Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Flower Collection and Measurements
2.3. Determination of C, N, and P Concentrations
2.4. Determination of NSC Concentrations
2.5. Statistical Analysis
3. Results
3.1. Variations of Morphological Indicators and Biomass Allocation
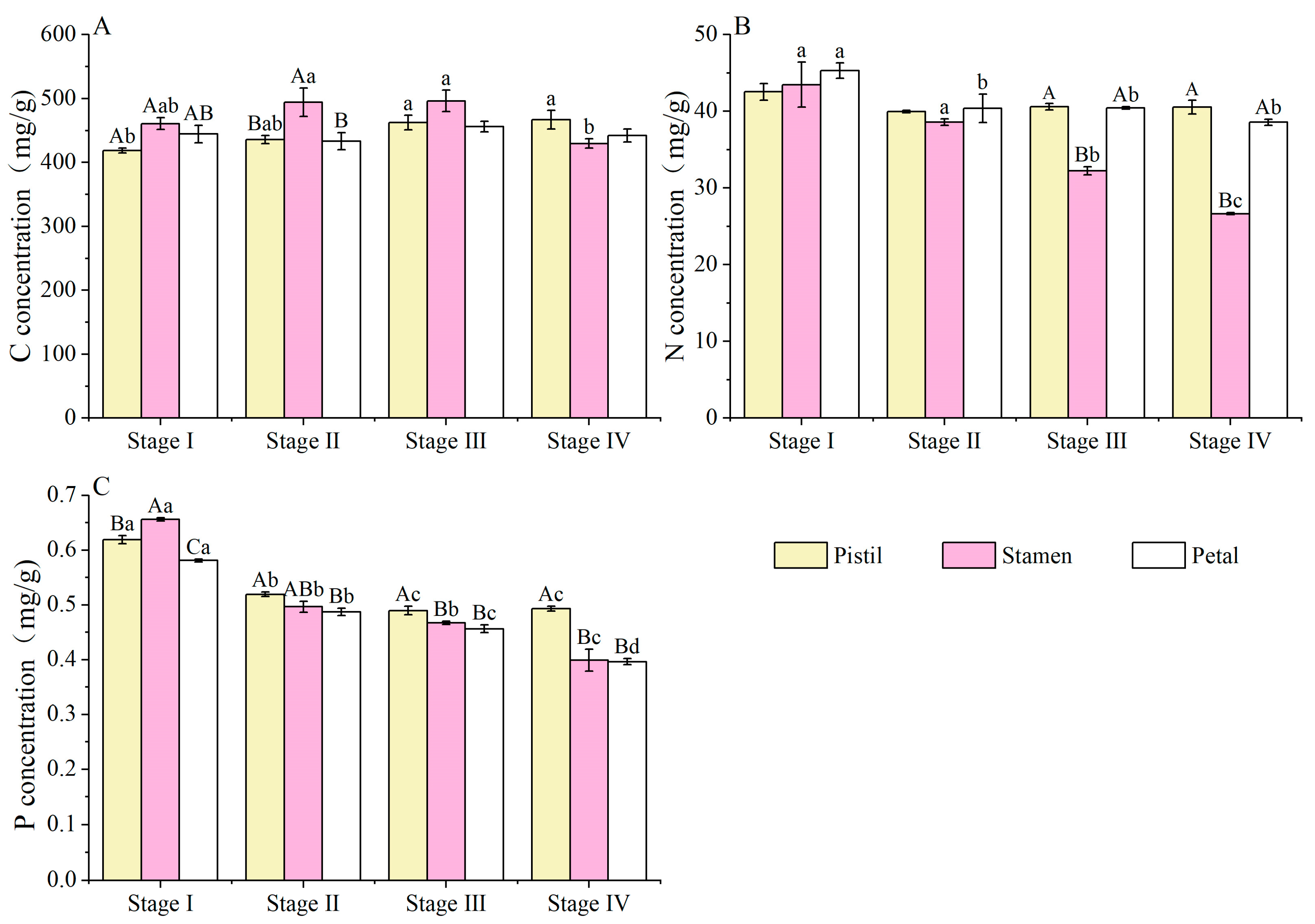
3.2. C, N and P Concentrations
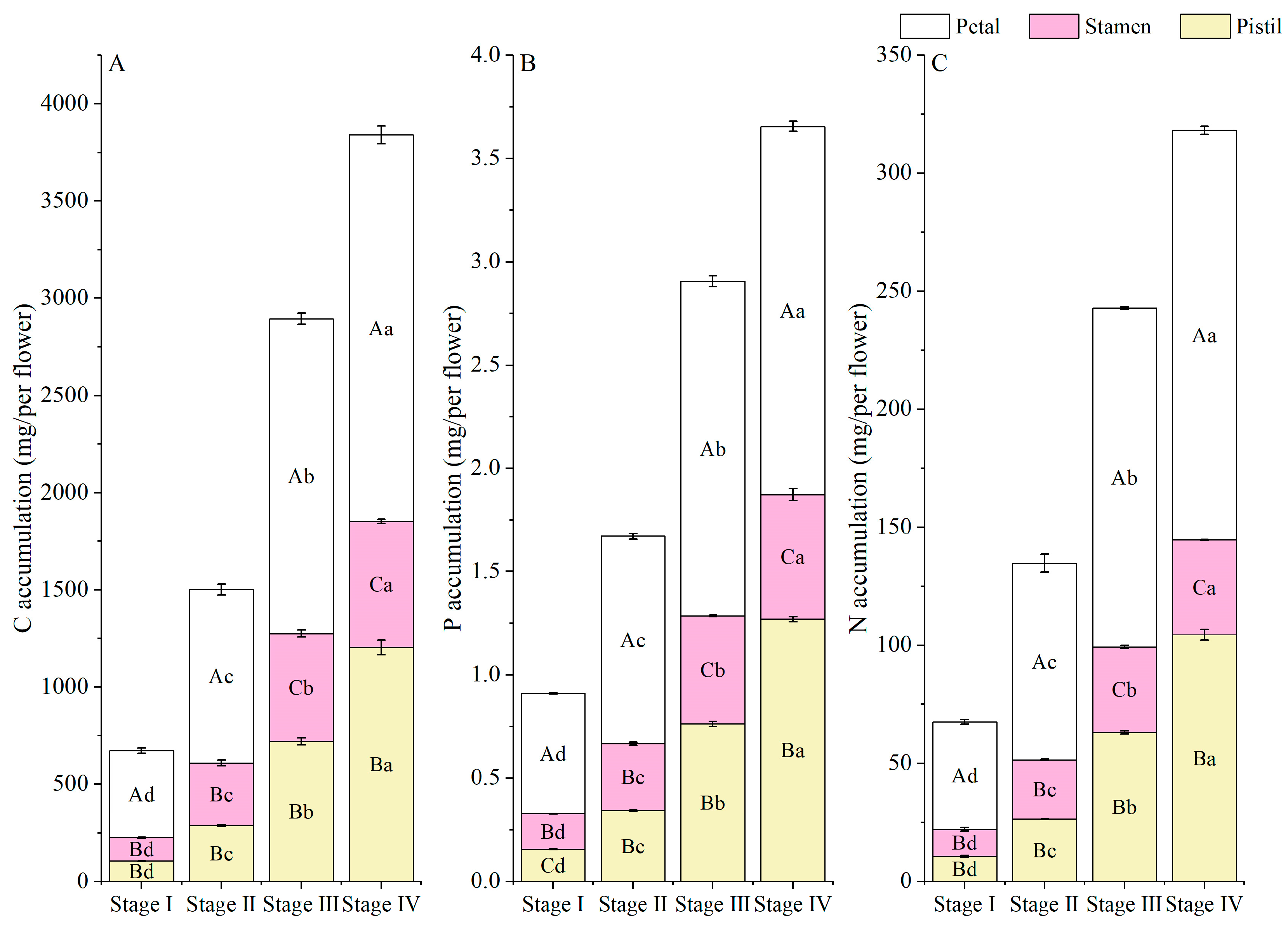
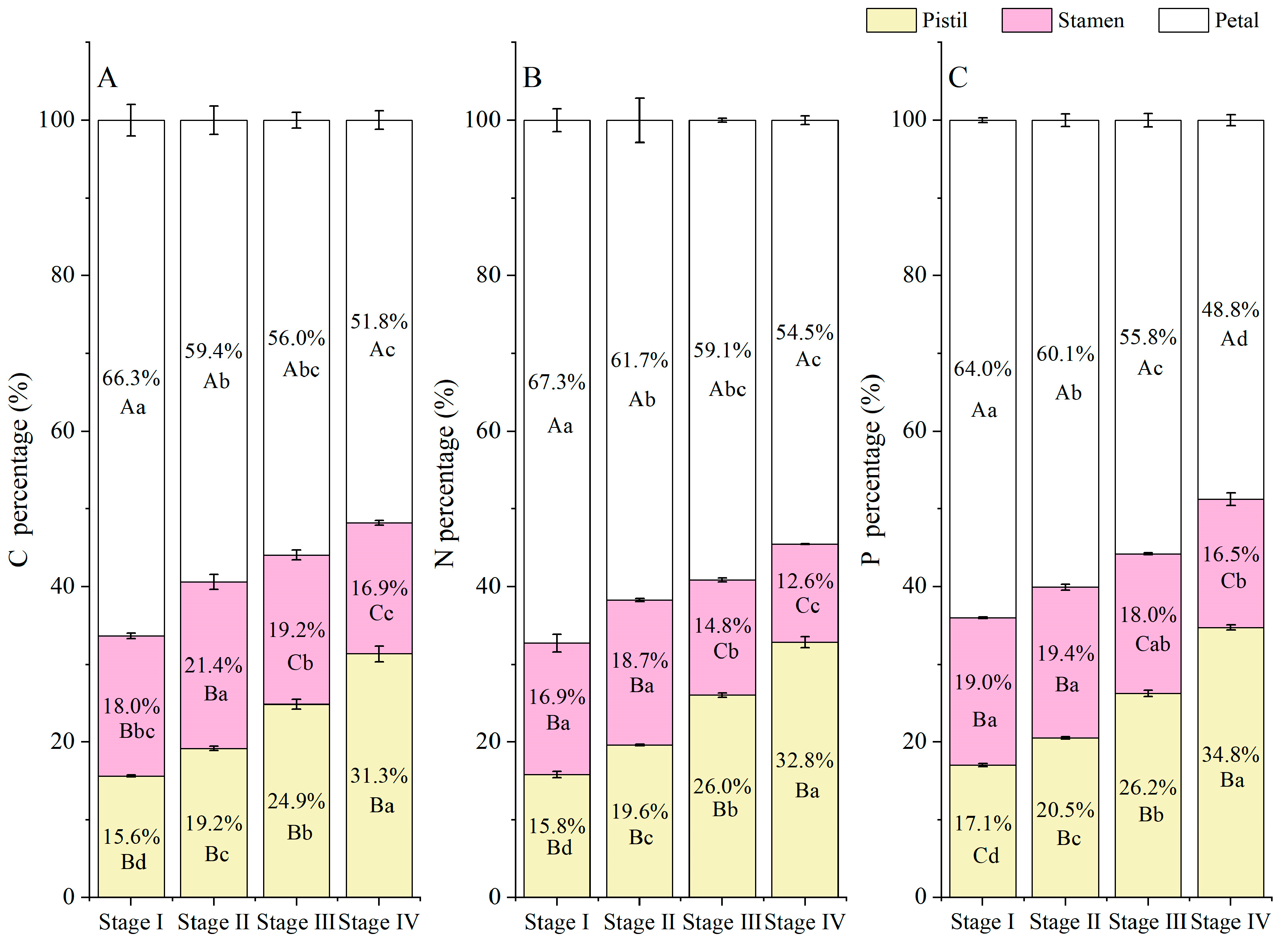
3.3. Element (C, N, and P) Accumulation and Allocation Proportion
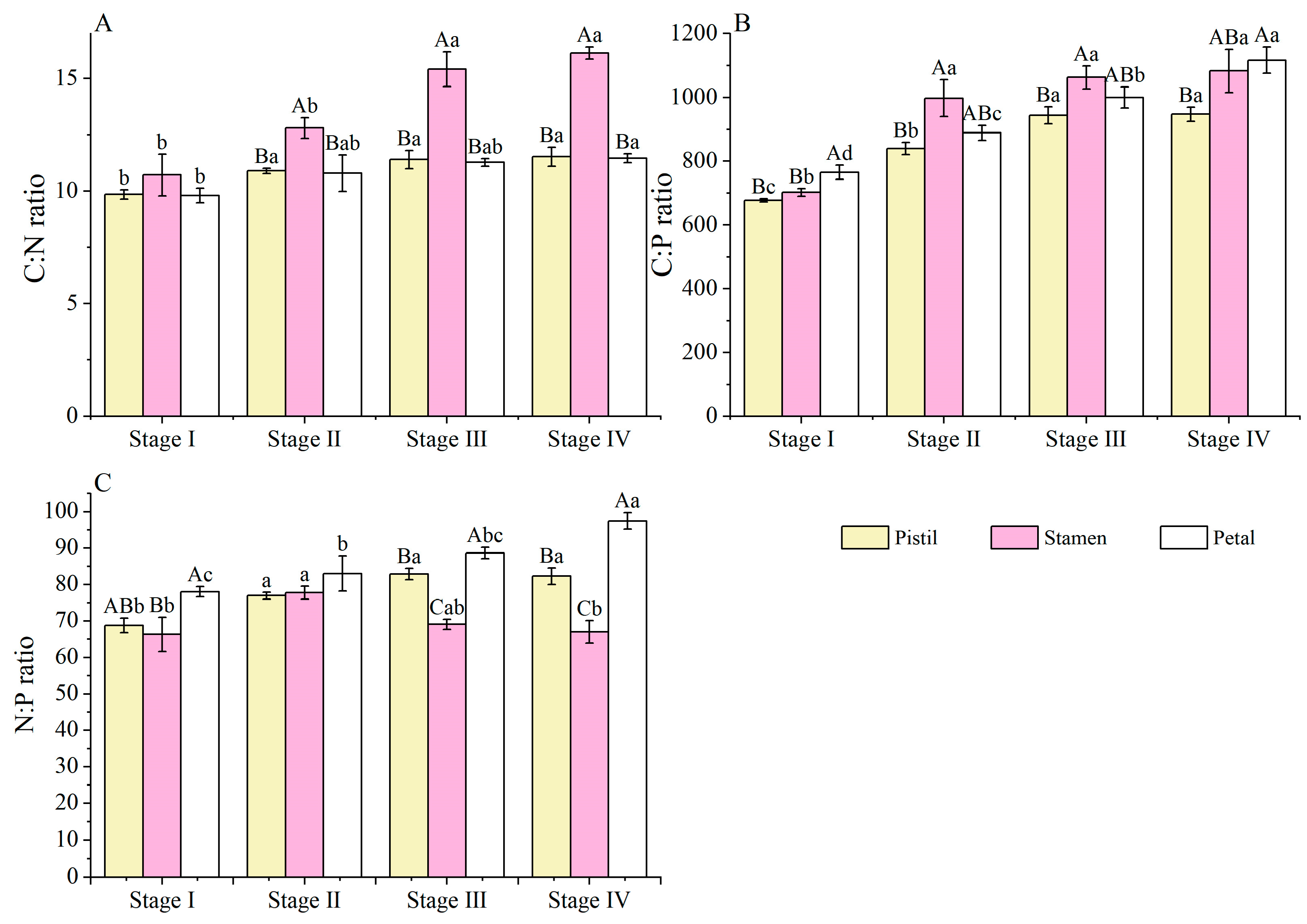
3.4. C, N, and P Stoichiometric Ratio
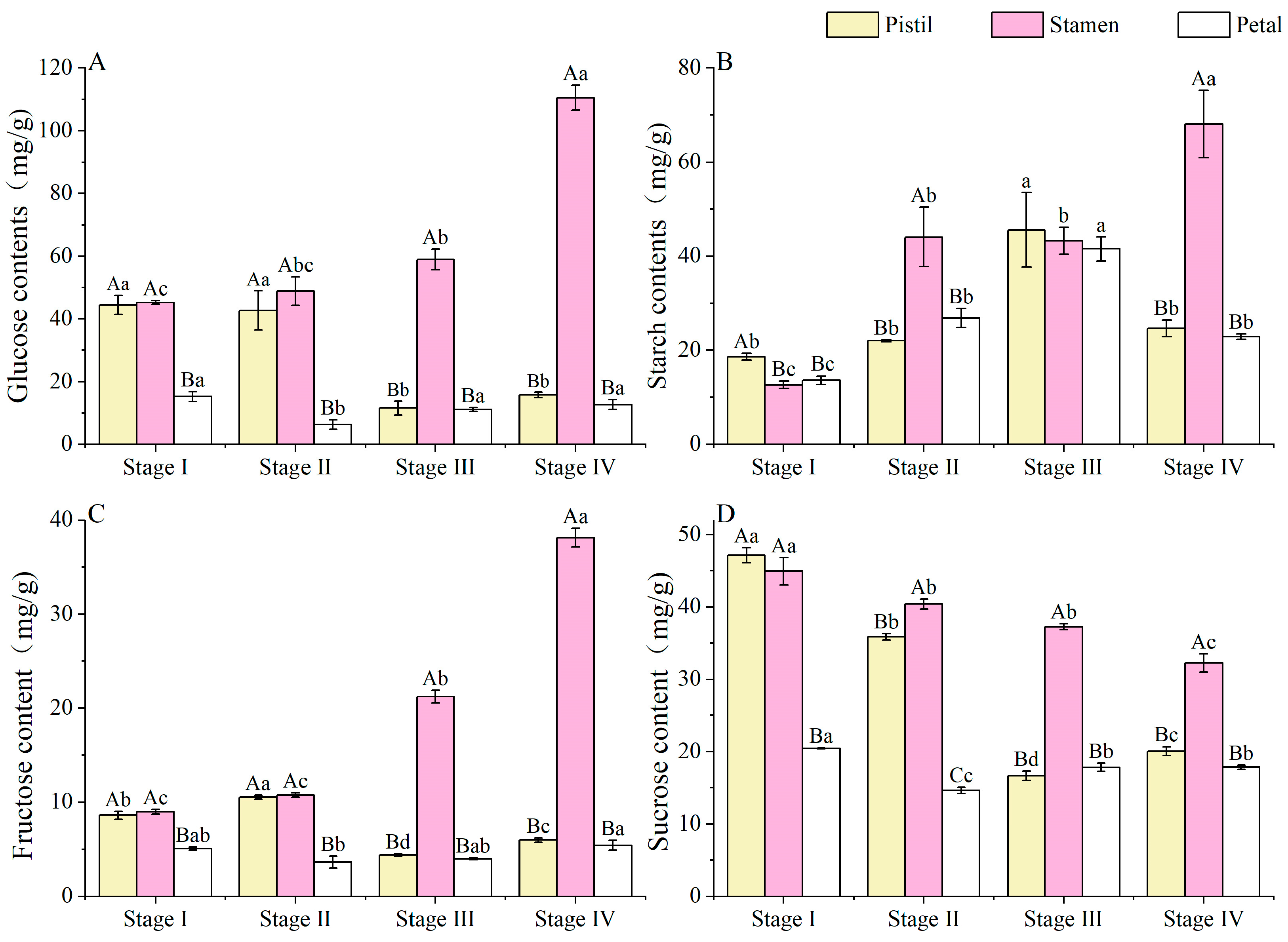
3.5. Non-Structural Sugar (NSCs) Contents
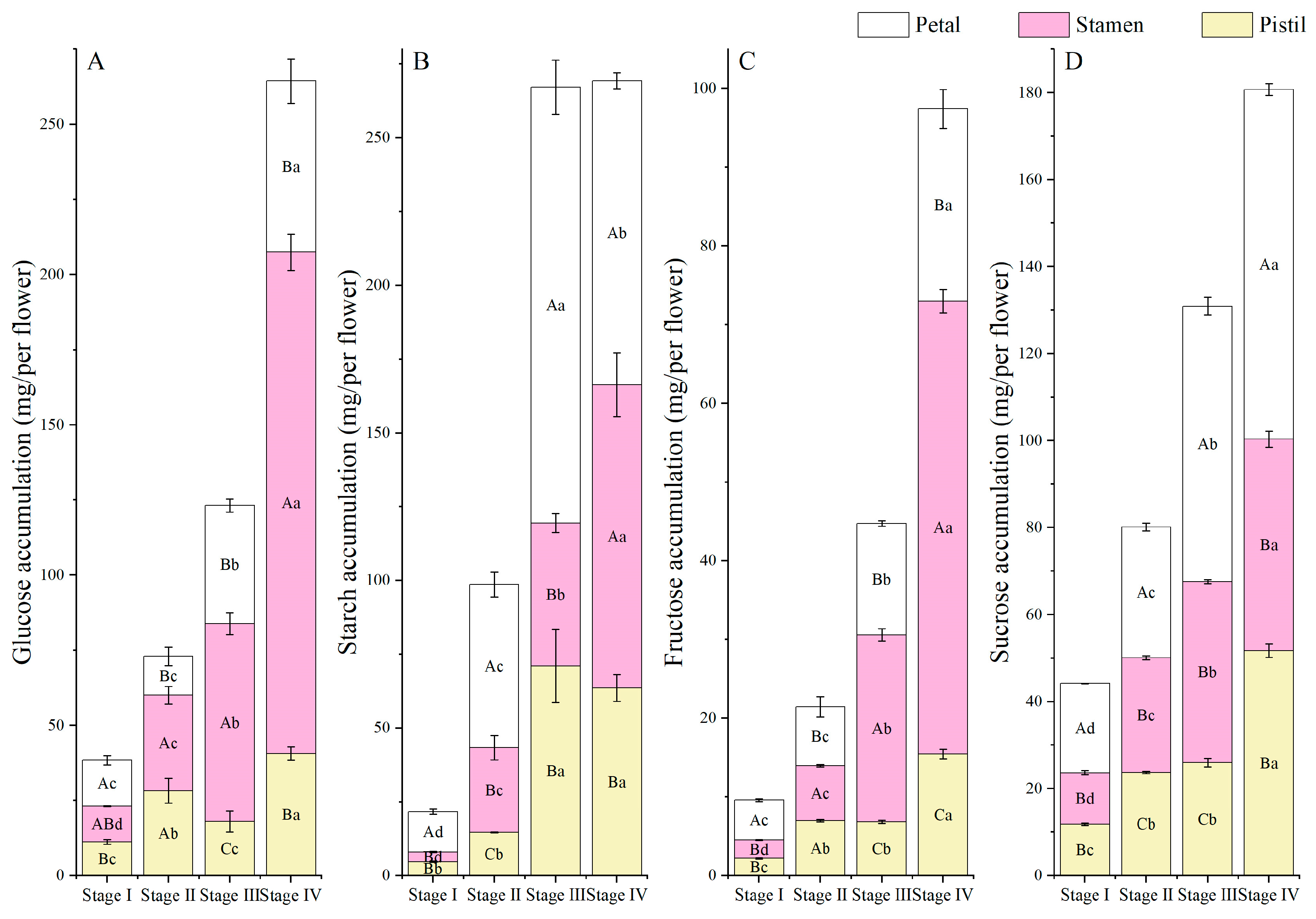
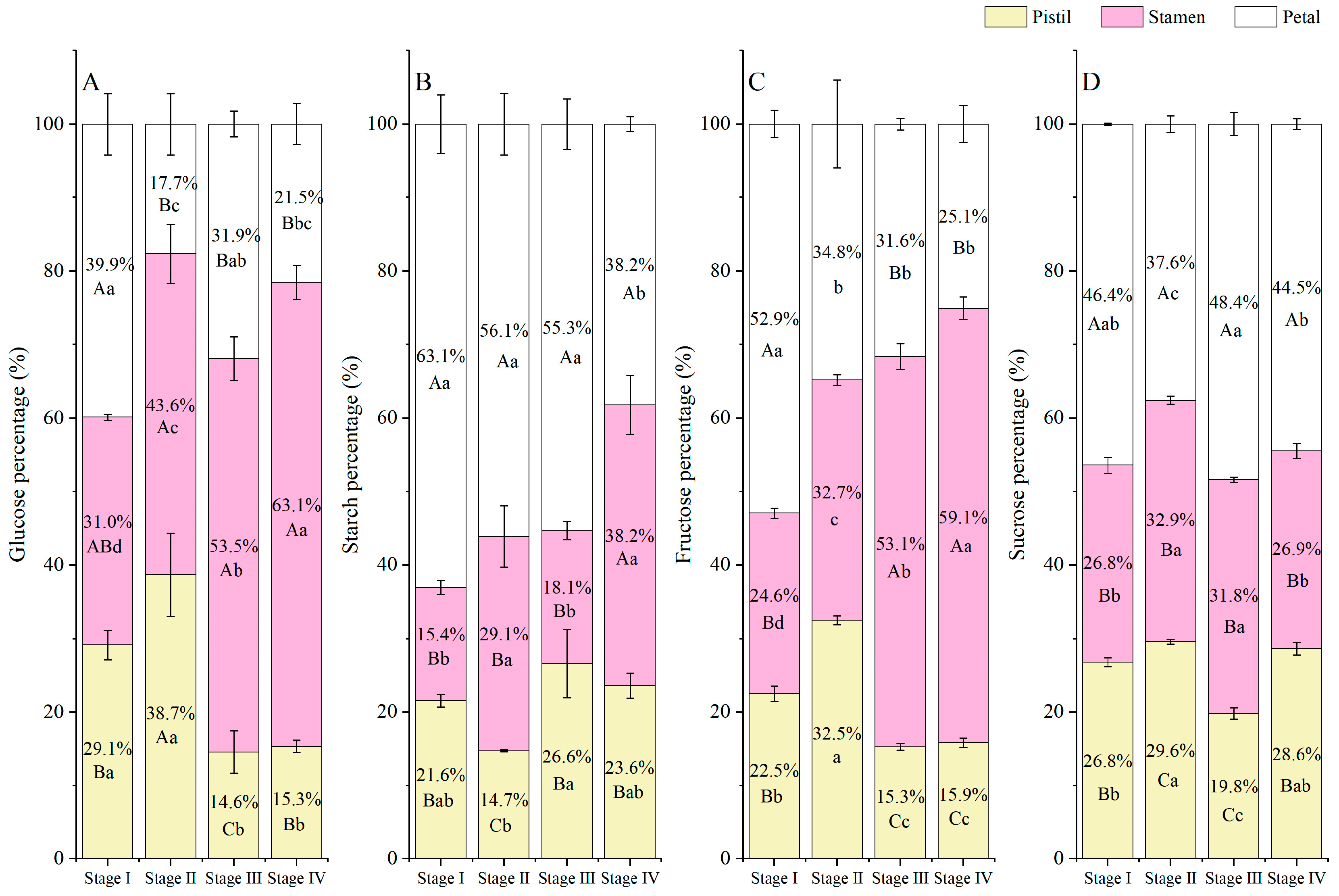
3.6. NSC Accumulation and Allocation Proportion
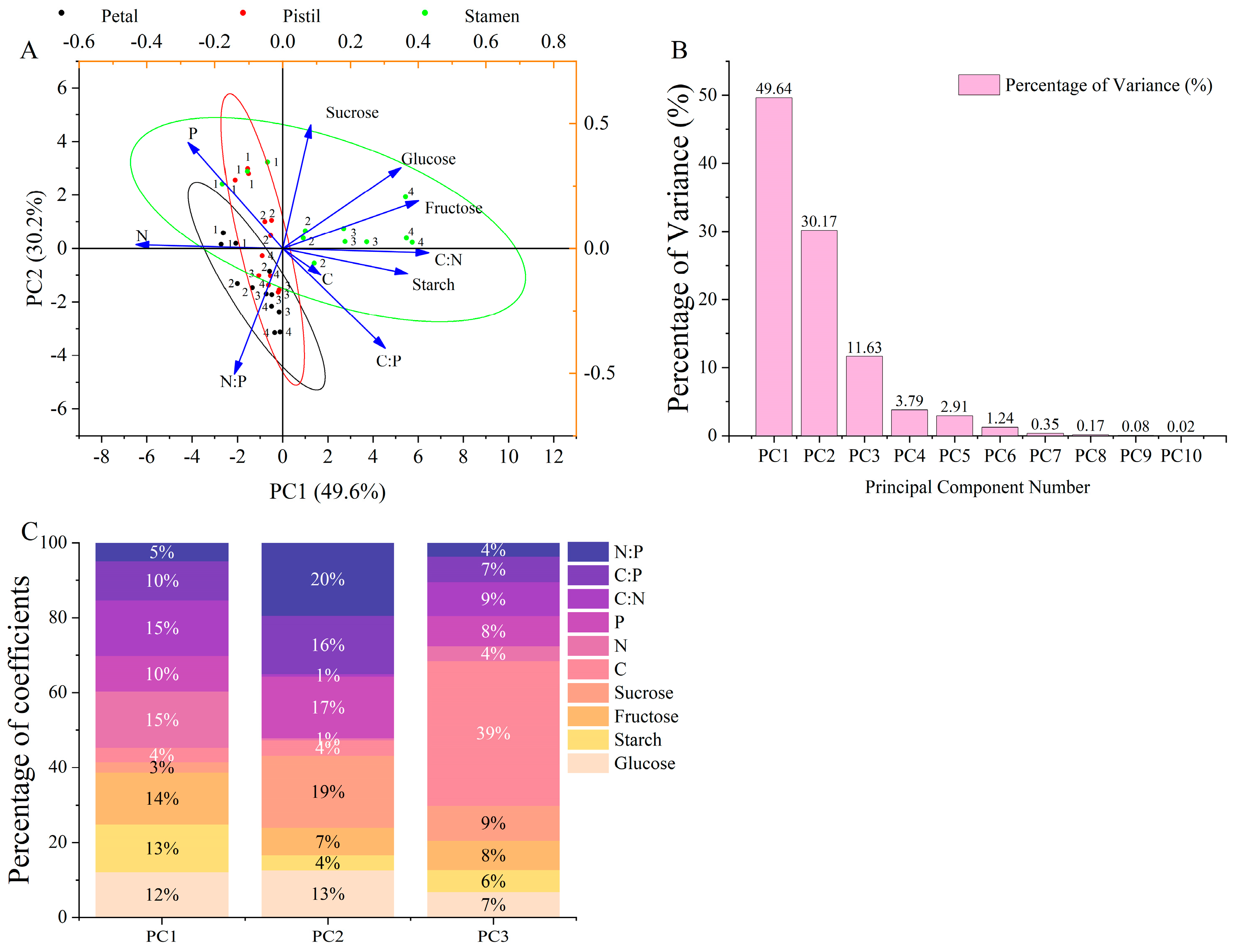
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of Phenological, Primary and Secondary Metabolites Changes during Flower Developmental of Rosa Damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Bischoff, M.; Lord, J.M.; Robertson, A.W. Flower Color Influences Insect Visitation in Alpine New Zealand. Ecology 2010, 91, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G.; Kamdee, C. Flower Opening and Closure: An Update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Y.; Wang, H.; Qian, W.; Hu, Y.; Gao, S.; Liao, H. The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Michelia maudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae 2023, 9, 1198. [Google Scholar] [CrossRef]
