Effects of Temperature Regulation on the Physiological Characteristics and Platycodin Synthesis of Platycodon grandiflorum
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Plants
2.2. Chemicals and Reagents
2.3. Temperature Regulation Scheme
2.4. Physiological Index Measurement
2.5. Quantitative Analysis of Platycodins
2.6. Key Genes Involved in the Platycodin Synthesis Pathway
2.7. Statistical Analysis
3. Results
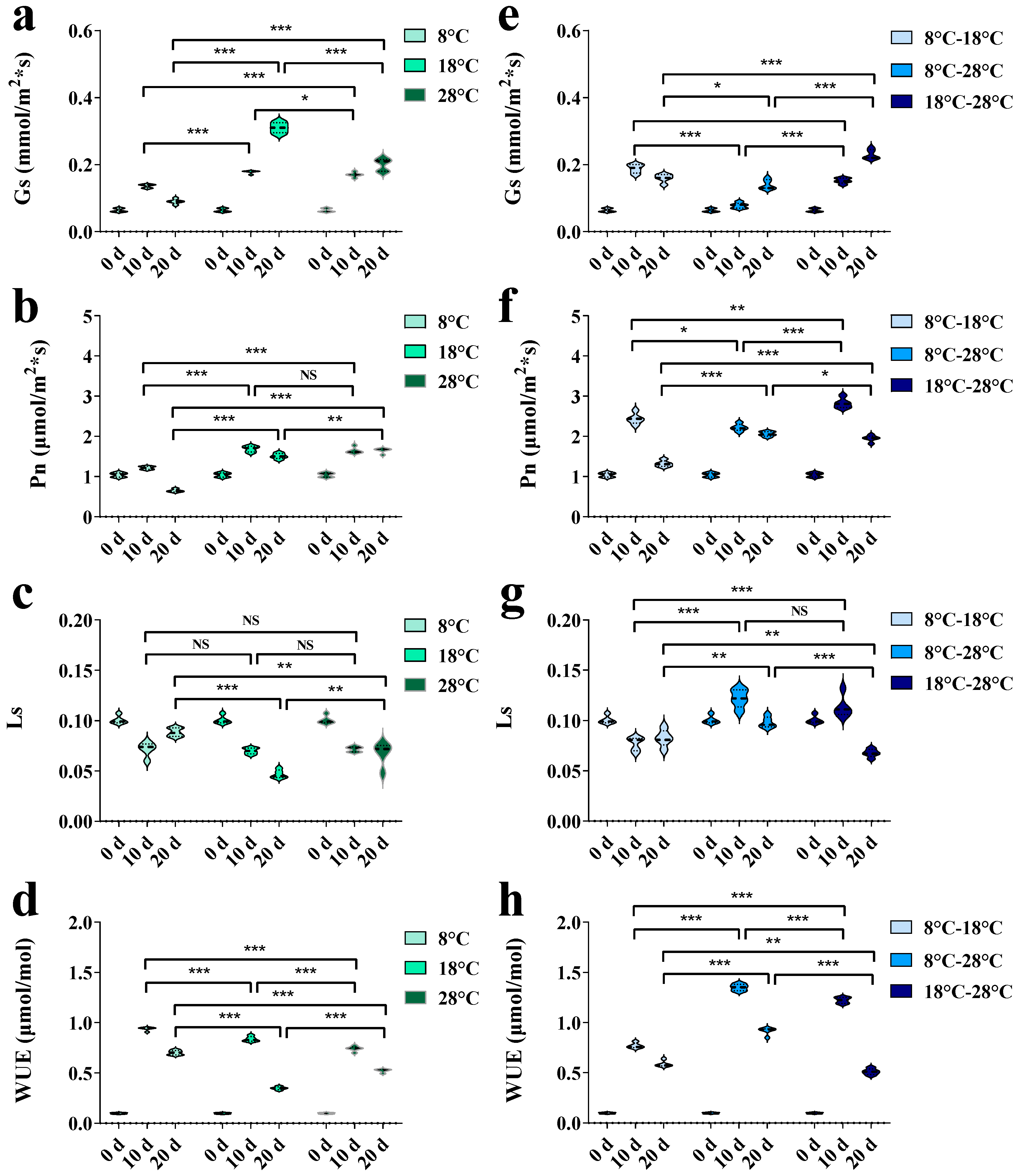
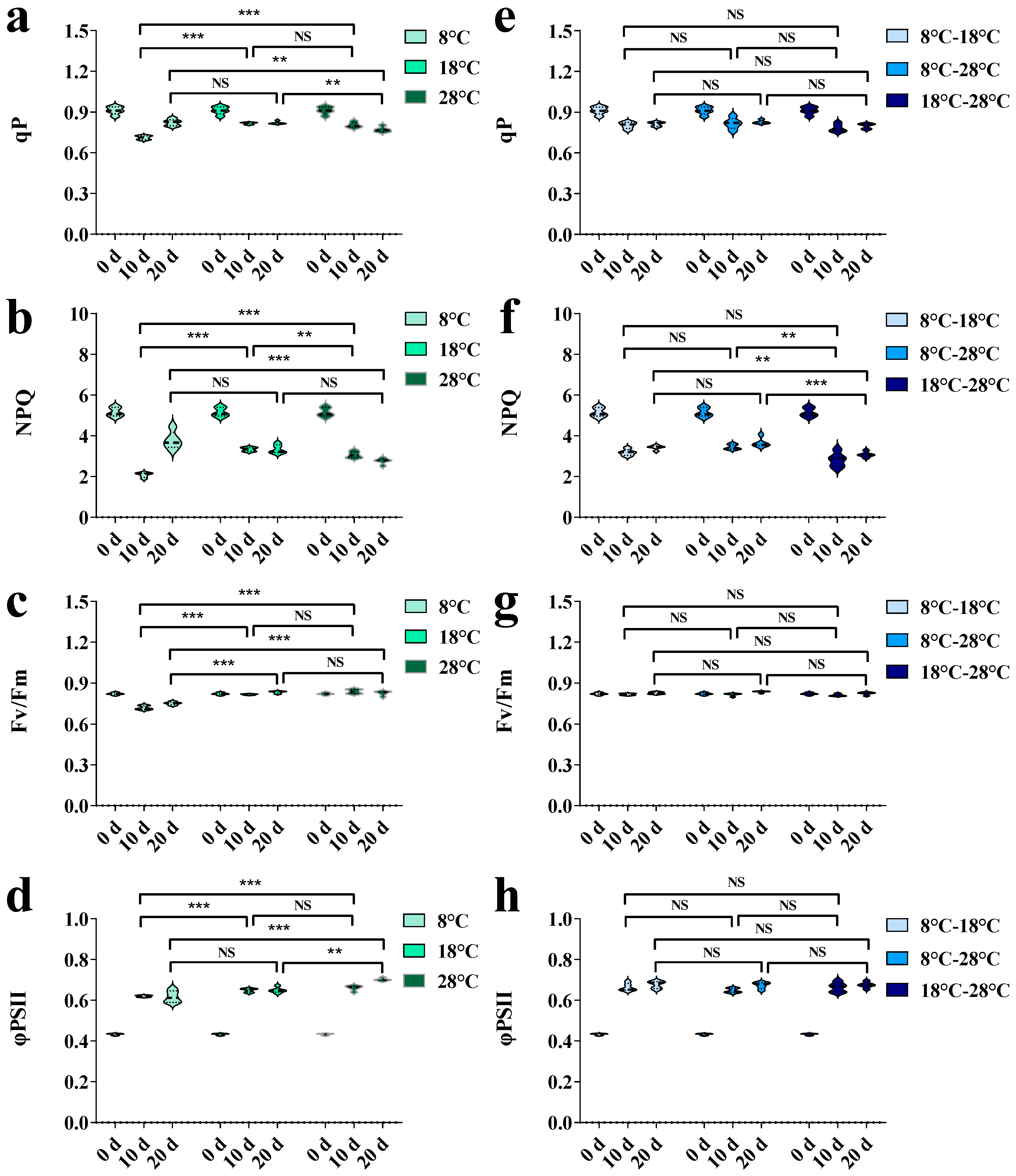
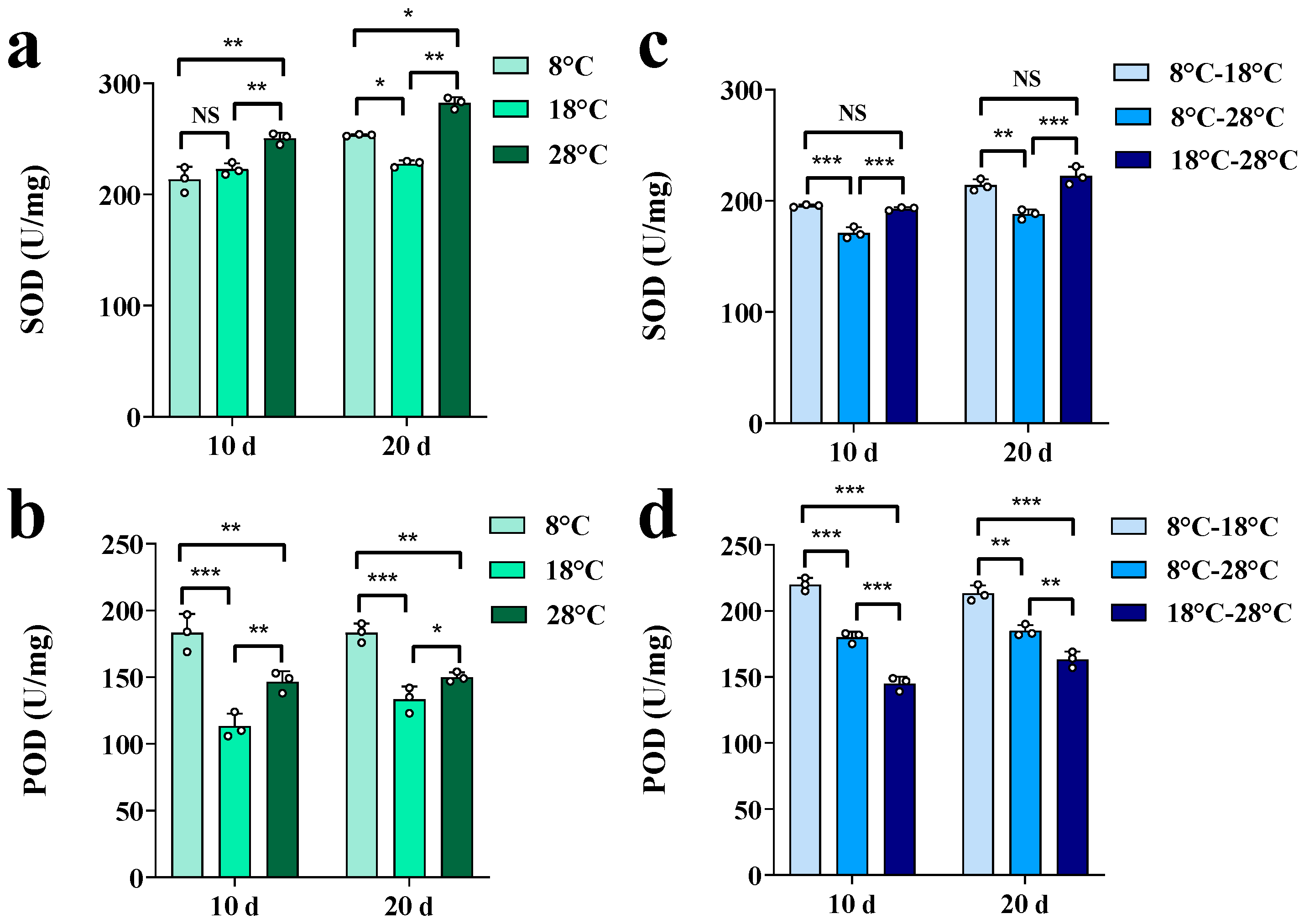
3.1. Effects of Temperature Regulation on Growth Characteristics and Physiological Indexes
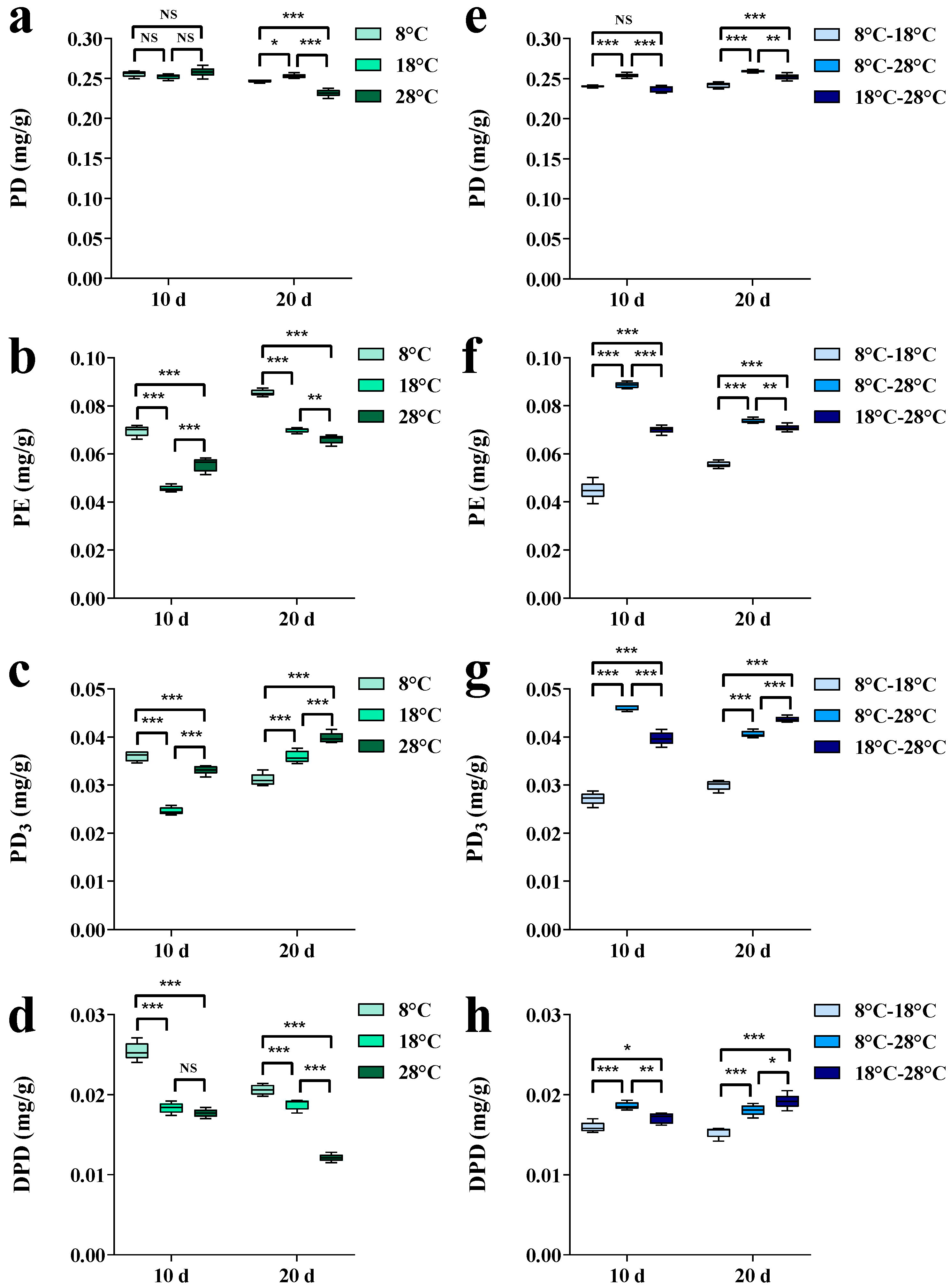
3.2. Effects of Temperature Regulation on Key Enzyme Gene Expressions and Platycodin Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Luca, D.; Omid, A.; Giles, E.; John, H.; Graham, O. The cannabinoid profile and growth of hemp (Cannabis sativa L.) is influenced by tropical daylengths and temperatures, genotype and nitrogen nutrition. Ind. Crop Prod. 2022, 178, 114605. [Google Scholar]
- Torabi, B.; Adibnya, M.; Rahimi, A.; Azari, A. Modeling flowering response to temperature and photoperiod in safflower. Ind. Crop Prod. 2020, 151, 112474. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, Y.; Han, M.; Yang, L. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress. Planta 2021, 253, 79. [Google Scholar] [CrossRef]
