Abstract
In the winter season, citrus nursery production faces challenges including shorter days, lower light levels, and lower temperatures that delay vegetative budbreak and scion shoot growth. With the goal of improving the production cycle in the citrus nursery, we investigated the effect of supplemental LED light on the production of bud-grafted citrus trees during short winter days. Three experiments were conducted under different temperature conditions. “Washington” Navel sweet orange (Citrus sinensis) was budded on Carrizo citrange (C. sinensis × Poncirus trifoliata) and Rubidoux trifoliate (P. trifoliata) rootstocks in early December. Light treatments included no supplemental light, day-length extension to 16 h with LED light, and night interruption (1 h of LED light at night). Day-length extension and night interruption were studied with and without preconditioning plants with the respective light treatments for 6 weeks before grafting. Day-length extension increased the scion growth, but only when supplemental heating was provided, implying that low temperatures are a limiting factor for plant growth. Growth effects were stronger when the Navel scion was grafted on Carrizo compared to Rubidoux, likely because of the higher dormancy of the latter rootstock. Night interruption did not affect scion budbreak or growth under any of the tested conditions. Preconditioning enhanced scion growth in some instances. The results suggest that the use of supplemental LED light to extend the day length may increase plant growth during the short winter days, but the effects are limited under low-temperature conditions.
Keywords:
light spectra; LED light; sweet orange; Citrus sinensis; Carrizo; Rubidoux; Poncirus trifoliata 1. Introduction
During the winter, the lower-light and lower-temperature conditions inhibit plant growth and development, slowing the production of new trees in the citrus nursery. Budbreak is delayed and budded plants grow slowly at this time, especially when they are grafted on trifoliate orange (Poncirus trifoliata) rootstocks [1]. Poncirus trifoliata and its hybrids are widely used as rootstocks in many citrus-growing regions [2] due to resistance to citrus tristeza virus (CTV) [3,4,5], gummosis root rot (Phytophthora spp.) [6], citrus nematode (Tylenchulus semipenetrans) [7], and tolerance to huanglongbing (HLB) associated with Candidatus Liberibacter asiaticus [8,9,10].
In commercial greenhouse production, supplemental light to increase day length has been used to improve growth of several horticultural crops, such as tomato, lettuce, and basil [11,12,13]. However, limited information is available for perennial fruit crops. Light is an important factor regulating many processes in the plant life cycle, driving photosynthesis and primary and secondary metabolism [14]. One way of comparing the total light available to plants is by measuring the daily light integral (DLI), which is the product of the photosynthetic photon flux density (PPFD) and the photoperiod, i.e., the sum of radiation in a 24 h period [13,15]. The use of supplemental light in the nursery increases the DLI and can influence plant growth, stem elongation, leaf anatomy, plant morphology, branch induction, leaf expansion, and leaf chlorophyll content [16]. Additionally, the duration of light and dark cycles and the light spectral composition can have unique effects on plant growth and development. In some instances, these effects are tied to the plant-growth stage during light exposure [17,18]. Plants have evolved complex mechanisms to perceive and respond to variations in light quality and quantity, initiating photosensory pathways essential for photomorphogenesis. The diverse spectra of light from both natural and artificial sources significantly affect plant behavior, leading to a variety of metabolic effects. Besides the influence of photosynthetic pigments, specialized photoreceptors, including phytochromes (absorbers of red light), cryptochromes (absorbers of blue light), and phototropins (absorbers of UV-A/blue light) play essential roles in how plants perceive and respond to light signals [14].
Previous studies described a positive effect on plant growth in the citrus nursery using supplemental light [1,19,20,21,22,23]. The use of supplemental light for day-length extension was effective in improving the growth of unbudded rootstock liners and budded “Valencia” on various rootstocks using high-pressure sodium (HPS) and light-emitting diode (LED) light [19]. Night interruption using low-intensity light, combined with short-day light, improved the vegetative growth of unbudded trifoliate orange hybrids and grafted sweet orange trees on the same rootstocks, and the response was suggested to be phytochrome-mediated [1]. These previous studies made use of different sources of light that contrast substantially in terms of light quality and quantity, including different spectra, intensities, and temperatures. LED lights are now the most-used lights for greenhouse production because of their low energy consumption, durability, cool-emitting temperature, and the option to control light spectra and intensity [24].
