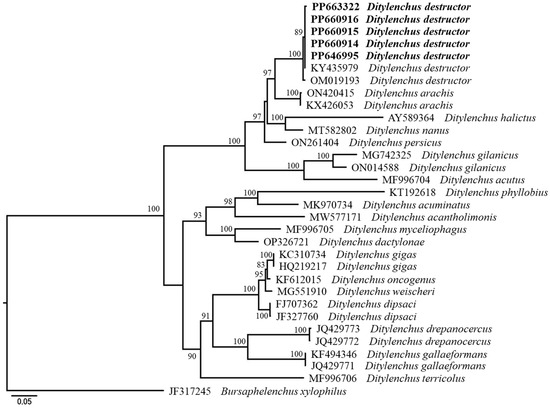
Figure 5.
Phylogenetic relationships within population and species of Ditylenchus. Bayesian 50% majority rule consensus tree from two runs, as inferred from the analysis of the D2D3 of 28S rDNA gene sequences under the GTR + I + G model. Posterior probability values more than 50% are given in appropriate clades. Newly sequenced samples are indicated in bold font.
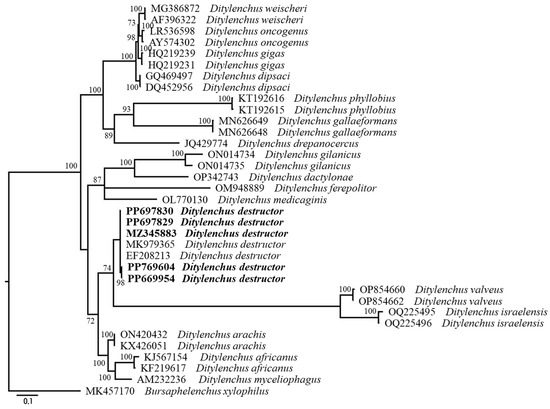
Figure 6.
Phylogenetic relationships within population and species of Ditylenchus. Bayesian 50% majority rule consensus tree from two runs, as inferred from the analysis of the ITS rRNA gene sequences under the GTR + I + G model. Posterior probability values more than 50% are given in appropriate clades. Newly sequenced samples are indicated in bold font.
4. Discussion
The results showed that, while Ditylenchus species were detected in only 6% of the total samples, all of these were identified as D. destructor based on morphological traits. Ditylenchus destructor was found in most provinces in Korea, indicating that despite its low infection rate, it is likely distributed nationwide. In Korea, D. destructor was first reported in ginseng (Panax ginseng C. A. Mayer) in 1976 and has not been reported in garlic [32]. Morphological characteristics were compared with those of the D. destructor population infesting ginseng in Korea. The population detected in garlic had a wide range of female body lengths (0.7–1.9 mm vs. 0.9–1.3 mm), and differed from the ginseng population in the ratio of body length to greatest body width of males (36–48 vs. 34.5) [33]. The population parasitizing the Codonopsis pilosula in China was characterized by significantly shorter body length in males (0.48–0.88 mm) and females (0.78–1.13 mm), suggesting that this species development may depend on the environment, including the host [34]. Furthermore, no D. dipsaci was identified based on morphological analysis in this study.
In garlic plants, D. dipsaci was first found in 1978, being detected in 81% of the garlic fields in 1986 [14,35]. In 1994, the infection rate of D. dipsaci was 10% in stored garlic from major production areas [15]. In this study, as a result of morphological analysis to identify the species of the genus Ditylenchus, no D. dipsaci were found, and all were identified as D. destructor. The characteristics of these populations are distinct from D. dipsaci, which has a sharp tail terminus, an esophagus that does not overlap the intestine, and a lateral field marked by four incisures. Thes traits are all consistent with D. destructor, a potato rot nematode [36]. The species of the Ditylenchus genus that are of concern for damage to garlic production in Korea changed from D. dipsaci to D. destructor. The first reason for this is that the supplied project of disease-free garlic seedlings that began around 2010 was successfully carried out, and healthy garlic that was not infected by D. dipsaci was distributed to farmers. The second reason is that, due to global warming, the cultivation area of the southern ecotype has expanded to the northward to regions such as in Gyeongbuk and Chungbuk, and the cultivation area of the northern ecotype has decreased [13].
In Japan, studies on the correlation between nematode density and garlic growth have been conducted since D. destructor was first reported in 1984 [16]. Ditylenchus destructor densities in the outer skins at harvest were consistently low when those in roots at harvest were lower than 80 individuals per 0.05 g garlic bulbs, and no damage to garlic bulbs after storage was observed when D. destructor densities in the outer skins were lower than 300 individuals per 0.05 g garlic bulbs [37]. Our study results showed that the infection rate of D. destructor was low at 6%, but the infection density was an average density of 494 individuals per garlic bulb. Although these control methods can prevent damage during the growing season, they do not provide successful control methods for rotting during post-harvest storage [37]. It is necessary to develop and set a control method against the potato rot nematode, D. destructor, in garlic cultivation. Therefore, it is estimated that garlic production in garlic cultivation areas where D. destructor occurs is likely to suffer severe damage.