- Allakhverdiev, S.; Kreslavski, V.; Klimov, V.; Los, D.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Gifford, R. Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature long-term vs short-term distinctions for modelling. Glob. Chang. Biol. 1995, 1, 385–396. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, W.; Zhao, Y.; Li, X.; Fang, Y.; Wu, J.; Zeng, X.; Yang, N.; Wang, Y.; He, L. Effects of low nocturnal temperature on photosynthetic characteristics and chloroplast ultrastructure of winter rapeseed. Russ. J. Plant Physiol. 2016, 4, 451–460. [Google Scholar] [CrossRef]
- Wise, R.; Olson, A.; Schrader, S.; Sharkey, T. Electron transport is the functional limitation of photosynthesis in field grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Li, Y.; Liu, P.; Zhao, S.; Gao, H.; Zhang, Z. Photoprotection by mitochondrial alternative pathway is enhanced at heat but disabled at chilling. Plant J. 2020, 104, 403–415. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Salvucci, S.; Crafts, B. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Plant Physiol. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, H.; Zhai, R.; Xu, K.; Fu, X. Phytochemical and pharmacological studies of natural saponins from Platycodon grandiflorum. Rec. Nat. Prod. 2023, 17, 963–985. [Google Scholar]
- Zhang, Y.; Sun, M.; He, Y.; Gao, W.; Wang, Y.; Yang, B.; Sun, Y.; Kuang, H. Polysaccharides from Platycodon grandiflorum: A review of their extraction, structures, modifications, and bioactivities. Int. J. Biol. Macromol. 2024, 271, 132617. [Google Scholar] [CrossRef]
- Zhang, S.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Platycodon grandiflorum (Jacq.) A. DC. A review of phytochemistry, pharmacology, toxicology and traditional use. Phytomedicine 2022, 106, 154422. [Google Scholar] [CrossRef]
