In Vitro Germination, Micropropagation and Addressing the Hyperhydricity of the Balkan Native Dianthus cruentus, a Plant with High Ornamental and Xeriscaping Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Germination
2.3. Establishment of In Vitro Cultures
2.4. Multiplication Stage and Addressing of Hyperhydricity
2.5. In Vitro Rooting and Ex Vitro Acclimatization
2.6. In Vitro Culture Conditions and Data Collection
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. In Vitro Germination
3.2. Establishment of In Vitro Cultures
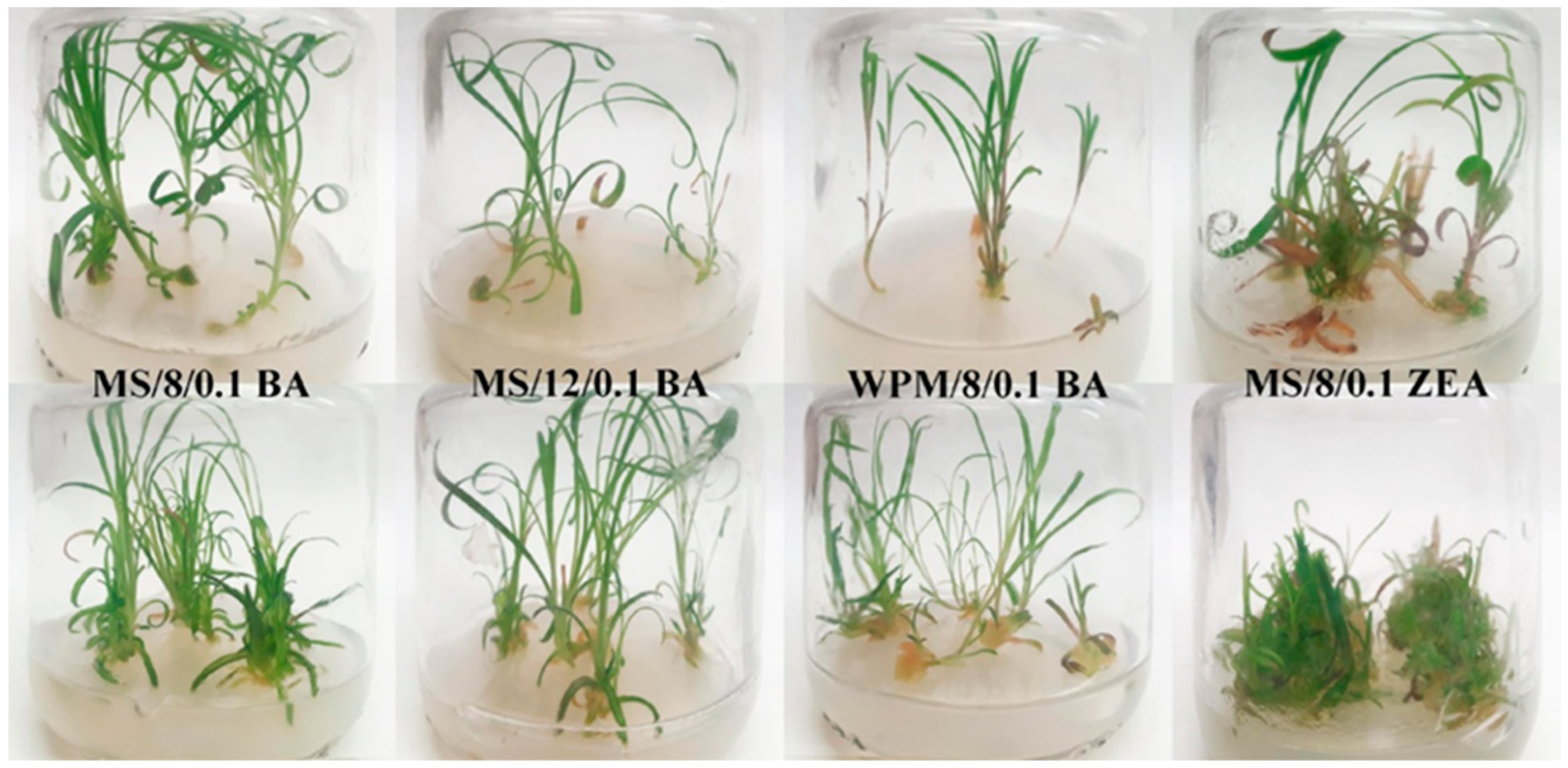
3.3. Effect of Explant Physiology on Multiplication and Addressing of Hyperhydricity
3.4. In Vitro Rooting and Ex Vitro Acclimatization
4. Discussion
4.1. In Vitro Seed Germination
4.2. In Vitro Clonal Propagation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bittrich, V. Caryophyllaceae. In Flowering Plants: Dicotyledons; Magnoliid, Hamamelid and Caryophyllid Families; The families and genera of vascular plants; Kubitzki, K., Rohwer, G., Bittrich, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 2, pp. 206–236. [Google Scholar]
- Fassou, G.; Korotkova, N.; Nersesyan, A.; Koch, M.A.; Dimopoulos, P.; Borsch, T. Taxonomy of Dianthus (Caryophyllaceae)—Overall Phylogenetic Relationships and Assessment of Species Diversity Based on a First Comprehensive Checklist of the Genus. PhytoKeys 2022, 196, 91–214. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.; Savolainen, V.; Vargas, P. Unparalleled Rates of Species Diversification in Europe. Proc. R. Soc. B Biol. Sci. 2010, 277, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Luqman, H.; Wegmann, D.; Fior, S.; Widmer, A. Climate-Induced Range Shifts Drive Adaptive Response via Spatio-Temporal Sieving of Alleles. Nat. Commun. 2023, 14, 1080. [Google Scholar] [CrossRef] [PubMed]
- Strid, A.; Tan, K. Flora Hellenica; Koeltz Scientific Books: Konigstein, Germany, 1997; Volume 1, pp. 369–370. ISBN 9783874293914. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea: Volume 1, Lycopodiaceae to Platanaceae, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; Volume 1, ISBN 9780521066617. [Google Scholar]
- Strid, A. Atlas of the Hellenic Flora; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2024; Volume 2, p. 499. ISBN 9789925351701. [Google Scholar]
- Cullen, J. The European Garden Flora Flowering Plants: A Manual for the Identification of Plants Cultivated in Europe, Both Out-of-Doors and under Glass, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011; Volume 1, ISBN 9780521761642. [Google Scholar]
- Dekić, B.R.; Ristić, M.N.; Mladenović, M.Z.; Dekić, V.S.; Ristić, N.R.; Ranđelović, V.; Radulović, N.S. Diethyl-Ether Flower Washings of Dianthus cruentus Griseb. (Caryophyllaceae): Derivatization Reactions Leading to the Identification of New Wax Constituents. Chem. Biodivers. 2019, 16, e1900153. [Google Scholar] [CrossRef] [PubMed]
- Kozuharova, E.K. Entomophilous Plant Species Inhabiting the Southern Limestone Slopes of Mt. Vitosa (SW Bulgaria) and Their Pollinators. Flora Mediterr. 2000, 10, 227–234. [Google Scholar]
