1. Introduction
Pyrus spinosa (syn.
amydaloformis; common name: almond-leaved pear) is one of the wild members of
Pyrus species originated from western and southwestern mountainous areas of China with tremendous evolutionary impact in entrepreneurial orchard farming and fruit production [
1,
2]. As a common wild habitat, almond-leaved pear forms small isolated populations with high levels of phenotypic plasticity, which are mainly dispersed by birds and mammals in woodland pastures [
3,
4]. The native profile of
P. spinosa reflects an extremely drought-resistant plant species with quite flexible physiology in environmental extremes [
5]. Agronomically, (a) it easily hybridizes with
P. communis,
P. elaeagrifolia and other genus
Pyrus members, offering novel sets of rootstocks especially resistant to lime chlorosis, (b) it plays a key role in apiculture due to it being rich in amino acid content of nectar and (c) it improves soil carbon storage under agroforestry cultivation schemes [
4,
6,
7,
8,
9,
10].
P. spinosa fruit pulp exhibits antibacterial and antifungal activities due to their richness in linoleic and oleic acids; bark extracts of the tree demonstrate antimicrobial and cytotoxic activities against malignant human cell lines, suggesting a promising spectrum of uses in pharmaceutical science [
11,
12].
Soil salinity is a time-lapse expansive phenomenon that affects food reserves and rural development around the globe. Advances in orchard irrigation and soil amelioration cannot solely counteract salinity consequences due to the fact that fresh and clean water availability is not guaranteed for many places on earth; physiologically resilient plant material must also be used to overcome fruit production yield losses [
13,
14,
15]. For perennial plants like trees, soil saline conditions form a complex of induced and acquired memory when intergenerationally and simultaneously exposed to the stress factor [
16]. Recorded up to now, non-wild, commercial
P. communis orchards exhibit salt tolerance with trade-off cost of reduced shoot growth at electrical conductivity values higher than 5.0 dS/m, regardless of quince rootstock genetic differentiations [
17].
Pyrus spinosa is a plant species resistant to drought and salinity, spatially distributed in coastal sand dunes as part of its native habitat [
18]. However, its adaptation mechanisms to saline stress are poorly documented.
Methionine is an essential amino acid affecting plant nutrition, plant defense to stresses and immunity; it is transcriptionally interdependent to threonine and isoleucine availability when abiotic stress conditions such as drought and salinity occur [
19,
20,
21]. Exogenous methionine application in horticultural species under saline stress conditions improves growth, fresh biomass and fruit yield, whereas jointly applied with l-phenylalanine affects positively free amino acid, carotenoid and total carbohydrate contents. This resilient-to-salinity, enriched methionine profile is also coupled with increased proline existence, higher plasma membrane stability and a plethora of osmolyte substrates present [
22]. Endogenously, methionine leads to synthesis of (a)
S-methymethionine (its mobile and storage forms), (b)
S-adenosylmethionine, which regulates ethylene, polyamines and biotin and (c) precursor molecules for secondary metabolite production with osmoprotectant properties like 3-dimethyl-sulfonioproprionate [
23]. Methionine oxidation is capable to modulate phosphatase and kinase activities, which are involved in cellular signaling under saline stress conditions; this process is fully reversible via methionine sulfoxide reductase enzymatic activity [
24,
25].
Soil-beneficial
Bacillus bacterial communities were found to optimize tree nutritional physiology and positively affect plant hormonal status, advancing resilience towards biotic and abiotic stress factors including salinity [
26,
27]. Furthermore, members of bacterial genus
Azotobacter provide rhizosphere counter-saline osmoprotectants like deaminases, salicyclic acid, proline and exopolysaccharides, attempting to restore unchallenged status for plants and farming activities in arid areas [
28].
Phenolic content of
P. spinosa consists of a front-line, interdisciplinary field in agriculture, medicine and pharmaceutical sciences [
29,
30,
31,
32].
P. spinosa leaves are richer in total phenolics in comparison to seeds and fruits where gallic acid, chlorogenic acid, rutin, coumaric acid, quercetin, apigenin and arbutin are abundant and dominant [
33,
34]. Hybrid rootstocks of
P. spinosa exudate phenols in the rhizosphere in order to become tolerant to soil-induced iron chlorosis [
35]. However, investigation of bark total phenolics and DPPH radicals of
P. spinosa reveal lower levels of response in comparison to
Pyrus communis subsp.
pyraster [
31].
