Assessment of the Impact of Annual Growing Conditions on the Physicochemical Properties of Mango Kernel Fat
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvest and Extraction
2.2. Fatty Acid Composition by Gas Chromatography
2.3. Triacylglycerol Composition
2.4. Melting Profiles by p-NMR
2.5. Melting Profiles by Differential Scanning Calorimetry (DSC)
2.6. Statistical Analysis
3. Results
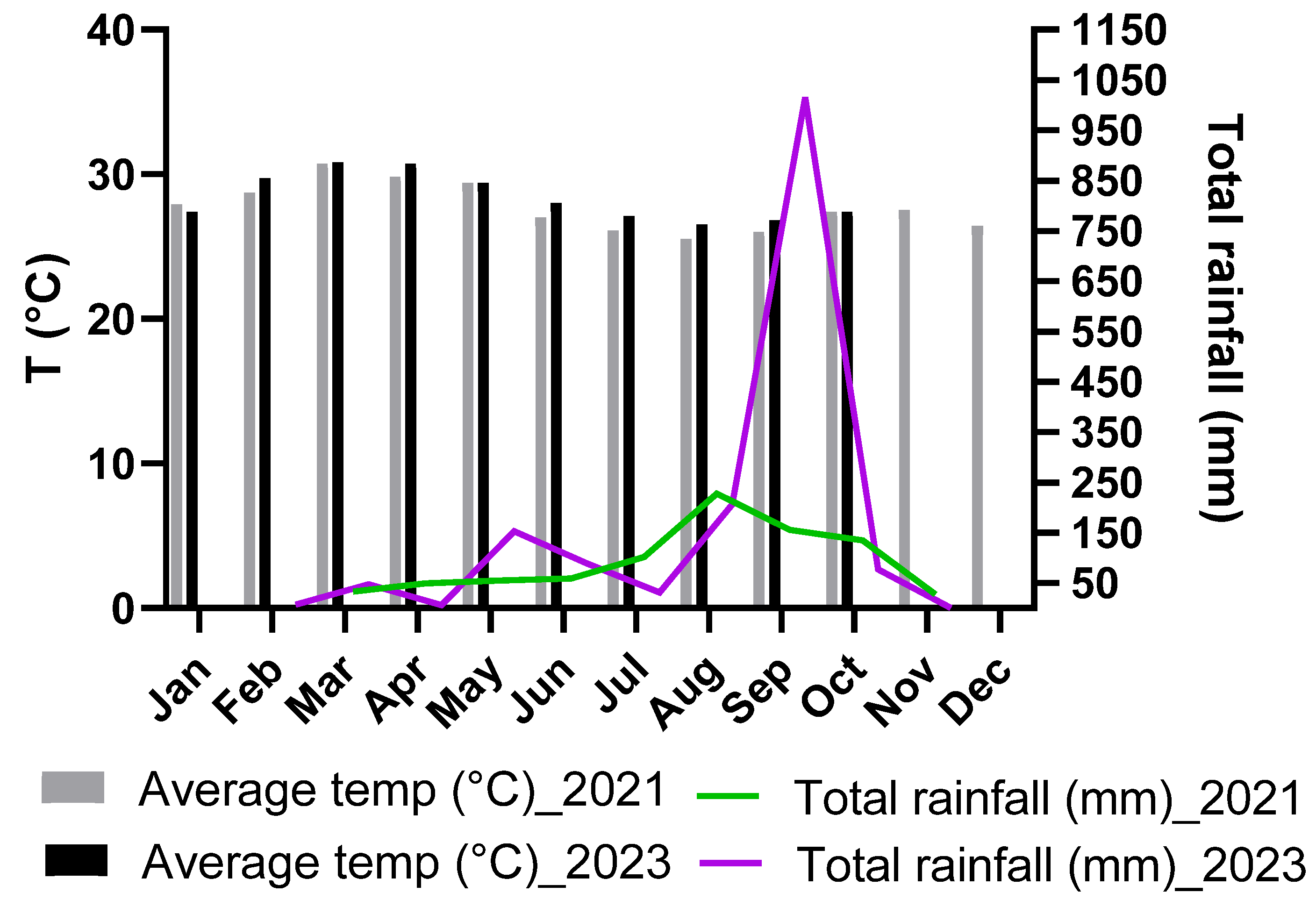
3.1. Weather Conditions and Plant Phenology
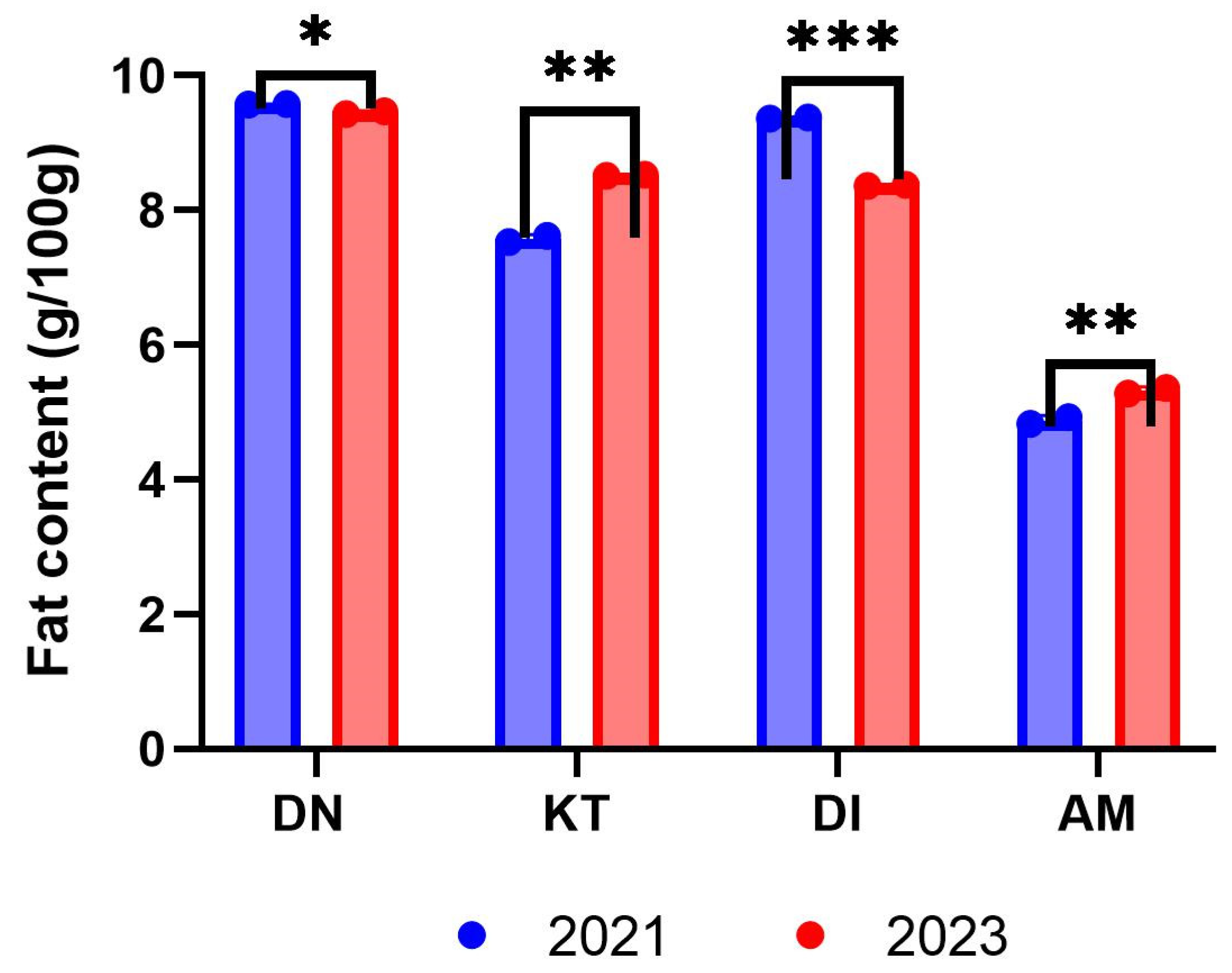
3.2. Fat Content
3.3. Fatty Acid Composition
3.4. Triacylglycerol Composition (TAG)
3.5. Physicochemical Properties of MKF
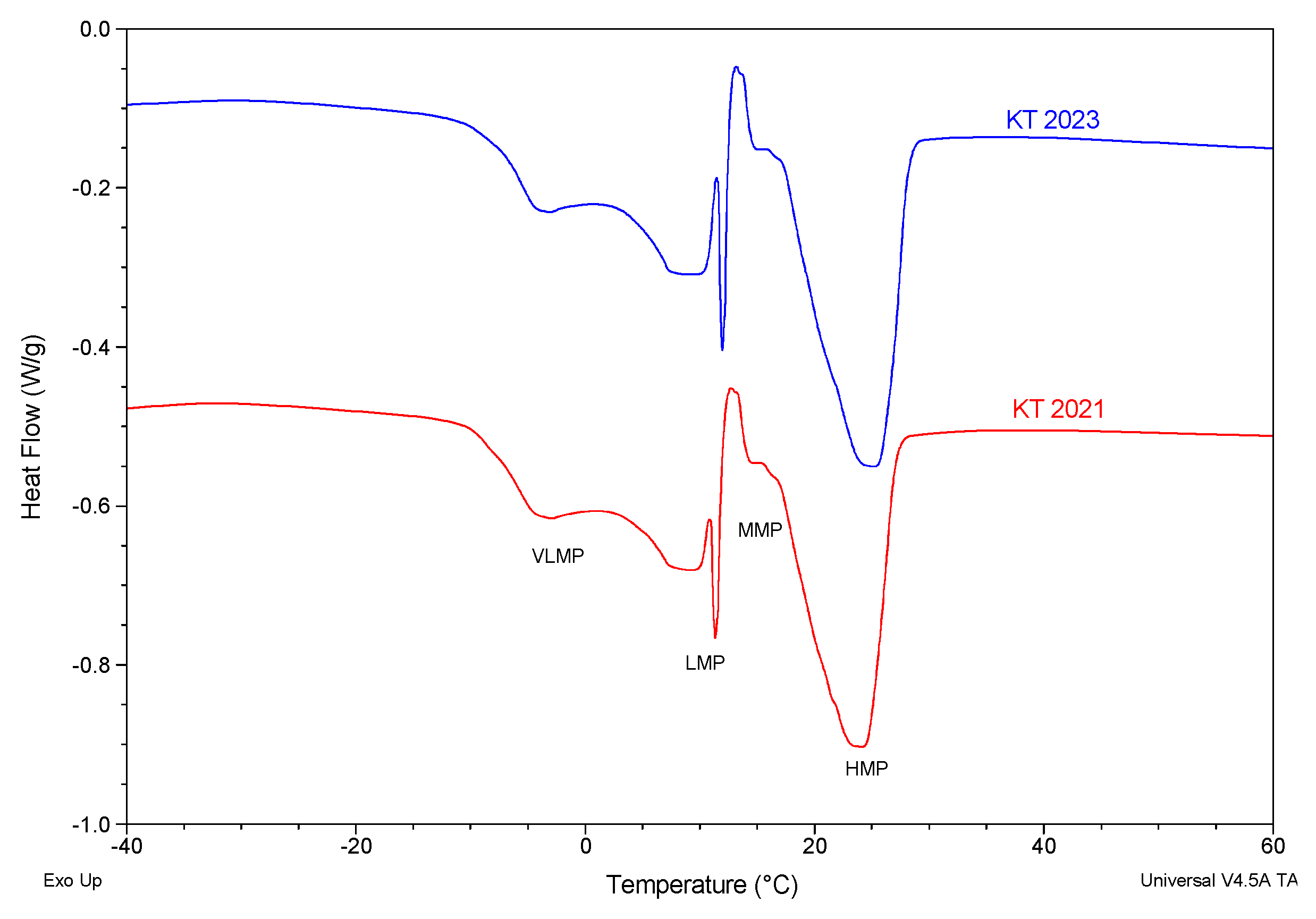
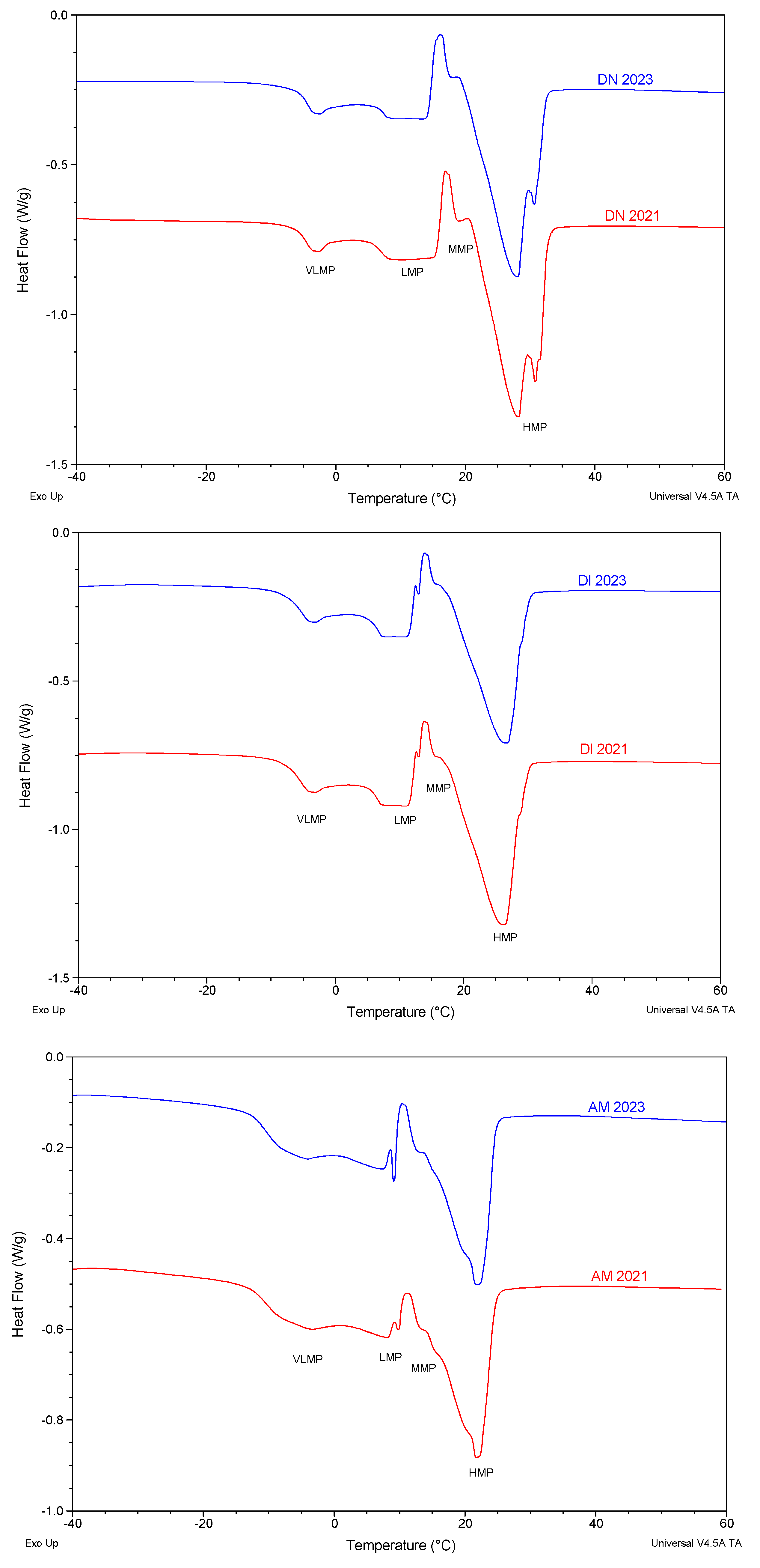
3.5.1. SFC Melting Profile by p-NMR
3.5.2. Melting Profile by Differential Scanning Calorimetry (DSC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonwai, S.; Kaphueakngam, P.; Flood, A. Blending of Mango Kernel Fat and Palm Oil Mid-Fraction to Obtain Cocoa Butter Equivalent. J. Food Sci. Technol. 