- Uysal, S.; Aktumsek, A.; Picot-Allain, C.M.N.; Unuvar, H.; Mollica, A.; Georgiev, M.I.; Zengin, G.; Mahomoodally, M.F. Biological, Chemical and in Silico Fingerprints of Dianthus calocephalus Boiss.: A Novel Source for Rutin. Food Chem. Toxicol. 2018, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Papafotiou, M.; Pergialioti, N.; Tassoula, L.; Massas, I.; Kargas, G. Growth of native aromatic xerophytes in an extensive Mediterranean green roof as affected by substrate type and depth and irrigation frequency. HortScience 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Çetin, N.; Mansuroğlu, S.; Önaç, A.K. Xeriscaping Feasibility as an Urban Adaptation Method for Global Warming: A Case Study from Turkey. Pol. J. Environ. Stud. 2018, 27, 1009–1018. [Google Scholar] [CrossRef]
- Caneva, G.; Kumbaric, A.; Savo, V.; Casalini, R. Ecological Approach in Selecting Extensive Green Roof Plants: A Data-Set of Mediterranean Plants. Plant Biosyst. 2013, 149, 374–383. [Google Scholar] [CrossRef]
- Ondoño, S.; Martínez-Sánchez, J.J.; Moreno, J.L. Evaluating the growth of several Mediterranean endemic species in artificial substrates: Are these species suitable for their future use in green roofs? Ecol. Eng. 2015, 81, 405–417. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Tassoula, L.; Stylias, E.G.; Kalantzis, A.; Dariotis, E. Acclimatization of Mediterranean Native Sages (Salvia spp.) and Interspecific Hybrids in an Urban Green Roof under Regular and Reduced Irrigation. Sustainability 2022, 14, 4978. [Google Scholar] [CrossRef]
- Azeñas, V.; Janner, I.; Medrano, H.; Gulías, J. Performance Evaluation of Five Mediterranean Species to Optimize Ecosystem Services of Green Roofs under Water-Limited Conditions. J. Environ. Manag. 2018, 212, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Water use efficiency, growth and anatomic-physiological parameters of Mediterranean xerophytes as affected by substrate and irrigation on a green roof. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12283. [Google Scholar] [CrossRef]
- Nektarios, P.A.; Amountzias, I.; Kokkinou, I.; Ntoulas, N. Green Roof Substrate Type and Depth Affect the Growth of the Native Species Dianthus fruticosus under Reduced Irrigation Regimens. HortScience 2011, 46, 1208–1216. [Google Scholar] [CrossRef]
- Wójcik, M.; Tukiendorf, A. Accumulation and Tolerance of Lead in Two Contrasting Ecotypes of Dianthus carthusianorum. Phytochemistry 2014, 100, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on Lead and Cadmium Toxicity in Dianthus carthusianorum Calamine Ecotype Cultivated in Vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Panwar, S.; Gupta, Y.C.; Kumari, P.; Thakur, N.; Mehraj, U. Carnation. In Floriculture and Ornamental Plants; Handbooks of Crop Diversity: Conservation and Use of Plant Genetic Resources; Datta, S.K., Gupta, Y.C., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Papafotiou, M.; Stragas, J. Seed Germination and in Vitro Propagation of Dianthus fruticosus. Acta Hortic. 2009, 813, 481–484. [Google Scholar] [CrossRef]
- Ivanovic, S.; Markovic, M.; Milutinovic, M.; Skocajic, D.; Dunisijevic-Bojovic, D. In Vitro Propagation of Dianthus cruentus and Acclimatization in Hydroponic Culture. Phyton 2023, 62–63, 107–114. [Google Scholar]
- Krawczyk, A.; Domagała-Świątkiewicz, I.; Lis-Krzyścin, A. The Effect of Substrate on Growth and Nutritional Status of Native Xerothermic Species Grown in Extensive Green Roof Technology. Ecol. Eng. 2017, 108, 194–202. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi- Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the Potential of Neglected Local Endemic Plants of Three Mediterranean Regions in the Ornamental Sector: Value Chain Feasibility and Readiness Timescale for Their Sustainable Exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. Propagation and ex-situ conservation of Lomelosia minoana subsp. minoana (Dipsacaceae) and Scutellaria hirta (Lamiaceae)—Two wild-growing endemic plants of Crete (Greece) with potential ornamental and medicinal value. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12168. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Using the Halophyte Crithmum maritimum in Green Roofs for Sustainable Urban Horticulture: Effect of Substrate and Nutrient Content Analysis Including Potentially Toxic Elements. Sustainability 2022, 14, 4713. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.T.; Tsarouchas, P.; Londra, P.A.; Kamoutsis, A.P. The Effect of Irrigation Treatment on the Growth of Lavender Species in an Extensive Green Roof System. Water 2020, 12, 863. [Google Scholar] [CrossRef]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Ntoulas, N.; Trigas, P.; Roukounakis, G.I. Sustainable Growth of Medicinal and Aromatic Mediterranean Plants Growing as Communities in Shallow Substrate Urban Green Roof Systems. Sustainability 2023, 15, 5940. [Google Scholar] [CrossRef]
- Casas, J.L.; Olmos, E.; Piqueras, A. In Vitro Propagation of Carnation (Dianthus caryophyllus L.). In Protocols for In Vitro Propagation of Ornamental Plants; Jain, S.M., Ochatt, S.J., Eds.; Springer Science+Business Media: Heidelberg, Germany, 2009; pp. 109–116. [Google Scholar]
- Onozaki, T.; Yagi, M. The Carnation Genome; Springer: Singapore, 2021; ISBN 9789811582615. [Google Scholar]
- Sreelekshmi, R.; Siril, E.A. Influence of Polyamines on Hyperhydricity Reversion and Its Associated Mechanism during Micropropagation of China Pink (Dianthus chinensis L.). Physiol. Mol. Biol. Plants 2020, 26, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Grbic, M.; Djukic, M. Micropropagation of Endangered and Decorative Species Dianthus pinifolius Sibth. et Sm. Braz. Arch. Biol. Technol. 2016, 59, e16150320. [Google Scholar] [CrossRef][Green Version]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K. Seed Germination, Micropropagation from Adult and Juvenile Origin Explants and Address of Hyperhydricity of the Cretan Endemic Herb Calamintha cretica. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1504–1518. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability 2023, 15, 4025. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. Studies on Seed Germination and Micropropagation of Clinopodium nepeta: A Medicinal and Aromatic Plant. HortScience 2019, 54, 1558–1564. [Google Scholar] [CrossRef]
- Bertsouklis, Κ.F.; Theodorou, P.; Aretaki, P.-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Vazaka-Vodena, D.; Bazanis, A.-E.; Papafotiou, M. Studies on Seed Germination and Micropropagation of Ebenus Sibthorpii, an Endemic Shrub of Greece with Potential Ornamental Use. Horticulturae 2023, 9, 1300. [Google Scholar] [CrossRef]
- Lantieri, A.; Salmeri, C.; Guglielmo, A.; Pavone, P. Seed Germination in the Sicilian Subspecies of Dianthus rupicola Biv. (Caryophyllaceae). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2012, 146, 906–909. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds, 2nd ed.; Elsevier: Heidelberg, Germany, 2014; ISBN 9780124166837. [Google Scholar]
- Kołodziejek, J.; Patykowski, J.; Wala, M. An Experimental Comparison of Germination Ecology and Its Implication for Conservation of Selected Rare and Endangered Species of Dianthus (Caryophyllaceae). Botany 2018, 96, 319–328. [Google Scholar] [CrossRef]