Herein, we examine P. spinosa seedlings for their growth resilience against soil salt stress, measuring changes in their shoot and root growth characteristics as well as leaf parameters (chlorophyll content, total phenolics, antioxidant activity) by exploring alternative alleviation stress techniques, administrating soil-formulated l-methionine biostimulant and bacterial inoculums of Bacillus and Azotobacter species.
2. Materials and Methods
2.1. Plant Materials
Mature fruits from wild pear trees (Pyrus spinosa) were collected from the coastal area of Central Greece (38°23′19.18″ N; 22°22′23.18″ E). Seeds were separated from fruit pomace manually with a knife and were washed several times with distilled water for pomace residual removal. In order to break seed dormancy, seeds were sterilized by sinking in 2% sodium hypochlorite solution for 5 min and then were placed in 500 ppm gibberellic acid (GA3) solution for 12 h. Finally, seeds were transferred to sterilized plastic box with wet cotton as a substrate and kept in low temperature (4 °C). After 30 days at cold storage, seeds were sowed in trays with substrate mixture of peat/perlite (1:2) and kept in greenhouse for germination and growth. For experimental purposes, sixty uniform 3-month-old plantlets were selected and transplanted to 1 L pots with the same substrate.
2.2. Biochemical and Microbial Saline Stress Alleviation Schemes
Formulated biostimulant rich in l-methionine (NPK 5-20-0+5% l-methionine; commercial name PHYTOAMINO®-PN produced by Karvelas S.A., Agrinio, Greece) and plant growth promoting bacteria (NPK 0.6-1.2-3+0.3% CaO, 0.1% MgO, 0.1% S; Bacillus subtilis, Bacillus pumillus, Bacillus licheniformis, Bacillus megaterium, Azotobacter sp.; commercial name RHI-ZOBAC produced by Humofert S.A., Metamorfosi, Athens, Greece) at 1 × 1011 cfu/L were applied in separate cultivation lines. Applications took place after seedling transplantation to 1 L pots once every 15 days for 3 months with standardized solutions of 2% and 1%, respectively.
2.3. Saline Exposure
Four levels of salinity stress were selected to challenge P. spinosa plantlets. A total of 200 mL of NaCl water solution in the following concentrations 0 mM, 50 mΜ, 75 mM and 100 mM were used to stress plantlets as irrigation regime, once every two days for 3 months (end of April–end of July). Months April to July were selected for experimentation in order to deliver robust data for (a) saline effect on P. spinosa seedlings, (b) P. spinosa plantlet limits of phenotypic and physiological plasticity in presence of alleviation schemes and (c) efficacy of the above applied schemes.
2.4. Measurement of Plant Growth Parameters
Shoot length from soil to shoot tip was recorded every 30 days coupled with calculation of growth increment percentage. At the end of experimental period, 3 months later, shoots separated from roots, both of them were washed carefully and their fresh weight was measured. The number and length of secondary shoots were also analyzed. Morphological parameters that reflect quantitative characteristics of root size and architecture, like median number of roots, total root length, root diameter, projected root volume, root orientation, root angle frequencies and root length per root thickness diameter, were determined using RhizoVision Explorer v2.0.3 open-source software for image analysis [
36]. Root and shoot biomasses were calculated after shade drying until their weights remained constant.
2.5. Measurement of Total Chlorophyll Content
The total chlorophyll content was determined during the experimental period using the CCM-200 Plus (OPTI-SCIENCES, Hudson, NH, USA) Chlorophyll Content Meter. Measurements were taken every 30 days. Five new fully expanded uppermost leaves per plant were chosen to be examined for their chlorophyll content, and changes were recorded under different levels of saline stress exposure and types of exogenous supplementations. Measurements taken every 30 days from end of April to end of July. From each leaf, five SPAD records were taken from the middle of leaf lamina so as to calculate the mean SPAD value per leaf.
