Abstract
Cold stress causes considerable damage to tender tea seedlings. Previous studies have explored changes in the physiological and biochemical factors of tea in response to cold stress; however, the mechanisms of cold resistance in ancient tea tree plants are unclear. The aim of this study was to analyze the effects of 0 °C cold stress for 15 days and 24 °C ambient temperature recovery for 5 days on the physiological and biochemical characteristics of two representative old tea varieties: Dali tea and Siqiu tea. The results revealed significant changes in antioxidant, photosynthetic efficiency, and physiological and biochemical indicators in response to cold stress, with the two species exhibiting different patterns. Cold stress decreased chlorophyll and carotene content, Fv/Fm, Y(II), non-photochemical quenching coefficient, photochemical quenching, and superoxide dismutase (SOD) activity, and increased intercellular CO2 concentration and ascorbate peroxidase activity. Siqiu tea showed a higher increase in soluble sugar content and antioxidant enzyme activity and a lower accumulation of malondialdehyde and minimal fluorescence (F0) than Dali, indicating a greater tolerance to cold stress. Based on partial least-squares discriminant analysis, six key differential physiological indicators of cold resistance—water-soluble sugar, F0, peroxidase, catalase, SOD, and gas conductance—were identified. Our findings provide technical support for identifying ways to protect ancient tea trees from extreme weather conditions.
1. Introduction
Tea is the second most consumed beverage in the world after water and is strongly favored by consumers because of its abundant beneficial compounds and unique flavor profile [1,2]. Tea plants originated in southwestern China and are sensitive to temperature, which is one of the strongest environmental influences on their distribution, yield, and economic benefits [3]. Among the various weather anomalies experienced by tea plants, the occurrence of “late spring frost” has the most significant negative effect on tea production and flavor. “Late spring frost” refers to a sudden drop in temperature after the initial warm-up in early spring, which can result in freezing, browning, and scorching damage to tender new shoots. Such damage can lead to a sharp decrease in yield and even complete crop failure, resulting in substantial economic losses for tea farmers.
Compared with low-altitude tea areas in the south, the aroma compounds and contents of tea in high-altitude areas are higher than those in low-altitude areas, but the phenomenon of “cold spring inversion” in high-altitude areas is more likely to cause the death of rare and ancient tea trees and the disease of tea trees seedlings [4]. Ancient tea trees are important but scarce germplasm resources and are widely considered to be valuable because of their elegant flavor, long-lasting aftertaste, and intense aroma [5]. Among them, Dali tea (Camellia taliensis (W. W. Sm.) Melch.) is predominantly distributed in Yunnan Province, China, at an altitude of 1500–2400 m. Dali, the largest wild ancient tea tree, is found at the highest altitude and listed as a second-class protected plant in China [6]. Siqiu tea (Camellia tetracocca Zhang) is a rare ancient tea tree distributed in southwestern Guizhou Province, China, at an altitude of 1700–1950 m. Ancient tea seed fossils were discovered in 1980, extending the history of tea by more than one million years [7].
Several studies have explored the cold resistance mechanisms of tea trees during winter [5]. Tea tree varieties with strong cold resistance have high antioxidant enzyme activities, such as superoxide dismutase (SOD), catalase (CAT), and SO [8]. Other studies have found that tea tree varieties with strong cold resistance have high peroxidase (POD) activity in fall shoots and high water-soluble sugar (WSS) content [9]. The primary light energy conversion efficiency of photosystem II (PSII) (Fv/Fm) is an indicator of its energy-capture efficiency. Differences in PSII (Fv/Fm) have been observed between tea varieties with different levels of cold tolerance during the recovery phase [10,11]. Research has shown that low temperatures lead to a decrease in Fv/Fm in tea plants, with a greater decrease observed in cold-sensitive varieties than in cold-tolerant varieties [12]. In a study on Yunwu gong tea, it was found that soluble protein content (SP) peaked at −2 °C and subsequently declined at lower temperatures, soluble sugar content significantly increased at −6 °C and −10 °C, malondialdehyde (MDA) content rose under all low-temperature treatments and was positively correlated with the degree of cold stress [13]. Low-temperature stress also decreases chlorophyll content in tea leaves, thereby affecting photosynthesis [14,15]. Cold temperatures can also significantly reduce the values of various gas exchange parameters, such as net photosynthesis rate (Pn), gas conductance (Gs), transpiration rate (Tr), and potential water use efficiency (RWUEi) in tea leaves [8,16].
This study makes up for the gaps in the previous studies on the cold resistance of ancient tea trees and deepens our understanding of the response mechanism of tea buds to “late spring frost” [3]. In this study, Dali tea and Siqiu tea, two representative ancient tea varieties, were selected as experimental objects. In an experimental environment simulating low-temperature stress, we investigated the cold resistance mechanism of these two varieties and identified the key biological indicators affecting their cold resistance. By comparing and analyzing the changes in 21 different physiological parameters over time, using correlation analysis and partial least squares discriminant analysis (PLS-DA), This study successfully identified several key indicators closely associated with the cold resistance of ancient tea trees. The research results not only lay a theoretical foundation for the in-depth understanding of the biological mechanism of tea trees against late spring frost but also provide a scientific basis for the protection of ancient tea tree resources and the optimization of cultivation and management practices.
2. Materials and Methods
2.1. Plant Materials and Treatments
One-year-old healthy and similarly sized Dali tea (Camellia taliensis (W. W. Sm.) Melch.) and Siqiu tea (Camellia tetracocca Zhang) seedlings were transferred to 5 L plastic pots. Each pot contained two-thirds of the soil used for growth. There were 25 pots of each variety. These seedlings were derived from tea seeds collected in June 2021 from Zhenyuan County in Yunnan Province and Puan County in Guizhou Province and cultivated in the Qiumu Garden of the Southwest Forestry University (Panlong District, Kunming City, Yunnan Province, China). Before the experiment, they were regularly managed in the shade of outdoor trees. The experiments were conducted at the Laboratory of Gardening and Horticulture at Southwest Forestry University, situated in the vibrant city of Kunming, Yunnan Province, China.