Temperature is an essential factor regulating phenological responses in the plant life cycle [25,26]. While most research has primarily been concentrated on comprehending how plants react to extreme temperature variations, there has been comparatively little exploration into unraveling the plant responses to minor fluctuations in ambient temperature. Both light and temperature exert control over similar developmental processes during various stages of the plant-life cycle, and there exists a complex crosstalk between light and temperature pathways to optimize plant development [25,26,27]. Depending on the specific temperature conditions, different phytochromes assume distinct functions in this intricate regulatory process [26,28,29,30]. With the objective of improving the production cycle in the citrus nursery, this study evaluated the effects and potential benefits of supplemental LED light on budbreak and shoot growth of the grafted sweet orange scion on two different rootstocks during the short winter days. Supplemental light treatments were applied as day-length extension or night interruption and studied in three different greenhouses with different temperature conditions to examine the relationship of temperature and supplemental lighting effects.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Carrizo (Citrus sinensis × Poncirus trifoliata) and Rubidoux (P. trifoliata) rootstock liners were grown from seeds in a soilless potting medium (Pro Mix BX; Premier Horticulture, Inc., Quakertown, PA, USA) by planting one seed each in 3.8 cm × 21 cm cone cells (Cone-tainers; Stuewe and Sons, Tangent, OR, USA). Off-type seedlings were discarded, and true-to-type seedlings were transplanted into 2.54 L pots (Treepots; Stuewe and Sons) using the soilless potting medium (Pro Mix BX).
Eight weeks before grafting (2 weeks before starting preconditioning treatments, see Section 2.2), rootstock trunk diameters were measured at 15 cm above the soil surface. Plants too small or too large were discarded for uniformity, and the remaining experimental plants were randomized by size across treatments.
At the beginning of December, the 6-month-old rootstock liners were budded with “Washington” Navel orange (C. sinensis; Florida DPI clone 052851) using the inverted T budding method. Grafted buds were wrapped with bud tape for 3 weeks. One week after unwrapping, the rootstock was trimmed at 65 cm and looped to force scion shoot growth. Plants were arranged on the bench by lighting treatment, with the grafted bud facing south to increase vegetative budbreak and scion shoot growth [31].
Plants were irrigated by hand to run-off as needed and fertilized every other week with a water-soluble fertilizer (20N-10P-20K Peters Professional, The Scotts Company, Marysville, OH, USA) at a rate of 400 mg N/L. Insecticides and miticides commonly used in citrus nursery production were applied as needed.
Three different experiments were conducted in different greenhouses and under different temperature conditions. In the first and second experiments, greenhouses were covered with 8 mm twin-wall Lexan™ Thermoclear 15 polycarbonate panels (General Electric Co., Norcross, GA, USA), and heaters, cooling fans, wet pads, and circulating fans were used to control the temperature and maintain uniformity. In the first and second experiments, cooling fans and wet pads were used to maintain high-temperature conditions below a set point; heaters were used to maintain low-temperature conditions above a set point. In the first experiment, plants were grown under controlled, high-temperature conditions (thermostat set to heat at 26 °C and to cool at 32 °C). In the second experiment, plants were grown under controlled, medium-temperature conditions (thermostat set to heat at 21 °C and to cool at 27 °C). In the third experiment, plants were grown with supplemental cooling, but without supplemental heating, temperatures dropped unregulated during periods of low-temperature events. The greenhouse was covered with a 6 mil clear plastic sheeting (Farm Plastic Supply, Addison, IL, USA) and an aluminized polyethylene shade cloth (Aluminet®, Polysack Plastic Industries Ltd., Negev, Israel, and it was fan ventilated, with the fan thermostat set to cool at 22 °C. In the following, greenhouses will be referred to as HTG (high-temperature greenhouse), MTG (medium-temperature greenhouse), and NHG (no-supplement-heat greenhouse).
2.2. Light Treatments
Plants received sunlight during the day and supplemental light according to treatment in each greenhouse. Supplemental light was provided either as a day-length extension (light was provided before sunrise until sunrise to extend the day length to 16 h) or as night interruption (1 h of light provided in the middle of the night). Supplemental light treatments were applied either with preconditioning or without. For preconditioning, light treatments were applied to rootstock liners 6 weeks before the budding. Without preconditioning, light treatments started immediately after budding and commenced 14 weeks after budding (wab). Control plants were grown without any supplemental light. In total, there were five treatments: (1) no supplemental light (NSL); (2) day-length extension to 16 h with preconditioning (DLE + P); (3) day-length extension to 16 h without preconditioning (DLE); (4) night interruption with preconditioning (NI + P); and (5) night interruption without preconditioning (NI).
The light sources were Elixia 600 W (Heliospectra, Göteborg, Sweden) containing multi-element LEDs and were used at full spectrum (red, blue, far-red, and white light) and at full intensity from the light fixture. Light fixtures were located 110 cm above the location where the buds were inserted into the rootstock seedlings. The timing of the light fixtures was adjusted according to the natural day-length change over the 20-week period of the experiment: every time there was a 15 min change in the length of natural daylight, the time of the supplemental lighting was adjusted to maintain the 16 h day length.