2014, 51, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, A.K.; Alabi, T.; Cissé, M.; Purcaro, G.; Moret, S.; Moret, E.; Blecker, C.; Danthine, S. Assessment of Composition, Color, and Oxidative Stability of Mango (Mangifera indica L.) Kernel Fats from Various Ivorian Varieties. J. Am. Oil Chem. Soc. 2024, 101, 283–295. [Google Scholar] [CrossRef]
- Vincenot, D.; Normand, F. Guide de Production Intégrée de Mangues à La Réunion. 2009, p. 121. Available online: https://agritrop.cirad.fr/552207/1/552207.pdf (accessed on 5 May 2024).
- Scuderi, D.; Gianguzzi, G.; Priola, F.; Farina, V. Phenological Cycle of Three Mango Cultivars in the Mediterranean Climate. Hortic. Environ. Biotechnol. 2024, 56, 433–441. [Google Scholar] [CrossRef]
- Clonan, M.; McConchie, C.; Hall, M.; Hearnden, M.; Olesen, T.; Sarkhosh, A. Effects of Ambient Temperatures on Floral Initiation in Australian Mango (Mangifera indica L.) Selections. Sci. Hortic. 2021, 276, 109767. [Google Scholar] [CrossRef]
- Magne, C. Effet de La Charge En Fruits Sur La Croissance Végétative de Plusieurs Variétés de Manguier à l’île de La Réunion; Université Blaise Pascal: Aubière, France, 2004. [Google Scholar]
- Normand, F.; Bello, A.K.P.; Trottier, C.; Lauri, P.É. Is Axis Position within Tree Architecture a Determinant of Axis Morphology, Branching, Flowering and Fruiting? An Essay in Mango. Ann. Bot. 2009, 103, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur Brar, J. Use of Mango Seed Kernels for the Development of Antioxidant Rich Health Foods. Food Sci. Res. J. 2017, 8, 368–374. [Google Scholar] [CrossRef]
- Ifesan, B.O.T. Chemical Properties of Mango Kernel and Seed and Production of Biscuit From Wheat-Mango Kernel Flour Blends. Int. J. Food Nutr. Res. 2017, 1, 5. [Google Scholar]
- Melo, P.E.F.; Silva, A.P.M.; Marques, F.P.; Ribeiro, P.R.V.; de sá Souza Filho, M.M.; Brito, E.S.; Lima, J.R.; Azeredo, H.M.C. Antioxidant Films from Mango Kernel Components. Food Hydrocoll. 2019, 95, 487–495. [Google Scholar] [CrossRef]
- EU. EU Directive 2000/36/EC of the European Parliament and of the Council of 23 June 2000 Relating to Cocoa and Chocolate Products Intended for Human Consumption. Off. J. Eur. Communities 2000, L197, 19–25. [Google Scholar]
- Jin, J.; Akoh, C.C.; Jin, Q.; Wang, X. Preparation of Mango Kernel Fat Stearin-Based Hard Chocolate Fats via Physical Blending and Enzymatic Interesterification. Food Sci. Technol. 2018, 97, 308–316. [Google Scholar] [CrossRef]
- Abdel-Razik, M.M.; Ashoush, I.S.; Nabih, M.N.Y. Characteristics of Mango Seed Kernel Butter and Its Effects on Quality Attributes of Muffins. J. Fd. Sci. Technol 2012, 9, 1–9. [Google Scholar]
- Nadeem, M.; Imran, M.; Khalique, A. Promising Features of Mango (Mangifera indica L.). J. Food Sci. Technol. 2016, 53, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical Characteristics, Bioactive Compounds and Industrial Applications of Mango Kernel and Its Products: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- PPAAO/WAAPP Côte d’Ivoire. Available online: http://www.waapp-ppaao.org/cotedivoire/ (accessed on 21 May 2024).
