Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation
Abstract
1. Introduction
2. Materials and Methods
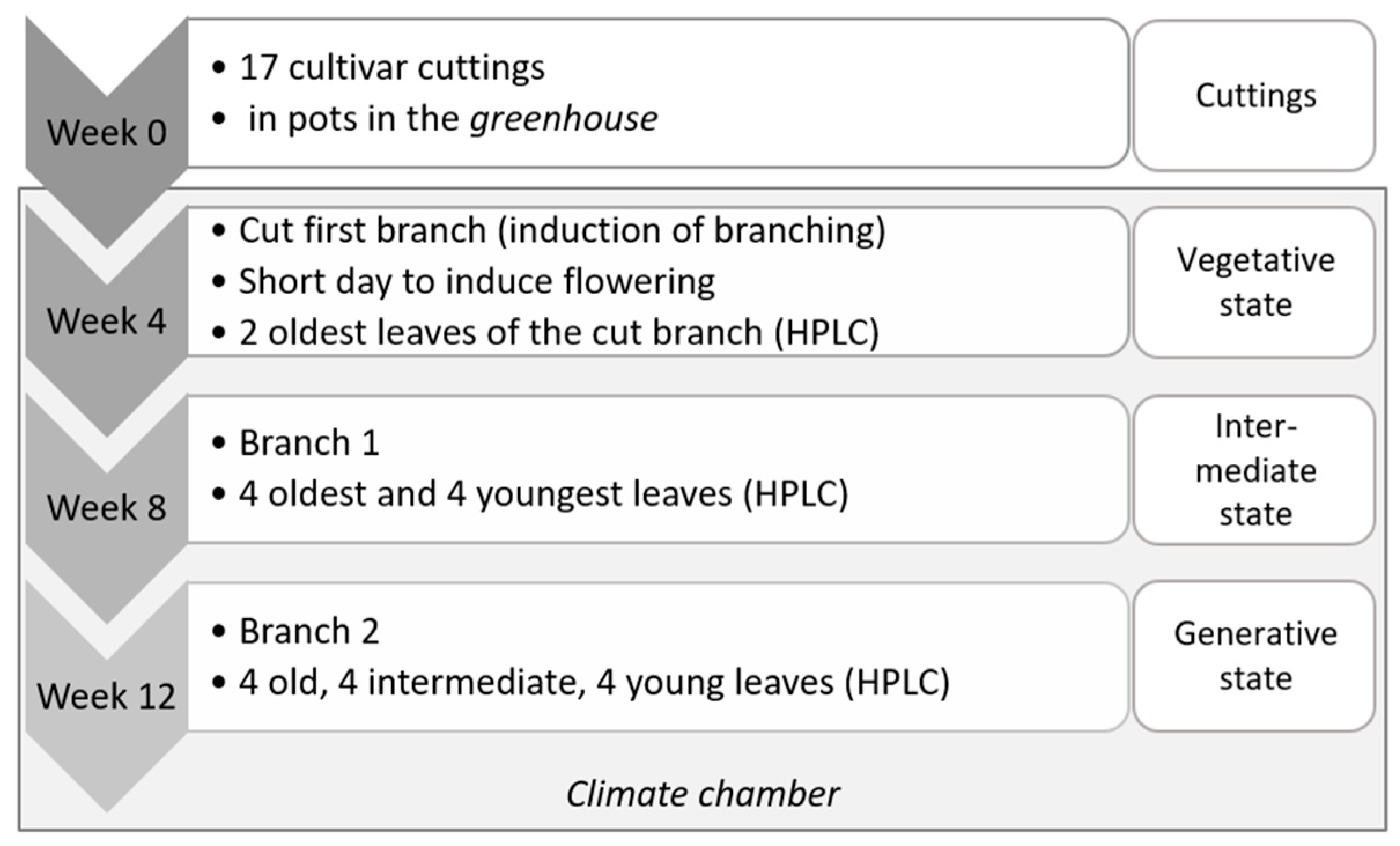
2.1. Plant Material, Growth Conditions and Leaf Analysis Setup
2.2. Method for the Analysis of Phenolic compounds in Leaves
2.3. Relative Epidermal Flavonol Content Measurement
2.4. Statistics
3. Results
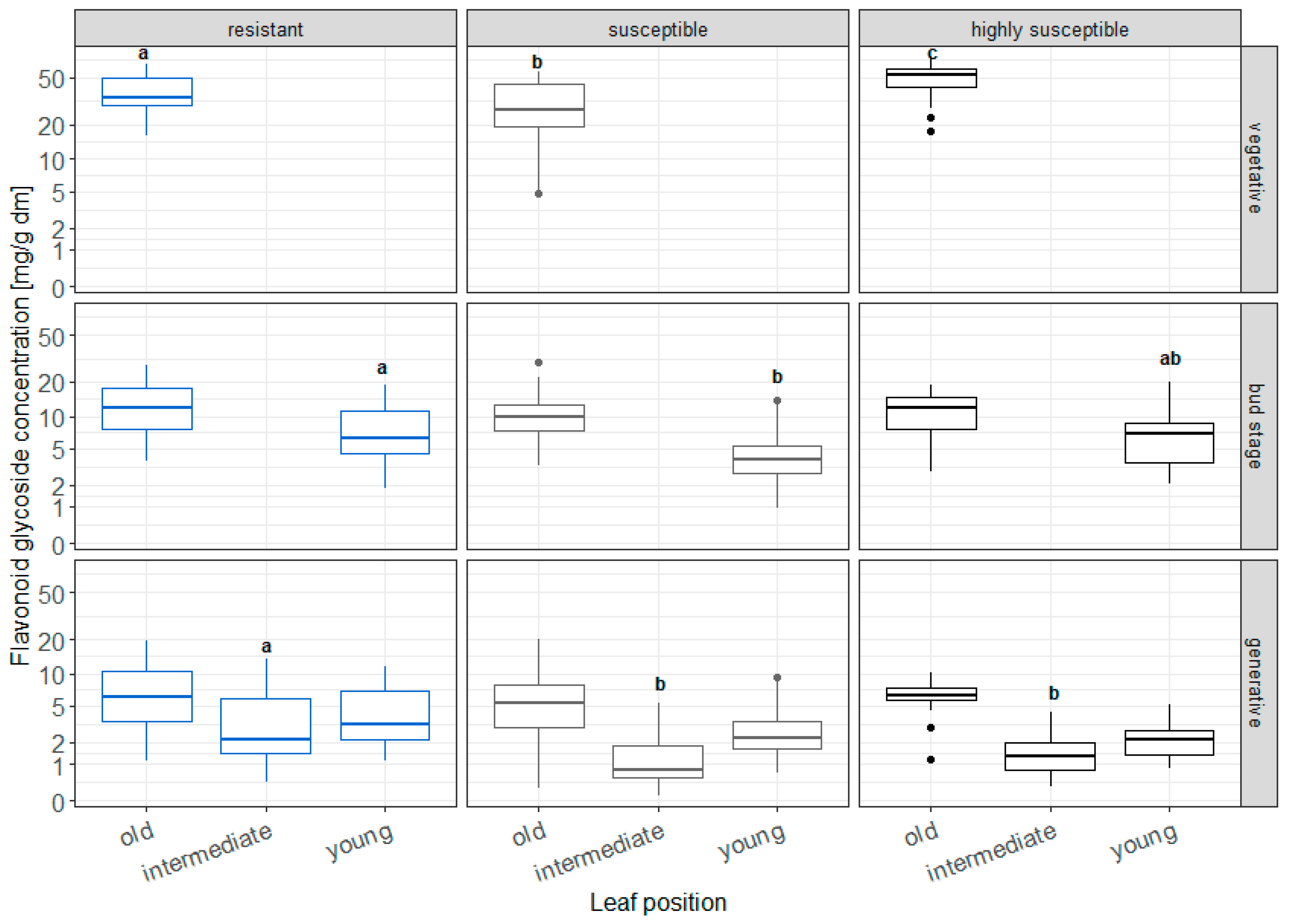
3.1. Analysis of Flavonoid Glycosides and Hydroxycinnamic Acid Derivatives in Chrysanthemum Leaves of Different Age and Ontogenetic Stage
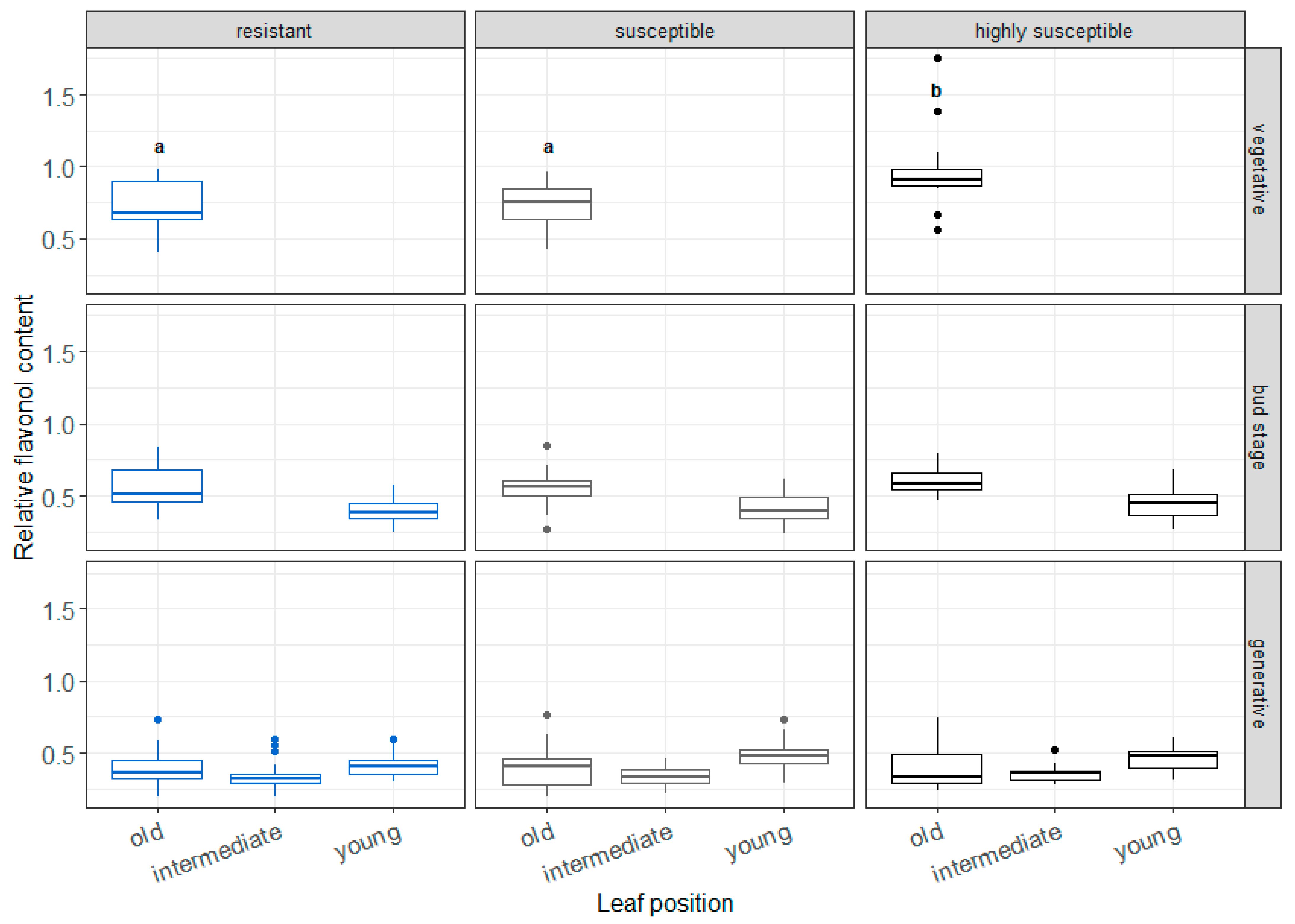
3.2. Relative Flavonol Content Measurements
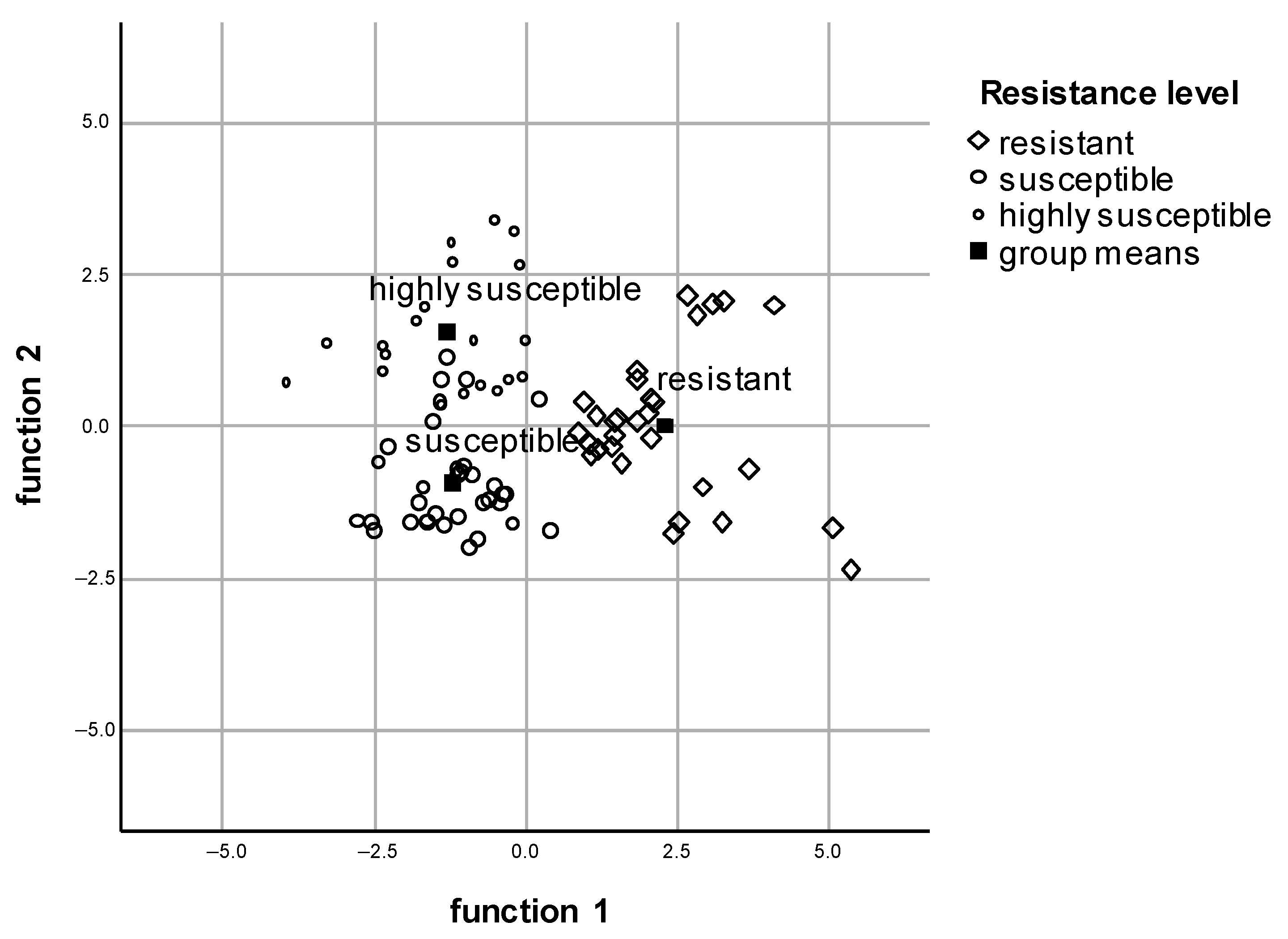
3.3. Multiple Discriminant Analysis Focusing on Old Leaves in Vegetative State
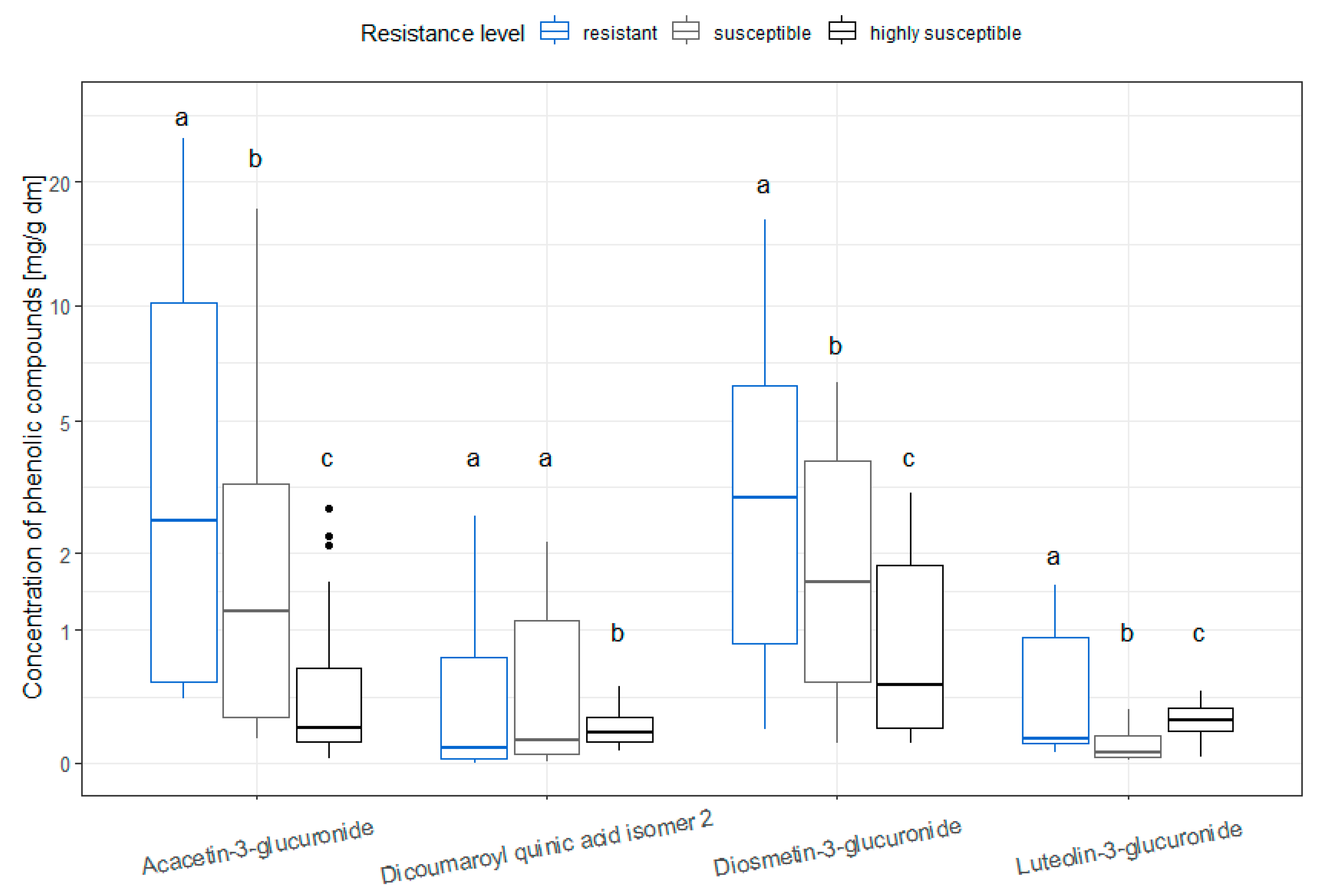
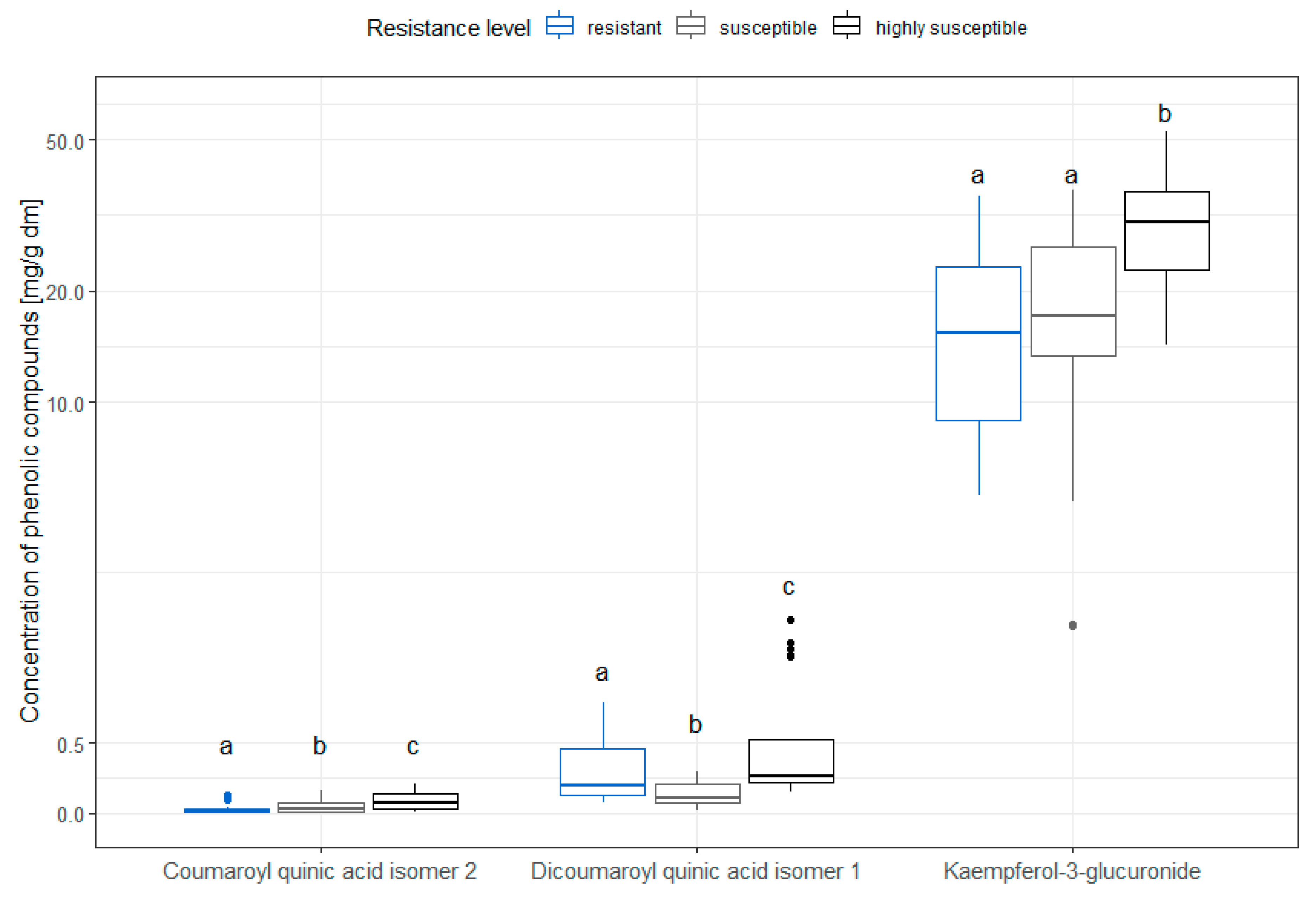
3.4. Distribution of Single Phenolic Compounds in Different Resistance Levels in Old Leaves in the Vegetative State
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaargaren, J.; van Geest, G. Chrysanthemum. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 319–348. ISBN 978-3-319-90697-3. [Google Scholar]
- Kos, S.P.; Klinkhamer, P.G.L.; Leiss, K.A. Cross-resistance of chrysanthemum to western flower thrips, celery leafminer, and two-spotted spider mite. Entomol. Exp. Appl. 2014, 151, 198–208. [Google Scholar] [CrossRef]
- Kindt, F.; Joosten, N.N.; Peters, D.; Tjallingii, W.F. Characterisation of the feeding behaviour of western flower thrips in terms of electrical penetration graph (EPG) waveforms. J. Insect Physiol. 2003, 49, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fiene, J.; Kalns, L.; Nansen, C.; Bernal, J.; Harris, M.; Sword, G.A. Foraging on individual leaves by an intracellular feeding insect is not associated with leaf biomechanical properties or leaf orientation. PLoS ONE 2013, 8, e80911. [Google Scholar] [CrossRef]
