Nitrates and Microbiome Components Engaged in Denitrification within Soil Regulate Morchella spp. Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Description and Soil Sampling
2.2. Soil Physical and Chemical Properties
2.3. DNA Extraction and Metagenomic Sequencing
2.4. Statistical Analysis and Visualization
3. Results
3.1. Soil Physical and Chemical Properties
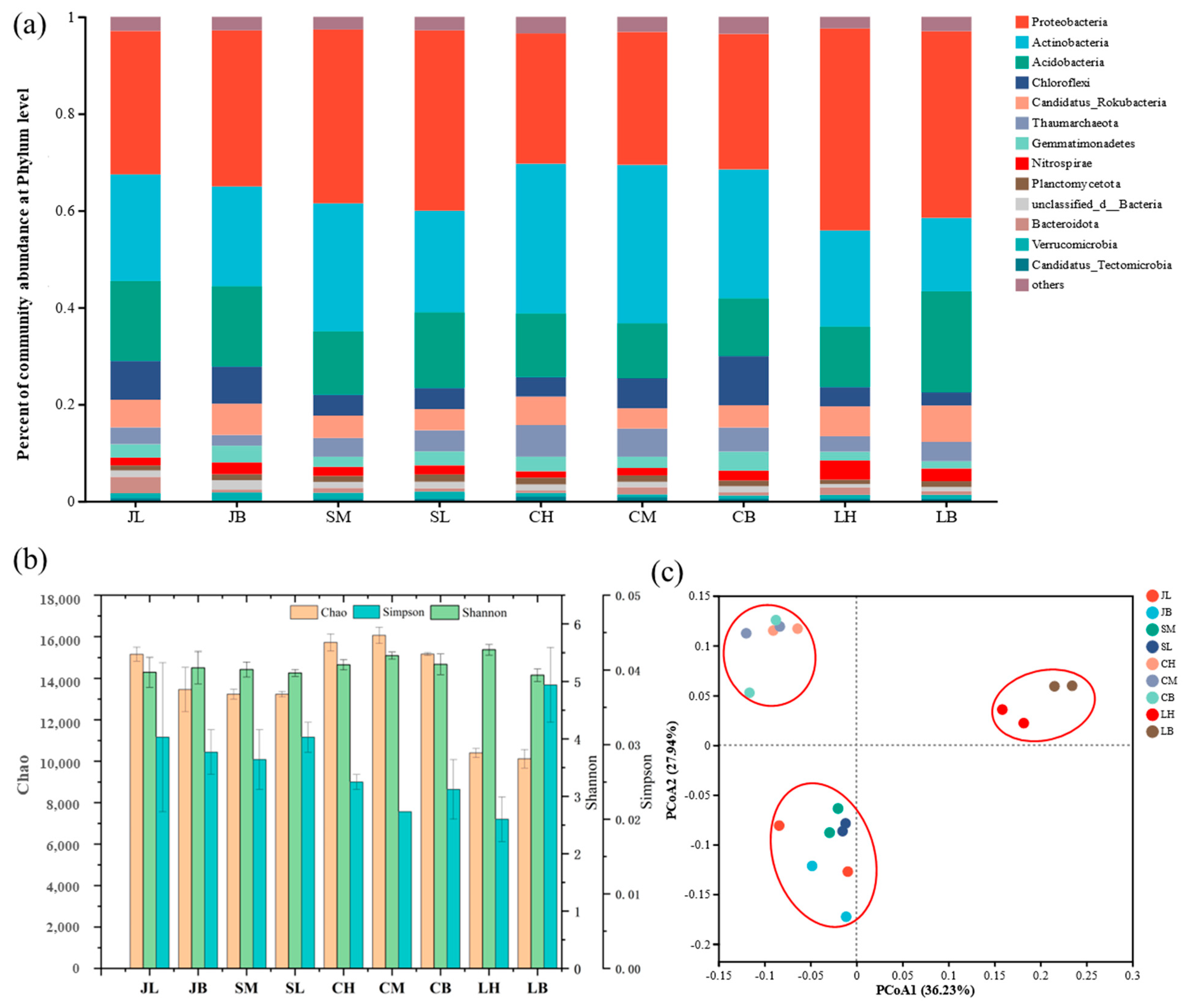
3.2. Soil Microbiome Community Structure and α Diversity from Different Sites
3.3. Key Microbial Biomarkers Associated with Different Growth Conditions of Morels
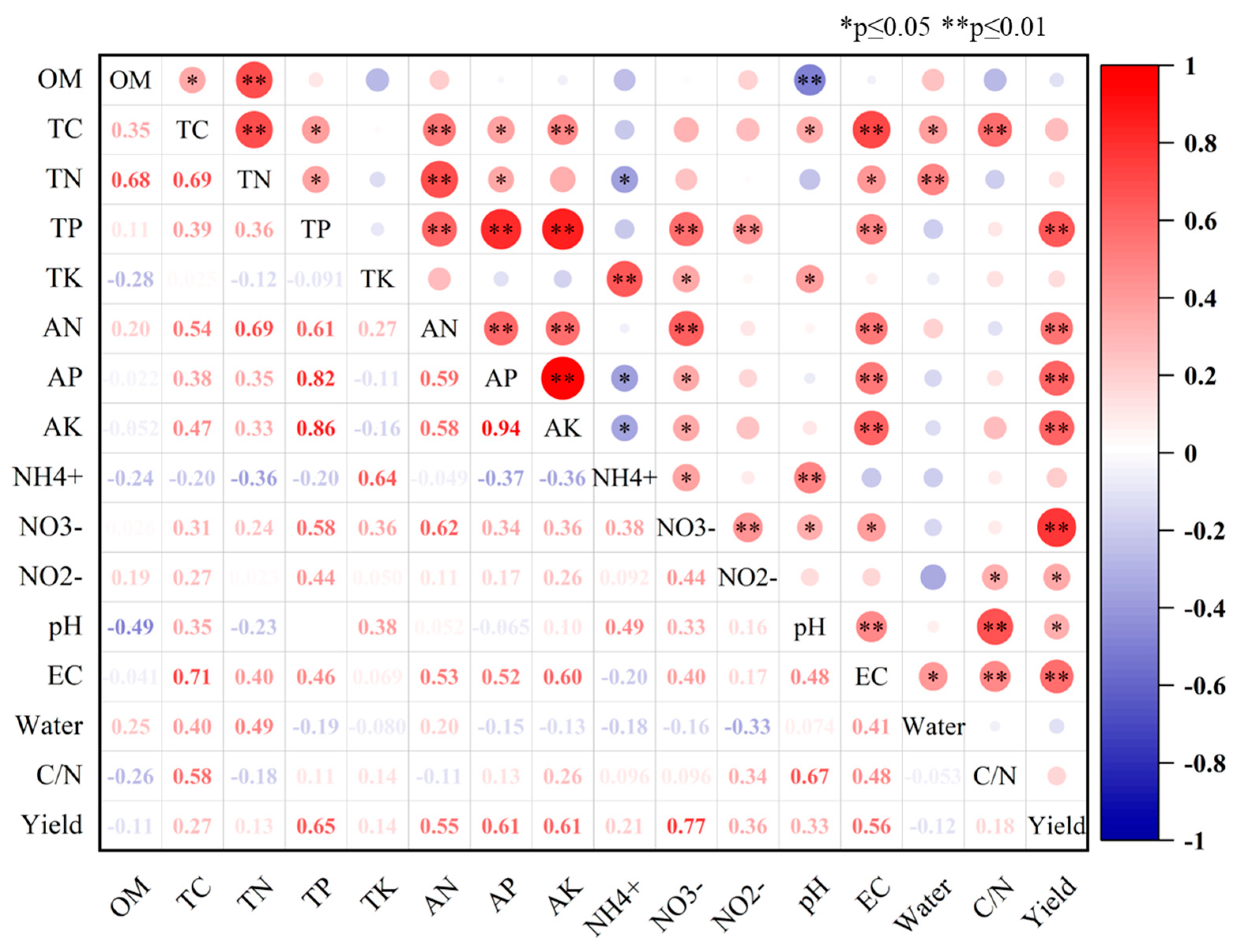
3.4. Relationship between Soil Physical and Chemical Properties and Microbiome Community
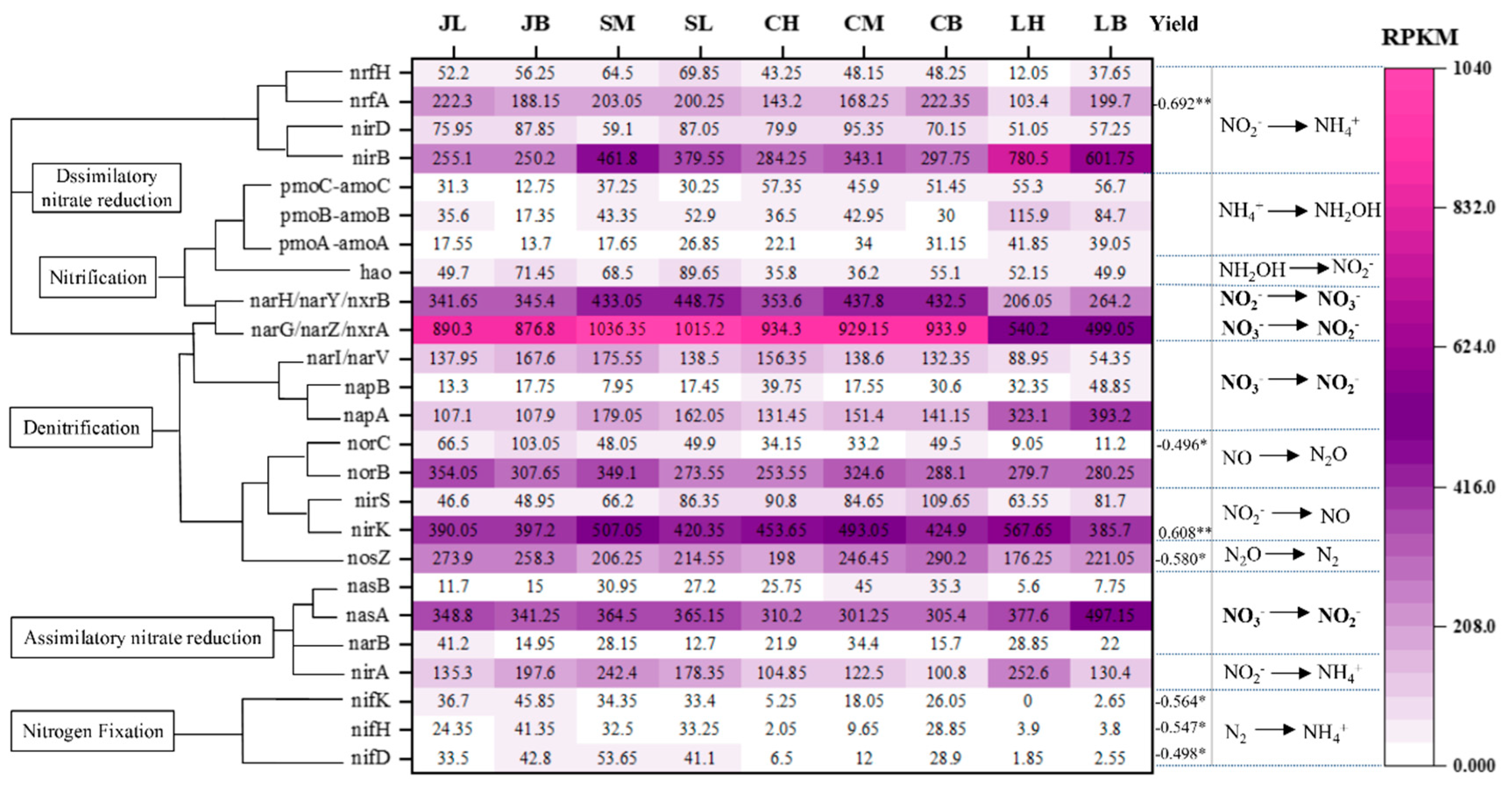
3.5. Nitrogen Cycling in Soil Microbiomes from Different Sites
4. Discussion
4.1. Potential Mechanisms of Soil Microbiome Impact on Morel Growth
4.2. Relationship between Soil Microbiomes, Soil Physical and Chemical Properties and Morel Growth
4.3. Microbial Mechanisms of Nitrogen Cycling Associated with Morel Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pilz, D.; McLain, R.; Alexander, S.; Villarreal-Ruiz, L.; Berch, S.; Wurtz, T.L.; Parks, C.G.; McFarlane, E.; Baker, B.; Molina, R.; et al. Ecology and Management of Morels Harvested from the Forests of Western North America; General Technical Report PNW-GTR-710; Pacific Northwest Research Station, USDA Forest Service: Corvallis, OR, USA, 2007. [Google Scholar]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Sambyal, K.; Singh, R.V. A comprehensive review on Morchella importuna: Cultivation aspects, phytochemistry, and other significant applications. Folia Microbiol. 2021, 66, 147–157. [Google Scholar] [CrossRef]