- Kouassi, A.K.; Alabi, T.; N’guessan, E.A.; Purcaro, G.; Moret, S.; Cissé, M.; Blecker, C.; Danthine, S. Comparative Study of Thermal Behavior of Mango Kernel Fat from Seven Ivorian Varieties Related to Their Chemical Composition. Eur. Food Res. Technol. 2024, 250, 1–13. [Google Scholar] [CrossRef]
- Fina, A.; Mascrez, S.; Beccaria, M.; De Luca, C.; Aspromonte, J.; Cordero, C.; Purcaro, G. A High Throughput Method for Fatty Acid Profiling Using Simultaneous Microwave-Assisted Extraction and Derivatization Followed by Reversed Fill/Flush Flow Modulation Comprehensive Multidimensional Gas Chromatography. Adv. Sample Prep. 2022, 4, 100039. [Google Scholar] [CrossRef]
- Danthine, S.; Closset, S.; Maes, J.; Mascrez, S.; Blecker, C.; Purcaro, G.; Gibon, V. Enzymatic Interesterification to Produce Zero- Trans and Dialkylketones-Free Fats from Rapeseed Oil. OCL-Oilseeds Fats Crops Lipids 2022, 29, 36. [Google Scholar] [CrossRef]
- IUPAC. Solid Content Determination in Fats by NMR (Low-Resolution Nuclear Magnetic Resonance); 2.323; IUPAC: Zürich, Switzerland, 1987. [Google Scholar]
- Danthine, S. Physicochemical and Structural Properties of Compound Dairy Fat Blends. Food Res. Int. 2012, 48, 187–195. [Google Scholar] [CrossRef]
- Lockwood, R. The Mango. Botany, Production and Uses. Exp. Agric. 2010, 46, 416. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. Mango (Mangifera indica L.) Flowering Physiology. Sci. Hortic. 2010, 126, 65–72. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Chandrasekhara Rao, T.; Ramalingaswamy, P.A. Varietal Variations in Content, Characteristics and Composition of Mango Seeds and Fat. J. Am. Oil Chem. Soc. 1983, 60, 88–89. [Google Scholar] [CrossRef]
- Nzikou, J.M.; Kimbonguila, A.; Matos, L.; Loumouamou, B.; Pambou-Tobi, N.P.G.; Ndangui, C.B.; Abena, A.A.; Silou, T.; Scher, J.; Desobry, S. Extraction and Characteristics of Seed Kernel Oil from Mango (Mangifera indica). Res. J. Environ. Earth Sci. 2010, 2, 31–35. [Google Scholar]
- Tan, C.X. Virgin Avocado Oil: An Emerging Source of Functional Fruit Oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Akusu, O.M.; Obinna-Echem, P.C.; Opurum, P.C.C.; Chibor, B.S. Comparative Analysis of the Physicochemical Characteristics, Phytochemical Components and Fatty Acid Profile of Avocado Pear (Persea americana L.) Pulp and Seed Oil. Eur. J. Agric. Food Sci. 2021, 3, 11–17. [Google Scholar] [CrossRef]
- Vanniere, H.; Rey, J.Y. P3 Document Élaboré Par Le PIP Avec La Collaboration Technique De. 2013. Available online: https://agritrop.cirad.fr/573091/1/document_573091.pdf (accessed on 21 May 2024).