To the best of our knowledge, this study is the first report of D. destructor in garlic from the Republic of Korea. Our results provide information on the morphological characteristics of a Korean population of D. destructor that infested garlic. Although sequence-based information is limited, this information can help in identifying the species. Recently, molecular and phylogenetic analyses based on DNA barcoding genes, such as the LSU D2-D3 segments and the ITS region [38], have been used to clarify the identification of PPNs.
5. Conclusions
This is the first record of D. destructor in garlic from the Republic of Korea. Ditylenchus destructor is distributed across several garlic cultivation regions and poses a new threat to garlic growing and storage in South Korea. Due to changes in agricultural or environmental conditions, the most damaging potential PPNs changed from D. dipsaci to D. destructor in garlic cultivation [14,15]. The host range of D. destructor includes some economic crops such as potatoes, ginseng, and groundnuts [31,39,40]. It is necessary to investigate the damage to economic crops in the Republic of Korea caused by this nematode and to develop a diagnosis method and controlling strategies.
Author Contributions
Conceptualization, S.H. and H.J.; methodology; S.H., S.P., and D.K.; formal analysis; S.H., S.P., and N.P.; data curation S.H. and D.K.; writing—original draft preparation, S.H. and H.K.; writing—review and editing, H.K. and I.C.; funding acquisition, I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Cooperative Research Program for Agriculture Science and Technology Department” (project no. PJ01565402), Rural Development Administration, Republic of Korea.
Data Availability Statement
The sequencing data reported in this study were deposited in GenBank and are available online at https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 20 April 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Major Statistics of Agriculture, Livestock and Food; Ministry of Agriculture, Food and Rural Affairs: Sejong, Republic of Korea, 2019; p. 345. Available online: https://lib.mafra.go.kr/skyblueimage/28195.pdf (accessed on 30 March 2020).
- Survey of Results of Garlic and Onion Cultivation Area; Statistics Korea: Daejeon, Republic of Korea, 2022; Available online: https://kostat.go.kr/boardDownload.es?bid=229&list_no=417923&seq=3 (accessed on 28 April 2022).
- Coke, M.C.; Bell, C.A.; Urwin, P.E. The use of Caenorhabditis elegans as a model for plant-parasitic nematodes: What have we learned? Annu. Rev. Phytopathol. 2024, 62, 7.1–7.16. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.W.; Moens, M. Migratory endoparasitic nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2006; pp. 123–152. [Google Scholar]
- Skwiercz, A.T.; Kornobis, F.W.; Winiszewska, G.; Przybylska, A.; Obrepalska-Steplowska, A.; Gawlak, M.; Subbotin, S.A. Ditylenchus laurae sp. n. (Tylenchida: Anguinidae) from Poland—A new species of the D. dipsaci complex associated with a water plant, Potamogeton perfoliatus L. Nematology 2017, 19, 197–209. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Madano, M.; Krall, E.; Sturhan, D.; Moens, M. Molecular diagnostics, taxonomy, and phylogeny of the stem nematode Ditylenchus dipsaci species complax based on the sequences of the internal transcribed spacer-rDNA. Phytopathology 2005, 95, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Subbotin, S.A.; Deimi, A.M.; Zheng, J.; Chizhov, V.N. Length variation and repetitive sequences of Internal Transcribed Spacer of ribosomal RNA gene, diagnostics and relationships of populations of potato rot nematode, Ditylenchus destructor Thorne, 1945 (Ttylenchida: Anguinidae). Nematology 2011, 13, 773–785. [Google Scholar] [CrossRef]
- Pan, F.; Li, F.; Mao, Y.; Liu, D.; Chen, A.; Zhao, D.; Hu, Y. First detection of Ditylenchus destructor parasitizing maize in Northeast China. Life 2021, 11, 1303. [Google Scholar] [CrossRef]
- Blauel, T.; Celetti, M.J.; Jordan, K.S.; Mcdonald, M.R. Optimizing methods to sample and quantify stem and bulb nematode, Ditylenchus dipsaci, in garlic, Allium sativum, field soil. Can. J. Plant Pathol. 2021, 43, 820–826. [Google Scholar] [CrossRef]
- Lin, Y.; Uchikawa, H.; Yoshiga, T. Propagation of Ditylenchus destructor on garlic storage leaf. Nematol. Res. 2020, 50, 21–26. [Google Scholar] [CrossRef]
- Song, W.; Dai, M.; Shi, Q.; Liang, C.; Duan, F.; Zhao, H. Diagnosis and characterization of Ditylenchus destructor isolated from Mazus japonicus in China. Life 2023, 13, 1758. [Google Scholar] [CrossRef]
- Bae, R.; Choi, S.; Hong, Y. The qualities of northern and southern ecotype garlic bulbs at different storage temperature. Korean J. Food Preserv. 2008, 15, 635–641. [Google Scholar]
- Garlic Cultivation Technique; Rural Development Administration: Jeonju, Republic of Korea, 2017; pp. 47–81. Available online: https://lib.rda.go.kr/search/mediaView.do?mets_no=000000316081 (accessed on 16 December 2022).
