The Effects of Different Postharvest Drying Temperatures on the Volatile Flavor Components and Non-Volatile Metabolites of Morchella sextelata
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. GC-IMS Analysis
2.3. LC-MS/MS Analysis
2.4. Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
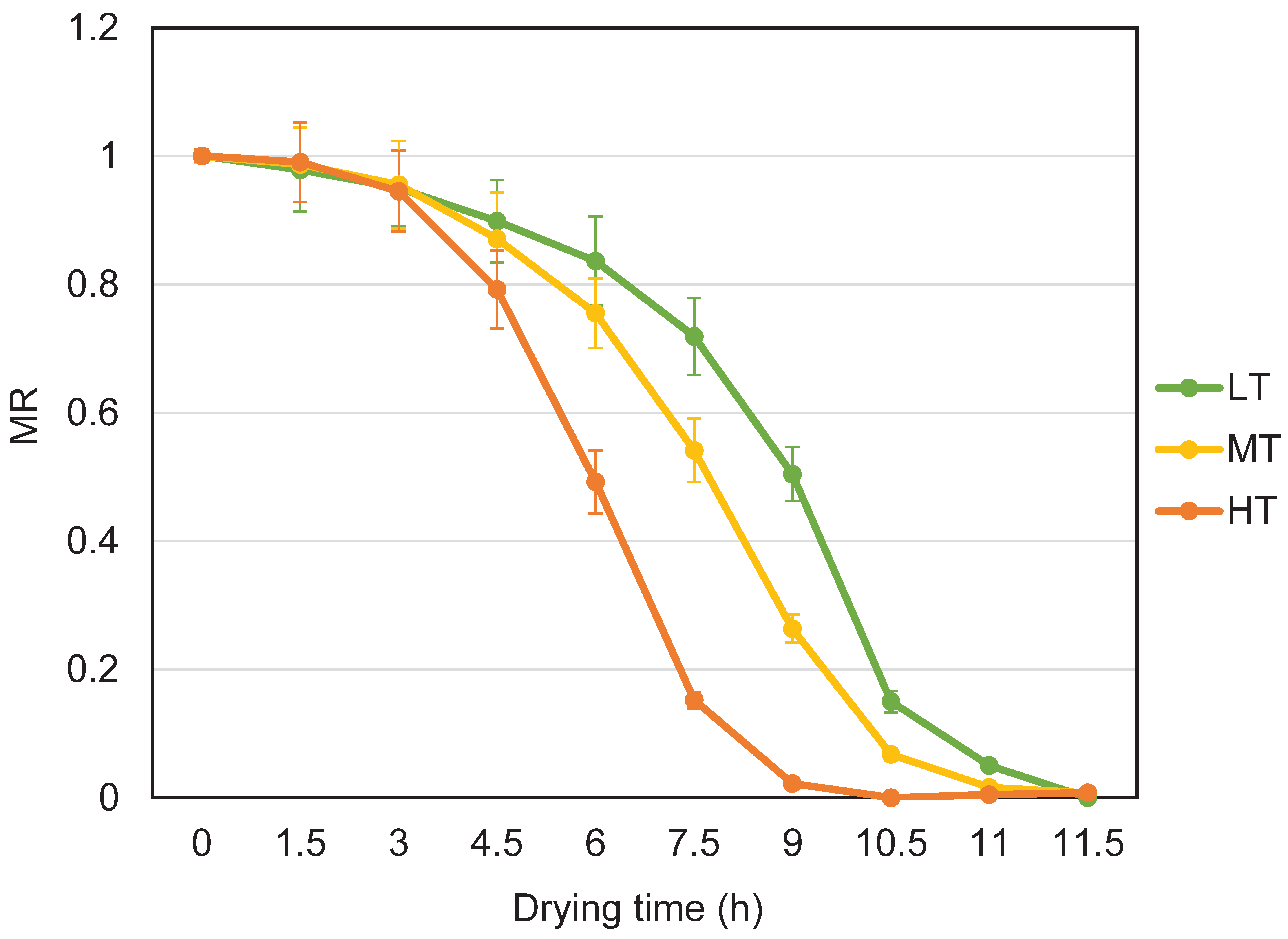
3.1. Effects of Different Temperatures on the Drying Rate of M. sextelata
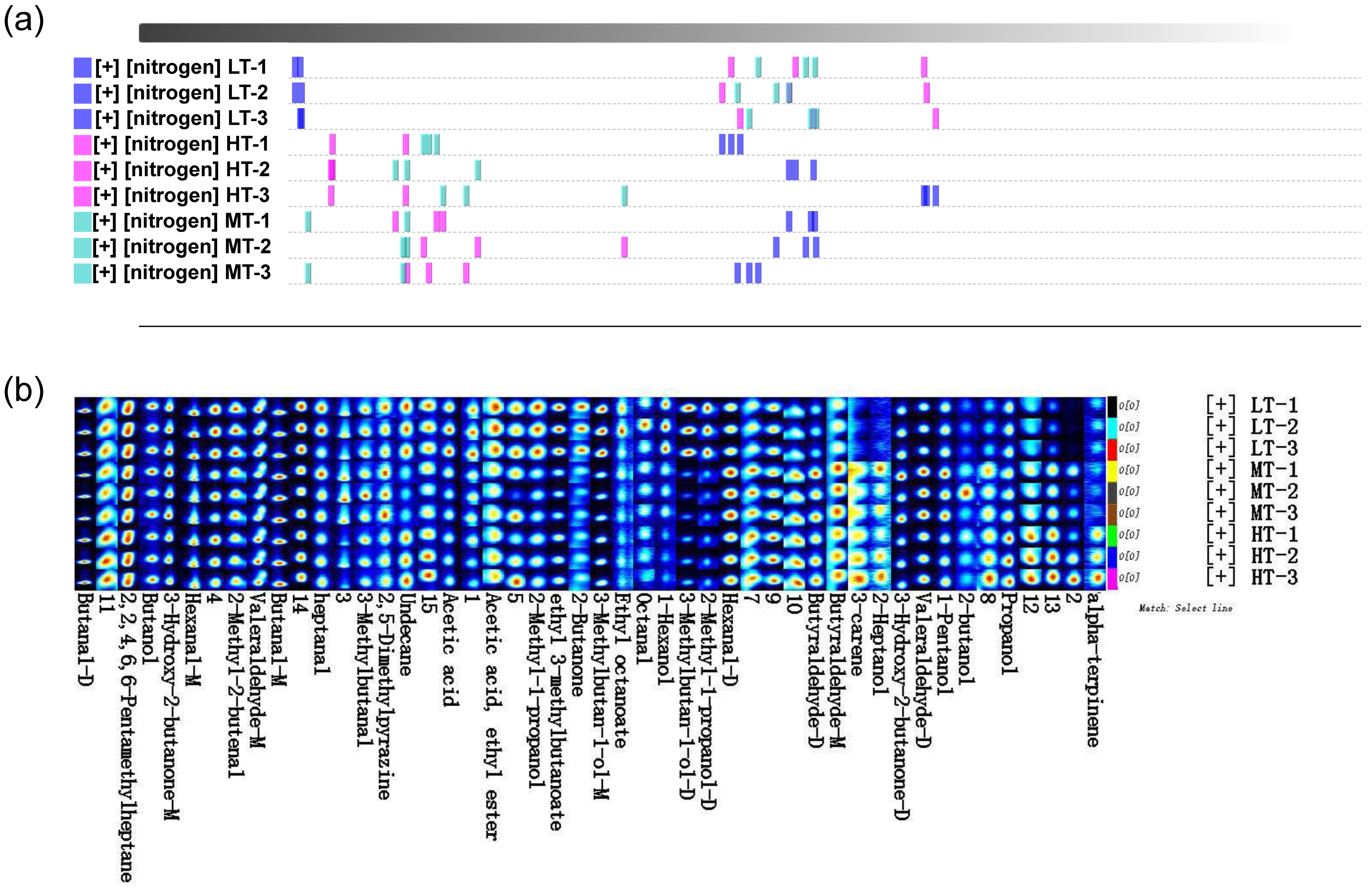
3.2. Composition of Volatile Flavor Compounds
3.3. Non-Volatile Metabolite Composition
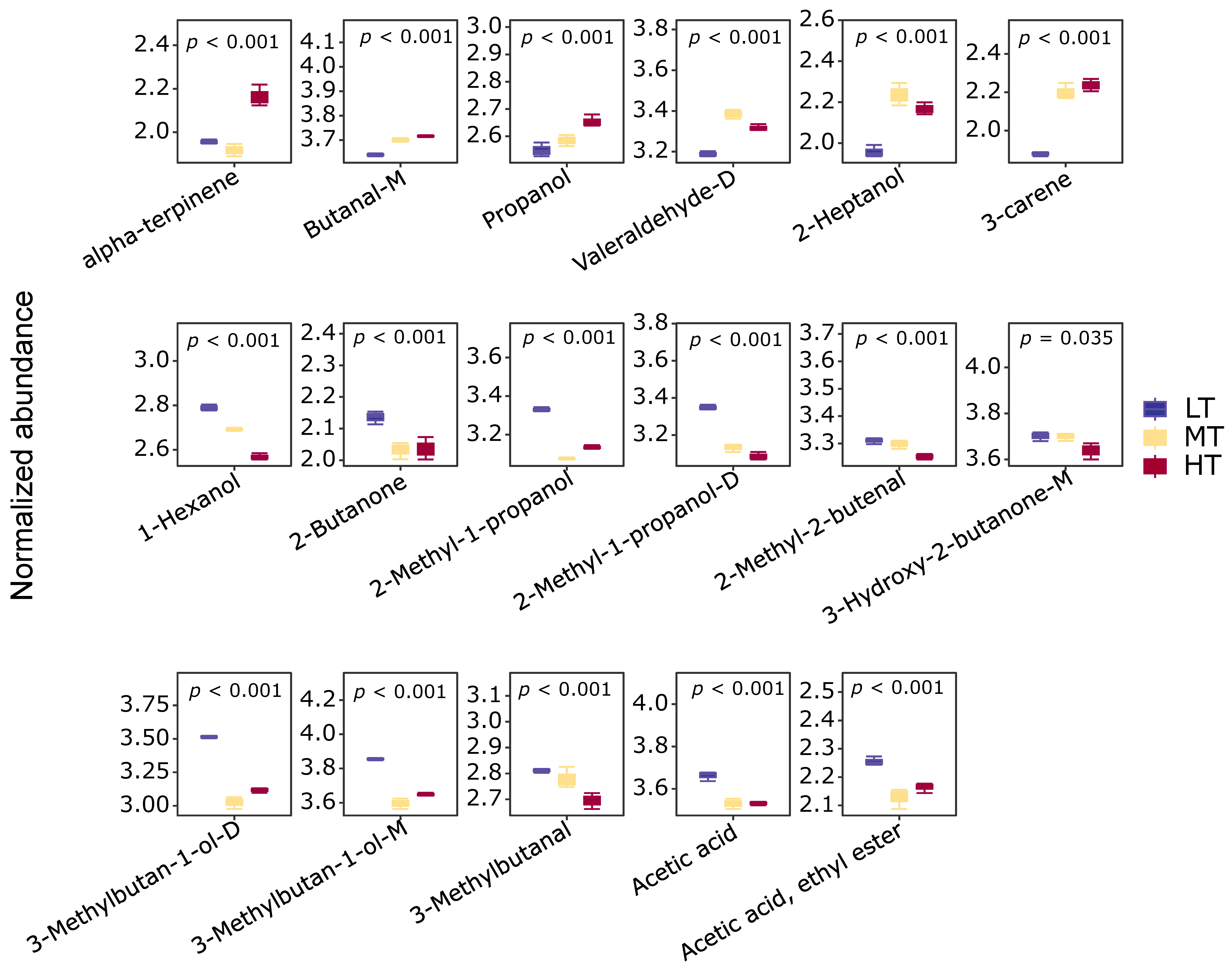
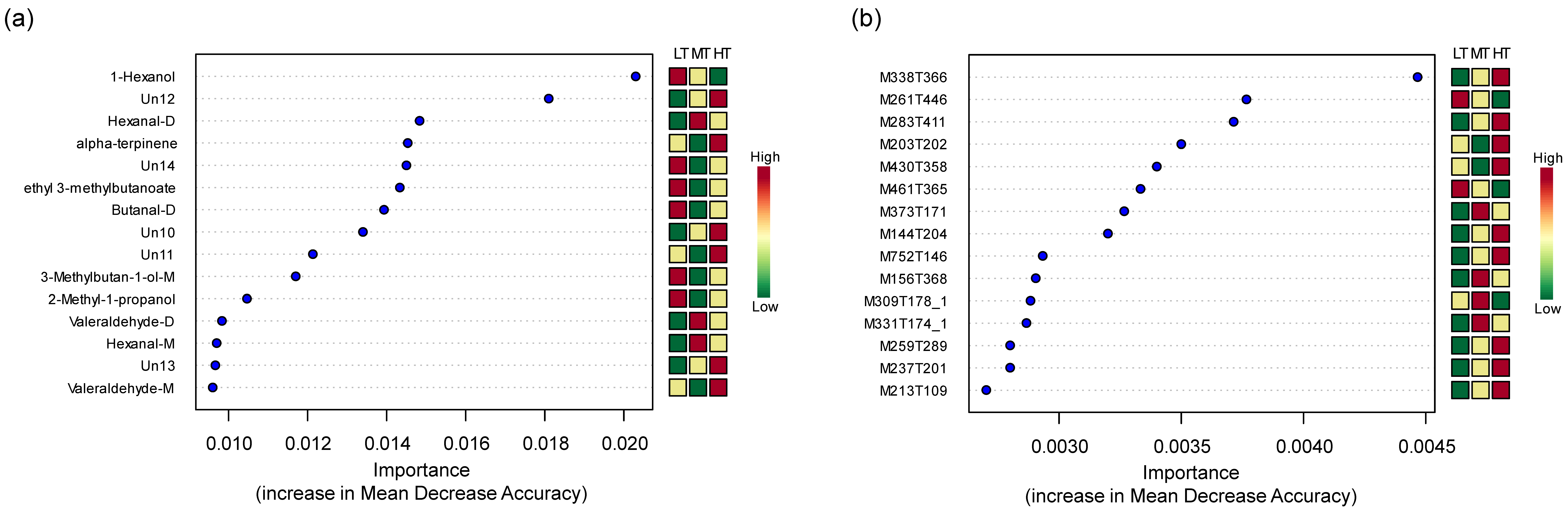
3.4. Characteristic Flavor Compounds and Metabolites at Different Drying Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Subramaniam, S.; Jiao, S.; Zhang, Z.; Jing, P. Impact of post-harvest processing or thermal dehydration on physiochemical, nutritional and sensory quality of shiitake mushrooms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2560–2595. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Le Port, G.; Pulvirenti, A.; Gormley, R. The Effect of Sodium Metabisulphite on the Whiteness and Keeping Quality of Sliced Mushrooms. LWT-Food Sci. Technol. 1999, 32, 460–463. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Cui, H.; Xia, S.; Zhang, X.; Yang, B. Effect of Temperature on Flavor Compounds and Sensory Characteristics of Maillard Reaction Products Derived from Mushroom Hydrolysate. Molecules 2018, 23, 247. [Google Scholar] [CrossRef] [PubMed]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2018, 98, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yagoub, A.E.A.; Sun, Y.; Arun, M.S.; Ma, H.; Zhou, C. Role of thermal and non-thermal drying techniques on drying kinetics and the physicochemical properties of shiitake mushroom. J. Sci. Food Agric. 