The parameters for the artificial climate chamber (German Binder climate chamber, KBWF240) were set as follows: temperature of 24 °C, relative humidity of 85%, the light intensity of 200 μmol·m−2·s−1 (equivalent to 16,000 lx), and a photoperiod of 12 h. Once the parameters were stabilized, all tea seedlings were placed in an artificial climate chamber for cultivation. The duration of the treatment was set to 18 days. Initially, the artificial climate chamber gradually decreased the temperature at a rate of 5 °C per hour until it reached the target temperature of 0 °C, where it was maintained constant. Subsequently, the samples underwent a 15-day period of continuous low-temperature stress, with physiological indices being measured every 5 days. Afterward, the temperature was raised back to 24 °C at a rate of 5 °C per hour and maintained for an additional 3 days before final measurements were taken. A comparative analysis was performed between the four sets of measurement data obtained post-treatment and the initial pre-treatment values to comprehensively assess the impacts of low-temperature stress on the samples (Figure 1).
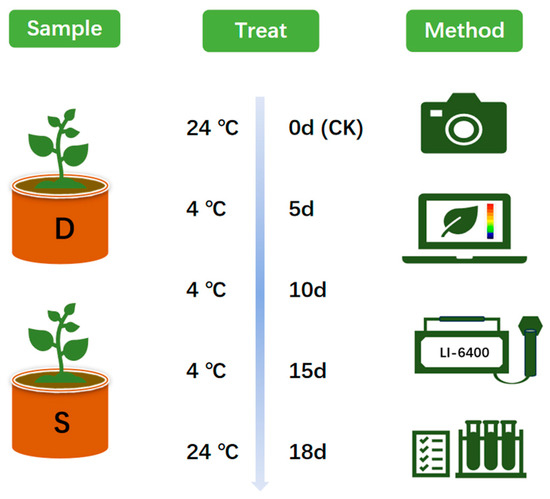
Figure 1.
Plant Materials and Treatments. D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). d, Day.
After each stage of the cold stress treatment, five pots were selected from each of the two varieties for measurements of plant growth, photosynthesis, and chlorophyll fluorescence parameters, including net photosynthesis rate (Pn), gas conductance (Gs), intercellular CO2 concentration (Ci), Tr, chlorophyll (Chl), carotene (CART), minimal fluorescence (F0), Conversion efficiency of primary light energy of PSII (Fv/Fm), light energy conversion efficiency (Y(II)), non-photochemical quenching coefficient (NPQ), and photochemical quenching (qp). Additionally, mature leaf tissue samples were collected from one-year-old seedlings of two ancient tea tree cultivars. Enzymatic activities of peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD), along with soluble protein (SP), water-soluble sugar (WSS), and malondialdehyde (MDA) content, were analyzed. Immediately following collection, the leaf samples were immersed in liquid nitrogen and stored at −80 °C in a refrigerator at the Jisico Lab (Seoul, Republic of Korea) until further measurements could be taken.
2.2. Photosynthetic Parameters
The portable photosynthesizer LI-6400 (LI-COR Biosciences, Lincoln, NE, USA) was adopted. The measurements were taken in clear weather from 9:00 to 11:00 [17]. When the plant leaves were first exposed to 1000 μmol m−2·s−1 saturated photosynthetically active radiation (PAR) at a relative humidity of 60% to 70%, the CO2 concentration of the sample was 400 μmol−1. The reaction continued for at least 3 min (min) until the photosynthetic rate and stomatal conductance were relatively stable. An instrument was then used to measure photosynthetic characteristics, and this was repeated 3 times for each treatment group.
2.3. Chlorophyll Fluorescence Parameters
The chlorophyll fluorescence parameters were measured using the modulated chlorophyll Imaging-PAMM-series fluorescence imaging system (WALZ, Effeltrich, Germany). The processed tea seedling leaves were first placed in the dark for at least half an hour, and the measurements were conducted under dark conditions. Parameters including Fv/Fm, Y(II), and qp were determined for tea tree leaves. Three replicates were conducted for each treatment, and the selected leaves were carefully marked. Subsequent measurements were performed on the same leaves, and the average values were analyzed until the end of the experiment.
2.4. Chlorophyll and Carotene Content
The experiment was carried out to determine the chlorophyll and carotene content in the leaves of ancient tea trees. First, 0.1–0.2 g of fresh leaves are removed from the ancient tea tree sample, using acetone or ethanol as the extraction agent, and the leaves are lightly ground with a mortar and pestle to further promote the extraction of pigments. After the extract was filtered or centrifuged, the absorbance was measured under a spectrophotometer, especially the absorption peak of chlorophyll in the blue (about 430–450 nm) and red (about 640–660 nm) regions and the absorption peak of carotene in the blue-violet (about 450–470 nm) region. Finally, the content of chlorophyll and carotene in the leaf per unit weight was calculated according to the standard curve, leaf mass, and extracted liquid volume.
2.5. Antioxidant Enzyme Activity
Fresh leaf samples (200 mg) were immersed in 1.6 mL 50 mM phosphate buffer (pH 7.8) on ice and then centrifuged at 12,000× g for 20 min at 4 °C. The supernatant was used to measure antioxidant enzyme activity.
SOD activity was measured by the reduction in Tetranitroblue tetrazolium chloride (NBT) [18]. In brief, 40 μL of enzyme extract was mixed with a reaction mixture consisting of 3 mL (14.5 mM Met, 30 μM Disodium edetate dihydrate (EDTA-Na2) solution, 50 mM phosphate buffer (pH 7.8), 2.25 mM NBT solution, and 6 μM riboflavin solution). The absorbance at 560 nm was recorded to determine SOD activity.
POD activity was estimated using the guaiacol oxidation colorimetric method [14]. In short, a reaction mixture of 3 mL containing 10 mM phosphate buffer, 40 mM H2O2, 20 mM guaiacol, and 0.01 mL enzyme solution was used. The absorbance was measured at 470 nm, with a 30 s interval for a total duration of 3 min.
CAT activity was determined using the UV analysis method [17]. In brief, a reaction mixture of 3 mL containing 0.1 mL enzyme extract, 50 mM potassium phosphate buffer (pH 7.0), and 33 mM H2O2 was used. CAT activity was measured at 240 nm.