- Csontos, P.; Kalapos, T.; Tamás, J. Comparison of Seed Longevity for Thirty Forest, Grassland and Weed Species of the Central European Flora: Results of a Seed Burial Experiment. Pol. J. Ecol. 2016, 64, 313–326. [Google Scholar] [CrossRef]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of Germination in the Aromatic Plants Thyme, Savory and Oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Cogoni, D.; Mattana, E.; Fenu, G.; Bacchetta, G. From Seed to Seedling: A Critical Transitional Stage for the Mediterranean Psammophilous Species Dianthus morisianus (Caryophyllaceae). Plant Biosyst. 2012, 146, 910–917. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef]
- Nelson, S.K.; Kanno, Y.; Seo, M.; Steber, C.M. Seed Dormancy Loss from Dry After-Ripening Is Associated with Increasing Gibberellin Hormone Levels in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1145414. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in Plant Tissue Culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term vitrification as used in micropropagation. Plant Cell Tissue Organ Cult. 1992, 30, 135–140. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Papafotiou, M.; Balotis, G. Effect of medium on in vitro growth and ex vitro establishment of Globularia alypum. Acta Hortic. 2003, 616, 177–180. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Studies on in vitro propagation of Lithodora zahnii. Acta Hortic. 2009, 813, 465–470. [Google Scholar] [CrossRef]
- Trigka, M.; Papafotiou, M. In Vitro Propagation of Anthyllis barba-Jovis from Seedling Tissues. Acta Hortic. 2017, 1189, 473–748. [Google Scholar] [CrossRef]
- Piqueras, A.; Cortina, M.; Serna, M.D.; Casas, J.L. Polyamines and Hyperhydricity in Micropropagated Carnation Plants. Plant Sci. 2002, 162, 671–678. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Prevention of Hyperhydricity in Micropropagated Carnation Shoots by Bottom Cooling: Implications of Oxidative Stress. Plant Cell Tissue Organ Cult. 2005, 81, 149–158. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A. Effective Reversal of Hyperhydricity Leading to Efficient Micropropagation of Dianthus chinensis L. 3 Biotech 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, R.; Siril, E.A.; Muthukrishnan, S. Role of Biogenic Silver Nanoparticles on Hyperhydricity Reversion in Dianthus chinensis L. An in Vitro Model Culture. J. Plant Growth Regul. 2021, 41, 23–39. [Google Scholar] [CrossRef]
- ENSCONET. Seed Collecting Manual for Wild Species; Royal Botanic Gardens, Kew (UK), Universidad Politécnica de Madrid (Spain), Eds.; ENSCONET: Richmond, UK, 2009; pp. 1–36. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing. Seed Sci. Tech. 1999, 27, 333. [Google Scholar]
- Lloyd, G.; McCown, B.H. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant Species. HortScience 1981, 16, 453. [Google Scholar]
- Raoudha, A.; Aymen, S.; Dhikra, Z.; Mohamed, N. Effects of Natural Long Storage Duration on Seed Germination Characteristics of Periploca angustifolia Labill. Afr. J. Biotechnol. 2013, 12, 1760–1768. [Google Scholar] [CrossRef]
- Picciau, R.; Pritchard, H.W.; Mattana, E.; Bacchetta, G. Thermal Thresholds for Seed Germination in Mediterranean Species Are Higher in Mountain Compared with Lowland Areas. Seed Sci. Res. 2018, 29, 44–54. [Google Scholar] [CrossRef]
- Fournaraki, C. Conservation of Threatened Plants of Crete: Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, University of Athens, Athens, Greece, 2010; pp. 191–194. [Google Scholar]
