In-Depth Understanding of Cytoplasmic Male Sterility by Metabolomics in Spring Stem Mustard (Brassica juncea var. tumida Tsen et Lee)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Electron Microscopy
2.3. Metabolite Extraction
2.4. UPLC–MS/MS Conditions
2.5. ESI-Q-TRAP-MS/MS
2.6. Qualitative and Quantitative Metabolite Analyses
2.7. Analysis of DEGs and Proteins
2.8. Quantitative Real-Time PCR Analysis
2.9. Functional Annotation
2.10. Data Processing and Statistical Analysis
3. Results
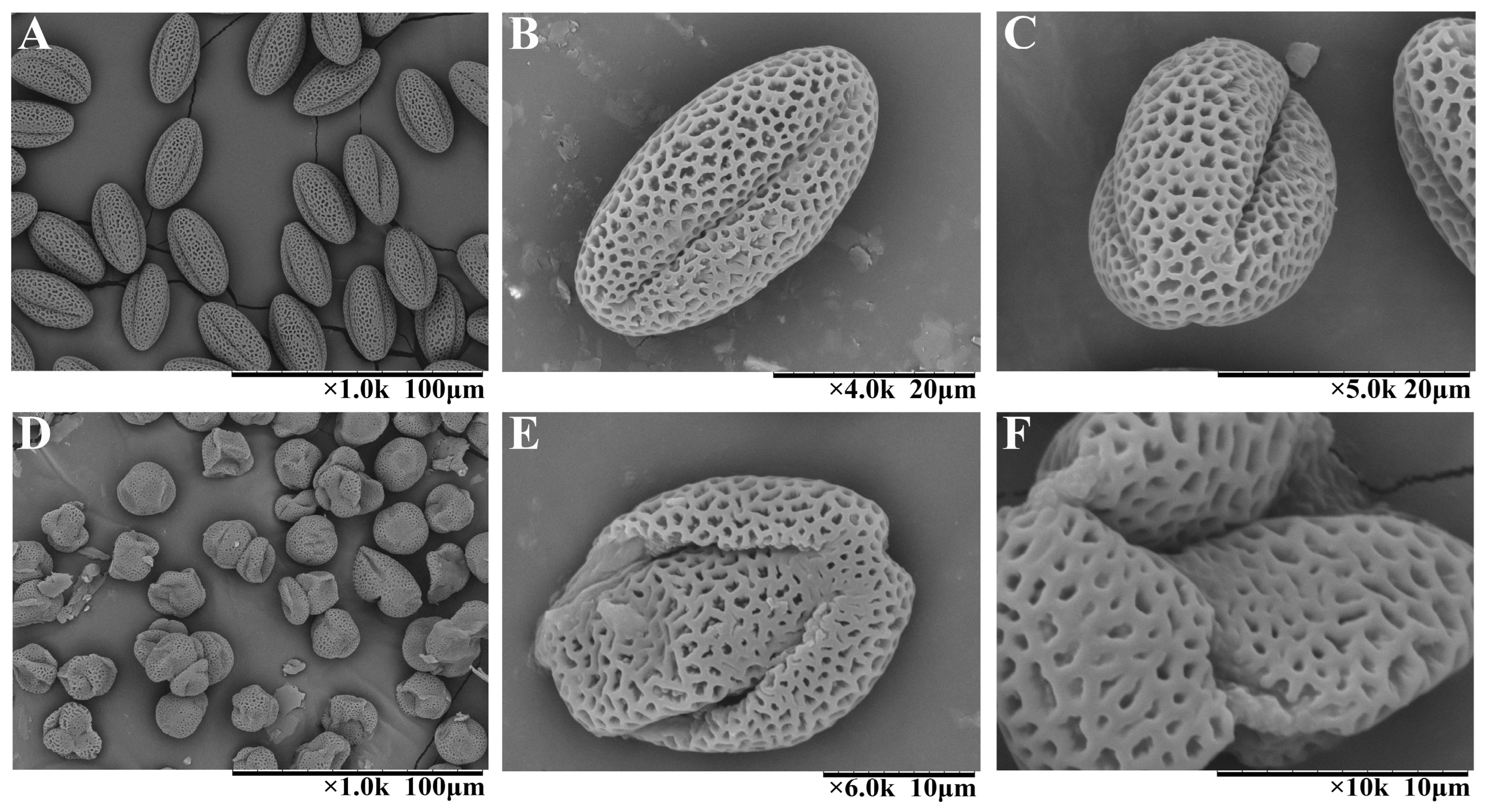
3.1. Phenotypic Characterization of Pollen in 09-05A/B Lines of B. juncea
3.2. Metabolic Characteristics of Metabolites Identified in 09-05A/B Lines of B. juncea
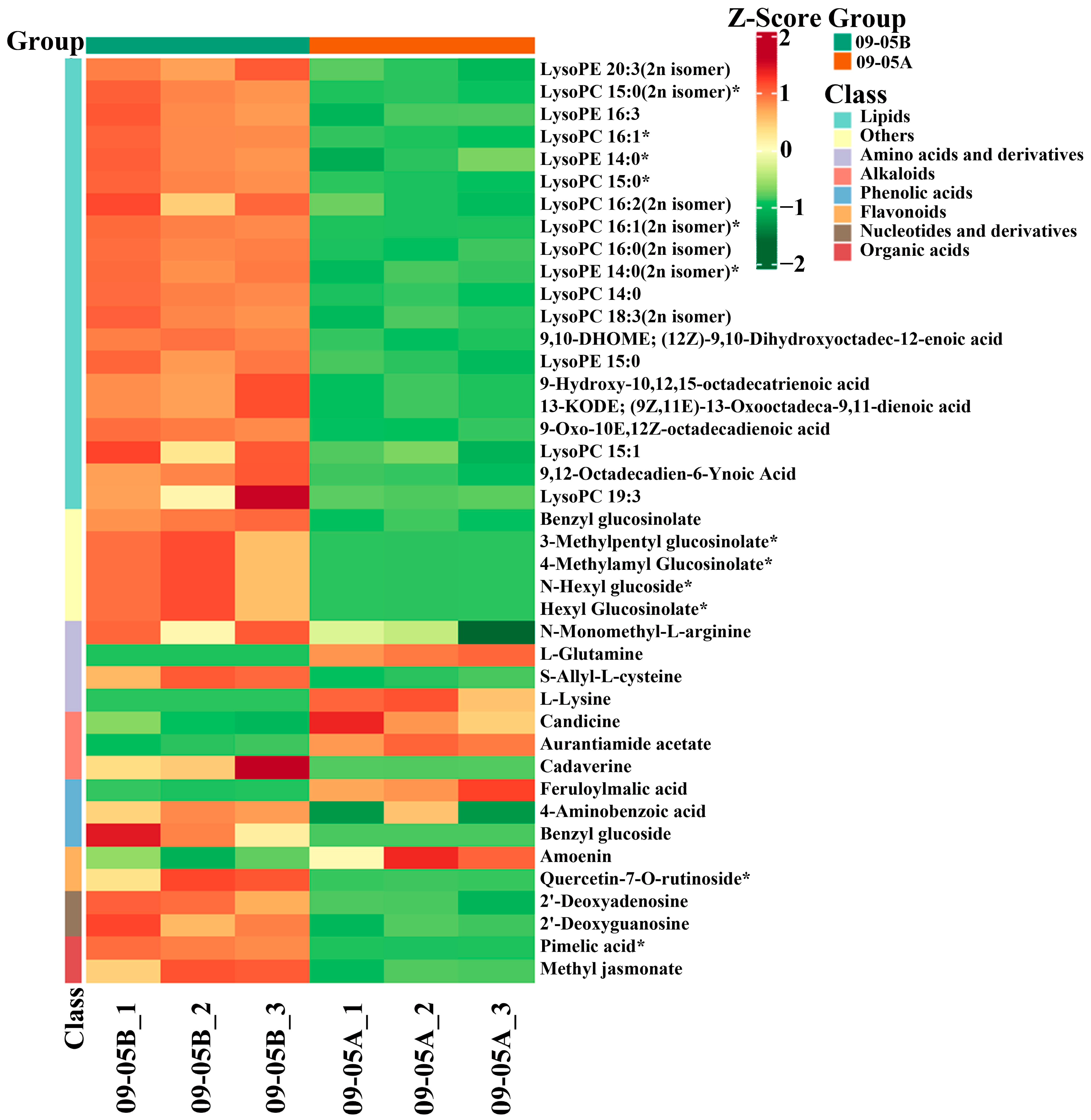
3.3. Comparative Analysis of DAMs in 09-05A/B Lines of B. juncea
3.4. KEGG Pathway Mapping of DAMs
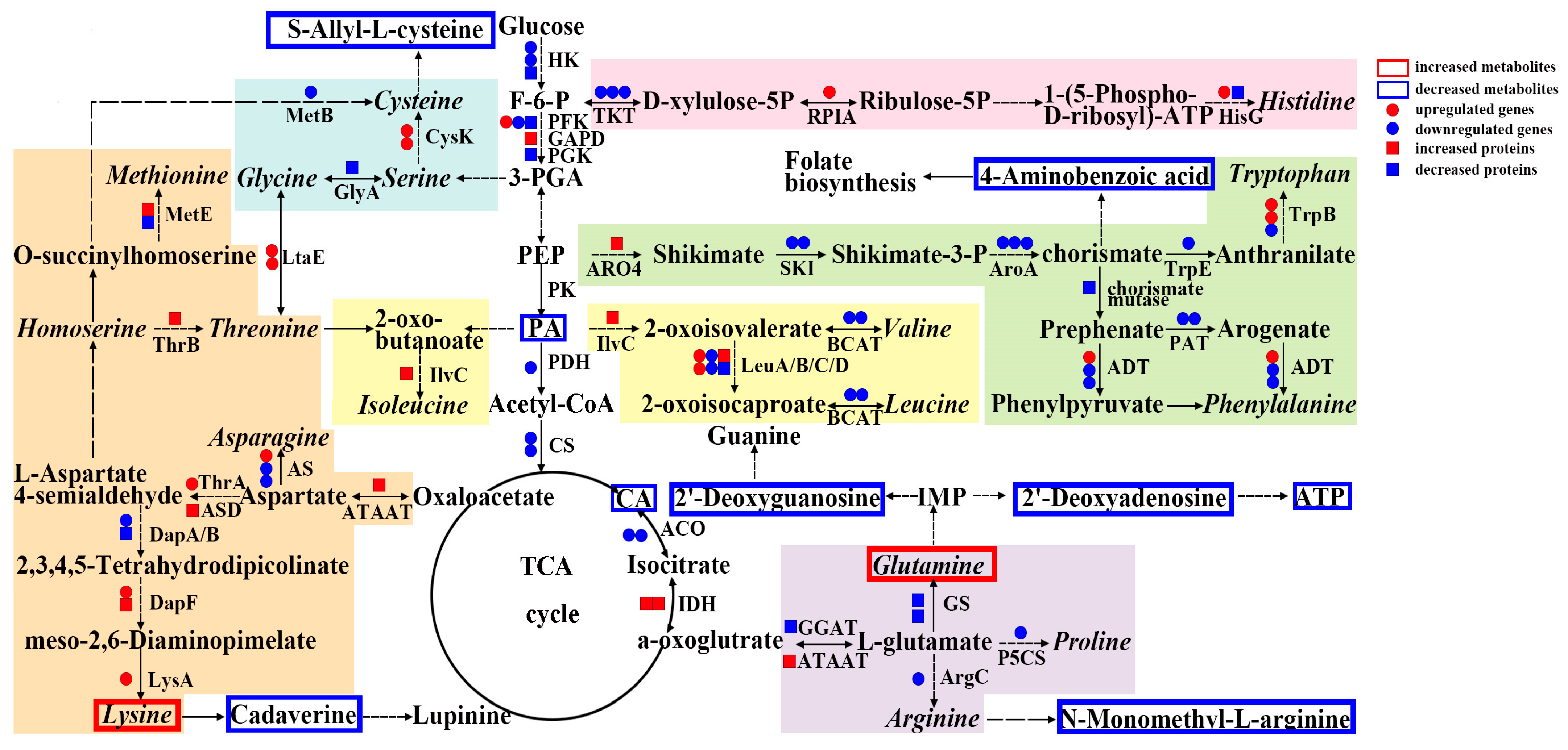
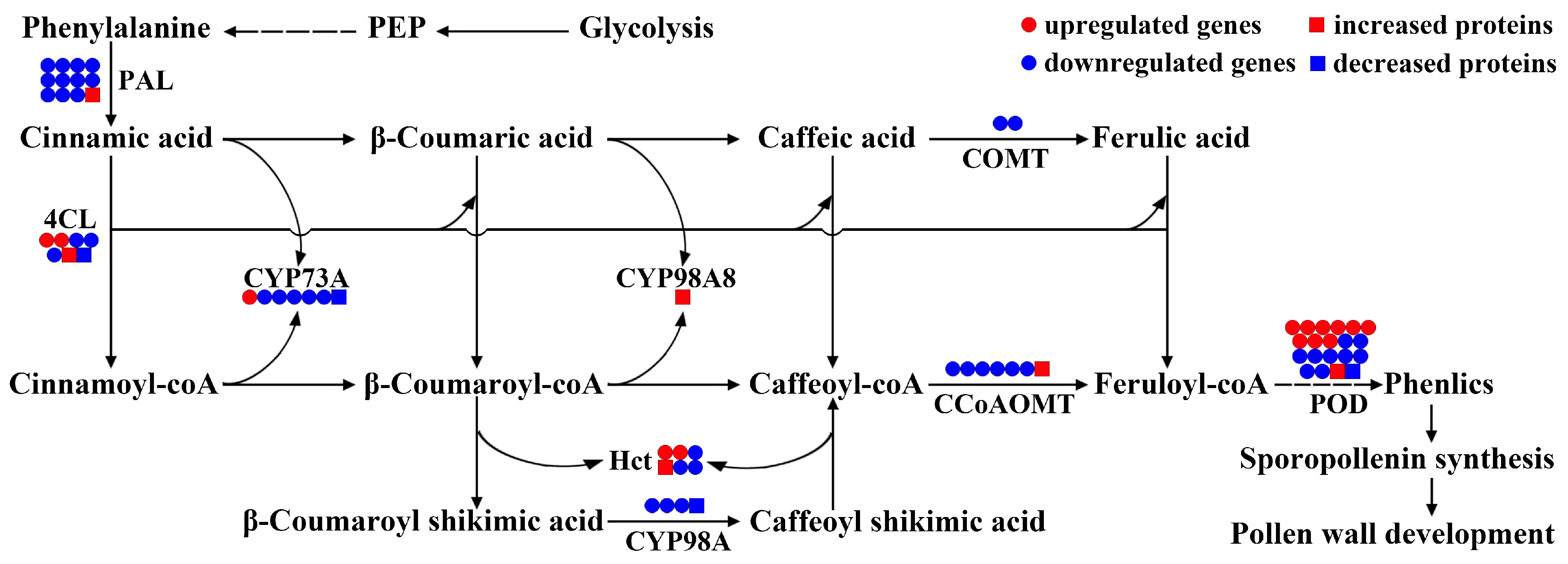
3.5. Key Biological Pathway Integration of DAMs, DEGs, and DAPs in 09-05A/B Lines of B. juncea
3.6. Identification of TFs in 09-05A/B Lines of B. juncea
3.7. QRT-PCR Validation of Genes Identified in 09-05A/B Lines of B. juncea
4. Discussion
4.1. Disrupted Amino Acid Homeostasis during Tapetum Development Is Responsible for MS in the 09-05A CMS Line
4.2. Suppression of Phenylpropanoid Metabolism Affects Sporopollenin Synthesis in the 09-05A CMS Line
4.3. Abnormal Expression of Key TFs Affects Tapetum Development and Pollen Wall Formation in the 09-05A CMS Line
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, K.B.; Sultana, R.; Mallikarjuna, N.; Saxena, R.K.; Kumar, R.V.; Sawargaonkar, S.L.; Varshney, R.K. Male-sterility systems in pigeonpea and their role in enhancing yield. Plant Breed. 2010, 129, 125–134. [Google Scholar] [CrossRef]
- Yi, G.; Shin, H.; Park, H.R.; Park, J.E.; Ahn, J.H.; Lim, S.; Lee, J.G.; Lee, E.J.; Huh, J.H. Revealing biomass heterosis in the allodiploid xBrassicoraphanus, a hybrid between Brassica rapa and Raphanus sativus, through integrated transcriptome and metabolites analysis. BMC Plant Biol. 2020, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.H.; Singh, G.; Singh, L.; Aftab, N.; Thakur, A.K. Delineation of inbred lines of Indian mustard into diverse gene pools based on agro-morphological traits. Czech J. Genet. Plant Breed. 2023, 59, 109–116. [Google Scholar] [CrossRef]
- Meena, O.P.; Dhaliwal, M.; Jindal, S. Heterosis breeding in chilli pepper by using cytoplasmic male sterile lines for high-yield production with special reference to seed and bioactive compound content under temperature stress regimes. Sci. Hortic. 2020, 262, 109036. [Google Scholar] [CrossRef]
- Aakanksha; Yadava, S.K.; Yadav, B.G.; Gupta, V.; Mukhopadhyay, A.; Pental, D.; Pradhan, A.K. Genetic analysis of heterosis for yield influencing traits in Brassica juncea using a doubled haploid population and its backcross progenies. Front. Plant Sci. 2021, 12, 721631. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.P.; Wang, R.F.; Ma, S.; Meng, Y.; Lu, Q.Q.; Zhang, L.G. Heterosis of plant gross weight and heterotic group classification of inbred lines in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Sci. Hortic. 2021, 280, 109938. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, S.Y.; Zhou, X.H.; Liu, J.; Zhuang, Y. The key genes and pathways related to male sterility of eggplant revealed by comparative transcriptome analysis. BMC Plant Biol. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Salava, J.; Lydiate, D. Mendelian inheritance of introrse orientated anthers in Brassica rapa. Czech J. Genet. Plant Breed. 2022, 58, 162–165. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawanabe, T.; Shimizu, M.; Okazaki, K.; Kaji, M.; Dennis, E.S.; Osabe, K.; Fujimoto, R. Genetic characterization of inbred lines of Chinese cabbage by DNA markers; towards the application of DNA markers to breeding of F1 hybrid cultivars. Data Brief. 2015, 6, 229–237. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wei, G.; Xia, Y.Y.; Liu, X.W.; Tang, J.; Lu, Y.L.; Lan, H.; Zhang, S.Z.; Li, C.; Cao, M.J. Comparative transcriptome analysis reveals that tricarboxylic acid cycle-related genes are associated with maize CMS-C fertility restoration. BMC Plant Biol. 2018, 18, 190. [Google Scholar] [CrossRef]
- Ze, L.; Li, G.L.; Li, F.; Zhang, S.F.; Wang, X.W.; Wu, J.; Sun, R.F.; Zhang, S.J.; Zhang, H. Development of ogura CMS fertility-restored interspecific hybrids for use in cytoplasm replacement of golden-heart materials in Brassica rapa. Genes 2023, 14, 1613. [Google Scholar] [CrossRef]
- Manjunath, K.S.; Singh, S.; Kalia, P.; Mangal, M.; Sharma, B.B.; Singh, N.; Ray, M.; Rao, M.; Tomar, B.S. Commercial suitability and characterization of newly developed Erucastrum canariense (Can) sterile cytoplasm based cytoplasmic male sterile (CMS) lines in Indian cauliflower. Sci. Rep. 2024, 14, 2346. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Rodriguez-Uribe, L.; Xu, J.N.; Zhang, J.F. Transcriptome analysis of cytoplasmic male sterility and restoration in CMS-D8 cotton. Plant Cell Rep. 2013, 32, 1531–1542. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, M.D.; Liu, S.J.; Teng, Q.; Li, S.H.; Jiang, Y.S. Functions of PPR proteins in plant growth and development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef]
- Du, K.; Xiao, Y.Y.; Liu, Q.; Wu, X.Y.; Jiang, J.J.; Wu, J.; Fang, Y.J.; Xiang, Y.; Wang, Y.P. Abnormal tapetum development and energy metabolism associated with sterility in SaNa-1A CMS of Brassica napus L. Plant Cell Rep. 2019, 38, 545–558. [Google Scholar] [CrossRef]
- Feng, J.X.; Wu, Z.; Wang, X.Q.; Zhang, Y.M.; Teng, N.J. Analysis of pollen allergens in Lily by transcriptome and proteome data. Int. J. Mol. Sci. 2019, 20, 5892. [Google Scholar] [CrossRef]
- Zhu, R.M.; Li, M.; Li, S.W.; Liang, X.; Li, S.; Zhang, Y. Arabidopsis ADP-RIBOSYLATION FACTOR-A1s mediate tapetum-controlled pollen development. Plant J. 2021, 108, 268–280. [Google Scholar] [CrossRef]
- Hao, M.M.; Yang, W.L.; Lu, W.W.; Sun, L.H.; Shoaib, M.; Sun, J.Z.; Liu, D.C.; Li, X.; Zhang, A.M. Characterization of the mitochondrial genome of a wheat AL-Type male sterility line and the candidate CMS gene. Int. J. Mol. Sci. 2021, 22, 6388. [Google Scholar] [CrossRef]
- Ge, X.Y.; Chen, J.L.; Li, O.Q.; Zou, M.; Tao, B.L.; Zhao, L.; Wen, J.; Yi, B.; Tu, J.X.; Shen, J.X. ORF138 causes abnormal lipid metabolism in the tapetum leading to Ogu cytoplasmic male sterility in Brassica napus. J. Integr. Agr. 2024, 3, 009. [Google Scholar] [CrossRef]