- Wei, S.; Liu, H.; Li, J.; Ren, T.; Xie, J. Metabolite Variations of Sugars, Organic Acids, Fatty Acids and Amino Acids in Flower Buds of Zingiber Mioga Roscoe at Different Developmental Stages. J. Food Compos. Anal. 2023, 116, 105050. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Önder, D.; Erbaş, S.; Mutlucan, M. Flower Color and Carbohydrate Metabolism Changes during the Floral Development of Rosa Damascena. S. Afr. J. Bot. 2023, 156, 234–243. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and Nutrition of Plant Growth in Natural Communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Li, L.; Sheen, J. Dynamic and Diverse Sugar Signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef]
- Prathap, V.; Kumar, A.; Maheshwari, C.; Tyagi, A. Phosphorus homeostasis: Acquisition, sensing, and long-distance signaling in plants. Mol. Biol. Rep. 2022, 49, 8071–8086. [Google Scholar] [CrossRef]
- Poorter, H.; Remkes, C.; Lambers, H. Carbon and Nitrogen Economy of 24 Wild Species Differing in Relative Growth Rate. Plant Physiol. 1990, 94, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yu, G.; Wang, Q.; Wang, R.; Zhang, J.; Liu, C.; He, N. Conservative Allocation Strategy of Multiple Nutrients among Major Plant Organs: From Species to Community. J. Ecol. 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Slik, J.W.F.; Cao, K. Leaf Element Concentrations of Terrestrial Plants across China Are Influenced by Taxonomy and the Environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global Patterns of Plant Leaf N and P in Relation to Temperature and Latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P Stoichiometry of Organisms and Ecosystems