2.6. Total Phenolic Content and Antioxidant Activity
Leaf extract: At the end of saline exposure period, plant shoots of all treatments were harvested and left to dry under natural conditions at room temperature. Leaves separated from shoots and grounded in grinding mill (KINEMATICA, POLYMIX PX-MFC 90 D Blade Grinding Mill, Malters, LU, Switzerland). A total of 1 g of grounded leaves per sample added in falcon tube with 20 mL of ethanol (80%) and left in shaker stirrer (170 rpm) for 24 h at room temperature. The extract was delivered by solvent filtration with Whatman filter paper No 1.
Determination of total phenolic content: The Folin–Ciocalteu assay was used to determine total phenolic content (TPC) according to Singleton and Rossi (1965) [
37] with some modifications. Briefly, 1 mL of leaf extract was mixed with 9 mL distilled water and 0.5 mL of Folin–Ciocalteu reagent (10%
v/
v FC reagent to distilled water). After 3 min, 1.5 mL aqueous Na
2CO
3 solution (20%
w/
v) was added, and the mixture was kept for 60 min in dark at room temperature. The absorbance was measured at 760 nm using a UV–VIS spectrophotometer (Shimadzu UV 1900i, Kyoto, Japan). Gallic acid (GAE) used as reference standard. A calibration curve was prepared using the absorptions of 5 different concentrations of gallic acid (10, 20, 30, 40 and 50 ppm). The linear equation obtained from gallic acid standard curve (y = 0.0317x + 0.0336, R
2 = 0.9923) was used for sample TPC quantification. The results were expressed as mg of gallic acid equivalent per gram dry weight of leaves (mg GAE/g d.w.).
Determination of antioxidant activity: Antioxidants of leaf extracts were accessed by DPPH radical scavenging assay (Brand-Williams et al., 1995) [
38]. An aliquot (0.1 mL) of leaf extract was added to 3.9 mL of DPPH ethanolic solution (0.06 mM). After a 30 min incubation period in dark at room temperature, absorbance was measured at 517 nm using a UV–VIS spectrophotometer (Shimadzu UV 1900i). Control consists of 3.9 mL of DPPH solution where 0.1 mL of ethanol (80%) was added. The free radical scavenging activity of samples was calculated using the following equation [
39]:
where A0 is the control absorbance, and A1 is the absorbance of sample.
2.7. Statistical Analysis
A factorial completely randomized design with 8 treatments—4 saline level and 2 alleviation schemes (amino acid and microbial)—was used in this study. A minimum of five replicates were used for each value, οr otherwise, it was stated.
Data were analyzed using the 95% confidence limits overlap protocol of Sokal and Rohlf (1969) [
40]. Table and graphic data were presented as means ± standard error of the mean. An α level of 0.05 was chosen. Prism 8.0 (GraphPad) was used for data analysis.
4. Discussion
Sodium chloride-based salinity affected negatively the number of shoots in many tree species. Six-month-old pomegranate plantlets dropped their lateral shoots number by 11.36%, 50% and 61.36% for 40 mM, 80 mM and 120 mM NaCl stress, respectively; herein,
P. spinosa declined faster under the same type of salt stress challenge [
41]. The osmotic effect of NaCl when salt is accumulated in soil can explain these biomass losses [
42]. Soil exogenous applications of l-methionine and microorganisms did not favor lateral shoot formation even at non-saline challenge condition. Methionine needs sulfur-rich proteins for long-distance transport and loading into phloem with complex adjustments in sulfur availability at organismal level; leaves are more competent organs for these tasks when induced systemic reactions take place [
43,
44]. In addition, when phloem contains elevated levels of methionine, it triggers an increased presence of sucrose, which is a major counter-saline osmolyte [
44]. Herein, soil application of methionine was possibly restricted towards nutritional absorption and stimulation of endogenous hormone homeostasis in roots; functional weakness of methionine sulfoxide reductase mechanism to reinstate methionine from its oxidative state under saline stress conditions could also be taken under consideration for low alleviation performance of the molecule [
19,
25].
In contrast, foliar application of methionine was found to (a) alleviate drought and saline osmotic-related stress in other plant species, enhancing shoot growth [
22,
45], and (b) induce l-methionine plant signaling to enhance salt tolerance, jointly with soil presence of
Bacillus subtilis strains [
46].