- Tan, H.; Yu, Y.; Tang, J.; Liu, T.; Miao, R.; Huang, Z.; Martin, F.M.; Peng, W. Build Your Own Mushroom Soil: Microbiota Succession and Nutritional Accumulation in Semi-Synthetic Substratum Drive the Fructification of a Soil-Saprotrophic Morel. Front. Microbiol. 2021, 12, 656656. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Tan, H.; Kohler, A.; Miao, R.; Liu, T.; Zhang, Q.; Zhang, B.; Jiang, L.; Wang, Y.; Xie, L.; Tang, J.; et al. Multi-omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 2019, 21, 3909–3926. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; He, P. Morel Biology and Cultivation; Jilin Science and Technology Press: Changchun, China, 2017. (In Chinese) [Google Scholar]
- Ge, S. Studies on the Molecular Identification, Biological Characteristics and Indoor Cultivation of Morchella spp.; Penn State University Libraries: University Park, PA, USA, 2019. [Google Scholar]
- Hussain, S.; Sher, H. Ecological characterization of Morel (Morchella spp.) habitats: A multivariate comparison from three forest types of district Swat, Pakistan. Acta Ecol. Sin. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Orlofsky, E.; Zabari, L.; Bonito, G.; Masaphy, S. Changes in soil bacteria functional ecology associated with Morchella rufobrunnea fruiting in a natural habitat. Environ. Microbiol. 2021, 23, 6651–6662. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Yang, R.; Wang, H.; Zhang, D.; Li, Y.; Qi, Z.; Lin, W. Evaluating the Effect on Cultivation of Replacing Soil with Typical Soilless Growing Media: A Microbial Perspective. Agronomy 2023, 13, 6. [Google Scholar] [CrossRef]