in a Changing World: A Review and Perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C: N: P Stoichiometry between Soil and Leaf and Their Response to Climatic Factors along Altitudinal Gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Qian, W.-Z.; Yi, L.; Ye, Y.-L.; Gu, T.; Gao, S.; Cao, G.-X. Nutrient Composition and Antioxidant Activity of Cercis Chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the Roles of Nonstructural Carbohydrates in Forest Trees—From What We can Measure to What We Want to Know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic Allocation and Transfer of Non-Structural Carbohydrates, a Possible Mechanism for the Explosive Growth of Moso Bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Wang, Y.; Funnell, K.A.; Eason, J.R.; Morgan, E.R.; Woolley, D.J. Osmotic Regulation via Carbohydrate Metabolism Drives Petal Expansion and Floret Opening in Gentian ‘Showtime Spotlight. ’ Sci. Hortic. 2016, 211, 19–25. [Google Scholar] [CrossRef]
- Cho, L.-H.; Pasriga, R.; Yoon, J.; Jeon, J.-S.; An, G. Roles of Sugars in Controlling Flowering Time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Yamada, K.; Norikoshi, R.; Suzuki, K.; Imanishi, H.; Ichimura, K. Determination of Subcellular Concentrations of Soluble Carbohydrates in Rose Petals during Opening by Nonaqueous Fractionation Method Combined with Infiltration–Centrifugation Method. Planta 2009, 230, 1115–1127. [Google Scholar] [CrossRef]