- Lichtenthaler, H. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Mahmoud, S.; Croteau, R. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Lange, B.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jeong, J.; Seo, J.; Shin, C.; Kim, Y.; In, J.; Yang, D.; Yi, J.; Choi, Y. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004, 45, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, W.; Yao, L.; Wang, J.; Gao, W. Effect of temperature on morphology, ginsenosides biosynthesis, functional genes, and transcriptional factors expression in Panax ginseng adventitious roots. J. Food Biochem. 2019, 43, e12794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Han, M.; Yang, L.; Han, Z.; Cheng, L.; Sun, Z.; Yang, L. The effects of environmental factors on ginsenoside biosynthetic enzyme gene expression and platycodin abundance. Molecules 2018, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Chang, X.; Dong, N.; Chen, B.; Wang, J.; Zha, L.; Gui, S. Genome-wide identification and expression profiling of the WRKY gene family reveals abiotic stress response mechanisms in Platycodon grandiflorus. Int. J. Biol. Macromol. 2024, 257, 128617. [Google Scholar] [CrossRef] [PubMed]
- The State Commission of Pharmacopoeia. Pharmacopoeia of People’s Republic of China; Part I; The Medicine Science and Technology Press: Beijing, China, 2020; p. 289.
- Shi, Y.; Ding, Y.; Yang, S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, H.; Roy, S.; Boo, H.; Woo, S.; Kim, H. Effects of temperature, light intensity and DIF on growth characteristics in Platycodon grandiflorum. J. Crop Sci. Biotechnol. 2019, 22, 379–386. [Google Scholar] [CrossRef]
- Nguyen, T.; Lee, J.; Lee, G.; Cho, K.; Cho, D.; Son, K. Optimization of cultivation type and temperature for the production of balloon flower (Platycodon grandiflorum A. DC) sprouts in a plant factory with artificial lighting. Horticulturae 2022, 8, 315. [Google Scholar] [CrossRef]
- Schwenkert, S.; Fernie, A.; Geigenberger, P.; Leister, D.; Moehlmann, T.; Naranjo, B.; Neuhaus, H. Chloroplasts are key players to cope with light and temperature stress. Trends Plant Sci. 2022, 27, 577–587. [Google Scholar] [CrossRef]
- Kitashova, A.; Adler, S.; Richter, A.; Eberlein, S.; Dziubek, D.; Klipp, E.; Nägele, T. Limitation of sucrose biosynthesis shapes carbon partitioning during plant cold acclimation. Plant Cell Environ. 2022, 46, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Ploschuk, E.; Bado, L.; Salinas, M.; Wassner, D.; Windauer, L.; Insausti, P. Photosynthesis and fluorescence responses of Jatropha curcas to chilling and freezing stress during early vegetative stage. Environ. Exp. Bot. 2014, 102, 18–26. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Ke, J.; Zhou, K.; Wang, J.; Dai, B.; Xue, S.; Zhou, Y.; Xie, Y.; Wang, Y. Evaluation of low-temperature adaptability of different of Lily varieties. Appl. Ecol. Environ. Res. 2022, 2101, 665679. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Maize seed germination under low-temperature stress impacts seedling growth under normal temperature by modulating photosynthesis and antioxidant metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Goh, H.; Khairudin, K.; Sukiran, N.; Normah, M.; Baharum, S. Metabolite profiling reveals temperature effects on the VOCs and flavonoids of different plant populations. Plant Biol. 2020, 95, 197–203. [Google Scholar] [CrossRef]
- Palaniyandi, K.; Wu, J. Low temperature enhanced the podophyllotoxin accumulation vis-a-vis its biosynthetic pathway gene(s) expression in Dysosma versipellis (Hance) M. Cheng-A pharmaceutically important medicinal plant. Process Biochem. 2016, 18, 130–139. [Google Scholar] [CrossRef]
- Vashisth, D.; Kumar, R.; Rastogi, S.; Patel, V.; Kalra, A.; Gupta, M.; Gupta, A.; Shasany, A. Transcriptome changes induced by abiotic stresses in Artemisia annua. Sci. Rep. 2018, 8, 3423. [Google Scholar] [CrossRef]
- He, J.; Yao, L.; Pecoraro, L.; Liu, C.; Wang, J.; Huang, L.; Gao, W. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2022, 43, 680–697. [Google Scholar] [CrossRef]
- Di, P.; Sun, Z.; Cheng, L.; Han, M.; Yang, L.; Yang, L. LED light irradiations differentially affect the physiological characteristics, ginsenoside content, and expressions of ginsenoside biosynthetic pathway genes in Panax ginseng. Agriculture 2023, 13, 807. [Google Scholar] [CrossRef]





| Gene | Primer Sequence (5′−3′) |
|---|---|
| PgGAPDH | F: CAGGGAGGCTTTTAGTTCAGGT |
| R: ATCACATCTACACCCCTCCAGC | |
| PgAACT | F: CCTCAATACCCCCAAGAGTGTC |
| R: AATGAAGCCTTTGCTGTCGTC | |
| PgPMK | F: ATCGTTGGCAGCCCTTCC |