- Xu, B.; Wu, R.; Shi, F.L.; Gao, C.P.; Wang, J. Transcriptome profiling of flower buds of male-sterile lines provides new insights into male sterility mechanism in alfalfa. BMC Plant Biol. 2022, 22, 199. [Google Scholar] [CrossRef]
- Fang, X.P.; Fu, H.F.; Gong, Z.H.; Chai, W.G. Involvement of a universal amino acid synthesis impediment in cytoplasmic male sterility in pepper. Sci. Rep. 2024, 6, 23357. [Google Scholar] [CrossRef]
- Ji, J.L.; Yang, L.M.; Fang, Z.Y.; Zhuang, M.; Zhang, Y.Y.; Lv, H.H.; Liu, Y.M.; Li, Z.S. Complementary transcriptome and proteome profiling in cabbage buds of a recessive male sterile mutant provides new insights into male reproductive development. J. Proteom. 2018, 179, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.B.; Shi, J.X.; Yang, X.J. Role of lipid metabolism in plant pollen exine development. Subcell Biochem. 2016, 86, 315–337. [Google Scholar]
- Li, H.; Pinot, F.; Sauveplane, V.; Werck-Reichhart, D.; Diehl, P.; Schreiber, L.; Franke, R.; Zhang, P.; Chen, L.; Gao, Y.W.; et al. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell. 2010, 22, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.X.; Cui, M.H.; Yang, L.; Kim, Y.J.; Zhang, D.B. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Wu, D.; Shi, J.X.; He, Y.; Pinot, F.; Grausem, B.; Yin, C.S.; Zhu, L.; Chen, M.J.; Luo, Z.J.; et al. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J. Integr. Plant Biol. 2014, 56, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.S.; Liang, W.Q.; Yin, C.S.; Zong, J.; Gu, F.W.; Zhang, D.B. OsC6, encoding a lipid transfer protein is required for postmeiotic anther development in rice. Plant Physiol. 2010, 154, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zou, T.; Yuan, G.Q.; He, Z.Y.; Li, W.J.; Tao, Y.; Liu, M.M.; Zhou, D.; Zhao, H.F.; Zhu, J.; et al. Less and shrunken pollen 1 (LSP1) encodes a member of the ABC transporter family required for pollen wall development in rice (Oryza sativa L.). Crop J. 2020, 8, 492–504. [Google Scholar] [CrossRef]
- Zhang, R.M.; Chang, J.J.; Li, J.Y.; Lan, G.P.; Xua, C.Q.; Li, H.; Ma, J.X.; Zhang, Y.; Yang, J.Q.; Tian, S.J.; et al. Disruption of the bHLH transcription factor Abnormal Tapetum 1 causes male sterility in watermelon. Hortic. Res. 2021, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Y.; Ding, X.L.; Zhang, R.J.; Yang, Y.H.; Wei, B.G.; Yang, S.P.; Gai, J.Y. Transcriptome analysis reveals the genes related to pollen abortion in a cytoplasmic male-sterile Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2022, 23, 12227. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, L.; Feng, P.; Liu, Y.; Yin, W.Z.; Shang, L.N.; Wang, N. OsMYB103 is essential for tapetum degradation in rice. Theor. Appl. Genet. 2022, 135, 929–945. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, X.; Huang, Z.; Xia, J.; Li, X.; Wu, J.; Yu, H.; Shahid, M.Q.; Liu, X. Transcriptome and gene editing analyses reveal MOF1 a defect alters the expression of genes associated with tapetum development and chromosome behavior at meiosis stage resulting in low pollen fertility of tetraploid rice. Int. J. Mol. Sci. 2020, 21, 7489. [Google Scholar] [CrossRef]