- Norikoshi, R.; Imanishi, H.; Ichimura, K. Changes in Cell Number, Osmotic Potential and Concentrations of Carbohydrates and Inorganic Ions in Tweedia caerulea during Flower Opening. J. Jpn. Soc. Hortic. Sci. 2013, 82, 51–56. [Google Scholar] [CrossRef]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clement, C. Sugars and Flowering in the Grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and Boron Content of Styles of ‘Hass’ Avocado (Persea americana Mill.) Flowers at Anthesis can Affect Final Fruit Set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A Review of the Phytochemistry and Pharmacological Activities of Magnoliae officinalis Cortex. J. Ethnopharmacol. 2019, 236, 412–442. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological Activity and Toxicity of the Chinese Herb Magnolia officinalis Rehder & E. Wilson (Houpo) and Its Constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef]
- Niu, L.; Hou, Y.; Jiang, M.; Bai, G. The Rich Pharmacological Activities of Magnolia officinalis and Secondary Effects Based on Significant Intestinal Contributions. J. Ethnopharmacol. 2021, 281, 114524. [Google Scholar] [CrossRef]
- Verlinden, S. Changes in Mineral Nutrient Concentrations in Petunia Corollas during Development and Senescence. HortScience 2003, 38, 71–74. [Google Scholar] [CrossRef]
- Wang, T.-W.; Tan, J.; Li, L.-Y.; Yang, Y.; Zhang, X.-M.; Wang, J.-R. Combined Analysis of Inorganic Elements and Flavonoid Metabolites Reveals the Relationship between Flower Quality and Maturity of Sophora japonica L. Front. Plant Sci. 2023, 14, 1255637. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Ren, H.; Wang, M.; Qian, W.; Hu, Y.; Yang, Y.; Yu, T.; Zhao, K.; Gao, S. Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae 2024, 10, 261. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Ho, T.-T.; Kwon, A.-R.; Yoon, Y.-J.; Paek, K.-Y.; Park, S.-Y. Endoreduplication Level Affects Flower Size and Development by Increasing Cell Size in Phalaenopsis and Doritaenopsis. Acta Physiol. Plant. 2016, 38, 190. [Google Scholar] [CrossRef]
- Steinacher, G.; Wagner, J. Flower Longevity and Duration of Pistil Receptivity in High Mountain Plants. Flora 2010, 205, 376–387. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, X.; Liang, L.; Gu, C.; Sun, X. Morphological Anatomy, Sporogenesis and Gametogenesis in Flowering Process of Jasmine (Jasminum sambac Aiton). Sci. Hortic. 2016, 198, 257–266. [Google Scholar] [CrossRef]
- Mittal, I.; Jhanji, S.; Dhatt, K.K. Efficacy of Sodium Nitroprusside, a Nitric Oxide Donor, on Vase Life and Postharvest Attributes of Gladiolus Spikes. Acta Physiol. Plant. 2021, 43, 108. [Google Scholar] [CrossRef]
- Jhanji, S.; Kaur, G.; Kaur, R.; Dhatt, U.K. Physiological and Biochemical Changes during Flower Development and Senescence in Chrysanthemum and Gladiolus. Acta Physiol. Plant. 2023, 45, 14. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; van Meeteren, U. Flower Opening and Closure: A Review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and Determinants of Carbon Content in Plants: A Global Synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Gong, Z.; Sheng, M.; Zheng, X.; Zhang, Y.; Wang, L. Ecological Stoichiometry of C, N, P and Si of Karst Masson Pine Forests: Insights for the Forest Management in Southern China. Sci. Total Environ. 2024, 912, 169490. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Kim, N.; Han, W.; Guo, Y.; Han, T.; Du, E.; Fang, J. Effects of Nitrogen and Phosphorus Supply on Growth Rate, Leaf Stoichiometry, and Nutrient Resorption of Arabidopsis thaliana. Plant Soil 2015, 388, 147–155. [Google Scholar] [CrossRef]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine Bud Fertility and Number of Berries per Bunch Are Determined by Water and Nitrogen Stress around Flowering in the Previous Year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Lau, T.-C.; Stephenson, A.G. Effects of Soil Phosphorus on Pollen Production, Pollen Size, Pollen Phosphorus Content, and the Ability to Sire Seeds in Cucurbita pepo (Cucurbitaceae). Sex. Plant Reprod. 1994, 7, 215–220. [Google Scholar] [CrossRef]
- Atasay, A.; Akgül, H.; Uçgun, K.; Şan, B. Nitrogen Fertilization Affected the Pollen Production and Quality in Apple Cultivars “Jerseymac” and “Golden Delicious”. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 460–465. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, Phosphorus, and Potassium Fertilization Affects the Flowering Time of Rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical Characterization of Borago officinalis L. and Centaurea cyanus L. during Flower Development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Tafazoli, E.; Dokhani, S.; Rahemi, M.; Emam, Y. Changes in Carbohydrate Contents in Shoot Tips, Leaves and Roots of Strawberry (Fragaria×ananassa Duch.) during Flower-Bud Differentiation. Sci. Hortic. 2007, 113, 255–260. [Google Scholar] [CrossRef]