Shoot fresh weights declined upon saline presence, which is in accordance with similar stress experimentation in apple rootstock MM.106 [
47]. Herein, soil application of methionine alleviated the negative effect on shoot weight as it is shown in foliar application in non-horticultural species (e.g., maize), suggesting a universal protecting role for this molecule no matter which way it will be exogenously administrated on the plant host [
48]. Bacterial counteraction to NaCl stress on shoot fresh weight was not successful as compared to in vitro apple tree saline challenge [
49].
P. spinosa inhibitory effect on shoot biomass at 75 mM and especially at 100 mM NaCl levels of soil stress were also demonstrated in at least three varieties of olive trees [
50].
Neither soil l-methionine nor microbial application were capable to ameliorate root fresh weight values. However, in proportionally less-lignified plant species (e.g., maize) than
P. spinosa, l-methionine tends to reverse ionic imbalance and restore roots fresh weight standards when administered foliarly in saline environments [
48]. Similarly, microbial soil support of
Bacillus species increases root weight characteristics under salt stress [
51,
52]. As seen above, there are many similar patterns of
P. spinosa seedling fresh weight changes with other plant species under salt stress; however, some researchers propose comparative data avoidance due to several abiotic and biotic uncontrolled conditions when data were collected [
53].
Lower performance in shoot dry weight formation of unchallenged plantlets in presence of l-methionine may be linked to cell membrane and protein synthesis functional disruption due to their overwhelming presence [
54]. Dry weight minimal losses under salinity stress have also been documented well in field crops where previous data are available [
55,
56,
57].
Root dry weight did not reveal changes among exogenous treatments in the presence of increased salt stress; however, percentage (%) losses in root weight were fewer than those in shoots. This is also in accordance with root/shoot ratio data examination, emerging a potential tissue plasticity survival mechanism. Above a value of 1.00, the root/shoot ratio of
P. spinosa seedlings in enriched bacterial and l-methionine saline soils solidifies a prioritized strategy of root developmental formation under NaCl stress conditions. This mechanism may not be a straight one for immediate restoration of salinity costs but it seems to be adequately resilient for survival under continuous stress, potentially via salt compartmentation and exclusion as it is observed in other tree species [