- Kumar, N.K.; Ullman, D.E.; Cho, J.J. Resistance Among Lycopersicon Species to Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 1995, 88, 1057–1065. [Google Scholar] [CrossRef]
- De Jager, C.M.; Butôt, R.P.; De Jong, T.J.; Klinkhamer, P.G.; Van der Meijden, E. Population growth and survival of western flower thrips Frankliniella occidentalis Pergande (Thysanoptera, Thripidae) on different chrysanthemum cultivars: Two methods for measuring resistance. J. Appl. Entomol. 1993, 115, 519–525. [Google Scholar] [CrossRef]
- De Jager, C.M.; Butôt, R.P.; Klinkhamer, P.G.; De Jong, T.J.; Wolff, K.; Van der Meijden, E. Genetic variation in chrysanthemum for resistance to Frankliniella occidentalis. Entomol. Exp. Appl. 1995, 77, 277–287. [Google Scholar] [CrossRef]
- Rogge, S.A.; Meyhöfer, R. Leaf age is important for assessment of resistance in chrysanthemum against Frankliniella occidentalis. J. Plant Dis. Prot. 2021, 128, 511–516. [Google Scholar] [CrossRef]
- Fung, S.Y.; Kuiper, I.; van Dijke-Hermans, C.M.; Van der Meijden, E. Growth damage and silvery damage in chrysanthemum caused by Frankliniella occidentalis is related to leaf food quality. In Thrips and Tospoviruses, Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy 2–7 July 2001; Australian National Insect Collection: Canberra, Australia, 2002; Volume 191. [Google Scholar]
- Rhainds, M.; Shipp, L. Dispersal of Adult Western Flower Thrips (Thysanoptera: Thripidae) on Chrysanthemum Plants: Impact of Feeding-Induced Senescence of Inflorescences. Environ. Entomol. 2003, 32, 1056–1065. [Google Scholar] [CrossRef]
- Van Haperen, P.; Voorrips, R.E.; van Loon, J.J.A.; Vosman, B. The effect of plant development on thrips resistance in Capsicum. Arthropod-Plant Interact. 2019, 13, 11–18. [Google Scholar] [CrossRef]
- Visschers, I.G.S.; Peters, J.L.; van de Vondervoort, J.A.H.; Hoogveld, R.H.M.; van Dam, N.M. Thrips resistance screening is coming of age: Leaf position and ontogeny are important determinants of leaf-based resistance in pepper. Front. Plant Sci. 2019, 10, 510. [Google Scholar] [CrossRef]