- Cho, H.J.; Han, S.C. Survey of plant parasitic nematodes on economic crops. Korean J. Appl. Entomol. 1986, 25, 175–182. [Google Scholar]
- La, S.Y.; Cho, M.R.; Kim, D.S.; Park, G.U.; Woo, J.G.; Kim, G.T. Survey on the pests of stored garlic. Korean J. Appl. Entomol. 1998, 37, 65–71. [Google Scholar]
- Fujimura, T.; Washio, S.; Nishizawa, T. Garlic as a new host of the potato-rot nematode, Ditylenchus destructor THORNE. Jpn. J. Nematol. 1986, 16, 38–47. [Google Scholar]
- Yu, Q.; Zaida, M.A.; Hughes, B.; Celetti, M. Discovery of potato rot nematode, Ditylenchus destructor, infesting garlic in Ontario, Canada. Plant Dis. 2012, 96, 297. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Gorney, A.; Hoogland, T.; Jones, L.; Hay, F.; Smart, C.; Abawi, G. Identification and characterization of Ditylenchus spp. populations from garlic in New York State, USA. Trop. Plant Pathol. 2016, 41, 193–197. [Google Scholar] [CrossRef]
- Seinhorst, J.W. On the killing, fixation and transferring to glycerin of nematodes. Nematologica 1962, 8, 29–32. [Google Scholar] [CrossRef]
- Cobb, N.A. Notes on nemas. Contrib. Sci. Nematol. 1917, 5, 117–128. [Google Scholar]
- Subbotin, S.A.; Sturhan, D.; Chizhov, V.N.; Vovlas, N.; Baldwin, J.G. Phylogenetic analysis of Tylenchida Thorne, 1949 as inferred from D2 and D3 expansion fragments of the 28S rRNA gene sequences. Nematology 2006, 8, 455–474. [Google Scholar] [CrossRef]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0a167; Sinauer: Sunderland, MA, USA, 2002. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2, efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Larget, B.; Simon, D.L. Markov Chasin Monte Carlo Algorithms for the Bayesian Analysis of Phylogenetic Trees. Mol. Biol. Evol. 1999, 16, 750. [Google Scholar] [CrossRef]
- Page, R.D. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar]
- Thorne, G. Ditylenchus destructor, n. sp., the potato rot nematode, and Ditylenchus dipsaci (Kühn, 1857) Filipjev, 1936, the teasel nematode (Nematoda: Tylenchidae). Proc. Helminthol. Soc. Wash. 1945, 12, 27–33. [Google Scholar]
- Choi, Y.E. A study of ginseng parasitic nematodes. In Monopoly Research Institute Report; Seoul, Republic of Korea, 1976; p. 39. [Google Scholar]
- Ohh, S.H.; Lee, S.K.; Lee, J.H.; Han, S.C. New root rot disease of Panax ginseng due to Ditylenchus destructor Thorne. Korean J. Plant Prot. 1983, 22, 181–185. [Google Scholar]
- Ni, C.; Zhang, S.; Li, H.; Liu, Y.; Li, W.; Xu, X.; Xu, Z. First report of potato rot nematode, Ditylenchus destructor Thorne, 1945 infecting Codonopsis pilosula in Gansu province, China. J. Nematol. 2020, 52, e2020-87. [Google Scholar] [CrossRef]
- Han, S.C.; Cho, H.J. Influence of bulb nematode, Ditylenchus dipsaci, on growth and yield of garlic. Korean J. Plant Prot. 1980, 19, 153–155. [Google Scholar]
- Sturhan, D.; Brzeski, M.W. Stem and bulb nematodes, Ditylenchus spp. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; Marcel Decker Inc.: New York, NY, USA, 1991; pp. 423–464. [Google Scholar]
- Cheng, Z.; Toyota, K.; Aoyama, R. Relationship among the potato rot nematode, Ditylenchus destructor, densities in soil, root and garlic (Allium sativum) bulbs, and rot damage in stored garlic bulbs. Nematology 2019, 21, 547–555. [Google Scholar] [CrossRef]
- Kang, H.; Ko, H.; Lim, Y.; Park, E.; Kim, E.; Park, S.; Park, B.; Han, H. Haplotype diversity of Heterodera koreana (Tylenchida: Heteroderidae), affecting bamboo in Korea. Eur. J. Plant Pathol. 2024, 169, 259–271. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ohh, S.H. In vitro culture and factors affecting population changes of Ditylenchus destructor of ginseng. Plant Pathol. J. 1995, 11, 39–46. [Google Scholar]
- Jones, B.L.; De Waele, D. First report of Ditylenchus destructor in pods and seeds of peanut. Plant Dis. 1988, 72, 453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).