2022, 102, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bi, J.; Yi, J.; Njoroge, D.M.; Peng, J.; Hou, C. Comparison of dynamic water distribution and microstructure formation of shiitake mushrooms during hot air and far infrared radiation drying by low-field nuclear magnetic resonance and scanning electron microscopy. J. Sci. Food Agric. 2019, 99, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- An, N.N.; Sun, W.H.; Li, B.Z.; Wang, Y.; Shang, N.; Lv, W.Q.; Li, D.; Wang, L.J. Effect of different drying techniques on drying kinetics, nutritional components, antioxidant capacity, physical properties and microstructure of edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, T.; Liu, L.; Chen, Y.; Tang, J.; Peng, W.; Tan, H. Application of the mushroom volatile 1-octen-3-ol to suppress a morel disease caused by Paecilomyces penicillatus. Appl. Microbiol. Biotechnol. 2022, 106, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, L.; Tang, J.; He, X.; Zhang, Z.; Chen, Y.; Zhou, J.; Gan, B.; Peng, W. Morchella importuna Flavones Improve Intestinal Integrity in Dextran Sulfate Sodium-Challenged Mice. Front. Microbiol. 2021, 12, 742033. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Zhang, X. Cultivation, nutritional value, bioactive compounds of morels, and their health benefits: A systematic review. Front. Nutr. 2023, 10, 1159029. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True morels (Morchella)-nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, J.; Wang, Y.; He, X.; Tan, H.; Yu, Y.; Chen, Y.; Peng, W. Large-scale commercial cultivation of morels: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 4401–4412. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef]
- Liu, W.; He, P.; Shi, X.; Zhang, Y.; Perez-Moreno, J.; Yu, F. Large-Scale Field Cultivation of Morchella and Relevance of Basic Knowledge for Its Steady Production. J. Fungi 2023, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Standard for Grade of Dried Morels. Storage and Processing [China Edible Mushroom Business Network]. 2020. Available online: http://jishu.mushroommarket.net/202002/26/14895.html (accessed on 26 February 2020).
- Li, X.; Zhang, Y.; Hengchao, E.; He, X.; Li, J.; Zhao, X.; Zhou, C. Characteristic fingerprints and comparison of volatile flavor compounds in Morchella sextelata under different drying methods. Food Res. Int. 2023, 172, 113103. [Google Scholar] [CrossRef]
- Gu, K.; Zhou, C. Effect of drying on nutrient composition of morels. Food Res. Dev. 2019, 40, 47–51. [Google Scholar]
- Morels drying methods. Rural New Technol. 2022, 62–63.
- Berbert; Queiroz, D.M.; Melo, E.C. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT 2021, 146, 111402. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Wang, P.; Zheng, Y.; Guo, Y.; Hong, Y.; Yang, R.; Ye, N. Metabolite profiling in albino tea mutant Camellia sinensis ‘Fuyun 6’ using LC–ESI–MS/MS. Trees 2022, 36, 261–272. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Seo, D.; Lee, M.-H.; Yu, S.J. Development of Network Analysis and Visualization System for KEGG Pathways. Symmetry 2015, 7, 1275–1288. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, M.; Zhang, H.; Yin, Y.; Cai, Y.; Zhu, Z.-J. Metabolite annotation from knowns to unknowns through knowledge-guided multi-layer metabolic networking. Nat. Commun. 2022, 13, 6656. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Delgado-Baquerizo, M.; Wang, J.-T.; Hu, H.-W.; Yang, Z.; He, J.-Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Xu, Q.; Jiang, Y.; Sun, B. Biochar induced negative priming effect on soil organic carbon mineralisation by changing the microbial community structure across plant growth stages. J. Soils Sediments 2020, 20, 3340–3350. [Google Scholar] [CrossRef]
- Guo, X.H.; Xia, C.Y.; Tan, Y.R.; Long, C.H.E.N.; Jian, M.I.N.G. Mathematical Modeling and Effect of Various Hot-Air Drying on Mushroom (Lentinus edodes). J. Integr. Agric. 2014, 13, 207–216. [Google Scholar]
- Zhang, Y.; Li, X.; Zhao, Z.; Hengchao, E.; Fan, T.; Dong, H.; He, X.; Zhao, X.; Tang, L.; Zhou, C. Comprehensive investigation on non-volatile and volatile flavor compounds in the Morchella sextelata and Morchella importuna by UPLC-MS/MS and GC × GC-TOF-MS. Food Chem. X 2023, 20, 100961. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, J.; Dong, H.; Liu, Q.; Li, X.; Zeng, X.; Bai, W. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food Chem. 2021, 342, 128381. [Google Scholar] [CrossRef]