- Kogan, M. Natural chemicals in plant resistance to insects. Iowa State J. Res. 1986, 60, 501–527. [Google Scholar]
- De Jager, C.M.; Butôt, R.P.; Klinkhamer, P.G.; Van der Meijden, E. Chemical characteristics of chrysanthemum cause resistance to Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 1995, 88, 1746–1753. [Google Scholar] [CrossRef]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B.; Pelgrom, K.; Wahyuni, Y.; de Vos, R.C.H.; Voorrips, R.E. Genetic variation in phytochemicals in leaves of pepper (Capsicum) in relation to thrips resistance. Arthropod-Plant Interact. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Sasía-Arriba, A.; Miñarro, M.; Antón, M.J.; Alonso-Salces, R.M.; Micheletti, D.; Gallo, B.; Dapena, E. Relationship between hydroxycinnamic acids and the resistance of apple cultivars to rosy apple aphid. Talanta 2018, 187, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Sprawka, I.; Łukasik, I. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochem. Syst. Ecol. 2014, 55, 137–144. [Google Scholar] [CrossRef]
- Goławska, S.; Łukasik, I.; Kapusta, I.; Janda, B. Do the contents of luteolin, tricin, and chrysoeriol glycosides in alfalfa (Medicago sativa L.) affect the behavior of pea aphid (Acyrthosiphon pisum)? Pol. J. Environ. Stud. 2012, 21, 1613–1619. [Google Scholar]
- Gómez, J.D.; Vital, C.E.; Oliveira, M.G.A.; Ramos, H.J.O. Broad range flavonoid profiling by LC/MS of soybean genotypes contrasting for resistance to Anticarsia gemmatalis (Lepidoptera: Noctuidae). PLoS ONE 2018, 13, e0205010. [Google Scholar]
- Rogge, S.A.; Meyhöfer, R. The role of plant physiology and cultivar of chrysanthemum in the resistance against Western flower thrips. Entomol. Exp. Appl. 2021, 169, 275–289. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Goulas, Y.; Meyer, S.; Moya, I. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Identification of complex, naturally occurring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode-array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Neugart, S.; Castagna, A.; Barilari, M.; Sarrocco, S.; Vannacci, G.; Schreiner, M.; Ranieri, A. UV-B pre-treatment alters phenolics response to Monilinia fructicola infection in a structure-dependent way in peach skin. Front. Plant Sci. 2018, 9, 1598. [Google Scholar]
- Bilger, W.; Veit, M.; Schreiber, L.; Schreiber, U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol. Plant. 1997, 101, 754–763. [Google Scholar] [CrossRef]
- Agati, G.; Pinelli, P.; Cortés Ebner, S.; Romani, A.; Cartelat, A.; Cerovic, Z.G. Nondestructive evaluation of anthocyanins in olive (Olea europaea) fruits by in situ chlorophyll fluorescence spectroscopy. J. Agric. Food Chem. 2005, 53, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Ben Ghozlen, N.; Meyer, S.; Cerovic, Z.G. Investigating UV screening in leaves by two different types of portable UV fluorimeter reveals in vivo screening by anthocyanins and carotenoids. Photosyn. Res. 2007, 93, 205–221. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P. Plant Phenolics; Oliver and Boyd: Edinburgh, UK, 1972; ISBN 0050025120. [Google Scholar]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Bioactive Natural Products, 1st ed.; Atta-ur-Rahman, Ed.; Digital Print; Elsevier: Amsterdam, The Netherlands, 2005; pp. 257–312. ISBN 9780444514158. [Google Scholar]
- Yoneyama, K.; Natsume, M. Allelochemicals for Plant–Plant and Plant–Microbe Interactions. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L.N., Liu, H., Eds.; Elsevier Science: Oxford, UK, 2010; pp. 539–561. ISBN 9780080453828. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Leszczyǹski, B.; Warchoł, J.; Niraz, S. The influence of phenolic compounds on the preference of winter wheat cultivars by cereal aphids. Insect Sci. Appl. 1985, 6, 157–158. [Google Scholar] [CrossRef]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.A.; Sonavane, U.; Thulasiram, H.V.; Joshi, R.; Gupta, V.S.; Giri, A.P. Way toward “dietary pesticides”: Molecular investigation of insecticidal action of caffeic acid against Helicoverpa armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef]
- Macel, M.; Visschers, I.G.S.; Peters, J.L.; Kappers, I.F.; de Vos, R.C.H.; van Dam, N.M. Metabolomics of Thrips Resistance in Pepper (Capsicum spp.) Reveals Monomer and Dimer Acyclic Diterpene Glycosides as Potential Chemical Defenses. J. Chem. Ecol. 2019, 45, 490–501. [Google Scholar] [CrossRef]
- Mirnezhad, M. Host Plant Resistance of Tomato Plants to Western Flower Thrips. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2011. [Google Scholar]
- Leiss, K.A.; Cristofori, G.; van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G.L. An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.L.; Felton, G.W.; Murphy, J.B.; Howles, P.A.; Dixon, R.A.; Lamb, C.J. Do Plant Phenolics Confer Resistance to Specialist and Generalist Insect Herbivores? J. Agric. Food Chem. 1997, 45, 4500–4504. [Google Scholar] [CrossRef]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between Plant Metabolites Affect Herbivores: A Study with Pyrrolizidine Alkaloids and Chlorogenic Acid. Front. Plant Sci. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef] [PubMed]
- Lanker, T.; King, T.G.; Arnold, S.W.; Flurkey, W.H. Active, inactive and in vitro synthesized forms of polyphenoloxidase during leaf development. Physiol. Plant. 1987, 69, 323–329. [Google Scholar] [CrossRef]
- Felton, G.W.; Donato, K.; Del Vecchio, R.J.; Duffey, S.S. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 1989, 15, 2667–2694. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Conn, E.E. Tissue distributions of chlorogenic acid and of enzymes involved in its metabolism in leaves of Sorghum bicolor. Plant Physiol. 