- Hedhly, A.; Vogler, H.; Schmid, M.W.; Pazmino, D.; Gagliardini, V.; Santelia, D.; Grossniklaus, U. Starch Turnover and Metabolism during Flower and Early Embryo Development. Plant Physiol. 2016, 172, 2388–2402. [Google Scholar] [CrossRef]
- Sood, S.; Vyas, D.; Nagar, P.K. Physiological and Biochemical Studies during Flower Development in Two Rose Species. Sci. Hortic. 2006, 108, 390–396. [Google Scholar] [CrossRef]
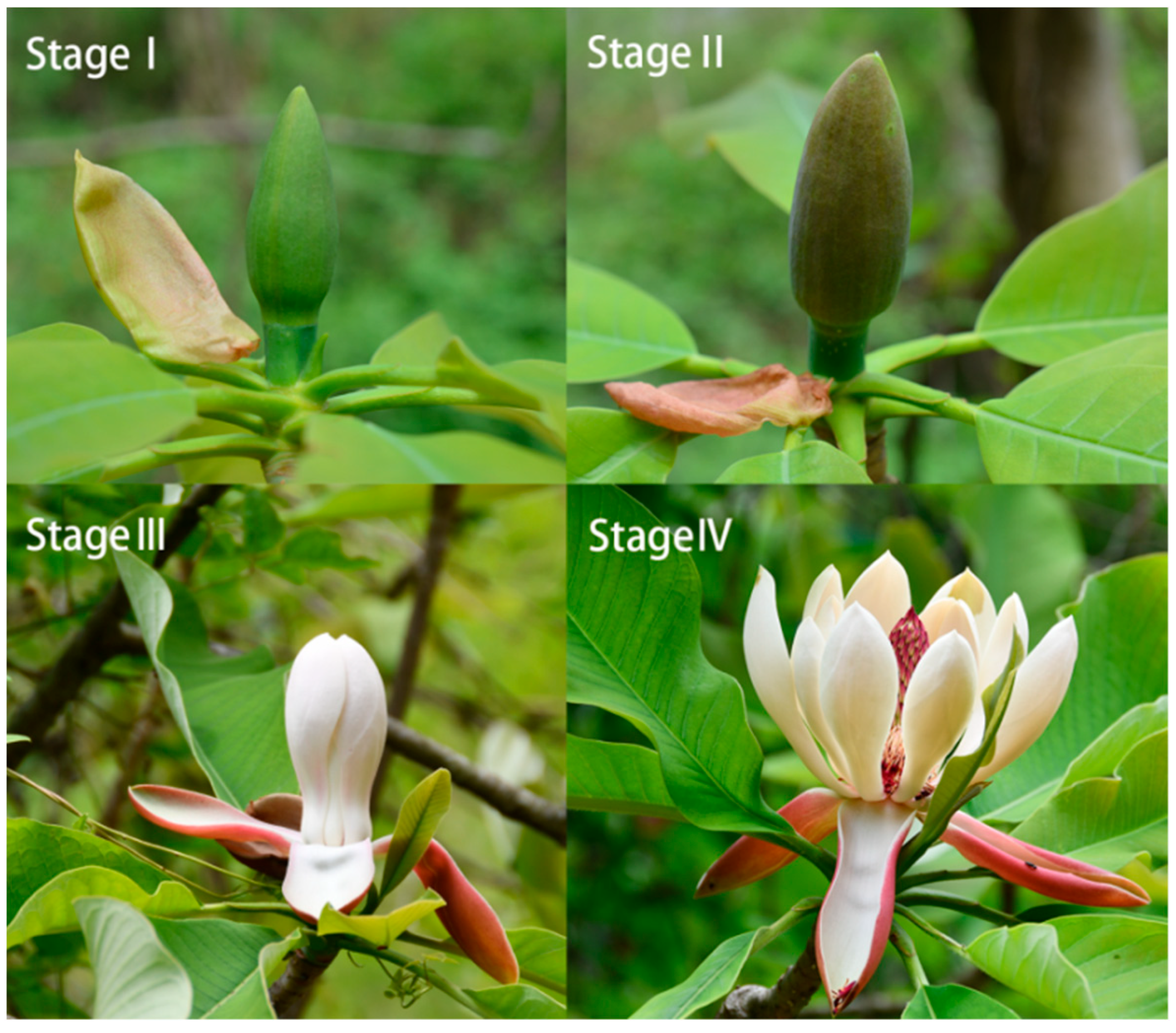
| Stage | Pistil | Stamen | Petal | |||
|---|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Length (cm) | Width (cm) | Length (cm) | Width (cm) | |
| I | 5.00 ± 0.15 b | 1.34 ± 0.08 a | 1.88 ± 0.04 c | 0.22 ± 0.01 c | 6.28 ± 0.14 d | 3.13 ± 0.13 c |
| II | 5.51 ± 0.16 b | 1.96 ± 0.06 a | 2.17 ± 0.06 b | 0.26 ± 0.02 bc | 7.40 ± 0.07 c | 3.49 ± 0.12 c |
| III | 7.08 ± 0.21 a | 2.09 ± 0.05 a | 2.74 ± 0.10 a | 0.35 ± 0.01 a | 8.70 ± 0.15 b | 4.80 ± 0.09 b |
| IV | 7.31 ± 0.24 a | 2.15 ± 0.07 a | 2.56 ± 0.12 a | 0.30 ± 0.03 ab | 11.3 ± 0.32 a | 5.62 ± 0.14 a |
| Stage | Pistil | Stamen | Petal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight/g | Dry Weight/g | RWC (%) | Fresh Weight/g | Dry Weight/g | RWC (%) | Fresh Weight/g | Dry Weight/g | RWC (%) | |
| I | 1.90 ± 0.09 c | 0.25 ± 0.01 c | 86.8 a | 1.74 ± 0.15 c | 0.26 ± 0.02 d | 84.8 a | 7.35 ± 0.50 d | 1.01 ± 0.08 d | 86.3 a |
| II | 4.15 ± 0.58 c | 0.65 ± 0.08 c | 84.1 b | 3.60 ± 0.51 b | 0.65 ± 0.09 c | 81.9 b | 16.3 ± 1.39 c | 2.07 ± 0.26 c | 87.4 a |
| III | 6.75 ± 0.87 b | 1.55 ± 0.19 b | 76.9 d | 4.15 ± 0.06 b | 1.12 ± 0.02 b | 73.0 d | 19.7 ± 1.20 b | 3.55 ± 0.21 b | 82.0 c |