- Xiong, C.; Li, X.; Li, Q. Zheng LinyongBacteria community structure and diversity in Morchella colonies. J. Hunan Agric. Univ. (Nat. Sci.) 2015, 41, 428–434. (In Chinese) [Google Scholar]
- Benucci, G.M.N.; Longley, R.; Zhang, P.; Zhao, Q.; Bonito, G.; Yu, F. Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses. PeerJ 2019, 7, e7744. [Google Scholar] [CrossRef]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Zhang, J.; Zhang, Y.; Wang, W. Dynamics of soil microbiome throughout the cultivation life cycle of morel (Morchella sextelata). Front. Microbiol. 2023, 14, 979835. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.L.; Wang, Y.; Reddy, K.R. Loss-on-Ignition Method to Assess Soil Organic Carbon in Calcareous Everglades Wetlands. Commun. Soil Sci. Plant Anal. 2008, 39, 3074–3083. [Google Scholar] [CrossRef]
- Duyck, P.F.; Dortel, E.; Tixier, P.; Vinatier, F.; Loubana, P.-M.; Chabrier, C.; Quénéhervé, P. Niche partitioning based on soil type and climate at the landscape scale in a community of plant-feeding nematodes. Soil Biol. Biochem. 2012, 44, 49–55. [Google Scholar] [CrossRef]
- Jansson, J.K.; Wu, R. Soil viral diversity, ecology and climate change. Nat. Rev. Microbiol. 