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on Mango (Mangifera indica L.) Kernel Fat of Some Kenyan Varieties in Meru. J. Am. Oil Chem. Soc. 2012, 89, 1567–1575. [Google Scholar] [CrossRef]
- Lieb, V.M.; Schuster, L.K.; Kronmüller, A.; Schmarr, H. Fatty Acids, Triacylglycerols, and Thermal Behaviour of Various Mango (Mangifera indica L.) Kernel Fats. Food Res. Int. 2019, 116, 527–537. [Google Scholar] [CrossRef]
- Anjani, K.; Yadav, P. High Yielding-High Oleic Non-Genetically Modified Indian Safflower Cultivars. Ind. Crops Prod. 2017, 104, 7–12. [Google Scholar] [CrossRef]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Effect of Sowing Dates on Fatty Acids and Phytosterols Patterns of Carthamus tinctorius L. Appl. Sci. 2019, 9, 2839. [Google Scholar] [CrossRef]
- Boydak, E.; Alpaslan, M.; Hayta, M.; Gerçek, S.; Simsek, M. Seed Composition of Soybeans Grown in the Harran Region of Turkey as Affected by Row Spacing and Irrigation. J. Agric. Food Chem. 2002, 50, 4718–4720. [Google Scholar] [CrossRef]
- Gao, J.; Hao, X.; Thelen, K.D.; Robertson, G.P. Agronomic Management System and Precipitation Eff Ects on Soybean Oil and Fatty Acid Profi Les. Crop Sci. 2009, 49, 1049–1057. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef]
- Attia, Z.; Pogoda, C.S.; Reinert, S.; Kane, N.C.; Hulke, B.S. Breeding for Sustainable Oilseed Crop Yield and Quality in a Changing Climate. Theor. Appl. Genet. 2021, 134, 1817–1827. [Google Scholar] [CrossRef]
- Braipson-Danthine, S.; Deroanne, C. Determination of Solid Fat Content (SFC) of Binary Fat Blends and Use of These Data to Predict SFC of Selected Ternary Fat Blends Containing Low-Erucic Rapeseed Oil. J. Am. Oil Chem. Soc. 2006, 83, 571–581. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; Gonçalves, L.A.G. Instrumental Methods for the Evaluation of Interesterified Fats. Food Anal. Methods 2009, 2, 282–302. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Nik Norulaini, N.A.; Sahena, F.; Abedin, M.Z.; Mohamed, A.; Mohd Omar, A.K. Hard Cocoa Butter Replacers from Mango Seed Fat and Palm Stearin. Food Chem. 2014, 154, 323–329. [Google Scholar] [CrossRef]
- Islam, M.; Bełkowska, L.; Konieczny, P.; Fornal, E.; Tomaszewska-Gras, J. Differential Scanning Calorimetry for Authentication of Edible Fats and OilseWhat Can We Learn from the Past to Face the Current Challenges? J. Food Drug Anal. 2022, 30, 185–201. [Google Scholar] [CrossRef]
- Sessa, D.J. Derivation of a Cocoa Butter Equivalent from Jojoba Transesterified Ester a Differential Scanning Calorimetry Index. J. Sci. Food Agric. 1996, 72, 295–298. [Google Scholar] [CrossRef]


| Variety | Dadiani (DI) | Kent (KT) | Djakoumankoun (DN) | Amelie (AM) | ||||
|---|---|---|---|---|---|---|---|---|
| FA (%) | 2021 | 2023 | 2021 | 2023 | 2021 | 2023 | 2021 | 2023 |
