Abstract
Salt stress is one of the major environmental problems in agricultural production, severely limiting crops’ germination, growth and yield. Silicon (Si) is a widely recognized beneficial element in plants, which can promote plant growth especially under stressful conditions. With the emergence of nanotechnology in agriculture, silicon nanoparticles (SiNPs) have been shown to be a promising tool in nano-enabled agricultural production. However, the comparative effects of Si and SiNPs in alleviating salt stress in plants remain unclear, which would limit the application of SiNPs in agricultural practice. In this study, the effects of SiNPs and conventional Si (silicate) on tomato (a typical low-Si accumulator) seed germination, reactive oxygen species (ROS) content, antioxidant enzyme activity, and the expression of genes related to hormone metabolism were investigated. The results showed that SiNPs more effectively promoted seed germination percentage, fresh weight, and Si content than conventional Si. Simultaneously, SiNPs more significantly modulated the activities of antioxidant enzymes and alleviated salt stress-induced oxidative damage in tomato seeds. Moreover, exogenous SiNPs addition promoted the expression of genes responsible for gibberellin (GA) synthesis and abscisic acid (ABA) catabolism, while downregulating the expression of genes related to GA deactivation and ABA synthesis in tomato seeds under salt stress. Overall, our results indicate that SiNPs are more effective than conventional Si in promoting tomato seed germination under salt stress via modulating antioxidant enzyme activity and key endogenous hormone metabolism, which could be based on the higher accumulation of SiNPs in tomato seeds than conventional Si.
1. Introduction
Salt stress is one of the most important environmental problems in agricultural production, which limits seed germination, plant growth, and crop yield severely [1,2]. With the background of global warming and an increasing occurrence of improper agricultural practices (such as excess chemical fertilizer usage and groundwater irrigation), the detrimental effects of salt stress on agricultural production are gradually expanding [3,4]. To combat salt stress in agricultural production, various efforts have been made including salt stress resistance breeding, rational irrigation, and application of plant growth regulators [5,6,7].
Silicon (Si) is the second most abundant element in earth’s crust [8,9]. Although, Si has not been proven to be an essential element for plants, it has been widely recognized that Si can promote plant growth and yield, especially for those grown under biotic and abiotic stresses including salt stress [10,11,12,13]. Under salt stress, the Si-induced promotion of seed germination, plant growth, and crop yield has been repeatedly documented in various plant species such as rice [14,15], maize [16,17], wheat [18,19], barley [20,21], tomato [22,23], and cucumber [24,25]. In general, silicic acid is the sole form of Si in nature that can be absorbed by plant roots; therefore, silicates such as sodium silicate are mostly used in laboratory experiments and agricultural production previously [10,11,26,27]. However, with the emergence of nanotechnology in agricultural production, the application of nanoparticles (NPs) has been shown to be a promising strategy in nano-enabled agriculture [28,29,30,31]. As a novel source of Si, silicon nanoparticles (SiNPs) share the beneficial effects of Si in plants, which can promote plant growth under various stresses including salt stress [32,33,34].
Although both Si and SiNPs can promote plant growth under stressful conditions, the regulatory effects of Si and SiNPs and their underlying mechanisms differ significantly among stress types and plant species [32,33,35,36]. For example, it has been reported that SiNPs are more effective in alleviating cadmium stress in tomato than silicate via modulating Cd uptake and antioxidant system [37]. Moreover, Zahedi et al. [38] found that Si and SiNPs application through foliar spray regulated morphological and physiological traits differentially in strawberry under drought stress. In addition, it has been documented that seed priming with Si and SiNPs promoted sweet pea growth and alleviated oxidative damage under salt stress [39]. It should be noted that the ability of Si uptake and accumulation differs significantly among plant species, while most plants are low Si accumulators. Therefore, aiming for the wide and reasonable usage of SiNPs in agriculture, the effects of Si and SiNPs on plant stress resistance in different plants, especially in low Si accumulators, deserve further investigation.