2023, 21, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Lv, J.; He, X.; Wang, J.; Teng, D.; Jiang, L.; Wang, H.; Lv, G. Rhizosphere effect alters the soil microbiome composition and C, N transformation in an arid ecosystem. Appl. Soil Ecol. 2022, 170, 104296. [Google Scholar] [CrossRef]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-M.; Jayawardena, R.S.; Thongklang, N.; Lv, M.-L.; Zhu, X.-T.; Zhao, Q. Morel Production Associated with Soil Nitrogen-Fixing and Nitrifying Microorganisms. J. Fungi 2022, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, H.K.; Reddy, M.S. Effect of carbon, nitrogen sources and inducers on ligninolytic enzyme production by Morchella crassipes. World J. Microbiol. Biotechnol. 2011, 27, 687–691. [Google Scholar] [CrossRef]
- Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130126. [Google Scholar] [CrossRef]
- Chen, M.; Pan, H.; Sun, M.; He, W.; Wei, M.; Lou, Y.; Wang, H.; Yang, Q.; Feng, H.; Zhuge, Y. Nitrosospira cluster 3 lineage of AOB and nirK of Rhizobiales respectively dominated N2O emissions from nitrification and denitrification in organic and chemical N fertilizer treated soils. Ecol. Indic. 2021, 127, 107722. [Google Scholar] [CrossRef]
- Kits, K.D.; Jung, M.Y.; Vierheilig, J.; Pjevac, P.; Sedlacek, C.J.; Liu, S.; Herbold, C.; Stein, L.Y.; Richter, A.; Wissel, H.; et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 2019, 10, 1836. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Uroz, S.; Gao, T.; Li, J.; He, F.; Rosazlina, R.; Martin, F.; Xu, L. The cultivation regimes of Morchella sextelata trigger shifts in the community assemblage and ecological traits of soil bacteria. Front. Microbiol. 2023, 14, 1257905. [Google Scholar] [CrossRef]