1982, 70, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.M.; Pinto, J.; Bertolucci, S.K.V.; Malta, M.R.; Cardoso, M.d.G.; de MSilva, F.A. Coumarin contents in young Mikania glomerata plants (Guaco) under different radiation levels and photoperiod. Acta Farm. Bonaer. 2007, 25, 387. [Google Scholar]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B 2018, 179, 66–73. [Google Scholar] [CrossRef]
- Saijo, R. Effect of shade treatment on biosynthesis of catechins in tea plants. Plant Cell Physiol. 1980, 21, 989–998. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Spektrum Akad. Verl./Springer: Heidelberg, Germany, 2007; ISBN 9783827418654. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Dillon, F.M.; Chludil, H.D.; Zavala, J.A. Solar UV-B radiation modulates chemical defenses against Anticarsia gemmatalis larvae in leaves of field-grown soybean. Phytochemistry 2017, 141, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, G.; Hedrich, R.; Sachs, G. Secondary phenolic products in isolated guard cell, epidermal cell and mesophyll cell protoplasts from pea (Pisum sativum L.) leaves: Distribution and determination. Protoplasma 1986, 134, 141–148. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef]
- Agati, G.; Galardi, C.; Gravano, E.; Romani, A.; Tattini, M. Flavonoid distribution in tissues of Phillyrea latifolia L. leaves as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochem. Photobiol. 2002, 76, 350–360. [Google Scholar] [CrossRef]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of ‘antioxidant’flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–861. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Kazlauskas, S.; Bagdonaitė, E. Paprastosios jonažolės (Hypericum perforatum L.) veikliųjų medžiagų kiekybinė analizė efektyviosios skysčių chromatografijos metodu. Medicina 2004, 40, 975–981. [Google Scholar] [PubMed]