- Qin, L.; Gao, J.X.; Xue, J.; Chen, D.; Lin, S.Y.; Dong, X.P.; Zhu, B.W. Changes in Aroma Profile of Shiitake Mushroom (Lentinus edodes) during Different Stages of Hot Air Drying. Foods 2020, 9, 444. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Ma, D.; Liu, Z.; Qi, P.; Wang, Z.; Di, S.; Wang, X. Review of fruits flavor deterioration in postharvest storage: Odorants, formation mechanism and quality control. Food Res. Int. 2024, 182, 114077. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lan, X.; Peng, W.; Gan, B. Effects of different ecological environments on non-volatile flavorful components of Morels. Sci. Technol. Food Ind. 2020, 41, 237–242. [Google Scholar]
- Xie, L.; Lan, X.; Tang, J.; Peng, W. Analysis and comprehensive evaluation of volatile components in different varieties of Morchella. Sci. Technol. Food Ind. 2021, 42, 227–233. [Google Scholar]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Suárez-Marina, I.; Abul-Haija, Y.M.; Turk-MacLeod, R.; Gromski, P.S.; Cooper, G.J.; Olivé, A.O.; Colón-Santos, S.; Cronin, L. Integrated synthesis of nucleotide and nucleosides influenced by amino acids. Commun. Chem. 2019, 2, 28. [Google Scholar] [CrossRef]
- Akune-Taylor, Y.; Kon, A.; Aoki-Kinoshita, K.F. In silico simulation of glycosylation and related pathways. Anal. Bioanal. Chem. 2024, 416, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Tietel, Z.; Masaphy, S. Chemotyping of three Morchella species reveals species- and age-related aroma volatile biomarkers. LWT 2022, 154, 112587. [Google Scholar] [CrossRef]