- Barbieri, E.; Ceccaroli, P.; Saltarelli, R.; Guidi, C.; Potenza, L.; Basaglia, M.; Fontana, F.; Baldan, E.; Casella, S.; Ryahi, O.; et al. New evidence for nitrogen fixation within the Italian white truffle Tuber magnatum. Fungal Biol. 2010, 114, 936–942. [Google Scholar] [CrossRef]
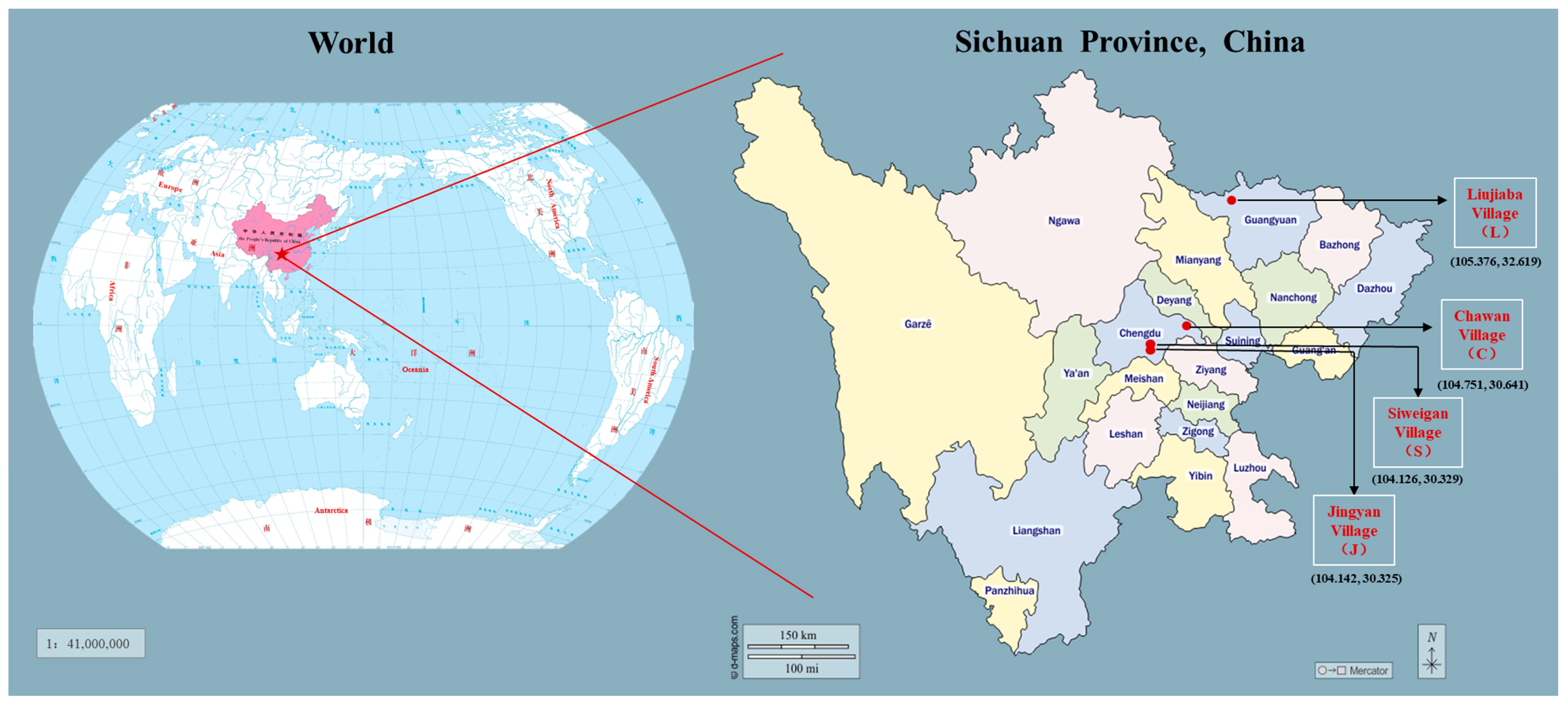


| Treatments | Sampling Location | Morel Yield a | Yield Classification | Classification |
|---|---|---|---|---|
| kg hm−2 | Standard b | |||
| JL | Jingyan village (J) | 800 ± 50 | Low-yield fields (L) |  |
| JB | 250 ± 100 | Barren fields (B) | ||
| SM | Siweigan village (S) | 4000 ± 300 | Medium-yield fields (M) | |
| SL | 1200 ± 200 | Low-yield fields (L) | ||
| CH | Chawan village (C) | 9000 ± 500 | High-yield fields (H) | |
| CM | 5000 ± 400 | Medium-yield fields (M) | ||
| CB | 500 ± 150 | Barren fields (B) | ||
| LH | Lujiaba village (L) | 8000 ± 500 | High-yield fields (H) | |
| LB | 200 ± 40 | Barren fields (B) |
| Treatments | SOM | TC | TN | TP | TK | AN | AP | AK | NH4+ | NO3− | NO2− | pH | EC | Water |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/kg | g/kg | g/kg | g/kg | g/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | μg/ms | % | ||
| JL | 27.5 ± 2.8 a | 18.2 ± 1.3 b | 1.8 ± 0.2 a | 0.7 ± 0.06 b | 17.3 ± 1.0 ab | 150.0 ± 15.7 b | 31.9 ± 5.5 b | 127.8 ± 2.6 cd | 12.8 ± 0.7 cd | 14.9 ± 2.6 cde | 0.1 ± 0.02 a | 7.2 ± 0.3 d | 62.5 ± 18.9 cd | 20 ± 2 cd |
| JB | 23.5 ± 3.3 ab | 16.2 ± 0.6 b | 1.6 ± 0.1 ab | 0.54 ± 0.05 b | 16.6 ± 0.7 ab | 129.6 ± 15.3 bc | 20.2 ± 2.1 cd | 108.1 ± 7.2 cd | 14.3 ± 1.4 bc | 6.2 ± 1.7 e | 0.07 ± 0.02 b | 7.6 ± 0.4 bc | 131.9 ± 67.0 bc | 27 ± 2 a |