Light energy was measured using a Spectrometer PG100N (UPRtek, Zhunan, Taiwan) for each treatment at 110 cm below the light (at bud height) during a moonless night and at noon inside the MTG on a sunny day (Figure 1). On a moonless night, the average PPFD (photosynthetic photon flux density) under the LED light was 364 µmol/m2/s, with a breakdown of PFD-blue: 68 µmol/m2/s, PFD-green: 65 µmol/m2/s, PFD-Red: 233 µmol/m2/s, and PFD-Far Red: 31 µmol/m2/s.
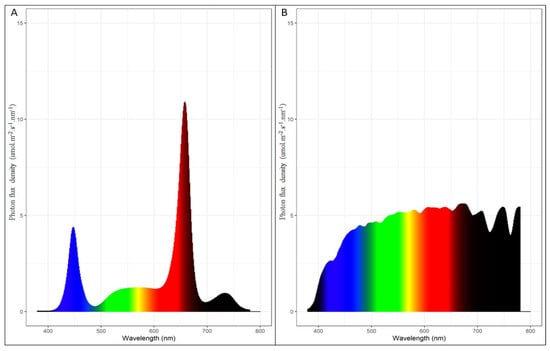
Figure 1.
LED light spectra at night (A), 110 cm below the light fixture and at noon in the MTG under natural sunlight (B).
2.3. Air Temperature
Air temperature was recorded every 10 min in each treatment from budding to 14 weeks after budding (wab), using a HOBO data logger MX2203 (Onset Computer Corporation, Bourne, MA, USA).
2.4. Leaf Surface Temperature
The leaf surface temperature of plants under supplemental light and no supplemental light was measured in each greenhouse. Leaf-surface temperatures were measured at 12 wab with a hand-held infrared radiometer MI-210 (Apogee Instruments, Logan, UT, USA). Measurements were conducted 60 min after turning on the lights. Plants receiving no supplemental light were assessed at the same time. Temperatures were measured on the first fully expanded leaf from the scion growth on seven different plants under light and under no light, respectively.
2.5. Daily Light Integral (DLI)
Photosynthetic active radiation (PAR) is the wavelengths of light within the visible range of 400–700 nm, which drives photosynthesis. The PPFD is the amount of PAR landing on the plant in a specific area and time. PPFD was monitored for each treatment using analog full-spectrum quantum sensors SQ-500 (Apogee Instruments, Logan, UT, USA). Each sensor was attached to a data logger, and PPFD was measured every 150 s and averaged every 900 s from preconditioning to 14 wab. DLI was calculated for each treatment using the formula:
2.6. Vegetative and Physiological Measurements
Plants were assessed for vegetative budbreak (scion bud initiation: shoot tip of bud at least 2 mm long) and scion growth (shoot length) at 4, 6, 8, 10, 12, and 14 wab. All other measurements were conducted at 14 wab. Scion stem diameters were measured at 5 cm above the graft union, and rootstock stem diameters were measured and compared with rootstock diameters that had been measured at 15 cm above the soil surface before starting the preconditioning treatments. The length of each internode was measured from the oldest to the youngest fully expanded leaf on the scion to determine the average internode length. The leaf chlorophyll content was measured on the most recent fully expanded leaf of each of six plants with a SPAD-502 portable chlorophyll meter (Minolta, Spectrum Technologies, Inc., Plainfield, IL, USA). The total leaf area on each plant was measured using a LI-3100C leaf area meter (LI-COR Biosciences, Lincoln, NE, USA). The scion shoot was excised at the graft union and dried in an oven at 70 °C to a constant weight to determine the scion dry biomass (g).
2.7. Statistical Analysis
The experimental design was a completely randomized block design with five different lighting treatments (NSL, DLE + P, DLE, NI + P, NI) and four replications (blocks). Each replication contained 22 plants from each rootstock (Carrizo and Rubidoux). Treatments were randomized in each greenhouse. A total of 2640 plants (880 per experiment) were assessed. Data were analyzed using a two-way ANOVA with light treatment and rootstock as main factors and block as a random factor. Differences were defined as statistically significant when the p-value was <0.05. If effects were significant, differences among means were analyzed by Tukey’s honestly significant difference (HSD) test, and significant differences (p < 0.05) were indicated by different letters. Data were analyzed using the statistical program R Version 4.0.2 (The R foundation for statistical computing, Vienna, Austria).
3. Results
3.1. Daily Light Integral (DLI)
The PPFD from daylight increased throughout the experiment and, consequently, the average of DLI increased (Figure 2 and Table 1). The average DLI was higher for the DLE treatment (20–32 mol/m2/day) than the NSL (11–18 mol/m2/day) and the NI (13–21 mol/m2/day) treatment in all three greenhouses. Overall, a higher DLI was measured in the NHG (18–32 mol/m2/day) than in the HTG (11–24 mol/m2/day) and the MTG (13–20 mol/m2/day) due to the different covering material and structure.