58]. Similar results in saline stress conditions have been reported for
Citrus species, olives and numerous vegetable and field crops [
59,
60,
61].
Ιn orchard soil saline conditions, phenolics play a protective role against stress-related reactive oxygens species (ROS), osmotic and ionic damages [
62].
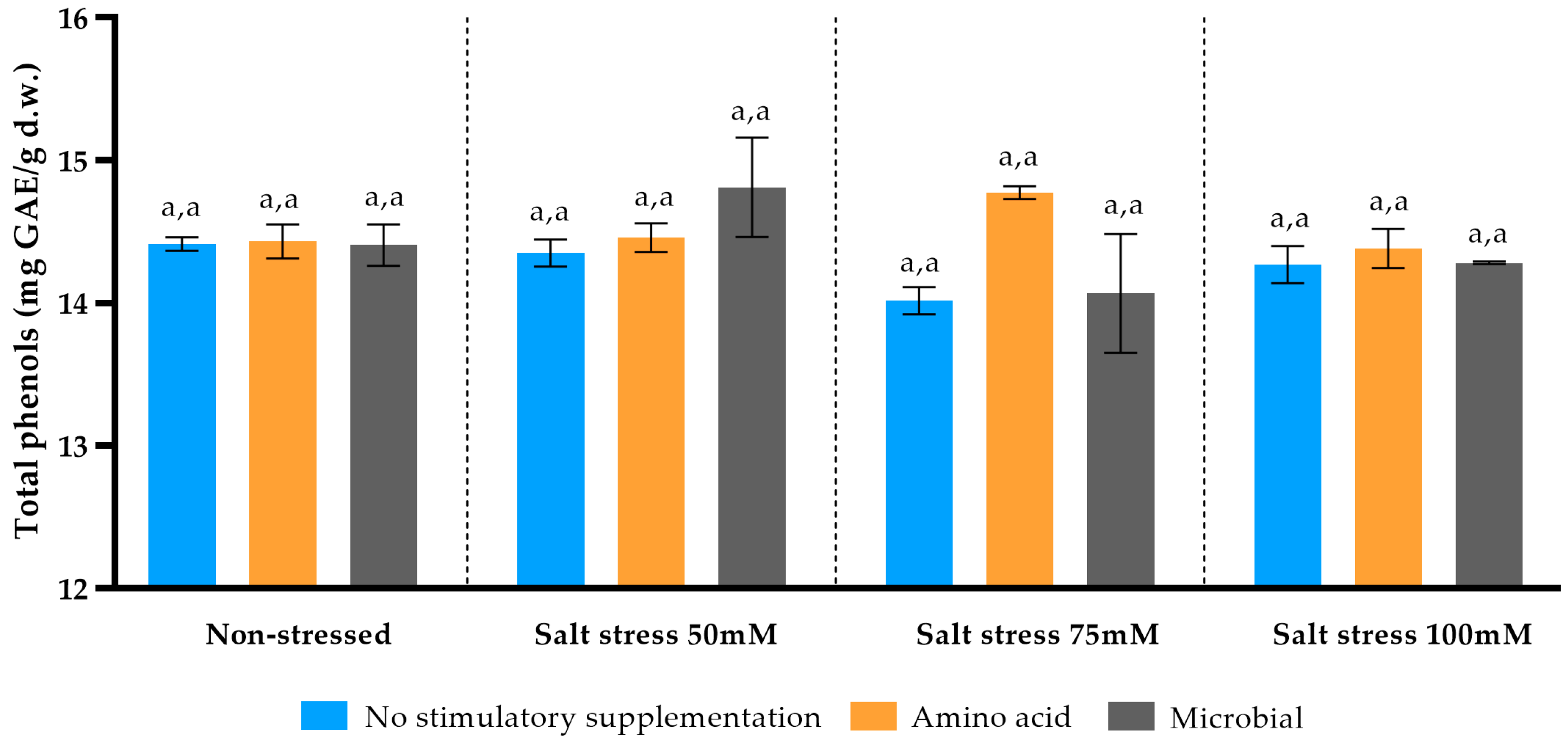
P. spinosa saline unchallenged plantlets and parentally survived in the wild provided total phenolic content similar to this work in the Lagadas, Pieria and Chalkidi areas of northern Greece [
29]. Values of total phenolic content in leaves did not change among soil treatments and salinity levels of applied stress. A similar total phenolics data trend under the same scaling stress factor was documented for new leaves in salt-sensitive Leccino var. olive trees and Brassicaceae plants [
63,
64]. In contrast, incremental changes in total phenolics were found in leaves of four olive tree varieties with content pick at 125 mM ΝaCl saline stress [
65]. For commercially valuable pear species, e.g.,
Pyrus communis, total phenolic high/low reaching concentrations are dependent to genetics, leaf age and environmental stress existence [
66]. Grafted and ungrafted
P. communis seedlings under drought stress, a physiological condition comparable to saline stress as used here, did not drastically change their total phenolic content through scaled up stress (moderate, severe), reaching a value plateau [
67]. Saline soil effect, alleviated via l-methionine and microbial enrichment, did not change total phenolics concentration; however, amino acid foliar application on saline-stressed plants increased their total phenolic content, whereas field microbial support is capable of such upregulation if exogenous microorganisms dominate on native soil microbial flora [
48,
68].
Three-year-old
P. spinosa seedlings decline their height growth rates in exposure to NaCl after 30 days; treatments with 75 mM and 150 mM salt concentrations conferred 28.9% and 43% inhibition effect, respectively [
69]. In this work, three-month-old
P. spinosa seedlings’ height growth rates declined in the presence of soil sodium chloride, which may due to a) low ability to cell elongation, b) suboptimal-to-detrimental osmotic regulation and c) counter growth hormonal status [
70]. Additionally, month by month data analysis (April–July) of seedling height growth responses interfered also with temperature increase and rainfall decline in addition to salt stress and/or exogenous treatments [
15,
71,
72].