| SM | 28.4 ± 1.6 a | 18.7 ± 1.6 b | 1.7 ± 0.1 ab | 0.86 ± 0.07 b | 12.5 ± 0.5 c | 125 ± 3.12 bc | 33.0 ± 7.2 b | 301 ± 45.7 b | 10.3 ± 1.0 de | 11.3 ± 0.3 cde | 0.1 ± 0.02 a | 7.4 ± 0.7 cd | 117.5 ± 22.4 bcd | 24 ± 2 ab |
| SL | 27.3 ± 1.1 a | 16.2 ± 0.7 b | 1.6 ± 0.2 ab | 0.85 ± 0.09 b | 12.4 ± 0.9 c | 120.5 ± 6.3 c | 27.1 ± 6.0 bc | 270.2 ± 31.8 b | 9.8 ± 0.1 e | 11.8 ± 0.9 cde | 0.1 ± 0.02 a | 7.5 ± 0.1 cd | 111.7 ± 12.5 bcd | 22 ± 1 bc |
| CH | 23.1 ± 2.5 ab | 24.5 ± 0.9 a | 1.8 ± 0.2 a | 1.74 ± 0.64 a | 15.9 ± 0.5 b | 179.1 ± 30.4 a | 83.5 ± 10.5 a | 1189.5 ± 101.5 a | 12.0 ± 0.8 cde | 26.0 ± 11.5 ab | 0.11 ± 0.02 a | 7.9 ± 0.2 ab | 310.8 ± 33.72 a | 21 ± 1 bc |
| CM | 24.4 ± 2.6 ab | 23.1 ± 1.4 a | 1.6 ± 0.2 ab | 0.6 ± 0.02 b | 16.6 ± 0.8 ab | 133.7 ± 8.9 bc | 22.9 ± 6.7 bcd | 158.1 ± 46.8 c | 12.8 ± 1.2 cd | 17.5 ± 6.2 bc | 0.11 ± 0.01 a | 8.1 ± 0.2 a | 308.3 ± 79.3 a | 27 ± 3 a |
| CB | 26.7 ± 3.5 a | 25.5 ± 2.7 a | 1.8 ± 0.2 a | 0.67 ± 0.03 b | 16.2 ± 0.4 ab | 144.1 ± 10.8 bc | 13.2 ± 0.6 d | 133.0 ± 18.9 cd | 15.8 ± 3.8 b | 16.7 ± 8.1 bcd | 0.11 ± 0 a | 8.1 ± 0.1 a | 175.3 ± 39.7 b | 27 ± 1 a |
| LH | 23.9 ± 0.5 ab | 15.4 ± 0.7 b | 1.4 ± 0.1 b | 0.83 ± 0.01 b | 17.9 ± 1.9 a | 145.2 ± 14.7 bc | 19.2 ± 5.9 cd | 82.6 ± 8.7 cd | 22.3 ± 1.1 a | 31.4 ± 0.2 a | 0.11 ± 0.01 a | 8.0 ± 0.1 a | 110.2 ± 8.7 bcd | 20 ± 3 c |
| LB | 19.9 ± 3.9 b | 13.9 ± 3.1 b | 0.8 ± 0.3 c | 0.57 ± 0.23 b | 16.3 ± 2 ab | 79.3 ± 21.0 d | 13.0 ± 1.5 d | 60.4 ± 5.2 d | 16.3 ± 0.5 b | 7.0 ± 0.2 de | 0.11 ± 0 a | 8.0 ± 0.1 ab | 50.9 ± 6.5 d | 16 ± 1 d |