APX activity followed the method [19]. The reaction mixture consisted of 250 μL phosphate buffer (pH = 7), EDTA, 10 μL H2O2 (1 mmol), 250 μL sodium ascorbate (0.25 mmol), and 50 μL enzyme solution. The absorbance at 290 nm was measured for 1 min. Enzyme activity was calculated using the molar absorption coefficient of 2.8 mmol−1 cm−1. The obtained value represented the activity of ascorbate peroxidase based on the micro-moles of ascorbate oxidized per min.
2.6. SP Content
The reaction solution consisted of 100 μL of enzyme extract, 200 μL of Bradford reagent, and 700 μL of deionized water (Bioroyee, Beijing, China). After 2 min of complex formation, the Bradford reagent exhibited the highest degree of binding with amino acids. The absorbance was measured at 535 nm. The protein content of the samples was calculated based on the standard curve obtained using a defined amount of bovine serum albumin [19].
2.7. WSS Content
The soluble sugar content was determined using the anthrone colorimetric method [20]. The soluble sugars in the leaf meat (Select leaves from consistent positions, avoid the main vein, and cut them into thin strips of 2 mm. Take 0.2 g of the strips) were extracted using anhydrous ethanol under 85 °C water bath conditions. To measure the soluble sugar content, the supernatant was added to an anthrone solution, and after incubation at 95 °C for 15 min, the absorbance at 620 nm was measured. The soluble sugar content (mg·g−1 FW) was calculated based on the absorbance value.
2.8. MDA Content
Using 2 mL homogenate of 20% trichloroacetic acid containing 0.05% thiobarbituric acid (TBA), 0.2 g of uniformly positioned leaves were taken, and the main vein was avoided. The samples were then incubated at 95 °C for 30 min before being transferred to ice. The sample was then centrifuged at 5180× g for 10 min, and absorbance was measured at 532 and 600 nm (UV-Vis spectrophotometer Model 1900i, Shimadzu, Japan). At the dark coefficient of 155 mmol cm−1, the lipid peroxidation range was obtained by the difference between absorption wavelengths [19].
2.9. Statistical Analysis
The experiment of physiological and biochemical indexes of each sample was repeated 3 times, and the results were expressed as mean ± standard error. Origin 2023 software (OriginLab, Northampton, MA, USA) was used to create bar charts, and SPSS 26 (International Business Machines Corporation, Armonk, NY, USA) was used to analyze the significance of the statistical analyses; p values < 0.05 indicated significant differences. SPSS 26 software was used to analyze the correlations between different physiological and biochemical indicators under low-temperature stress. Differences between the two groups were determined with a two-tailed student’s t-test. They were analyzed by Pearson correlation p ≤ 0.05 was considered to be significant. SIMCA 13.0 software (Umetrics, Umea, Sweden) was used to select the key indicators of low-temperature stress for the two ancient tree varieties. The processed data were input into SIMCA V14.1 (Umetrics AB, Umea, Sweden) software for orthogonal projections to latent structure discriminant analysis (OPLS-DA). The OPLS-DA model was validated by the permutation test consisting of 200 times repetitions.
3. Results
3.1. Effects of Low-Temperature Stress on Phenotypic Changes in Tea Seedlings
The phenotypic characteristics of the two tea plants varied under low-temperature stress (Figure 2). Specifically, after 5 d of low-temperature treatment, there were no noticeable changes in leaf shape in either variety, and no symptoms of dehydration were observed. After 10 d of treatment, both varieties showed faint red spots at the edges of the young leaves. After 15 d of treatment, Dali tea exhibited more pronounced damage than Siqiu tea, which manifested as browning, wilting, and dehydration of the leaves. Three days after stress (18 d), a significant difference was observed in the cold-resistance recovery ability of the two varieties. Dali tea continued to exhibit noticeable leaf browning, wilting, and dehydration, whereas the symptoms of damage in Siqiu tea improved.

Figure 2.
Effects of low-temperature stress on phenotypic changes in tea seedlings. D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). d, Day.
3.2. Effects of Low-Temperature Stress on Photosynthetic Characteristics
Pn, Gs, Ci, and Tr are important indicators of photosynthesis and represent the ability of plants to absorb CO2 and produce carbohydrates [21]. As the duration of low-temperature stress increased, the Pn, Gs, and Tr values of the leaves of the two tea varieties decreased (Figure 3). The first five days were characterized by drastic changes, followed by a leveling off and then rapid recovery during the warming period. The Pn, Gs, and Tr values of Siqiu tea were significantly higher than those of Dali tea at various stress points. Gs and Tr values in Dali tea leaves decreased to their lowest after 15 d of treatment (p < 0.05); Pn, Gs, and Tr decreased by 98.5%, 87.0%, and 81.7%, respectively, from their values at 0 d (Figure 3b,d). In contrast, Gs and Tr values in Siqiu tea leaves reached their lowest at 10 d and showed a slight increase at 15 d; however, this difference was not significant (p > 0.05). The Ci values for both tea species increased with prolonged low-temperature stress and then decreased after returning to normal temperatures (Figure 3c).
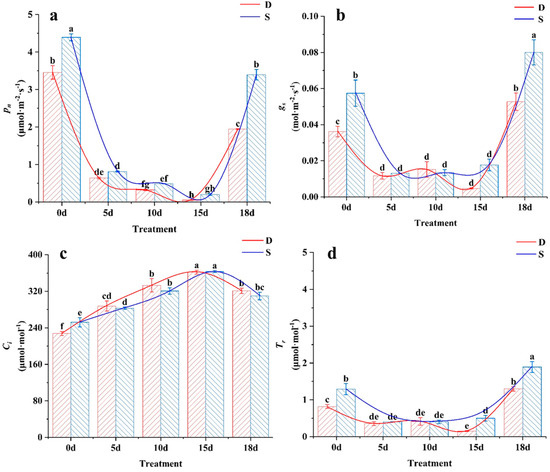
Figure 3.