- Cai, C.F.; Zhu, J.; Lou, Y.; Guo, Z.L.; Xiong, S.X.; Wang, K.; Yang, Z.N. The functional analysis of OsTDF1 reveals a conserved genetic pathway for tapetal development between rice and Arabidopsis. Sci. Bull. 2015, 60, 1073–1082. [Google Scholar] [CrossRef]
- Distelfeld, A.; Pearce, S.P.; Avni, R.; Scherer, B.; Uauy, C.; Piston, F.; Slade, A.; Zhao, R.R.; Dubcovsky, J. Divergent functions of orthologous NAC transcription factors in wheat and rice. Plant Mol. Biol. 2012, 78, 515–524. [Google Scholar] [CrossRef]
- Zou, T.; He, Z.Y.; Qu, L.Y.; Liu, M.X.; Zeng, J.; Liang, Y.L.; Wang, T.; Chen, D.; Xiao, Q.; Zhu, J.; et al. Knockout of OsACOS12 caused male sterility in rice. Mol. Breed. 2017, 37, 126. [Google Scholar] [CrossRef]
- Gibalová, A.; Reňák, D.; Matczuk, K.; Dupl’áková, N.; Cháb, D.; Twell, D.; Honys, D. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Mol. Biol. 2009, 70, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Gibalová, A.; Steinbachová, L.; Hafidh, S.; Bláhová, V.; Gadiou, Z.; Michailidis, C.; Műller, K.; Pleskot, R.; Dupľáková, N.; Honys, D. Characterization of pollen-expressed bZIP protein interactions and the role of ATbZIP18 in the male gametophyte. Plant Reprod. 2017, 30, 1–17. [Google Scholar] [CrossRef]
- Wan, Z.J.; Shi, D.Y.; Zou, R.C.; Huang, Y.; Bie, Z.L.; Shi, Z.; Xu, Y.J.; Fu, T.D. Development and utilization of one new cytoplasmic male sterile line of chinese leaf mustard (Brassica juncea var. rugosa bailey). Sci. Hortic. 2014, 165, 211–217. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, F.M.; Zhao, L.Y.; Du, H.P.; Li, T.; Lai, D.N.; Zhou, T.C.; Qing, Z.X. Characterization of extractable components of fresh and fermented huarong large-leaf mustard and their inhibitory effects on human colon cancer cells. Food Biosci. 2021, 43, 101280. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Xia, R.; Tong, M.Y.; Huang, Y.P.; Xu, L.A.; Zhu, Z.J.; Meng, Q.F.; Yu, Y.J. Integrative analysis of transcriptomic and proteomic changes related to cytoplasmic male sterility in spring stem mustard (Brassica juncea var. tumida Tsen et Lee). Int. J. Mol. Sci. 2022, 23, 6248. [Google Scholar] [CrossRef]
- Tang, M.; Li, Z.; Luo, D.; Wei, F.; Kashif, M.H.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z.; Tan, W.; et al. A comprehensive integrated transcriptome and metabolome analyses to reveal key genes and essential metabolic pathways involved in CMS in kenaf. Plant Cell Rep. 2021, 40, 223–236. [Google Scholar] [CrossRef]
- Wang, B.; Farooq, Z.; Chu, L.; Liu, J.; Wang, H.; Guo, J.; Tu, J.; Ma, C.; Dai, C.; Wen, J.; et al. High-generation near-isogenic lines combined with multi-omics to study the mechanism of polima cytoplasmic male sterility. BMC Plant Biol. 2021, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, M.; Hu, J.; Lan, M.; He, J. Lateral metabolome study reveals the molecular mechanism of cytoplasmic male sterility (CMS) in Chinese cabbage. BMC Plant Biol. 2023, 23, 128. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Liu, S.; Xia, C.; Tang, H.; Xie, F.; Fu, T.; Wan, Z. Morphological and genetic characterization of a new cytoplasmicsterile line system (oxa CMS) in stem mustard (Brassica juncea). Theor. Appl. Genet. 