- Yadav, R.; Prasad, R. Identification and functional characterization of sorbitol-6-phosphate dehydrogenase protein from rice and structural elucidation by in silico approach. Planta 2014, 240, 223–238. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Lee, M.; Park, H.Y.; Jung, K.H.; Kim, D.H.; Rho, H.S.; Choi, K. Anti-melanogenic Effects of Kojic Acid and Hydroxycinnamic Acid Derivatives. Biotechnol. Bioprocess. Eng. 2020, 25, 190–196. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wu, X.; Long, W.; Xu, Y.; Yu, Y.; Wang, H. The Effects of Different Postharvest Drying Temperatures on the Volatile Flavor Components and Non-Volatile Metabolites of Morchella sextelata. Horticulturae 2024, 10, 812. https://doi.org/10.3390/horticulturae10080812
Liu T, Wu X, Long W, Xu Y, Yu Y, Wang H. The Effects of Different Postharvest Drying Temperatures on the Volatile Flavor Components and Non-Volatile Metabolites of Morchella sextelata. Horticulturae. 2024; 10(8):812. https://doi.org/10.3390/horticulturae10080812
Chicago/Turabian StyleLiu, Tianhai, Xiang Wu, Weiwei Long, Yingying Xu, Yang Yu, and Haixia Wang. 2024. "The Effects of Different Postharvest Drying Temperatures on the Volatile Flavor Components and Non-Volatile Metabolites of Morchella sextelata" Horticulturae 10, no. 8: 812. https://doi.org/10.3390/horticulturae10080812
APA StyleLiu, T., Wu, X., Long, W., Xu, Y., Yu, Y., & Wang, H. (2024). The Effects of Different Postharvest Drying Temperatures on the Volatile Flavor Components and Non-Volatile Metabolites of Morchella sextelata. Horticulturae, 10(8), 812. https://doi.org/10.3390/horticulturae10080812