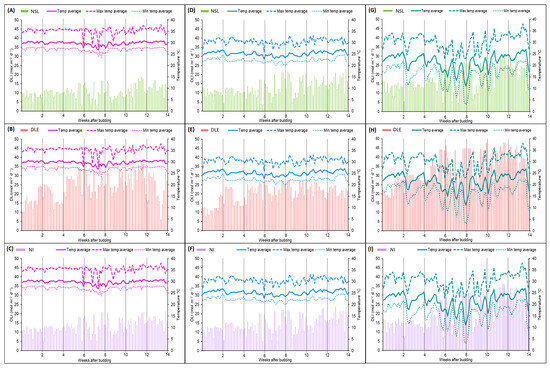
Figure 2.
Daily light integral (DLI), temperature average, maximum temperature average, and minimum temperature average in the high-temperature greenhouse (A–C), medium-temperature greenhouse (D–F), and no-heat greenhouse (G–I), under no supplemental light (A,D,G), 16 h day-length extension (B,E,H) and night interruption (C,F,I).

Table 1.
Daily light integral (mol/m2/day) averages from pre-conditioning to 14 weeks after budding.
3.2. Greenhouse Temperature
The average temperature in each greenhouse over the 14-week experimental period was 29.7 °C, 25.2 °C, and 21.6 °C for the HTG, MTG, and NHG, respectively (Figure 2 and Table 2). The amplitude of the temperature was more than 2-fold higher in the NHG (25.7 °C) compared to the other greenhouses (11.2 °C and 11.3 °C). The average minimum and maximum temperatures were 25.7 °C and 36.9 °C, respectively, in the HTG, 21.1 °C and 32.4 °C, respectively, in the MTG, and 8.9 °C and 34.6 °C, respectively, in the NHG.

Table 2.
Average greenhouse temperature (°C), including maximum and minimum average and amplitude, from 2 to 14 weeks after budding.
3.3. Leaf Surface Temperature
The leaf surface temperature measured at 12 wab was significantly higher for plants under supplemental light (23.0–29.1 °C) than for plants with no supplemental light (20.8–26.9 °C) regardless of the greenhouse (Table 3). The average leaf-surface temperatures also differed among greenhouses regardless of the light treatment. The highest leaf-surface temperatures were measured in the HTG (29.1 °C and 26.9 °C) and the lowest in the NHG (23.0 °C and 20.8 °C).

Table 3.
Leaf-surface temperature of the “Washington” Navel scion under supplemental light and no supplemental light.
3.4. Vegetative and Physiological Measurements
3.4.1. Bud Survival
Bud survival was 88–99% and not significantly affected by the light treatment (Table 4). The percentage of bud survival on Carrizo was significantly higher (98.2% and 99.3%, respectively) than on Rubidoux (94.0% and 92.5%, respectively) in the MTG and NHG, while there was no significant difference between rootstocks in the HTG.

Table 4.
Percentage of bud survival and vegetative budbreak of “Washington” Navel on Carrizo and Rubidoux rootstock.
3.4.2. Vegetative Budbreak
At 4 wab, no buds were growing, and at this time, plants were looped to stimulate budbreak. Budbreak was first observed at 6 wab (Table 4). The percentage of budbreak was significantly affected by the light treatment only in the HTG and MTG and only at 6 wab. At that time, budbreak in the HTG occurred at a significantly higher frequency under day-length extension with preconditioning (81.3%) than under no supplement light (59.1%). In the MTG, budbreak occurred at a higher frequency under day-length extension with preconditioning (55.7%) than under night interruption with preconditioning (30.1%). In the HTG, more buds grew on Rubidoux (73.9–87.5%) than on Carrizo (64.3–76.8%). The reverse was true in the MTG and NHG. The percentage of buds growing on Carrizo was 55.7–80.0% (MTG) and 72.3–97.5% (NHG), while it was 37.7–67.0% (MTG) and 60–85.5% (NHG) for Rubidoux.
3.4.3. Scion Growth
Scion growth (shoot length) was significantly affected by light treatment and rootstock, but there was no interaction (Table 5 and Figure 3). In the heated greenhouses (HTG and MTG), day-length extension with or without preconditioning generally increased scion growth (HTG: 6.5–44.4 cm, MTG: 2.7–36.8 cm) compared to the no-supplemental light (HTG: 5.1–34.2 cm, MTG: 1.9–29.0 cm) and night-interruption treatments (HTG: 4.5–39.0 cm, MTG: 1.6–28.3 cm), although in the MTG, this effect was not evident until 10 wab. There was significantly more growth on Carrizo (6.6–45.3 cm and 1.6–37.6 cm, respectively) than on Rubidoux (5.2–33.9 cm and 2.7–25.1 cm, respectively) in both the HTG and MTG from 6 wab to 14 wab. In the NHG, day-length extension without preconditioning increased scion growth significantly (14.4 cm) compared to night interruption (11.1–11.2 cm) treatments at 12 wab and compared to all other treatments at 14 wab (41.0 cm compared to 33.3–35.4 cm, respectively). The scion was significantly longer on Carrizo (11.4–44.8 cm) than on Rubidoux (7.4–27.1 cm) from 10 wab to 14 wab, although there was a significant interaction between light treatment and rootstock at 10 wab with no supplemental light and preconditioning treatments producing less growth on Rubidoux (6.3–6.9 cm) compared to the other treatment combinations (8.1–12.9 cm).