2018, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, H.; Zhou, D.; Li, M.; Liu, Y.H.; Zhang, F.; Feng, Y.Y.; Yu, D.L.; Lin, S.; Cao, J.S. Systematic identification of long non-coding RNAs during pollen development and fertilization in Brassica rapa. Plant J. 2018, 96, 203–222. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2022, 375, 131908. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Lv, Q.Y.; Liu, A.; Wang, J.R.; Sun, X.Q.; Deng, J.; Chen, Q.F.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kong, Z.; Huan, X.; Liu, Y.; Liu, Y.; Wang, Q.; Liu, J.; Zhang, P.; Guo, Y.; Qin, P. Transcriptomics integrated with widely targeted metabolomics reveals the mechanism underlying grain color formation in wheat at the grain-filling stage. Front. Plant Sci. 2021, 12, 757750. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J.G. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, S.; Wang, C.; Wang, F.; Wang, F.; Zhao, K. BjMYB1, a transcription factor implicated in plant defence through activating BjCHI1 chitinase expression by binding to a W-box-like element. J. Exp. Bot. 2016, 67, 4647–4658. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Puentes-Romero, A.C.; González, S.A.; González-Villanueva, E.; Figueroa, C.R.; Ruiz-Lara, S. AtZAT4, a C2H2-type zinc finger transcription factor from Arabidopsis thaliana, is involved in pollen and seed development. Plants 2022, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Liu, L.; Sun, L.P.; Yu, P.; Zhang, P.P.; Abbas, A.; Xiang, X.J.; Wu, W.X.; Zhang, Y.X.; Cao, L.Y.; et al. OsMS1 functions as a transcriptional activator to regulate programmed tapetum development and pollen exine formation in rice. Plant Mol. Biol. 2019, 99, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Wu, S.W.; Li, Z.W.; An, X.L.; Tian, Y.H. Lipid metabolism: Critical roles in male fertility and other aspects of reproductive development in plants. Mol. Plant. 2020, 13, 955–983. [Google Scholar] [CrossRef]
- Hu, J.F.; Lan, M.; Xu, X.Z.; Yang, H.L.; Zhang, L.Q.; Lv, F.X.; Yang, H.J.; Yang, D.; Li, C.J.; He, J.M. Transcriptome profiling reveals molecular changes during flower development between male sterile and fertile Chinese Cabbage (Brassica rapa ssp. pekinensis) Lines. Life 2021, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Ma, L.G. Comprehensive insight into tapetum-mediated pollen development in Arabidopsis thaliana. Cells. 2023, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.C.; Hu, F.; Guan, W.D.; Yuan, F.C.; Lai, Z.P.; Zhong, J.; Liu, J.; Wu, Z.M.; Cheng, J.W.; Hu, K.L. A 163-bp insertion in the Capana10g000198 encoding a MYB transcription factor causes male sterility in pepper (Capsicum annuum L.). Plant J. 2023, 113, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.M.; Sun, C.; Li, H.L.; Hu, S.L.; Lei, L.; Kang, J.G. Integrated analysis of transcriptome and proteome changes related to the ogura cytoplasmic male sterility in cabbage. PLoS ONE 2018, 13, e0193462. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004, 40, 979–995. [Google Scholar] [CrossRef]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef]