- Mattana, E.; Daws, M.I.; Baccheta, G. Comparative Germination Ecology of the Endemic Centranthus amazonum (Valerianaceae) and Its Widespread Congener Centranthus ruber. Plant Species Biol. 2010, 25, 165–172. [Google Scholar] [CrossRef]
- Carruggio, F.; Onofri, A.; Catara, S.; Impelluso, C.; Castrogiovanni, M.; Lo Cascio, P.; Cristaudo, A. Conditional Seed Dormancy Helps Silene hicesiae Brullo & Signor. Overcome Stressful Mediterranean Summer Conditions. Plants 2021, 10, 2130. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.N.; Papafotiou, M. Micropropagation as Means for the Conservation of the Rare and Endangered × Malosorbus florentina Zucc. (Rosaceae). Acta Hortic. 2013, 990, 409–414. [Google Scholar] [CrossRef]
- Markovic, M.; Grbic, M.; Djukic, M. An Efficient in Vitro Propagation Protocol of Dianthus giganteiformis Borbas subsp. kladovanus (Degen) Soo. Bull. Fac. For. 2018, 118, 77–85. [Google Scholar] [CrossRef]
- Frabetti, M.; Gutiérrez-Pesce, P.; Mendoza-de Gyves, E.; Rugini, E. Micropropagation of Teucrium fruticans L., an Ornamental and Medicinal Plant. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 129–134. [Google Scholar] [CrossRef]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of Explant Origin and Medium Plant Growth Regulators on in Vitro Shoot Proliferation and Rooting of Salvia tomentosa, a Native Sage of the Northeastern Mediterranean Basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Cretan Dittany (Origanum dictamnus L.), a Valuable Local Endemic Plant: In Vitro Regeneration Potential of Different Type of Explants for Conservation and Sustainable Exploitation. Plants 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the Effects of the Explant Type and Different Plant Growth Regulators on Micropropagation of Five Mediterranean Salvia spp. Native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Bethge, H.; Nakhjiri, Z.M.; Rath, T.; Winkelmann, T. Towards Automated Detection of Hyperhydricity in Plant in Vitro Culture. Plant Cell Tissue Organ Cult. 2023, 154, 551–573. [Google Scholar] [CrossRef]
- Lee, J.; Naing, A.H.; Park, K.I.; Kim, C.K. Silver Nitrate Reduces Hyperhydricity in Shoots Regenerated from the Hypocotyl of Snapdragon Cv. Maryland Apple Blossom. Sci. Hortic. 2023, 308, 111593. [Google Scholar] [CrossRef]
- Cartabia, A.; Sarropoulou, V.; Grigoriadou, K.; Maloupa, E.; Declerck, S. In Vitro Propagation of Alkanna tinctoria Tausch.: A Medicinal Plant of the Boraginaceae Family with High Pharmaceutical Value. Ind. Crop. Prod. 2022, 182, 114860. [Google Scholar] [CrossRef]
- Majada, J.P.; Tadeo, F.; Fal, M.A.; Sánchez-Tamés, R. Impact of Culture Vessel Ventilation on the Anatomy and Morphology of Micropropagated Carnation. Plant Cell Tissue Organ Cult. 2000, 63, 207–214. [Google Scholar] [CrossRef]
- Fal, M.A.; Majada, J.P.; Sánchez Tamés, R. Physical Environment in Non-Ventilated Culture Vessels Affects in Vitro Growth and Morphogenesis of Several Cultivars of Dianthus caryophyllus L. Vitr. Cell. Dev. Biol.-Plant 2002, 38, 589–594. [Google Scholar] [CrossRef][Green Version]
- Muneer, S.; Park, Y.G.; Jeong, B.R. Red and Blue Light Emitting Diodes (LEDs) Participate in Mitigation of Hyperhydricity in in Vitro-Grown Carnation Genotypes (Dianthus caryophyllus). J. Plant Growth Regul. 2017, 37, 370–379. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of Hyperhydricity in Pink (Dianthus chinensis L.) Plantlets by AgNO3 and Its Associated Mechanism during in Vitro Culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xu, P.; Li, J.; Ji, H.; An, L.; Xia, X. AgNO3 Prevents the Occurrence of Hyperhydricity in Dianthus chinensis L. By Enhancing Water Loss and Antioxidant Capacity. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 561–570. [Google Scholar] [CrossRef]