Effects of low-temperature stress on photosynthetic characteristics: D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). (a) Pn, net photosynthesis rat; (b) Gs, gas conductance; (c) Ci, intercellular CO2 concentration; (d) Tr, transpiration rate. Data are presented as mean ± standard deviation (n = 3). Mean values with different lowercase letters indicate significant differences based on the least significant difference (LSD) test (p < 0.05). Red represents Dali tea, blue represents Siqiu tea.
3.3. Effects of Low-Temperature Stress on Chlorophyll Content
Chl and CART are photosynthetic pigments involved in the absorption, transfer, and conversion of light energy and are key factors affecting the photosynthetic capacity of plants [22]. As shown in Table 1, before treatment (0 d), the contents of Chl a and CART in Dali tea were higher than those in Siqiu tea, while the contents of Chl b and Chl a+b in Dali tea were lower. Under low-temperature stress, there was a significant decrease in Chl a, Chl b, and CART, indicating that the photosynthetic pigments underwent degradation reactions in response to long-term low-temperature exposure but then recovered rapidly during the warming period [23]. Chl a/b showed an initial decrease with low-temperature stress, followed by an increase, indicating that the rate of Chl a degradation was faster than that of Chl b under prolonged low-temperature stress. The degradation of Chl a and Chl a+b in Siqiu tea was significantly higher (p < 0.05) than that in Dali tea under low-temperature stress, but the recovery of Siqiu tea was significantly better (p < 0.05) than that of Dali tea during the recovery period, with higher levels of Chl a, Chl b, and Chl a+b.

Table 1.
Effects of low-temperature stress on the photosynthetic pigment content of seedlings leaves in Dali Tea (D) and Siqiu Tea (S).
3.4. Impact of Low-Temperature Stress on Chlorophyll Fluorescence Characteristics
The chlorophyll fluorescence parameters F0, Fv/Fm, Y(II), NPQ, and qp serve as effective indicators of the photosynthetic physiological status of plants under non-biological stressors [24]. Higher NPQ and Fv/Fm values indicate stronger resistance to low temperatures. As shown in Table 2, under low-temperature stress, Fv/Fm, Y(II), NPQ, and qp all showed a decreasing trend in both varieties. During the stress recovery period (18 d), these parameters exhibited an increasing trend. There were no significant differences in Fv/Fm, Y(II), NPQ, and qp of primary leaves between the tea varieties. However, the performance of the varieties under low-temperature stress differed significantly (p < 0.05), with Siqiu tea showing significantly better preservation of Fv/Fm, Y(II), and qp compared with Dali tea. These results indicate that Dali tea was more sensitive to stress, with low temperatures leading to a significant decrease in the maximum photosynthetic efficiency of PSII and Fv/Fm values [25].

Table 2.
Effects of low-temperature stress on chlorophyll fluorescence characteristics in the leaves of Dali tea (D) and Siqiu tea (S) seedlings.
3.5. Effects of Low-Temperature Stress on Antioxidant Enzyme Activity
POD is widely present in plant tissues and can work with flavin proteins to alleviate the toxicity of H2O2, stabilize membrane structures, and enhance plant cold resistance. The POD activity of the tea varieties increased under low-temperature stress (Figure 4a), which is consistent with the results of previous studies [26]. The POD activity of Siqiu tea was significantly higher (p < 0.05) than that of Dali tea.
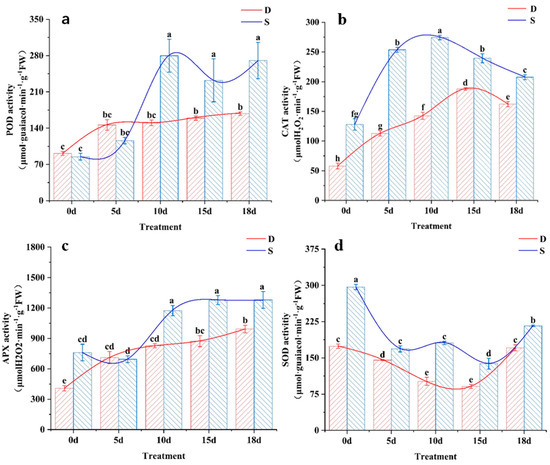
Figure 4.
Effects of low-temperature stress on antioxidant enzyme activity. D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). (a) POD, peroxidase; (b) CAT, catalase; (c) APX, ascorbate peroxidase; (d) SOD, superoxide dismutase. Data are presented as mean ± standard deviation (n = 3). Mean values with different lowercase letters indicate significant differences based on the least significant difference (LSD) test (p < 0.05). Red represents Dali tea, blue represents Siqiu tea.
CAT plays a crucial role in cold resistance in plants and influences their metabolic intensity and disease resistance. Under continuous low-temperature stress, the CAT activity of Dali and Siqiu tea leaves increased significantly and then decreased after returning to normal temperatures. Furthermore, the CAT activity of Siqiu tea was significantly higher (p < 0.05) than that of Dali tea at all cold stress stages (Figure 4b).
APX is an important antioxidant enzyme involved in the clearance of H2O2 from chloroplasts during the metabolism of plant reactive oxygen species. During the low-temperature stress and recovery periods, the APX activity of Dali showed an increasing trend, followed by stabilization and another significant increase after 10 d of stress (Figure 4c). At 10–18 d, the APX activity of Siqiu tea was significantly higher than that of Dali tea (p < 0.05) and showed different changes, first decreasing, rapidly increasing, and then stabilizing within 5–10 d. SOD catalyzes superoxide radicals and is induced by reactive oxygen species under low-temperature conditions, thereby alleviating damage to cell membranes [27]. During the low-temperature stress and recovery period, the SOD activity of both tea varieties showed an overall decrease during the stress period, reaching the lowest point at 15 d of cold stress, followed by an increase during recovery (Figure 4d). In each treatment stage (0–18 d), the SOD maintenance ability of Siqiu tea was significantly higher than that of Dali tea (p < 0.05).
Overall, Siqiu tea exhibited significantly higher POD, CAT, APX, and SOD activities than Dali tea under low-temperature stress, indicating its superior cold resistance. These results are consistent with the findings for photosynthetic characteristics, pigment content, and fluorescence properties (Table 1 and Table 2; Figure 2 and Figure 3).