- Qian, Z.H.; Shi, D.X.; Zhang, H.X.; Li, Z.Z.; Huang, L.; Yan, X.F.; Lin, S. Transcription factors and their regulatory roles in the male gametophyte development of flowering plants. Int. J. Mol. Sci. 2024, 25, 566. [Google Scholar] [CrossRef]
- Hao, M.M.; Yang, W.L.; Li, T.D.; Shoaib, M.; Sun, J.Z.; Liu, D.C.; Li, X.; Nie, Y.B.; Tian, X.M.; Zhang, A.M. Combined transcriptome and proteome analysis of anthers of AL-type cytoplasmic male sterile line and its maintainer line reveals new insights into mechanism of male sterility in common wheat. Front. Genet. 2021, 12, 762332. [Google Scholar] [CrossRef]
- Wang, D.G.; Wang, Y.N.; Zhang, L.; Yang, Y.; Wu, Q.; Hu, G.Y.; Wang, W.H.; Li, J.K.; Huang, Z.P. Integrated transcriptomic and proteomic analysis of a cytoplasmic male sterility line and associated maintainer line in soybean. Front. Plant Sci. 2023, 75, 4891–4905. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Shimada, A.; Nagai, T. Effects of glutamine synthetase inhibitors on rice sterility. Biosci. Biotechnol. Biochem. 1994, 58, 669–673. [Google Scholar] [CrossRef][Green Version]
- Ribarits, A.; Mamun, A.N.K.; Li, S.P.; Resch, T.T.; Fiers, M.T.; Heberle-Bors, E.; Liu, C.M.; Touraev, A. Combination of reversible male sterility and doubled haploid production by targeted inactivation of cytoplasmic glutamine synthetase in developing anthers and pollen. Plant Biotech. J. 2007, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Fucile, G.; Garcia, C.; Carlsson, J.; Sunnerhagen, M.; Christendat, D. Structural and biochemical investigation of two Arabidopsis shikimate kinases: The heat-inducible isoform is thermostable. Protein Sci. 2011, 20, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.Y.; Hou, B.Z.; Qi, X.Q. Biosynthesis and transport of pollen coat precursors in angiosperms. Nat. Plants 2023, 9, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.S.; Qiu, S.; Jia, X.L.; Shen, S.Y.; Shen, C.W.; Wang, S.; Xu, P.; Tong, Q.; Lou, Y.X.; Yang, N.Y.; et al. Stepwise changes in flavonoids in spores/pollen contributed to terrestrial adaptation of plants. Plant Physiol. 2023, 193, 627–642. [Google Scholar] [CrossRef]
- Wu, B.L.; Xia, Y.; Zhang, G.S.; Wang, J.W.; Ma, S.C.; Song, Y.L.; Yang, Z.Q.; Dennis, E.S.; Niu, N. The transcription factors TaTDRL and TaMYB103 synergistically activate the expression of TAA1a in wheat, which positively regulates the development of microspore in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 7996. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, F.Y.; Zhou, L.; Zhou, Y.; Liu, Z.Y.; Ji, R.Q.; Feng, H. iTRAQ-based proteomic analysis of fertile and sterile flower buds from a genetic male sterile line ‘AB01’ in Chinese cabbage (Brassica campestris L. ssp. pekinensis). J. Proteom. 2019, 204, 103395. [Google Scholar] [CrossRef]