- Soundararajan, P.; Manivannan, A.; Cho, Y.M.; Jeong, B.R. Exogenous Supplementation of Silicon Improved the Recovery of Hyperhydric Shoots in Dianthus caryophyllus L. By Stabilizing the Physiology and Protein Expression. Front. Plant Sci. 2017, 8, 738. [Google Scholar] [CrossRef]
- Thu, H.T.M.; Naing, A.H.; Jeong, H.Y.; Kim, C.K. Regeneration of Genetically Stable Plants from in Vitro Vitrified Leaves of Different Carnation Cultivars. Plants 2020, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Maloupa, E. Micropropagation and Ex Situ Conservation of Three Rare and Endemic Ornamental Dianthus Taxa (Caryophyllaceae). Bot. Serbica 2022, 46, 49–60. [Google Scholar] [CrossRef]
- Casanova, E.; Moysset, L.; Trillas, M.I. Effects of Agar Concentration and Vessel Closure on the Organogenesis and Hyperhydricity of Adventitious Carnation Shoots. Biol. Plant. 2008, 52, 1–8. [Google Scholar] [CrossRef]
- Ling, M.; Jiang, F.; Kong, X.; Tian, J.; Wu, Z.; Wu, Z. Effects of Multiple Factors on Hyperhydricity of Allium sativum L. Sci. Hortic. 2017, 217, 285–296. [Google Scholar] [CrossRef]
- Gerszberg, A. Tissue Culture and Genetic Transformation of Cabbage (Brassica oleracea var. capitata): An Overview. Planta 2018, 248, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Van Staden, J. Nitrogen Source, Concentration, and NH4+:NO3—Ratio Influence Shoot Regeneration and Hyperhydricity in Tissue Cultured Aloe polyphylla. Plant Cell Tissue Organ Cult. 2009, 99, 167–174. [Google Scholar] [CrossRef]
- Ivanova, M.; van Staden, J. Effect of Ammonium Ions and Cytokinins on Hyperhydricity and Multiplication Rate of in Vitro Regenerated Shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2007, 92, 227–231. [Google Scholar] [CrossRef]
- Jan, T.; Gul, S.; Khan, A.G.; Pervez, S.; Mohd, W.; Amin, H.; Bibi, S.; Nawaz, M.A.; Rahim, A.; Ahmad, M.; et al. Range of Factors in the Reduction of Hyperhydricity Associated with in Vitro Shoots of Salvia santolinifolia Bioss. Braz. J. Biol. 2023, 83, e246904. [Google Scholar] [CrossRef]
- Mohamed, S.M.; El-Mahrouk, M.E.; El-Banna, A.N.; Hafez, Y.M.; El-Ramady, H.; Abdalla, N.; Dobránszki, J. Optimizing Medium Composition and Environmental Culture Condition Enhances Antioxidant Enzymes, Recovers Gypsophila paniculata L. Hyperhydric Shoots and Improves Rooting in Vitro. Plants 2023, 12, 306. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. In Vitro Propagation Of Ballota acetabulosa. Acta Hortic. 2016, 1113, 171–174. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In Vitro Propagation and NaCl Tolerance of the Multipurpose Medicinal Halophyte Limoniastrum monopetalum. HortScience 2020, 55, 436–443. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Wegrzynowicz-Lesiak, E.; Dolezal, K.; Krekule, K.; Strnad, M.; Saniewski, M. New Cytokinin—Meta-Methoxytopolins in Micropropagation of Cotinus coggygria Scop. “Royal Purple”. Propag. Ornam. Plants 2012, 12, 220–228. [Google Scholar]
- Ozel, C.A.; Khawar, K.M.; Unal, F. Factors Affecting Efficient in Vitro Micropropagation of Muscari muscarimi Medikus Using Twin Bulb Scale. Saudi J. Biol. Sci. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kereša, S.; Stanković, D.; Batelja Lodeta, K.; Habuš Jerčić, I.; Bolarić, S.; Barić, M.; Bošnjak Mihovilović, A. Efficient Protocol for the in Vitro Plantlet Production of Caper (Capparis orientalis Veill.) from the East Adriatic Coast. Agronomy 2019, 9, 303. [Google Scholar] [CrossRef]