| IV | 13.3 ± 1.31 a | 2.58 ± 0.27 a | 80.6 c | 6.52 ±0.64 a | 1.51 ± 0.15 a | 76.9 c | 28.1 ± 0.84 a | 4.50 ± 0.18 a | 84.0 b |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Glucose concentration | 0.34909 | 0.3236 | −0.15173 |
| Starch concentration | 0.36917 | −0.10128 | −0.13488 |
| Fructose concentration | 0.40093 | 0.19085 | −0.17745 |
| Sucrose concentration | 0.08265 | 0.4945 | 0.21313 |
| C concentration | 0.11207 | −0.10532 | 0.87582 |
| N concentration | −0.43173 | 0.01485 | 0.08933 |
| P concentration | −0.27832 | 0.42472 | 0.18284 |
| C:N ratio | 0.43122 | −0.01701 | 0.206 |
| C:P ratio | 0.30272 | −0.39901 | 0.15451 |
| N:P ratio | −0.14236 | −0.50285 | −0.08417 |
| Percentage of variance (%) | 44.4% | 35.9% | 13.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Qiu, Y.; Cheng, Y.; Yu, T.; Zhu, M.; Qian, W.; Gao, S.; Zhuang, G. Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae 2024, 10, 784. https://doi.org/10.3390/horticulturae10080784
Yang Y, Qiu Y, Cheng Y, Yu T, Zhu M, Qian W, Gao S, Zhuang G. Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae. 2024; 10(8):784. https://doi.org/10.3390/horticulturae10080784
Chicago/Turabian StyleYang, Yao, Yuxian Qiu, Yu Cheng, Ting Yu, Maoyuan Zhu, Wenzhang Qian, Shun Gao, and Guoqing Zhuang. 2024. "Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages" Horticulturae 10, no. 8: 784. https://doi.org/10.3390/horticulturae10080784
APA StyleYang, Y., Qiu, Y., Cheng, Y., Yu, T., Zhu, M., Qian, W., Gao, S., & Zhuang, G. (2024). Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae, 10(8), 784. https://doi.org/10.3390/horticulturae10080784