- Yang, X.T.; Ye, J.L.; Zhang, L.L.; Song, X.Y. Blocked synthesis of sporopollenin and jasmonic acid leads to pollen wall defects and anther indehiscence in genic male sterile wheat line 4110S at high temperatures. Funct. Integr. Genom. 2020, 20, 383–396. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.L.; Preuss, D. CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Fan, J.; Du, W.; Chen, Q.L.; Zhang, J.G.; Yang, X.P.; Hussain, S.B.; Hu, H.J. Comparative transcriptomic analyses provide insights into the enzymatic browning mechanism of fresh-cut sand pear fruit. Horticulturae 2021, 7, 502. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, M.; Guo, L.P.; Qi, T.X.; Tang, H.N.; Li, Y.Q.; Zuo, Z.D.; Shahzad, K.; Feng, J.J.; Zang, R.; et al. Integrated analysis of metabolome and transcriptome reveals the cytoplasmic effects of CMS-D2 on pollen fertility resulting from disrupted lipid metabolism. Front. Plant Sci. 2022, 13, 998203. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.L.; Duan, Y.; Hu, G.; Geng, X.X.; Zhang, G.M.; Yan, P.J.; Liu, Z.H.; Zhang, L.L.; Song, X.Y. Identification of candidate genes and biosynthesis pathways related to fertility conversion by wheat KTM3315A transcriptome profiling. Front. Plant Sci. 2017, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, D.H.; Ke, F.Z.; Zhu, M.M.; Xu, J.G.; Zhang, M. Seedless mutant ‘Wuzi Ougan’ (Citrus suavissima Hort. Ex Tanaka ‘seedless’) and the wild type were compared by iTRAQ-based quantitative proteomics and integratedly analyzed with transcriptome to improve understanding of male sterility. BMC Genet. 2018, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.X.; Xie, H.; Wang, X.M.; Yan, X.J.; Wang, B.; Feng, H.P.; Zhao, Y.X.; Gao, J.X.; Gao, J. Proteomic analysis of differential anther development from sterile/fertile lines in Capsicum annuum L. Peer J. 2022, 10, e13168. [Google Scholar] [CrossRef]
- Zou, J.Q.; Dong, S.Y.; Fang, B.; Zhao, Y.; Song, G.X.; Xin, Y.; Huang, S.N.; Feng, H. BrACOS5 mutations induced male sterility via impeding pollen exine formation in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet. 2023, 136, 6. [Google Scholar] [CrossRef]
- Zhao, J.; Long, T.; Wang, Y.F.; Tong, X.H.; Tang, J.; Li, J.L.; Wang, H.M.; Tang, L.Q.; Li, Z.Y.; Shu, Y.Z.; et al. Rms2 encoding a gdsl lipase mediates lipid homeostasis in anthers to determine rice male fertility. Plant Physiol. 2020, 182, 2047–2064. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Z.; Li, H.X.; Sun, Y.J.; Zeng, H.L.; He, Y. Lipid metabolism is involved in male fertility regulation of the photoperiod- and thermo sensitive genic male sterile rice line peiai 64s. Plant Sci. 2020, 299, 110581. [Google Scholar] [CrossRef]
- Lyu, T.Q.; Cao, J.S. Cys₂/His₂ zinc-finger proteins in transcriptional regulation of flower development. Int. J. Mol. Sci. 2018, 19, 2589. [Google Scholar] [CrossRef]
- Wiese, A.J.; Torutaeva, E.; Honys, D. The transcription factors and pathways underpinning male reproductive development in Arabidopsis. Front. Plant Sci. 2024, 15, 1354418. [Google Scholar] [CrossRef]