3.6. Effects of Low-Temperature Stress on Osmoregulation Substances and MDA Content
SP is an important osmoregulatory substance in plants and is closely related to plant cold tolerance. Under the low-temperature treatment, SP showed a similar increase in both tea varieties with prolonged stress, reaching a peak at 15 d (p < 0.05) (Figure 5a). After the stress was removed (18 d), there was a significant decrease in SP, with a greater decrease in Dali tea than in Siqiu tea, with a greater decrease in Dali tea than in Siqiu tea. In this study, it was found that SP content in Dali tea was higher than that in Siqiu tea with high resistance, and SP content was greatly increased under low-temperature treatment. As for the SP difference between the two varieties in the leaves without low-temperature treatment, this part needs to be further studied in the relevant molecular biology so as to clarify the internal relevant gene expression differences to determine and explain. The WSS of Dali tea was significantly lower than that of Siqiu tea, both in the original leaves and under low-temperature stress (p < 0.05). In addition, a slight increase in WSS in Dali tea over the experimental period was not significant (p > 0.05) (Figure 5b). In contrast, the WSS in Siqiu tea significantly increased after 5 d, reaching its peak at 15 d, with a 252.2% increase over the value at 0 d.
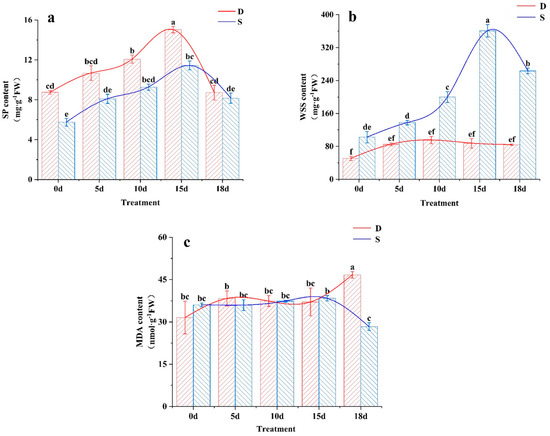
Figure 5.
Effects of low-temperature stress on osmoregulation substances and MDA content. D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). (a) SP, soluble protein; (b) WSS, water-soluble sugars; (c) MDA, malondialdehyde. Data are presented as mean ± standard deviation (n = 3). Mean values with different lowercase letters indicate significant differences based on the least significant difference (LSD) test (p < 0.05). Red represents Dali tea, blue represents Siqiu tea.
MDA is directly related to the degree of lipid peroxidation under low-temperature stress, and higher MDA levels indicate more severe damage to tea trees [28]. As shown in Figure 5c, the MDA content in both tea varieties fluctuated with the duration of cold stress, but there was no significant difference between the varieties (p > 0.05). After stress recovery, the MDA content in Dali tea leaves was significantly higher (p < 0.05) than that in Siqiu tea leaves, indicating that Dali tea leaves had weaker cold resistance (p < 0.05).
3.7. Correlation Analysis of Physiological and Biochemical Characteristics
There were significant correlations between the different physiological and biochemical characteristics under low-temperature stress (p < 0.05; Figure 6). F0, Fv/Fm, Y(II), NPQ, and qp showed significant positive correlations with Chl a, Chl b, Chl a+b, and CART contents (p < 0.05). The results for chlorophyll fluorescence and photosynthetic characteristics showed that Ci was negatively correlated with other indicators and significantly negatively correlated with Fv/Fm, Y(II), NPQ, qp, and Pn content (p < 0.05). The NPQ index showed a significant positive correlation with all measured photosynthetic indicators (p < 0.05). Except for a significant negative correlation with Ci, Pn showed significant positive correlations with all other measured photosynthetic pigments and photosynthetic indicators (p < 0.05). Chlorophyll showed mostly negative correlations with osmotic adjustment and antioxidant enzyme activity indicators (p < 0.05). Only the WSS index showed a significant positive correlation with POD, CAT, and APX (p < 0.05).
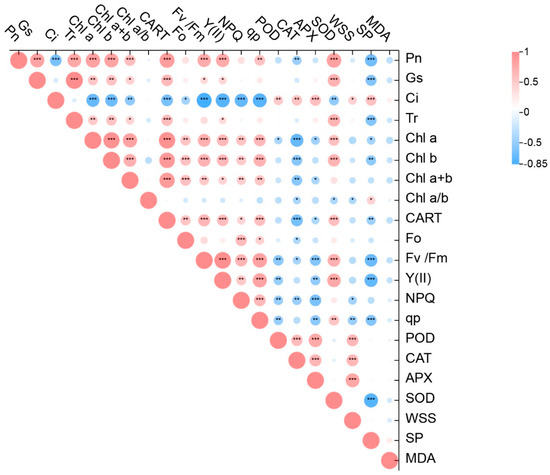
Figure 6.
Correlation analyses of physiological and biochemical characteristics in seedlings of two tea varieties. Pn, net photosynthesis rat; Gs, gas conductance; Ci, intercellular CO2 concentration; Tr, transpiration rate; Chl, chlorophyll; CATR, carotene; F0, minimal fluorescence; Fv/Fm, conversion efficiency of primary light energy of PSII; Y(II), light energy conversion efficiency; NPQ, non-photochemical quenching coefficient; qp, photochemical quenching; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; SOD, superoxide dismutase; SP, soluble protein; WSS, water soluble sugars; MDA, malondialdehyde. Data are presented as mean ± standard deviation (n = 3). After (LSD) test, * means significant difference (p < 0.05), ** means significant difference (p < 0.01), *** means significant difference (p < 0.001). Red represents Dali tea, blue represents Siqiu tea.