- D’Arth, S.M.; Simpson, S.I.; Seelye, J.F.; Jameson, P.E. Bushiness and Cytokinin Sensitivity in Micropropagated Zantedeschia. Plant Cell Tissue Organ Cult. 2002, 70, 113–118. [Google Scholar] [CrossRef]
- Valero-Aracama, C.; Kane, M.E.; Wilson, S.B.; Philman, N.L. Substitution of Benzyladenine with Meta-Topolin during Shoot Multiplication Increases Acclimatization of Difficult- and Easy-To-Acclimatize Sea Oats (Uniola paniculata L.) Genotypes. Plants 2009, 60, 43–49. [Google Scholar] [CrossRef]
- Katel, S.; Mandal, H.R.; Kattel, S.; Yadav, S.P.S.; Lamshal, B.S. Impacts of Plant Growth Regulators in Strawberry Plant: A Review. Heliyon 2022, 8, e11959. [Google Scholar] [CrossRef]

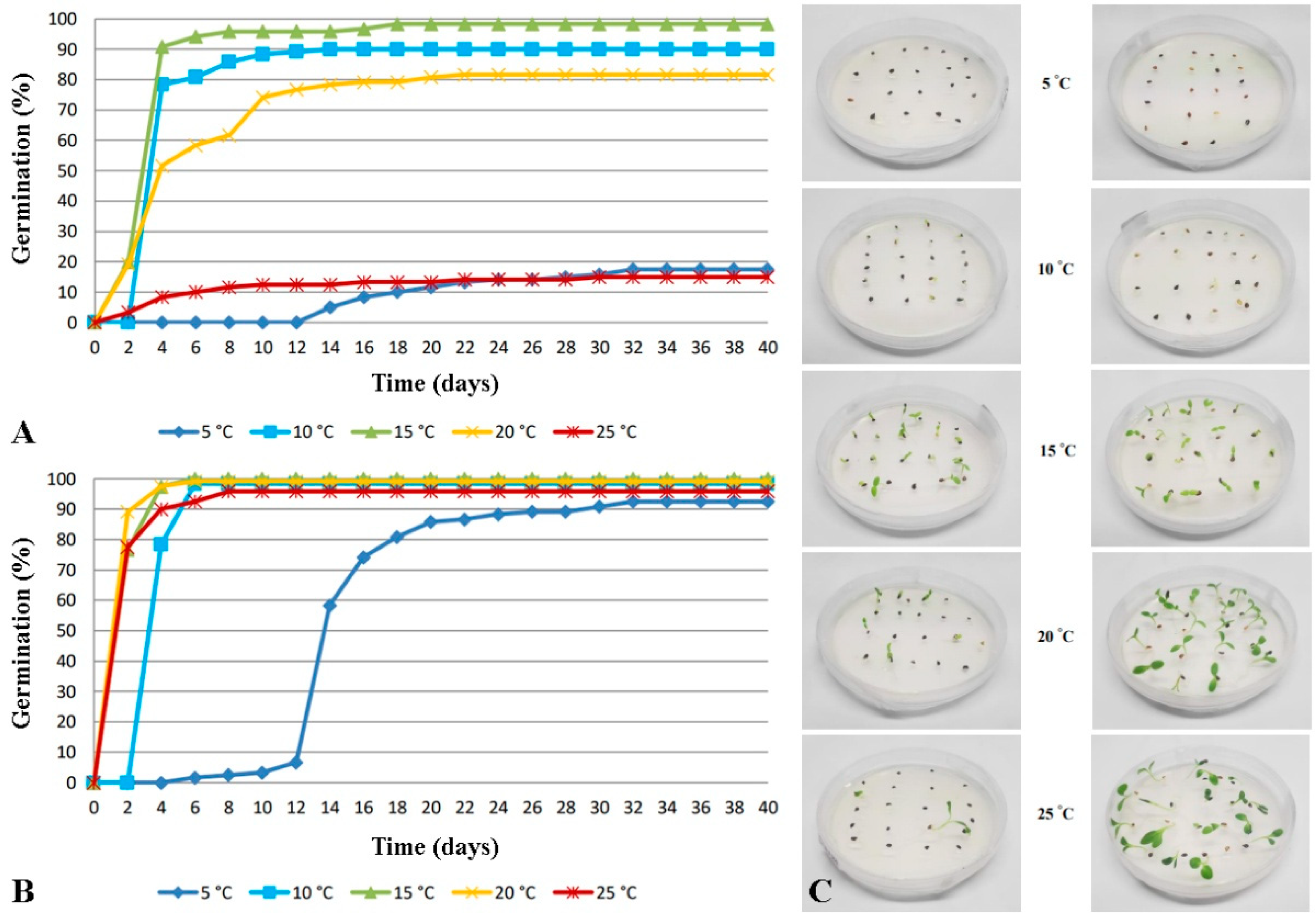




| Seed Age (Months) | Temperature (°C) | Germination (%) ± SE | T50 † (d) | Time for Full Germination (d) |
|---|---|---|---|---|
| 0 | 5 | 18.0 d | 18 | 32 |
| 0 | 10 | 90.0 b | 4 | 14 |
| 0 | 15 | 98.0 a | 4 | 8 |
| 0 | 20 | 82.0 c | 4 | 22 |
| 0 | 25 | 15.0 d | 4 | 30 |
| 12 | 5 | 91.0 b | 14 | 30 |
| 12 | 10 | 98.0 a | 4 | 6 |
| 12 | 15 | 100.0 a | 4 | 6 |
| 12 | 20 | 99.0 a | 2 | 6 |
| 12 | 25 | 96.0 ab | 2 | 8 |
| Fseed age | *** | - | - | |
| Ftemperature | *** | - | - | |
| Fseed age × temperature | *** | - | - | |
| Fone-way | *** | - | - |
| Cytokinin (mg L−1) | Reaction (%) | Shoot Formation (%) † | NS †† Number | Node Number per NS | HS ††† Number | MI †††† |
|---|---|---|---|---|---|---|
| BA 0.1 | 76.0 b | 55.0 a | 2.3 a | 2.2 b | 2.7 a | 2.8 a |
| BA 0.5 | 33.0 c | 16.0 b | 1.8 ab | 2.1 b | 2.4 a | 0.6 a |
| 2iP 0.1 | 72.0 b | 34.0 a | 1.1 bc | 2.6 a | 4.1 a | 1.0 a |
| 2iP 0.5 | 87.0 a | 40.0 a | 0.7 c | 2.9 a | 3.8 a | 0.8 a |
| Fone-way ANOVA § | *** | ** | *** | *** | NS | NS |
| Explant Physiology | Cytokinin (mg L−1) | NAA (mgL−1) | Reaction (%) | Shoot Formation (%) † | NS †† Number | Node Number per NS | HS ††† Number | MI †††† |
|---|---|---|---|---|---|---|---|---|
| Normal | - | - | 76.0 de | 58.0 cde | 1.7 cd | 2.4 b | 2.4 ef | 2.4 cde |
| BA 0.1 | - | 85.0 b | 80.0 ab | 2.5 ab | 2.5 ab | 2.0 f | 5.0 ab | |
| BA 0.1 | 0.01 | 85.0 b | 82.0 a | 2.0 abc | 2.6 ab | 1.5 f | 4.3 ab | |
| BA 0.1 | 0.05 | 83.0 b | 78.0 a | 2.6 a | 2.5 ab | 1.8 f | 5.1 a | |
| 2iP 0.1 | - | 80.0 bc | 62.0 cde | 1.5 cde | 2.4 ab | 2.6 def | 2.2 de | |
| 2iP 0.1 | 0.01 | 72.0 e | 54.0 de | 1.7 cd | 2.5 ab | 2.2 ef | 2.3 de | |
| 2iP 0.1 | 0.05 | 76.0 cde | 70.0 ab | 1.8 bcd | 2.6 a | 2.2 ef | 3.3 bcd | |
| Hyperhydric | - | 91.0 ab | 69.0 abc | 1.4 de | 2.5 ab | 4.1 cd | 2.4 de | |
| BA 0.1 | - | 100.0 a | 67.0 bcd | 1.4 cde | 2.5 ab | 7.1 ab | 2.3 de | |
| BA 0.1 | 0.01 | 100.0 a | 73.0 abc | 1.5 cde | 2.6 ab | 6.1 abc | 2.9 abc | |
| BA 0.1 | 0.05 | 100.0 a | 87.0 a | 2.1 abc | 2.8 a | 5.6 bc | 5.1 ab | |
| 2iP 0.1 | - | 100.0 a | 40.0 e | 0.8 e | 2.3 b | 8.3 a | 0.7 e | |
| 2iP 0.1 | 0.01 | 87.0 bc | 53.0 de | 1.1 de | 2.4 ab | 5.8 abc | 1.4 e | |
| 2iP 0.1 | 0.05 | 100.0 a | 60.0 bcd | 1.2 cde | 2.4 ab | 4.1 cde | 1.7 de | |
| Fphysiology | *** | NS | *** | NS | *** | * | ||
| Fcytokinin | * | *** | *** | NS | NS | *** | ||
| FNAA | NS | * | NS | NS | ** | NS | ||
| Fphys × NAA § | NS | NS | NS | NS | * | NS | ||
| Fone-way ANOVA | *** | * | *** | NS | *** | *** | ||
| Explant Physiology | Medium Composition (mg L−1) | Reaction (%) | Shoot Formation (%) † | NS †† Number | Node Number per NS | HS ††† Number | MI †††† |