Table 5.
“Washington” Navel growth (cm shoot length) on Carrizo and Rubidoux rootstock.

Figure 3.
“Washington” Navel growth on Carrizo and Rubidoux rootstock under (A) no-supplemental light, (B) day-length extension, and (C) night-interruption treatments in the medium-temperature greenhouse 14 weeks after budding. In each photo, the two plants on the left are budded on Carrizo, and the two plants on the right on Rubidoux.
3.4.4. Scion Diameter
The “Washington” Navel scion stem diameter was significantly affected by the light treatment and the rootstock (Table 6). Diameters were significantly larger under day-length extension (4.3–4.4 mm) compared to the no-supplemental light (3.4–3.7 mm) and night-interruption treatments (3.5–3.7 mm) in both heated greenhouses (HTG and MTG), regardless of preconditioning. In the NHG, diameters were significantly larger under daylength extension without preconditioning (4.0 mm) compared to night interruption with or without preconditioning (3.5 and 3.7 mm, respectively). The scion diameter was significantly larger on Carrizo (4.2–4.5 mm) than on Rubidoux (3.1–3.5 mm) regardless of the greenhouse.

Table 6.
Scion and rootstock stem diameters, scion dry biomass, leaf area, internode length, and chlorophyll index of “Washington” Navel on Carrizo and Rubidoux rootstock 14 weeks after budding.
3.4.5. Rootstock Diameter
There was a significant effect of light treatment and rootstock on the rootstock stem diameter in all greenhouses (Table 6). In the heated greenhouses (HTG and MTG), rootstock diameters were significantly larger under day-length extension with or without preconditioning (7.4–7.9 mm) compared to no supplemental light and night-interruption treatments (6.6–7.0 mm). In the HTG, day-length extension with preconditioning increased the diameter more (7.7 mm) than day-length extension without preconditioning (7.4 mm). Similar results were found in the NHG, but here, differences were only significant between day-length extension with or without preconditioning (7.6 mm) and night interruption with or without preconditioning (7.2 mm and 7.3 mm, respectively). The rootstock diameter was significantly larger for Carrizo (7.5–8.0 mm) than for Rubidoux in all greenhouses (6.6–6.7 mm).
3.4.6. Scion Dry Biomass
The scion dry biomass was significantly affected by light treatment and rootstock in all three greenhouses (Table 6). In the heated greenhouses (HTG and MTG), the biomass was significantly larger under day-length extension with or without preconditioning (7.7–9.2 g) compared to the other treatments (4.4–5.9 g). Similar results were found in the NHG, but here, day-length extension without preconditioning produced more biomass (6.7 g) than no supplement light (5.3 g) and night interruption with or without preconditioning (4.9 g and 4.8 g, respectively). Significantly more scion dry biomass was produced on Carrizo (7.5–8.3 g) than on Rubidoux (3.5–5.2 g) in all three greenhouses.
3.4.7. Scion Leaf Area
The scion total leaf area was significantly affected by light treatment and rootstock in all three greenhouses (Table 6). In general, day-length extension increased the total leaf area (647–752 cm2) compared to the other light treatments (456–592 cm2). However, in the NHG, day-length extension with preconditioning did not produce any significant increase compared to no supplemental light and night-interruption treatments. The scion leaf area was significantly larger on Carrizo (700–734 cm2) than on Rubidoux (368–490 cm2) in all three greenhouses.
3.4.8. Internode Length
There was no significant effect of light treatments on the scion internode length in any of the greenhouses, but there was a significant rootstock effect (Table 6). Internodes were significantly longer when budded on Carrizo (16.9–19.4 mm) compared to Rubidoux (14.8–15.3 mm) in all three greenhouses.
3.4.9. Leaf Chlorophyll Index
There was a significant effect of light treatment on the leaf chlorophyll index in the heated greenhouses (HTG and MTG). The leaf chlorophyll index was significantly higher under day-length extension with or without preconditioning (74.8–80.4) compared to the other treatments (68.3–73.5) (Table 6). No significant difference was observed among the light treatments in the NHG. There was no significant effect from the rootstock in any of the greenhouses.