3.8. Screening Key Indicators to Characterize Cold Resistance in Two Tea Varieties
To further identify the key indicators that characterize the cold resistance/sensitivity of Dali and Siqiu teas, PLS-DA was applied based on 10 stress treatment points and 21 low-temperature stress-related physiological and biochemical indicators. The score plot (Figure 7a) shows that cold stress treatment had a significant differential impact on the cold resistance of different tea varieties. Dali tea showed a transformation trend of “lower right → lower left → upper right”, while Siqiu tea showed a transformation trend of “upper right → lower right → lower left → upper left → upper right”. Based on the loading plot (Figure 7b), F0, Chl, NPQ, and CART had the highest contents in tea leaves without low-temperature stress treatment, whereas CAT, POD, WSS, and APX were significantly enriched after prolonged (15 d) low-temperature stress in Siqiu tea. Variable importance in projection (VIP) analysis was performed to screen key indicators that could characterize low-temperature stress in tea plants (Figure 7c). Based on the VIP > 1 criterion, nine key differential indicators—WSS, MDA, F0, qp, POD, CAT, Tr, SOD, and Gs—were identified. These indicators can be used to identify cold resistance in ancient tea tree varieties. The higher the WSS value, the stronger the cold resistance, whereas the higher the F0 value, the weaker the cold resistance.
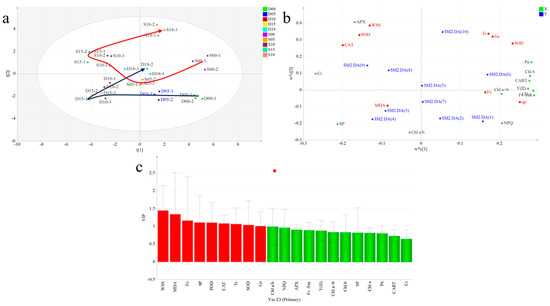
Figure 7.
Screening key indicators for characterizing cold-resistance ability in seedlings of two tea varieties: (a) dynamic trajectory plot drawn using PLS-DA; (b) PCA score scatter plot; (c) variable importance in projection (VIP) plot based on partial least squares-discriminant analysis (PLS-DA). Red bars represent volatile compounds with VIP > 1, while green represents VIP < 1. D, Dali tea (Camellia taliensis (W. W. Sm.) Melch.); S, Siqiu tea (Camellia tetracocca Zhang). Pn, net photosynthesis rat; Gs, gas conductance; Ci, intercellular CO2 concentration; Tr, transpiration rate; Chl, chlorophyll; CATR, carotene; F0, minimal fluorescence; Fv/Fm, conversion efficiency of primary light energy of PSII; Y(II), light energy conversion efficiency; NPQ, non-photochemical quenching coefficient; qp, photochemical quenching; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; SOD, superoxide dismutase; SP, soluble protein; WSS, water soluble sugars; MDA, malondialdehyde. Red represents Dali tea, blue represents Siqiu tea.
4. Discussion
The changes in cell structure, morphology, and some substances in tea under low-temperature stress lead to internal physiological disorders [29]. Under cold stress at 4 °C for 15 d, Pn, Gs, and Tr of two tea varieties significantly decreased before quickly rebounding during the recovery period. These findings indicate that cold stress has a significant inhibitory effect on stomata in the leaf of tea seedlings, which is consistent with previous studies on the low-temperature stress tolerance of cucumber [30]. Simultaneously, Ci increased significantly, indicating that lower temperatures caused stomatal blockage and affected CO2 assimilation and gas exchange, thereby inhibiting photosynthesis. Correlation analysis revealed a significant negative correlation (p < 0.05) between Ci and Pn, suggesting that as stress increased, the photosynthetic apparatus of plants gradually deteriorated, leading to a decrease in Pn and intracellular CO2 utilization and an increase in Ci. During the 10–15 d period of cold stress, the Pn, Gs, and Tr of Siqiu tea showed a slight increase, which was opposite to that of Dali tea. These increases indicated an enhanced photosynthetic respiration capacity of these plants, suggesting that Siqiu tea has a stronger adaptability to cold stress than Dali tea.
Previous studies have found that compared with the control, the chlorophyll content of “Longjing 43” and “Baiye 1” both decreased at low temperatures at 4 °C-4 d and 4 °C-6 d, which is consistent with this study, there is a significant positive correlation between the chlorophyll and carotenoid content of the two tea varieties [15]. At 18 d, the values of Chl a+b, Chl a/b, and Pn in the leaves of both tea varieties were lower than those at 0 d. These results indicate that prolonged cold temperatures inhibited chlorophyll synthesis and subsequently reduced light absorption and conversion efficiency, which resulted in ineffective utilization of the excitation energy and a decrease in Pn. Dali tea leaves showed noticeable browning and a significant decline in photosynthetic capacity, and the chloroplasts in Dali tea leaves had already disintegrated owing to severe damage [31]. Even with increasing temperatures, the chloroplasts could not recover, which was a key factor contributing to the later browning of some Dali tea leaves.
CART concentration decreases with decreasing temperature, but the response of CART and Chl a+b to temperature is largely varietal dependent. In this study, the CART concentration of Dali tea was significantly lower than that of Siqiu tea at the early 5 days of stress, decreased by 64.5% and 24.8%, respectively, but at 10 days, CART concentration of Dali tea was higher than that of Siqiu tea, which was 193.20 μg·g−1 FW and 155.59 μg·g−1 FW, respectively. As in Du’s study, different varieties respond differently to temperature. “xiaoxueya” is lower than “fudingdabai” at 25 °C but 94.4% lower than “fudingdabai” at 15 °C. Compared with 25 °C, CART concentration of ‘xiaoxueya’ and ‘fudingdabai’ decreased by 97.1% and 50.1%, respectively at 15 °C [32].
Additionally, this study found that Pn had a highly significant positive correlation (correlation coefficient of 0.83) with the Chl a/b ratio and chlorophyll content, which is consistent with previous research results on grapevines [33]. Cold-sensitive varieties produced more carotenoids than cold-tolerant varieties, indicating that they needed to dissipate more excitation energy. Our study indicated that during the three-day recovery process, the increase in carotenoids was significantly higher (p < 0.05) in Dali tea leaves than in Siqiu tea leaves, which also indicates that Dali tea is a cold-sensitive variety and that Siqiu tea is a cold-tolerant variety.