Data of l-methionine supplementation provided resilient aspects in saline stress conditions; however, amino acid soil application in this work cannot be directly correlated to potential photosynthetic enhancement due to non-foliar administration, as it is shown in other experimental conditions [
19,
73]. Exogenous bacterial rhizosphere depositions improved growth traits in accordance with other tree species, such as olives and bananas, where microorganisms offer photo-oxidative protectant and photosynthetic advancement roles [
26,
74].
Under saline conditions,
P. spinosa exhibits higher photosynthetic rates with lower Na and Cl ion concentrations in leaves in comparison to native Asian pear species,
P. betulaefolia,
P. pyrifolia and
P. xerophila, emerging as a protective pattern for chlorophyll functionality as observed to other plants [
69,
75]. Herein, leaf chlorophyll index reduced gradually when salinity concentration increased for all treatment lines. This attribute was also shown in olive trees where stress was mitigated by foliar use of gibberellic acid [
76]. Exogenous foliar application of methionine on C4 plants (maize) ameliorated the content of chlorophylls; however, this alleviation effect was not achieved in our C3
P. spinosa plants [
48]. In comparison to
P. spinosa, maize as a C4 plant with NADP-dependent malic enzyme (NADP-ME) and phosphoenolpyruvate carboxykinase (PCK) type of photosynthetic capacity provides within bundle sheath and mesophyll cells a developmentally and functionally resilient grana physiology under saline conditions [
77]. Plateau chlorophyll index values of 75 mM and 100 mM NaCl saline stress for—all to none—soil enrichments have an underlying more toxic effect of the pressure factor due to plantlet potential failure in osmotic adjustment, regulation of ion homeostasis and cytoplasm metabolism inhibition [
78].
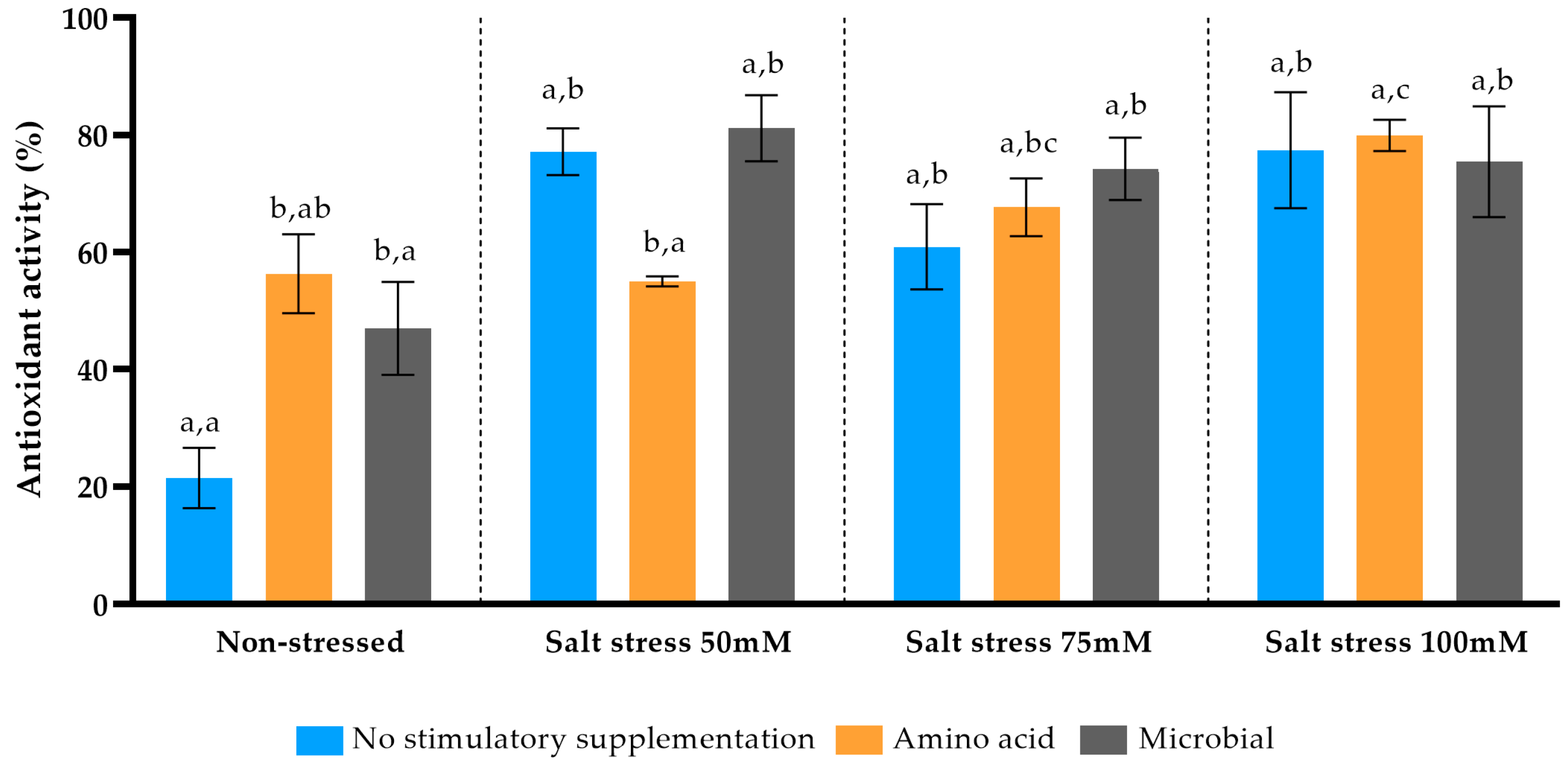
Antioxidant capacity found in
P. spinosa leaves was increased in saline environment, reaching plateau in 75 mM/100 mM NaCl soil stress; however, this phenomenon took place as a non-specific one towards these two types of diverse basis (biochemical and microbial) soil enrichments. Many tree species like olives and
Moringa oleifera advance their antioxidant capacity when exposed to saline [
65,
79]. Advanced antioxidant activity in
Pyrus species under saline conditions was recorded in exogenous administration of sodium nitroprusside as nitro oxide donor in
P. communis [
80].
Νo differences in root orientation (angles 30°–90°) and their frequencies were observed for soil saline exposure of