|---|---|---|---|---|---|---|---|
| Normal | MS/8/0.1 BA | 90.0 abc | 77.0 a | 2.0 ab | 2.5 ab | 1.4 de | 3.8 ab |
| MS/12/0.1 BA | 87.0 bc | 87.0 a | 2.0 ab | 2.3 b | 0.1 e | 4.0 ab | |
| WPM/8/0.1 BA | 93.0 ab | 87.0 a | 1.6 b | 2.3 b | 0.2 e | 3.2 b | |
| MS/8/0.1 ZEA | 80.0 c | 57.0 bc | 1.5 b | 2.4 ab | 4.0 bc | 2.0 bc | |
| Hyperhydric | MS/8/0.1 BA | 93.0 ab | 43.0 cd | 0.7 c | 2.6 ab | 5.3 b | 0.8 c |
| MS/12/0.1 BA | 93.0 ab | 83.0 a | 2.4 a | 2.6 a | 2.5 cd | 5.2 a | |
| WPM/8/0.1 BA | 90.0 abc | 67.0 ab | 2.0 ab | 2.2 b | 2.1 cde | 2.9 b | |
| MS/8/0.1 ZEA | 100.0 a | 30.0 d | 0.5 c | 2.6 ab | 12.3 a | 0.4 c | |
| Fphysiology | * | ** | NS | NS | *** | NS | |
| Fsubstrate | NS | *** | *** | NS | *** | *** | |
| Fphysiology × substrate § | NS | NS | ** | NS | *** | * | |
| Fone-way | NS | *** | *** | NS | *** | *** | |
| IBA (mg L−1) | Cytokinin (mg L−1) | Rooting (%) | Root Number | Root Length (cm) | Callus (%) |
|---|---|---|---|---|---|
| 0.0 | 0.0 | 90.0 ab | 5.7 bc | 4.0 a | 53.0 bc |
| 0.0 | 0.1 BA | 86.0 b | 4.6 cd | 3.0 b | 44.0 bc |
| 0.0 | 0.1 ZEA | 60.0 c | 4.0 d | 3.2 b | 33.0 bc |
| 0.5 | 0.0 | 100.0 a | 7.6 a | 2.6 bc | 83.0 a |
| 0.5 | 0.1 BA | 89.0 ab | 4.6 cd | 2.1 c | 52.0 b |
| 0.5 | 0.1 ZEA | 83.0 bc | 6.1 b | 2.1 c | 44.0 bc |
| FIBA | * | ** | *** | * | |
| Fcytokinin | *** | *** | ** | ** | |
| FIBA × cytokinin § | NS | NS | NS | NS | |
| Fone-way ANOVA | *** | *** | *** | *** | |
| IBA in Rooting (mg L−1) | Cytokinin (mg L−1) | Acclimatization (%) | Stem Number | Stem Length (cm) | Node Number | Max Leaf Length (cm) | Max Leaf Width (mm) |
|---|---|---|---|---|---|---|---|
| 0.0 | 0.0 | 65.0 b | 1.1 b | 2.2 b | 6.3 a | 4.8 c | 2.6 a |
| 0.0 | 0.1 BA | 100.0 a | 1.0 b | 3.0 a | 6.2 a | 7.4 ab | 2.6 a |
| 0.0 | 0.1 ZEA | 92.0 a | 1.5 a | 1.2 c | 5.2 b | 6.4 b | 2.3 b |
| 0.5 | 0.0 | 83.0 a | 1.2 b | 2.4 b | 6.0 a | 8.4 a | 2.6 a |
| 0.5 | 0.1 BA | 100.0 a | 1.3 ab | 2.3 b | 5.8 a | 6.2 bc | 2.3 b |
| 0.5 | 0.1 ZEA | 96.0 a | 1.1 b | 1.5 c | 5.9 a | 7.6 ab | 2.6 a |
| FIBA | NS | NS | NS | NS | *** | NS | |
| Fcytokinin | *** | NS | *** | * | NS | * | |
| FIBA × cytokinin | * | * | ** | *** | *** | *** | |
| Fone-way | *** | * | *** | ** | *** | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazanis, A.-E.; Papafotiou, M. In Vitro Germination, Micropropagation and Addressing the Hyperhydricity of the Balkan Native Dianthus cruentus, a Plant with High Ornamental and Xeriscaping Potential. Horticulturae 2024, 10, 813. https://doi.org/10.3390/horticulturae10080813
Bazanis A-E, Papafotiou M. In Vitro Germination, Micropropagation and Addressing the Hyperhydricity of the Balkan Native Dianthus cruentus, a Plant with High Ornamental and Xeriscaping Potential. Horticulturae. 2024; 10(8):813. https://doi.org/10.3390/horticulturae10080813
Chicago/Turabian StyleBazanis, Apostolos-Emmanouil, and Maria Papafotiou. 2024. "In Vitro Germination, Micropropagation and Addressing the Hyperhydricity of the Balkan Native Dianthus cruentus, a Plant with High Ornamental and Xeriscaping Potential" Horticulturae 10, no. 8: 813. https://doi.org/10.3390/horticulturae10080813
APA StyleBazanis, A.-E., & Papafotiou, M. (2024). In Vitro Germination, Micropropagation and Addressing the Hyperhydricity of the Balkan Native Dianthus cruentus, a Plant with High Ornamental and Xeriscaping Potential. Horticulturae, 10(8), 813. https://doi.org/10.3390/horticulturae10080813