The five chlorophyll fluorescence parameters (F0, Y(II), Fv/Fm, NPQ, and qp) decreased with prolonged exposure to low-temperature stress in both tea varieties, eventually leading to a decrease in photosynthetic enzyme activity and photosynthetic efficiency. The Y(II) value rapidly decreased with the duration of cold stress, and the Y(II) value of Siqiu tea at all stages was higher than that of Dali tea, allowing Siqiu tea to accumulate more energy for dark reactions and promote carbon assimilation and organic matter accumulation. The Fv/Fm values of both tea varieties were above 0.75 at 0 d (0.76 and 0.76), while at 5 d, both Fv/Fm values were below 0.75 (0.50 and 0.65), indicating that leaf photosynthesis was inhibited by low-temperature stress. The Pn, Gs, Tr, Fv/Fm, and qp values significantly decreased with prolonged low-temperature exposure in both tea varieties, which is consistent with previous results for “Huashuo” and “Huaxin” Camellia seedlings [34].
With low-temperature stress, POD and CAT activities in the two tea varieties showed an initial increase, followed by a decrease. There was a significant difference in CAT activity between the two varieties, indicating that the cold resistance of the varieties varied. The decrease in temperature caused a decrease in CAT and SOD activity, leading to irreversible damage to the plants. Compared with Dali tea, Siqiu tea exhibited higher CAT and SOD activities, indicating better cold resistance.
This study demonstrated a close correlation between WSS content and cold resistance; higher WSS content corresponds to stronger cold resistance in plants, and the Siqiu tea variety with stronger cold resistance showed a greater increase in WSS content; the Dali tea variety, with weaker cold resistance, showed a smaller increase. The SP content in both tea varieties increased with prolonged exposure to low temperatures, and an increase in SP content enhanced plant resistance to low temperatures, which is consistent with previous findings [35]. The MDA content in both tea varieties fluctuated with prolonged low-temperature stress, and the variation from 0 to 15 d was not significant, indicating that low temperatures did not cause noticeable changes in the degree of cell membrane lipid peroxidation in tea trees. This result indicates that both tea varieties have good anti-lipid peroxidation capabilities.
Different tea varieties showed significant differences in cold resistance under low-temperature stress, and Siqiu tea exhibited greater cold resistance than Dali tea. The contents of F0, Chl, NPQ, and CART were the highest in the original leaves (before treatment), whereas CAT, POD, WSS, and APX were highly enriched after a prolonged period (15 d) of low-temperature stress in Siqiu Tea. Via a systematic analysis of evolutionary patterns, significant differences, correlation analysis, and PLS-DA, nine key indicators (WSS, MDA, F0, qp, POD, CAT, Tr, SOD, Gs) were identified to characterize the cold resistance of ancient tea tree varieties. Among these, WSS, POD, and CAT were positively correlated with cold resistance in ancient tea tree varieties.
5. Conclusions
This study investigated the physiological responses of two ancient tea tree saplings to simulated low-temperature stress and subsequent natural recovery conditions. It was found that early in the low-temperature stress, both types of tea saplings could effectively enhance the activity of antioxidant enzymes and regulate the content of osmotic regulators to mitigate the adverse effects of increased membrane permeability. As the duration of stress extended, the physiological indicators presented different dynamic patterns of change. The levels of Chl a, CART, Fv/Fm, Y(II), NPQ, qp, and SOD activity all showed a significant downward trend, reaching the lowest point on the 15th day. Conversely, the activities of Ci and APX exhibited a significant upward trend. The soluble sugar content of Siqiu tea increased with the prolongation of stress, while that of Dali tea was irregular. Siqiu tea had higher accumulation levels of physiological indicators such as CAT, POD, WSS, and APX under low-temperature stress, which was significantly superior to Dali tea, indicating that Siqiu tea possesses stronger cold resistance. Furthermore, the soluble sugar content of Siqiu tea significantly increased under low-temperature stress, while that of Dali tea did not change much. The higher accumulation levels of physiological indicators such as CAT, POD, WSS, and APX in Siqiu tea also suggested its stronger cold resistance. The PLS-DA results further confirmed this finding, highlighting the importance of physiological indicators like WSS, F0, POD, CAT, SOD, and Gs in distinguishing the cold resistance of the two tea tree species. The findings not only reveal the physiological adaptive mechanisms of the two ancient tea tree saplings under low-temperature stress but also provide a scientific foundation for assessing and comparing the cold resistance of different tea tree varieties. The research outcomes also offer crucial theoretical support for the conservation and management of ancient tea trees.
Author Contributions
Conceptualization, J.H. and X.H.; methodology, M.C. and M.H.; software, X.Z.; formal analysis, W.L. and Y.Y.; validation, J.W.; investigation, M.C. and X.Z.; resources, Y.J.; data curation, W.L. and M.C.; writing—original draft preparation, M.C. and Y.Y.; writing—review and editing, J.H.; visualization, X.H.; supervision, Y.J.; project administration, H.Y.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovation Project of the CAAS [grant number CAAS-ASTIP-TRICAAS], Yunnan Province high-level talent “Young Top Talent” project [grant number YNWR-QNBJ-2020-222], National Natural Science Regional Fund project [grant number 31760197], and Agricultural Joint Project of Yunnan Province [grant number 202101BD070001-04].