| R: CCAGTCTTTGCTACTTCAGGCTT | |
| PgMVK | F: CCTTTAGCATCATCATTTCGCA |
| R: CCATACTCTAAAACTGTTGTTCGCTA | |
| PgMVD | F: ACATCTCCTTTGGATTTCTGCG |
| R: CTTCAGAGGCTGCTTTTTCACTT | |
| PgSS | F: CGGATGATTTCTACCCGTTGTT |
| R: CTGTTGAATAACGAGGGCGAAG | |
| PgSE | F: CACCACGACTTCTATCAACGGA |
| R: GAGATAGCCGCCTGGTTGTAG | |
| Pgβ-AS | F: GTTGGTCGTCTCCCACAATCAC |
| R: CCAGCAGTGACTCCCTAAACCA | |
| PgUGT2 | F: AGAGCGTGTGGTGTGGGGT |
| R: CACCGTTCTGAAATCCCTCCTAT | |
| PgUGT4 | F: CCCACAATGAGTCACGAATCC |
| R: TTCATTTGGGTAATAAGGAAAGTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yan, Y.; Han, M.; Yang, L. Effects of Temperature Regulation on the Physiological Characteristics and Platycodin Synthesis of Platycodon grandiflorum. Horticulturae 2024, 10, 848. https://doi.org/10.3390/horticulturae10080848
Wang Z, Yan Y, Han M, Yang L. Effects of Temperature Regulation on the Physiological Characteristics and Platycodin Synthesis of Platycodon grandiflorum. Horticulturae. 2024; 10(8):848. https://doi.org/10.3390/horticulturae10080848
Chicago/Turabian StyleWang, Zhuang, Yan Yan, Mei Han, and Limin Yang. 2024. "Effects of Temperature Regulation on the Physiological Characteristics and Platycodin Synthesis of Platycodon grandiflorum" Horticulturae 10, no. 8: 848. https://doi.org/10.3390/horticulturae10080848
APA StyleWang, Z., Yan, Y., Han, M., & Yang, L. (2024). Effects of Temperature Regulation on the Physiological Characteristics and Platycodin Synthesis of Platycodon grandiflorum. Horticulturae, 10(8), 848. https://doi.org/10.3390/horticulturae10080848