P. spinosa, independently of exogenous l-methionine and bacterial alleviation treatment; this suggests low levels of root architectural plasticity under salt stress, which is not commonly observed in resilient and halophytic plants due to advance hormonal management [
81,
82,
83]. L-methionine-supplemented seedlings on NaCl-free soil increased total root length, a response that is also reported in other horticultural species [
84]. However, it should be taken into account that saline soil integration of methionine did not always confer a counteract salinity effect; this may also be due to occurring changes in its thermodynamic properties and solubility in the presence of NaCl [
85,
86]. Bacterial supplementation supported root expansion in length as recorded in other plant species due to positive spatial interaction between plant host and microorganisms [
55].
Supplementation of bacterial complex population (
Bacillus subtilis,
B. pumillus,
B. licheniformis,
B. megaterium,
Azotobacter sp.) promoted
P. spinosa survival strategy via improved stress root management.
Bacillus subtilis,
B. pumilus,
B. licheniformis and
B. megaterium salinity alleviation properties were reported extensively for field crops [
57,
87,
88,
89,
90,
91] and vegetable crops [
92,
93,
94,
95]; however, research data for tree species are still few. Limitations for sustainable use of
B. subtilis as soil orchard inoculant exist due to low iron solubility and downregulation of iron-related genes in NaCl-enriched environments [
96].
Azotobacter sp., a root colonized with biofilm formation attribute bacterial species, provided contributory alleviation support to saline stress; however, most of the comparably documented work was performed on annual plant species where root–shoot hardiness and tissue development differ from perennial ones [
97,
98,
99].
Plant species with parental growth in coastal wild conditions like
P. spinosa are possible to carry extensive intergenerational and transgenerational stress memory due to continuous soil and air droplet saline challenge status; thus, examination of resilience mechanisms may not be too apparent and distinctive due to pre-conditioned abiotic exposure [
16,
100]. Therefore, it is not known if minimal to non-significant response differences found among treatments on
P. spinosa seedlings were due to preformed salinity stress memory [
16,
101,
102]. Salinity stress-related memory for tree species in agricultural practice has been reported for olives [
103].