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Jiang, Y.W.; Yuan, H.B.; Hua, J.J. 40 years of tea processing in China. China Tea 2019, 8, 1–5. [Google Scholar]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.T.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.L.; Huang, Y.H. Responses and resistance mechanisms of tea plants to stresses—A review. Acta Tea Sin. 2021, 62, 185–190. [Google Scholar]
- Wang, M.; Yang, J.; Li, J.L.; Zhou, X.C.; Xiao, Y.Y.; Liao, Y.Y.; Tang, J.C.; Dong, F.; Zeng, L. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, L.; Hao, X.Y.; Li, N.N.; Ding, C.Q.; Huang, J.Y.; Yang, Y.J. Progress and prospective of cold resistance mechanism in tea plant. J. Tea Commun. 2022, 49, 139–148. [Google Scholar]
- Chen, M.; Li, N.; Zhu, H.T.; Zhang, M.; Duan, Z.H.; Wang, D.; Yang, C.R.; Zhang, Y.J. New Hydrolyzable Tannin with Potent Antioxidant and α-Glucosidase Inhibitory Activity from Black Tea Produced from Camellia taliensis. Foods 2023, 12, 2512. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xing, H.; Shen, T. Analysis of SSR information in Camellia tetracocca Zhang transcriptome. J. Anhui Agric. Univ. 2017, 44, 558–562. [Google Scholar]
- Vyas, D.; Kumar, S. Tea (Camellia sinensis (L.) O. Kuntze) clone with lower period of winter dormancy exhibits lesser cellular damage in response to low temperature. Plant Physiol. Biochem. 2005, 43, 383–388. [Google Scholar] [CrossRef]
- Huang, H.T.; Yu, J.Z.; Wang, X.B.; Zhang, W.; Zhou, T.F.; Ao, C. Study on physiological characters of new shoot in different cold-resistant tea varieties in autumn. Acta Agric. Zhejiangensis 2014, 4, 925–928. [Google Scholar]
- Rapac, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- Han, N.; Tang, Q.; Lai, Y.S.; Ao, T.M.; Peng, M. Photosynthetic Characteristics of Introduced Tea Cultivars Huangjinya, Jinguang and Yujinxiang in Sichuan Tea-are. Southwest China J. Agric. Sci. 2015, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wang, S.; Zhao, D.G. Effect of low temperature on biochemical characteristics of Camellia sinensi (L.) Kuntze var. niaowangensis Q.H. Chen. in Giuzhou province. Hubei Agric. Sci. 2019, 58, 70–74. [Google Scholar]
- Mao, Y.; Li, H.; Wang, Y.; Fan, K.; Shen, J.; Zhang, J.; Han, X.; Song, Y.; Bi, C.; Sun, L.; et al. Low temperature response index for monitoring freezing injury of tea plant. Front. Plant Sci. 2023, 14, 1096490. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Li, T.; Teng, R.M.; Han, M.H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Zhang, Z.; Yang, Y.; Zhen, J.; Chen, W.; Tang, D.; Wei, C.; Tang, Q. Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings. Int. J. Mol. Sci. 2023, 24, 6718. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Cheng, X.M.; Huang, X.X. Effects of cadmium stress on growth and cadmium enrichment of Chlorophytum comosum and Chlorophytum comosum var. variegatum. Chin. J. Appl. Ecol. 2021, 32, 1835–1844. [Google Scholar]
- Miao, B.H.; Han, X.G.; Zhang, W.H. The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann. Bot. 2010, 105, 967–973. [Google Scholar] [CrossRef]
- Aazami, M.A.; Asghari-Aruq, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Baron, M.; Sochor, J. Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. cultivars. Plants 2021, 10, 1877. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Kuppusamy, S.; Cho, K.M.; Kim, P.J.; Kwack, Y.B.; Lee, Y.B. Influence of cold stress on contents of soluble sugars, vitamin C and free amino acids including gamma-aminobutyric acid (GABA) in spinach (Spinacia oleracea). Food Chem. 2017, 215, 185–192. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Yang, S.L.; Li, J.Y.; Ma, J.H.; Pang, T.; Zou, C.M.; He, B.; Gong, M. Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Bot. Stud. 2018, 59, 5. [Google Scholar] [CrossRef]
- Amri, M.; Abbes, Z.; Trabelsi, I.; Ghanem, M.E.; Mentag, R.; Kharrat, M. Chlorophyll content and fluorescence as physiological parameters for monitoring Orobanche foetida Poir. infection in faba bean. PLoS ONE 2021, 16, e0241527. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Cai, Z.Y.; Li, D.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Effect of freezing on photosystem II and assessment of freezing tolerance of tea cultivar. Plants 2019, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y. Effects of low temperature stress on cold tolerance of broomcorn millet varieties with different cold tolerance at seedling stage. Crops 2023. Available online: https://link.cnki.net/urlid/11.1808.S.20231206.1534.002 (accessed on 3 June 2024).
- Zhang, Z.; Gu, Y.; Mao, Q.; Wang, J. Physiological Response to Low Temperature of Four Genotypes of Cyclocarya paliurus and Their Preliminary Evaluation to Cold Resistance. Forests 2023, 14, 1680. [Google Scholar] [CrossRef]
- Han, B.; Ma, X.; Cui, D.; Wang, Y.; Geng, L.; Cao, G.; Zhang, H.; Han, L. Comprehensive evaluation and analysis of the mechanism of cold tolerance based on the transcriptome of weedy rice seedlings. Rice 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Z.; Hu, Y.G.; Zhang, X.L.; Li, P.P. Responses of electrical properties of tea leaves to low-temperature stress. Int. J. Agric. Biol. Eng. 2015, 10, 170–175. [Google Scholar]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef]
- Domínguez, F.; Cejudo, F.J. Chloroplast dismantling in leaf senescence. J. Exp. Bot. 2021, 72, 5905–5918. [Google Scholar] [CrossRef]
- Du, Y.Y.; Shin, S.; Wang, K.R.; Lu, J.L.; Liang, Y.R. Effect of temperature on the expression of genes related to the accumulation of chlorophylls and carotenoids in albino tea. J. Hortic. Sci. Biotechnol. 2009, 84, 365–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.X.; Xu, P.L.; Yang, Y.M.; Liu, Y.X.; Liu, H.S.; Ai, J. The characteristics of chlorophyll fluorescence and metabolism of reactive oxygen species in relation to the cold injury of Vitis amurensis ‘Shuangfeng’ and ‘Zuoyouhong’. Hortic. Plant J. 2018, 45, 650–658. [Google Scholar]
- Wu, L.L.; Li, J.A.; Wang, N.; Gu, Y.Y.; Zhang, F.H.; Tan, X.F. The effects of low temperature stress on the flowering, fruiting and physiological characteristics of two Camellia oleifera cultivars. Plant Physiol. J. 2020, 56, 681–692. [Google Scholar]
- Wu, W.J.; Qi, J.L.; Jin, G.M.; Zhang, R.; Yao, Y.F.; Ma, C.Y.; Jiang, C.Y. Evaluation of cold tolerance of eight olive varieties in cuttage seedlings. For. Res. 2023, 36, 87–96. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).