- Hosni, K.; Msaada, K.; Taârit, M.B.; Marzouk, B. Phenological variations of secondary metabolites from Hypericum triquetrifolium Turra. Biochem. Syst. Ecol. 2011, 39, 43–50. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Camas, N.; Caliskan, O.; Odabas, M.S. Changes in the contents of main secondary metabolites in two Turkish Hypericum species during plant development. Pharm. Biol. 2013, 51, 391–399. [Google Scholar] [CrossRef]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.V.; Sazanova, K.V.; Medvedeva, N.A.; Shavarda, A.L. Features of Metabolomic Profiles in Different Stages of Ontogenesis in Prunella vulgaris (Lamiaceae) Grown in a Climate Chamber. Russ. J. Bioorganic Chem. 2019, 45, 906–912. [Google Scholar] [CrossRef]
- Popović, Z.; Milošević, D.K.; Stefanović, M.; Vidaković, V.; Matić, R.; Janković, J.; Bojović, S. Variability of six secondary metabolites in plant parts and developmental stages in natural populations of rare Gentiana pneumonanthe. Plant Biosyst. 2021, 155, 816–822. [Google Scholar] [CrossRef]
- Achakzai, A.K.K.; Achakzai, P.; Masood, A.; Kayani, S.A.; Tareen, R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak. J. Bot. 2009, 41, 2129–2135. [Google Scholar]
- Kowalski, R.; Wolski, T. Evaluation of phenolic acid content in Silphium perfoliatum L. leaves, inflorescences and rhizomes. Electron. J. Pol. Agric. Univ. 2003, 6, 3. [Google Scholar]
- Rogge, S.; Neugart, S.; Schreiner, M.; Meyhöfer, R. Dataset: Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation; LUH Data Repository: Hannover, Germany, 2023. [Google Scholar] [CrossRef]

| Cultivar Name | Resistant Cultivars | Cultivar Name | Susceptible Cultivars | Cultivar Name | Highly Susceptible Cultivars |
|---|---|---|---|---|---|
| Aviso (8) | r | Kowloon (30) | s | Kanok (9) | ss |
| Colombo Apricot (3) | r | Mumbai Orange (42) | s | Mumbai Red (10) | ss |
| Dragona (4) | r | Palm Green (7) | s | Pemba Canari (6) | ss |
| Luzon Pink (2) | r | Pemba Purple (36) | s | Yala (1) | ss |