| Palmitic acid (p) | 9.50 c ± 0.02 | 8.59 e ± 0.03 | 9.39 d ± 0.04 | 7.72 g ± 0.05 | 8.28 f ± 0.12 | 7.28 h ± 0.01 | 13.44 a ± 0.9 | 12.10 b ± 0.16 |
| Stearic acid (St) | 40.62 c ± 0.08 | 40.36 d ± 0.05 | 35.07 f ± 0.01 | 37.64 e ± 0.14 | 48.33 a ± 0.02 | 46.18 b ± 0.12 | 30.26 h ± 2.27 | 31.76 g ± 0.06 |
| Oleic acid (O) | 41.44 f ± 0.01 | 43.29 e ± 0.12 | 47.00 a ± 0.03 | 46.95 b ± 0.12 | 35.92 h ± 0.09 | 38.94 g ± 0.08 | 43.93 d ± 0.66 | 44.25 c ± 0.15 |
| Linoleic acid (L) | 6.39 c ± 0.02 | 5.43 e ± 0.02 | 6.23 d ± 0.03 | 5.20 f ± 0.04 | 4.88 g ± 0.02 | 4.75 h ± 0.02 | 9.58 a ± 1.91 | 8.79 b ± 0.16 |
| Linolenic (Ln) | 0.32 g ± 0.02 | 0.44 f ± 0.01 | 0.49 d ± 0.07 | 0.52 c ± 0.01 | 0.31 h ± 0.02 | 0.45 e ± 0.01 | 1.11 a ± 0.36 | 1.10 b ± 0.01 |
| Arachidic acid (A) | 1.72 g ± 0.05 | 1.91 e ± 0.01 | 1.82 f ± 0.08 | 1.99 d ± 0.01 | 2.29 b ± 0.02 | 2.42 a ± 0.01 | 1.67 h ± 0.23 | 2.02 c ± 0.08 |
| DN | KT | DI | AM | |||||
|---|---|---|---|---|---|---|---|---|
| Triacylglycerol (TAG) % | 2021 | 2023 | 2021 | 2023 | 2021 | 2023 | 2021 | 2023 |
| LLL | 0.01 c ± 00 | 0.08 bc ± 0.04 | 0.08 bc ± 0.01 | 0.21 ab ± 0.01 | 0.12 bc ± 0.03 | 0.10 bc ± 0.01 | 0.22 ab ± 0.03 | 0.35 a ± 0.01 |
| OLLn | 0.03 c ± 00 | 0.13 abc ± 0.02 | 0.11 abc ± 0.01 | 0.24 abc ± 0.03 | 0.14 abc ± 0.09 | 0.06 bc ± 0.02 | 0.27 a ± 0.01 | 0.26 ab ± 0.09 |
| PLLn | 0.03 b ± 00 | 0.13 b ± 0.1 | 0.07 b ± 0.01 | 0.65 a ± 0.04 | 0.08 b ± 0.07 | 0.09 b ± 0.01 | 0.22 b ± 0.01 | 0.23 b ± 0.16 |
| OLL | 0.16 c ± 0.01 | 0.24 c ± 0.01 | 0.54 abc ± 24 | 0.50 abc ± 0.04 | 0.44 abc ± 0.04 | 0.42 bc ± 0.07 | 0.90 ab ± 0.01 | 0.97 a ± 0.29 |
| PLL | 0.01 b ± 0.01 | 0.07 b ± 0.01 | 0.15 a ± 0.16 | 0.18 a ± 0.03 | 0.08 b ± 0.05 | 0.10 b ± 0.01 | 0.35 a ± 0.02 | 0.36 a ± 0.01 |
| POLn | 0.09 a ± 0.06 | 0.19 a ± 0.03 | 0.33 a ± 0.21 | 0.46 a ± 0.02 | 0.21 a ± 0.16 | 0.30 a ± 0.01 | 0.91 a ± 0.03 | 0.83 a ± 0.09 |
| PLnP | 0.02 c ±0.01 | 0.04 c ± 0.01 | 0.02 c ± 0.01 | 0.14 b ± 0.05 | 0.01 c ± 0.01 | 0.03 c ± 0.01 | 0.08 bc ± 0.01 | 0.30 a ± 0.01 |
| OOL | 0.85 d ± 0.04 | 1.01 cd ± 0.02 | 2.21 b ± 0.02 | 1.97 b ± 0.05 | 1.70 b ± 0.10 | 1.66 bc ± 0.17 | 3.15 a ± 0.13 | 3.07 a ± 0.41 |
| StLL + POL | 1.24 b ± 0.01 | 1.22 b ± 0.01 | 1.90 b ± 0.03 | 1.77b ± 0.04 | 1.84 b ± 0.16 | 1.62 b ± 0.24 | 3.57 a ± 0.08 | 3.54 a ± 0.55 |
| PLP | 0.44 b ± 0.02 | 0.47 b ± 0.01 | 0.38 b ± 0.01 | 0.44 b ± 0.01 | 0.37 b ± 0.08 | 0.32 b ± 0.13 | 1.03 a ± 0.02 | 1.20 a ± 020 |
| OOO | 2.29 d ± 0.02 | 3.20 c ± 0.01 | 8.06 a ± 0.05 | 7.76 a ± 0.09 | 4.66 b ± 0.09 | 4.67 b ± 0.30 | 7.99 a ± 0.12 | 7.67 a ± 0.23 |
| StLO | 2.91 e ± 0.06 | 3.32 de ± 0.03 | 3.77 cd ± 0.04 | 3.71 cd ±0.06 | 4.67 a ± 0.05 | 4.38 ab ± 0.29 | 4.05bc ± 0.26 | 4.21 abc ± 0.10 |
| OOP | 2.16 e ± 0.03 | 2.28 e ± 0.02 | 6.08 b ± 0.03 | 5.15 c ± 0.01 | 4.16 d ± 0.02 | 3.91 d ± 0.37 | 7.31 a ± 0.29 | 7.60 a ± 0.30 |