| Location | 0.376 | <0.01 ** | <0.01 ** | 0.011 * | <0.01 ** | <0.01 ** | <0.01 ** | <0.01 ** | <0.01 ** | 0.198 | 0.076 | 0.01 * | <0.01 ** | <0.01 ** |
| Yield | 0.802 | 0.925 | 0.034 * | <0.01 ** | 0.554 | <0.01 ** | <0.01 ** | <0.01 ** | 0.308 | <0.01 ** | 0.39 | 0.276 | <0.01 ** | <0.01 ** |
| Location * Yield | 0.089 | 0.091 | 0.011 * | 0.014 * | 0.33 | 0.142 | <0.01 ** | <0.01 ** | <0.01 ** | 0.02 * | 0.444 | 0.211 | <0.01 ** | <0.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lin, W.; Chen, J.; Lin, J.; Feng, R.; Yan, J.; Miao, R.; Gan, B. Nitrates and Microbiome Components Engaged in Denitrification within Soil Regulate Morchella spp. Growth. Horticulturae 2024, 10, 905. https://doi.org/10.3390/horticulturae10090905
Li Y, Lin W, Chen J, Lin J, Feng R, Yan J, Miao R, Gan B. Nitrates and Microbiome Components Engaged in Denitrification within Soil Regulate Morchella spp. Growth. Horticulturae. 2024; 10(9):905. https://doi.org/10.3390/horticulturae10090905
Chicago/Turabian StyleLi, Yujia, Wei Lin, Jie Chen, Junbin Lin, Rencai Feng, Junjie Yan, Renyun Miao, and Bingcheng Gan. 2024. "Nitrates and Microbiome Components Engaged in Denitrification within Soil Regulate Morchella spp. Growth" Horticulturae 10, no. 9: 905. https://doi.org/10.3390/horticulturae10090905
APA StyleLi, Y., Lin, W., Chen, J., Lin, J., Feng, R., Yan, J., Miao, R., & Gan, B. (2024). Nitrates and Microbiome Components Engaged in Denitrification within Soil Regulate Morchella spp. Growth. Horticulturae, 10(9), 905. https://doi.org/10.3390/horticulturae10090905