The inhibition of seed germination is one of the major toxic symptoms of salt stress, which would lead to economic loss and successively limit plant growth and crop yield [40]. In agricultural practices, both Si and SiNPs can be applied via seed priming and can enhance seed germination and seedling growth under different stress conditions [33]. However, the comparative effects of Si and SiNPs on seed germination under salt stress and this mechanism, especially in low Si accumulation plants, remain unclear. Therefore, based on the backgrounds mentioned above, the effects of Si and SiNPs on the germination of tomato seeds under salt stress were investigated in this study. In addition, the regulation of these two Si sources on Si content, oxidative damage, ROS accumulation, antioxidant enzyme activity, and the expression of genes responsible for endogenous hormones metabolism including abscisic acid (ABA) and gibberellin (GA) was evaluated.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Tomato seeds (Solanum lycopersicum L. cv. Hezuo 903) were surface sterilized with deionized water at 55 °C for 15 min, and then germinated at 25 °C in darkness for 7 d. Each 100 seeds were neatly placed in one germination box (12 cm × 12 cm × 5 cm) as one replication, and three individual replications were conducted in this study.
Four treatments were applied via seed immersion in this study including control (CK), salt stress (NaCl), salt stress and 1 mmol L−1 silicon nanoparticles (NaCl + SiNPs), and salt stress and 1 mmol L−1 silicate (NaCl + Si). Salt stress was applied with 125 mmol L−1 NaCl. SiNPs were added in the form of nano magnesium silicate which was supplied by Henan University. SiNPs were synthesized from silicon dioxide, sodium acetate, and magnesium sulfate at 180 °C for 8 h and the average diameter was 40 nm (Figure S1). Conventional silicon source was added in the form of sodium silicate (Na2SiO3).
2.2. Germination Percentage and Germination Index
Germination percentage (GP) and germination index (GI) was calculated as follows:
GP (%) = number of germinated seeds/number of total seeds × 100
GI = ∑(number of germinated seeds at time t/days of germination at time t)
2.3. Fresh Weight and Si Content
Tomato seeds were collected after 7 d of treatment, and the total fresh weight was determined and recorded. As for the determination of Si content, seeds samples were ground into fine powder and then digested using 5 mL nitric acid and 1 mL 30% H2O2 at 140 °C for 15 min, 180 °C for 20 min, and 160 °C for 70 min on a microwave digestion system (MARS6, CEM, Charlotte, NC, USA). The content of Si was measured using molybdenum blue method at 660 nm using a spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan).
2.4. Malondialdehyde (MDA) and Proline Content
The content of MDA was measured using trichloroacetic acid-thiobarbituric acid (TCA-TBA) method according to Heath and Lester [41]. Briefly, samples were homogenized with 0.1% (w/v) TCA solution and the supernatant was mixed with 0.5% (w/v) TBA solution. After incubation in boiling water for 20 min, the absorbance of the mixture was determined at 450, 532, and 600 nm. The content of proline was determined with ninhydrin method as described by Bates et al. [42]. Seed samples were homogenized with 3% (w/v) aqueous sulfosalicylic acid solution, and then mixed with glacial acetic and ninhydrin solution. After incubation in boiling water for 40 min, toluene was added and the absorptance at 520 nm of the upper phase was measured.
2.5. Superoxide Anion (O2·−) and Hydrogen Peroxide (H2O2) Content and Visualization
The content of O2·− was determined with the hydroxylamine oxidation method as described by Elstner and Heupel [43]. Fresh samples were homogenized with phosphate butter (65 mmol L−1, pH 7.8), supernatant was mixed with hydroxylamine hydrochloride (10 mmol L−1), sulfanilamide (58 mmol L−1), naphthylamine (7 mmol L−1), and trichloromethane. The absorptance was measured at 530 nm. H2O2 was extracted using perchloric acid (1 mol L−1) and assayed according to Cheeseman [44] using eFOX reagent. For the visualization of O2·−, chemical staining was conducted with nitroblue tetrazolium (NBT) method according to Jabs et al. [45].
2.6. Antioxidant Enzyme Activity
Seed samples were homogenized with phosphate butter (50 mmol L−1, pH 7.8, containing 0.2 mmol L−1 EDTA and 2% PVP). After centrifugation at 10,000 rpm for 15 min, the supernatant was collected, and the content of protein was measured according to Bradford [46]. Then, the activities of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POD) were determined and calculated on a protein basis according to Giannopolitis and Ries [47]; Cakmak and Marschner [48]; Nakano and Asada [49]; Yan et al. [50], respectively.