4. Discussion
The results from this study demonstrate that day-length extension to 16 h with supplemental LED light combined with heat to maintain the average daily temperature above 24 °C increased the growth of the “Washington” Navel orange scion during the short winter days. Previous studies also reported the benefits of supplemental light to extend the day length during the short day-length season in the citrus nursery [19,20,21,22,23,32], although those previous studies did not identify the temperature requirement to obtain the beneficial effects.
Budbreak was promoted by day-length extension with preconditioning in the heated greenhouses, but this effect was only significant 6 weeks after budding. Budbreak was also affected by the rootstock genotype, but effects varied by greenhouse. Under the high-temperature conditions of the HTG, budbreak was promoted more on Rubidoux rootstock than on Carrizo, while the reverse was true under the lower-temperature conditions of the MTG and NHG. True Citrus species hold their leaves throughout the winter, with only limited dormancy, while Poncirus trifoliata (trifoliate orange) typically defoliates during the winter months and enters a dormancy similar to deciduous trees [33]. The different responses of the two rootstocks found in our study are likely associated with their different genetic backgrounds, with Rubidoux (P. trifoliata) likely possessing a higher level of winter dormancy compared to Carrizo (Citrus sinensis × P. trifoliata) [34,35], resulting in a lower budbreak frequency under lower-temperature conditions.
The light effect on vegetative budbreak observed in the present study was only temporary, although a previous study [19] reported that day-length extension with LED lighting increased budbreak 14 weeks after budding. The different findings could be explained by the differences in the light spectrum and intensity used in the two studies. The light intensity used in the previous experiment was 92.7 µmol·m−2·s−1 PPFD with the highest intensity in the blue spectrum. In the present experiment, the average PPFD under the light was 364 µmol·m−2·s−1 and with the highest intensity in the red spectrum. Further studies on manipulating light intensity and spectrum are necessary to understand the potential to improve vegetative budbreak through light manipulation.
Night-interruption treatments did not increase scion growth in the present study. In contrast, Singh et al. [36], working with “Clementine” mandarin on Carrizo observed significantly more plant growth under both night interruption (NI) (10 h at 500 µmol·m−2·s−1 + 1 h of NI at 10 µmol·m−2·s−1) and 14 h day-length extension (10 h at 500 µmol·m−2·s−1 + 4 h at 10 µmol·m−2·s−1). The authors suggested a phytochrome-mediated response as the reason for the observed effects. We propose that the different results in the present study may have been due to the plants growing under natural daylight, which was longer in duration (11 h) and higher in intensity than the light used in the growth-chamber study by [36]. In addition, the intensity of the supplemental light for NI in our study was higher (PPFD = 364 µmol·m−2·s−1) and the spectrum was broader.
Although research to develop light and temperature strategies has expanded in recent years for horticultural production in controlled environments [24], little information on the use of light and temperature has been developed for citrus nursery production. Bowman and Albrecht [19] observed that extending day length to 16 h with HPS or LED lighting was effective in increasing the growth of scion shoots and unbudded rootstock liners under controlled temperature conditions (26.5 to 29.7 °C). Wu and Zou [37], who compared citrus growth in the nursery at 15 °C and at 25 °C, observed that the lower temperature severely decreased stem diameter, plant height, leaf area, and plant biomass. However, the effects of supplemental light were not explored in that study. Our study is the first report to describe the effects of supplemental light under different temperature conditions in the citrus nursery.
A significant impact of the relationship between light and temperature on plant quality was observed by Liu and Heins [38], who described the ratio of radiant to thermal energy as a useful parameter to understand the combined effects of light and temperature on plant growth, development, and quality. In our study, stem diameter and leaf area were improved by day-length extension regardless of the temperature conditions. However, day-length extension increased the scion shoot length and chlorophyll content only under high- and medium-temperature conditions despite a higher daily light integral (DLI) in the greenhouse without supplemental heating (NHG). In the MTG, plants grew overall slower than in the HTG until 10 wab despite a similar DLI. In the NHG, plants grew slower until 12 wab despite a higher DLI. This suggests that the lower temperatures were a more limiting factor for plant growth than the DLI. After 12 wab, when the average temperature increased, plants tripled in size in the NHG within a short period, further supporting the limiting effects of low temperatures. That the extent of DLI effects is temperature dependent was also observed in different herbaceous crops [39], but there is little information on tree crops.
No light effect on the “Washington” Navel internode length was observed for either rootstock, which is similar to a study conducted with Redblush grapefruit (C. paradisi) on sour orange (C. aurantium) rootstock [23]. Our previous study with HPS and LED light found a significant effect on internode length only for the combination of HPS light and one rootstock, and this effect was relatively small [19]. Piringer et al. [22] found that day-length extension increased internode length and number of nodes in seedlings of several Citrus species, P. trifoliata, and grafted grapefruit trees with sour orange rootstock. The internode elongation found in both previous studies could be because of a temperature effect from the light source, since under HPS light the leaf temperature is usually higher than under LED light [40,41]. The LED light in this study also increased the leaf temperature, but only by less than two degrees.