| Mega Time Gold (5) | r | Pemba Red (38) | s | ||
| Robinho (26) | r | Solta (44) | s | ||
| Vyking (35) | s |
| Metabolite | Wilks-Lambda | F | df1 | df2 | p-Value |
|---|---|---|---|---|---|
| caffeoyl quinic acid | 0.866 | 6.362 | 2 | 82 | 0.003 |
| coumaroyl quinic acid isomer 1 | 0.837 | 7.983 | 2 | 82 | 0.001 |
| coumaroyl quinic acid isomer 2 | 0.843 | 7.639 | 2 | 82 | 0.001 |
| luteolin-3-glucuronide | 0.703 | 17.327 | 2 | 82 | <0.001 |
| kaempferol-3-glucuronide | 0.805 | 9.947 | 2 | 82 | <0.001 |
| dicaffeoyl quinic acid isomer 1 | 0.967 | 1.404 | 2 | 82 | 0.251 |
| dicaffeoyl quinic acid isomer 2 | 0.975 | 1.050 | 2 | 82 | 0.355 |
| apigenin-3-glucuronide | 0.766 | 12.555 | 2 | 82 | <0.001 |
| diosmetin-3-glucuronide | 0.846 | 7.456 | 2 | 82 | 0.001 |
| dicoumaroyl quinic acid isomer 1 | 0.665 | 20.630 | 2 | 82 | <0.001 |
| dicoumaroyl quinic acid isomer 2 | 0.985 | 0.625 | 2 | 82 | 0.538 |
| acacetin-3-rutinoside | 0.957 | 1.836 | 2 | 82 | 0.166 |
| acacetin-3-glucuronide | 0.746 | 13.940 | 2 | 82 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogge, S.A.; Neugart, S.; Schreiner, M.; Meyhöfer, R. Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation. Horticulturae 2024, 10, 822. https://doi.org/10.3390/horticulturae10080822
Rogge SA, Neugart S, Schreiner M, Meyhöfer R. Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation. Horticulturae. 2024; 10(8):822. https://doi.org/10.3390/horticulturae10080822
Chicago/Turabian StyleRogge, Sina Alexandra, Susanne Neugart, Monika Schreiner, and Rainer Meyhöfer. 2024. "Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation" Horticulturae 10, no. 8: 822. https://doi.org/10.3390/horticulturae10080822
APA StyleRogge, S. A., Neugart, S., Schreiner, M., & Meyhöfer, R. (2024). Phenolic Compounds in Different Stages of Ontogenesis in Chrysanthemum—A Potential for Thrips-Resistance Characterisation. Horticulturae, 10(8), 822. https://doi.org/10.3390/horticulturae10080822