| StLP | 1.97 bc ± 0.07 | 2.05 bc ± 0.02 | 1.61 c ±0.02 | 1.56 c ± 0.01 | 1.90 bc ± 0.01 | 1.59 c ± 0.03 | 2.29 b ± 0.01 | 2.90 a ± 0.23 |
| POP | 1.41 c ± 0.01 | 1.46 c ± 0.02 | 1.88 bc ± 0.03 | 1.55 c ± 0.01 | 1.73 c ± 0.01 | 1.63 c ± 0.15 | 3.03 ab ± 0.48 | 3.89 a ± 0.88 |
| StOO | 15.33 g ± 0.14 | 18.17 f ± 0.09 | 25.50 ab ± 0.01 | 26.45 a ± 0.02 | 23.02 cd ± 0.01 | 24.63 bc ± 0.06 | 21.05e ± 0.48 | 21.64 de ± 0.80 |
| POSt | 4.18 a ± 0.09 | 4.03 a ± 0.01 | 2.64 a ± 0.16 | 2.75 a ± 0.17 | 2.92 a ± 0.07 | 3.69 a ± 0.83 | 2.99 a ± 0.02 | 3.62 a ± 0.07 |
| StLSt | 13.88 ab ± 0.06 | 13.20 bc ± 0.23 | 11.61 d ± 0.05 | 11.26 d ± 0.16 | 14.45 a ± 0.17 | 14.31 a ± 0.36 | 13.03 c ± 0.32 | 12.96 c ± 0.23 |
| AOO | 1.91 a ± 0.17 | 1.92 a ± 0.02 | 2.08 a ± 0.01 | 2.14 a ± 0.03 | 1.85 a ± 0.06 | 1.82 a ± 0.02 | 1.71 a ± 0.42 | 1.68 a ± 0.28 |
| StOSt | 46.20 a ± 0.08 | 42.61 b ± 0.23 | 28.34 d ± 0.19 | 28.58 d ± 0.01 | 32.77 c ± 0.07 | 32.43 c ± 0.46 | 23.39 e ± 0.80 | 21.36 f ± 0.37 |
| StOA | 4.18 a ± 0.03 | 3.44 b ± 0.19 | 2.22 cd ± 0.02 | 1.93 cd ±0.17 | 2.46 c ± 0.28 | 2.18 cd ± 0.01 | 1.99 cd ± 0.03 | 1.71 d ± 0.07 |
| OAA | 0.72 a ± 0.01 | 0.62 b ± 0.03 | 0.42 cd ± 0.04 | 0.40 cd ± 0.01 | 0.42 cd ± 0.03 | 0.34 d ± 0.01 | 0.47 c ± 0.01 | 0.00 e ± 0.00 |
| SUS (mono-unsaturated TAGs) | 73.00 a ± 0.02 | 67.91 b ± 0.09 | 49.12 d ± 0.32 | 48.61 d ± 0.17 | 57.04 c ± 0.01 | 56.51 c ± 0.04 | 48.30 d ± 0.49 | 47.94 d ± 0.23 |
| SUU (di-unsaturated TAGs) | 24.40 d ± 0.17 | 27.91 c ± 0.16 | 40.31 a ± 0.20 | 40.91 a ± 0.06 | 36.33 b ±0.06 | 37.18 b ± 0.44 | 39.64 a ± 0.21 | 40.08 a ± 0.69 |
| UUU (tri-unsaturated TAGs) | 3.32 c ± 0.03 | 4.65 c ± 0.09 | 10.99 a ± 0.16 | 10.67 a ± 0.07 | 7.06 b ± 0.02 | 6.91 b ± 0.64 | 12.53 a ± 0.29 | 12.31 a ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouassi, A.K.; Alabi, T.; Purcaro, G.; Blecker, C.; Danthine, S. Assessment of the Impact of Annual Growing Conditions on the Physicochemical Properties of Mango Kernel Fat. Horticulturae 2024, 10, 814. https://doi.org/10.3390/horticulturae10080814
Kouassi AK, Alabi T, Purcaro G, Blecker C, Danthine S. Assessment of the Impact of Annual Growing Conditions on the Physicochemical Properties of Mango Kernel Fat. Horticulturae. 2024; 10(8):814. https://doi.org/10.3390/horticulturae10080814
Chicago/Turabian StyleKouassi, Alfred Kouakou, Taofic Alabi, Giorgia Purcaro, Christophe Blecker, and Sabine Danthine. 2024. "Assessment of the Impact of Annual Growing Conditions on the Physicochemical Properties of Mango Kernel Fat" Horticulturae 10, no. 8: 814. https://doi.org/10.3390/horticulturae10080814
APA StyleKouassi, A. K., Alabi, T., Purcaro, G., Blecker, C., & Danthine, S. (2024). Assessment of the Impact of Annual Growing Conditions on the Physicochemical Properties of Mango Kernel Fat. Horticulturae, 10(8), 814. https://doi.org/10.3390/horticulturae10080814