The LED light fixtures used in this study provided light at a broad spectrum, with wavelengths corresponding to blue (445–500 nm), green (500–580 nm), red (620–700 nm), and far-red (700–775 nm) and at full intensity. However, different spectra received from a natural or artificial source of light can induce different physiological responses, including photosynthesis rate, photomorphogenesis, and chlorophyll production [42,43], and they strongly influence plant behavior [14], affecting budbreak and plant development. Variations in light spectra and intensity influence the excitation of Photosystems I and II, affecting the photosynthetic rates [44,45]. In addition, light intensity plays an important role in the plant’s response to light because photoreceptors are usually activated by a lower intensity than the intensity required for photosynthetic processes [46]. The results from the present study suggest that the use of supplemental LED light to extend the day length to 16 h can improve the growth of budded citrus trees during the short days of winter, but that average temperatures of 25 °C or higher may be required. Additional studies to evaluate the effects of different wavelengths and intensities to enhance plant growth and extend the citrus nursery production cycle in the winter are in progress.
5. Conclusions
The results from this study suggest that the use of supplemental LED lighting to extend the day length may be an effective strategy to increase the growth of grafted citrus trees in the citrus nursery during the short days of winter. However, supplemental light at a broad spectrum and high intensity may not affect the rate or frequency of budbreak. Supplemental LED light appears to be only effective during the winter when combined with supplemental heating, as lower temperatures are a limiting factor for plant growth. Nighttime light interruption at a broad spectrum and full-intensity light do not seem to affect citrus scion shoot growth, while preconditioning trees with light may enhance scion shoot growth to some extent. Optimizing light spectrum and intensities may further increase the beneficial effects of day-length extension and warm temperatures in the winter citrus nursery.
Author Contributions
Conceptualization, U.A. and K.D.B.; methodology, R.B.B., U.A. and K.D.B.; software, R.B.B.; validation, R.B.B., U.A. and K.D.B.; formal analysis, R.B.B.; investigation, R.B.B.; resources, U.A. and K.D.B.; data curation, R.B.B.; writing—original draft preparation, R.B.B.; writing—review and editing, R.B.B., U.A. and K.D.B.; visualization, R.B.B., U.A. and K.D.B.; supervision, U.A. and K.D.B.; project administration, U.A. and K.D.B.; funding acquisition, U.A. and K.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the California Citrus Nursery Board (ALB-21-23 and BOW-21-23).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Sailindra Patel, Diane Helseth, and Kerry Worton for their help with tree manipulations, data collection, and sample collection and analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brar, G.R.P.S.; Spann, T.M. Photoperiodic phytochrome-mediated vegetative growth responses of container-grown citrus nursery trees. Sci. Hort. 2014, 176, 112–119. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus Rootstocks. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 105–127. [Google Scholar]
- Garnsey, S.M.; Barrett, H.C.; Hutchson, D.J. Identification of citrus tristeza virus resistance in citrus relatives and its potential applications. Phytophylactica 1987, 19, 187–192. [Google Scholar]
- Gmitter, F.G.; Xiao, S.Y.; Huang, S.; Hu, X.L.; Garnsey, S.M.; Deng, Z. A localized linkage map of the citrus tristeza virus resistance gene region. Theor. Appl. Genet. 1996, 92, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Mestre, P.F.; Asins, M.J.; Pina, J.A.; Carbonell, E.A.; Navarro, L. Molecular markers flanking citrus tristeza virus resistance gene from Poncirus trifoliata (L.) Raf. Theor. Appl. Genet. 1997, 94, 458. [Google Scholar] [CrossRef]
- Graham, J.; Feichtenberger, E. Citrus phytophthora diseases: Management challenges and successes. J. Cit. Pathol. 2015, 2, 27203. [Google Scholar] [CrossRef]
- Verdejo-Lucas, S.; Kaplan, D.T. The citrus nematode: Tylenchulus semipenetrans. In Plant Resistance to Parasitic Nematodes; Starr, J.L., Cook, R., Bridge, J., Eds.; CABI: Wallingford, UK, 2002; pp. 207–219. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-term field evaluation reveals Huanglongbing resistance in Citrus relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 2011, 46, 16–22. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G. Five new citrus rootstocks with improved tolerance to huanglongbing. HortScience 2015, 50, 1731–1734. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2020, 53, 1250–1257. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, A.; Cheng, Z.M.M. Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. Acclimations to light quality on plant and leaf level affect the vulnerability of pepper (Capsicum annuum L.) to water deficit. J. Plant Res. 2015, 128, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Improving winter growth in the citrus nursery with LED and HPS supplemental lighting. HortScience 2021, 56, 21–27. [Google Scholar] [CrossRef]