- Shen, X.P.; Xu, L.A.; Liu, Y.H.; Dong, H.; Zhou, D.; Zhang, Y.Z.; Lin, S.; Cao, J.S.; Huang, L. Comparative transcriptome analysis and ChIP-sequencing reveals stage-specific gene expression and regulation profiles associated with pollen wall formation in Brassica rapa. BMC Genom. 2019, 20, 264. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.J.; Timofejeva, L.M.; Chen, C.B.; Grossniklaus, U.; Ma, H. Regulation of Arabidopsis tapetum development and function by Dysfunctional Tapetum1 (DYT1) encoding a putative bHLH transcription factor. Development 2006, 133, 3085–3095. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Li, H.; Gao, J.F.; Jiang, H.; Wang, C.; Guan, Y.F.; Yang, Z.N. Defective in tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008, 55, 266–277. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.Q.; Chang, Y.H.; Li, X.C.; Yang, J.; Huang, X.Y.; Yu, Q.B.; Chen, H.; Wu, T.L.; Yang, Z.N. AtMYB103 is a crucial regulator of several pathways affecting Arabidopsis anther development. Sci. China Life Sci. 2010, 53, 1112–1122. [Google Scholar] [CrossRef]
- Xu, J.; Ding, Z.W.; Vizcay-Barrena, G.; Shi, J.X.; Liang, W.Q.; Yuan, Z.; Werck-Reichhart, D.; Schreiber, L.; Wilson, Z.A.; Zhang, D.B. ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell 2014, 26, 1544–1556. [Google Scholar] [CrossRef]
- Tianpei, X.Z.; Li, D.; Qiu, P.; Luo, J.; Zhu, Y.G.; Li, S.Q. Scorpion peptide LqhIT2 activates phenylpropanoid pathways via jasmonate to increase rice resistance to rice leafrollers. Plant Sci. 2015, 230, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Zhang, S.; Liu, J.Y.; Zhao, C.H.; Wu, J.; Cao, Y.P.; Fu, C.X.; Han, X.; He, H.; Zhao, Q. Myb20, myb42, myb43, and myb85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- Fernández-Gómez, J.; Talle, B.; Wilson, Z.A. Increased expression of the MALE STERILITY1 transcription factor gene results in temperature-sensitive male sterility in barley. J. Exp. Bot. 2020, 71, 6328–6339. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Xiong, S.X.; Yin, W.Z.; Teng, X.D.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.N.; Wilson, Z.A.; Yang, Z.N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Li, X.L.; Ma, Z.B.; Lv, Y.; Hu, Y.R.; Yu, D.Q. Arabidopsis WRKY2 and WRKY34 transcription factors interact with VQ20 protein to modulate pollen development and function. Plant J. 2017, 91, 962–976. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shen, Y.; Huang, Y.; Ren, X.; Gao, T.; Yu, Y.; Wang, Y.; Meng, Q. In-Depth Understanding of Cytoplasmic Male Sterility by Metabolomics in Spring Stem Mustard (Brassica juncea var. tumida Tsen et Lee). Horticulturae 2024, 10, 896. https://doi.org/10.3390/horticulturae10090896
Wang J, Shen Y, Huang Y, Ren X, Gao T, Yu Y, Wang Y, Meng Q. In-Depth Understanding of Cytoplasmic Male Sterility by Metabolomics in Spring Stem Mustard (Brassica juncea var. tumida Tsen et Lee). Horticulturae. 2024; 10(9):896. https://doi.org/10.3390/horticulturae10090896
Chicago/Turabian StyleWang, Jie, Ying Shen, Yunping Huang, Xiliang Ren, Tianyi Gao, Youjian Yu, Yuhong Wang, and Qiufeng Meng. 2024. "In-Depth Understanding of Cytoplasmic Male Sterility by Metabolomics in Spring Stem Mustard (Brassica juncea var. tumida Tsen et Lee)" Horticulturae 10, no. 9: 896. https://doi.org/10.3390/horticulturae10090896
APA StyleWang, J., Shen, Y., Huang, Y., Ren, X., Gao, T., Yu, Y., Wang, Y., & Meng, Q. (2024). In-Depth Understanding of Cytoplasmic Male Sterility by Metabolomics in Spring Stem Mustard (Brassica juncea var. tumida Tsen et Lee). Horticulturae, 10(9), 896. https://doi.org/10.3390/horticulturae10090896