- Inoue, H. Effects of day length and temperature on the vegetative growth and flower bud differentiation of satsuma mandarin. J. Japan. Soc. Hortic. Sci. 1989, 58, 563–567. [Google Scholar] [CrossRef]
- Nauer, E.M.; Boswell, S.B.; Holmes, R.C. Chemical treatments, greenhouse temperature, and supplemental day length affect forcing and growth of newly budded orange trees. HortScience 1979, 14, 229–231. [Google Scholar] [CrossRef]
- Piringer, A.A.; Downs, R.J.; Borthwick, H.A. Effects of photoperiod and kind of supplemental light on the growth of three species of citrus and Poncirus trifoliata. Proc. Am. Soc. Hortic. Sci. 1961, 77, 202–210. [Google Scholar]
- Young, R.H. Influence of day length, light intensity and temperature on growth, dormancy and cold hardiness of red-blush grapefruit trees. Proc. Am. Soc. Hortic. Sci. 1961, 78, 174–180. [Google Scholar]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1661. [Google Scholar] [CrossRef]
- Janda, T.; Prerostová, S.; Vanková, R.; Darkó, É. Crosstalk between light-and temperature-mediated processes under cold and heat stress conditions in plants. Intern. J. Mol. Sci. 2021, 22, 8602. [Google Scholar] [CrossRef]
- Franklin, K.A. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M.; Kim, R.J.A.; Moore, C.M.; Chen, M. Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat. Commun. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef]
- Dechaine, J.M.; Gardner, G.; Weinig, C. Phytochromes differentially regulate seed germination responses to light quality and temperature cues during seed maturation. Plant Cell Environ. 2009, 32, 1297–1309. [Google Scholar] [CrossRef]
- Niedz, R.P.; Bowman, K.D. Improving citrus bud grafting efficiency. Sci. Rep. 2023, 13, 17807. [Google Scholar] [CrossRef]
- Warner, R.M.; Worku, Z.; Silva, J.A. Effect of photoperiod on growth responses of citrus rootstocks. J. Am. Soc. Hortic. Sci. 1979, 104, 232–235. [Google Scholar] [CrossRef]
- Ortiz, J.M. Botany: Taxonomy, morphology and physiology of fruits, leaves and flowers. In The Genus Citrus; Dugo, G., Di Giacomo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 30–49. [Google Scholar]
- Primo-Millo, E.; Agustí, M. Vegetative growth. In The Genus Citrus, 1st ed.; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Sawston, UK, 2020; Chapter 10; pp. 193–244. [Google Scholar]
- Yelenosky, G. Cold hardiness in citrus. Hortic. Rev. 1985, 7, 201–238. [Google Scholar]
- Singh, H.; Khezri, M.; Bushoven, J.; Benes, S.; Hadavi, F.; Brar, G. Carbohydrate partitioning and vegetative growth of citrus nursery trees influenced by varying photoperiods under LED lighting. Hortic. J. 2022, 91, 467–475. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Beneficial roles of arbuscular mycorrhizas in citrus seedlings at temperature stress. Sci. Hortic. 2010, 125, 289–293. [Google Scholar] [CrossRef]
- Liu, B.; Heins, R.D. Is plant quality related to the ratio of radiant energy to thermal energy? In II Workshop on Environmental Regulation of Plant Morphogenesis; Acta Horticulturae: Leuven, Belgium, 1996; Volume 435, pp. 171–182. [Google Scholar]
- Walters, K.J.; Lopez, R.G. Modeling growth and development of hydroponically grown dill, parsley, and watercress in response to photosynthetic daily light integral and mean daily temperature. PLoS ONE 2021, 16, e0248662. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of yield, lycopene, and lutein content in tomatoes grown under continuous PAR spectrum LED lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; Bugbee, B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE 2015, 10, e0138930. [Google Scholar] [CrossRef]
- Fukuda, N. Advanced light control technologies in protected horticulture: A review of morphological and physiological responses in plant to light quality and its application. J. Dev. Sustain. Agric. 2013, 8, 32–40. [Google Scholar] [CrossRef]
- Weller, J.L.; Kendrick, R.E. Photomorphogenesis and photoperiodism in plants. In Photobiology: The Science of Light and Life; Bjorn, L.O., Ed.; Springer: New York, NY, USA, 2008; pp. 299–321. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Far-red photons increase light capture but have lower photosynthetic capacity than red photons. J. Am. Soc. Hortic. Sci. 2023, 148, 253–265. [Google Scholar] [CrossRef]
- Costa-Galvão, V.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).