2.7. Gene Expression
Total RNA was extracted from frozen samples using TRIzol reagent (Invitrogen, Thermo Fisher, Waltham, MA, USA), and then converted to cDNA using HiScriptIII 1st strand cDNA synthesis kit (Vazyme, Nanjing, China) following the instruction. Quantitative PCR was performed using SYBR Green PCR Master Mix (Vazyme, Nanjing, China) on qTower3 quantitative PCR system (Analytik jena, Jena, Germany). The expression of genes was calculated using ΔΔCt method, and Actin was used as reference gene. The sequences of primes used in this study are listed as follows: 5′-AGGCAACAGTGAAACTTCCATCAAG-3′ and 5′-TCCATTAAAGAGGATATTACCGGGGAC-3′ for SlNCED1, 5′-TGGTTTTCATGGGACATTCATTAGC-3′ and 5′-ATCTCCCTTCTCAACTCCCTATTCC-3′ for SlNCED2, 5′-AGTTTAGTGCAAGGCGGGTT-3′ and 5′-TCTATCTCCACCTCGCTGACA-3′ for SlAAO3, 5′-AGAGAGGCTGTAGCTGAGTGG-3′ and 5′-TTGGCAAGTTCATTCCCTGGAC-3′ for SlCYP707A1, 5′-TTCTTTCTGGTGACCCCGAC-3′ and 5′-AACGCCATTCATCGTCCACA-3′ for SlGA20ox1, 5′-GGGGCATTCCTTTCTGGTGA-3′ and 5′-GTGGTCCAGTTCCTAACGCA-3′ for SlGA20ox2, 5′-CACCTCACCCGAATACTGCT-3′ and 5′-ACGAGTGTCCCCAAGTCTCA-3′ for SlGA2ox1, 5′-ACCCGACTCCAAGAACCTCA-3′ and 5′-TGTTCGACCCGACCACAATC-3′ for SlGA2ox2, and 5′-TTCAAAGGGCGAGTACGACG-3′ and 5′-ACTTGCCTAACAGCAGACCC-3′ for Actin.
2.8. Statistical Analysis
All the data were analyzed using Excel (2019 version, Microsoft, Redmond, WA, USA), and subjected to one-way analysis of variance (one-way ANOVA). The mean values were compared using the least significant difference (LSD) method and a significant difference was defined at the 0.05 probability level.
3. Results
3.1. SiNPs Enhanced Tomato Seed Germination under Salt Stress
Under normal condition (CK), the germination percentage (GP) reached 53.3% and 86.8% at 48 h and 96 h, respectively (Figure 1A). The germination index (GI) under CK treatment was 37.8 (Figure 1B). The germination of tomato seeds was severely inhibited by salt stress. The germination of tomato seeds was first observed after 96 h of incubation, and the final GP and GI were 43.0% and 7.2, respectively (Figure 1). Under salt stress, SiNPs treatment significantly promoted tomato seeds germination under salt stress, and the final GP and GI were 75.1% and 13.1, respectively. However, in contrast with the positive regulation effects of SiNPs on tomato seed germination under salt stress, no significant promotion of germination percentage and germination index was observed under exogenous Si addition (Figure 1).
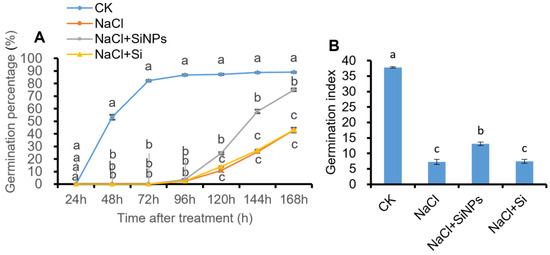
Figure 1.
Effects of Si and SiNPs on (A) germination percentage and (B) germination index of tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
3.2. SiNPs Promoted Fresh Weight and Si Content
In addition to germination parameters including GP and GI, total fresh weight (FW) and Si concentration were measured after 7 d of germination. As shown in Figure 2A, salt stress caused a significant decline of FW, and SiNPs treatment significantly promoted FW while Si treatment induced no obvious change in FW. In addition, the Si concentration in tomato seeds significantly increased under SiNPs treatment, while no significant change in Si concentration was observed under NaCl and Si treatment (Figure 2B).
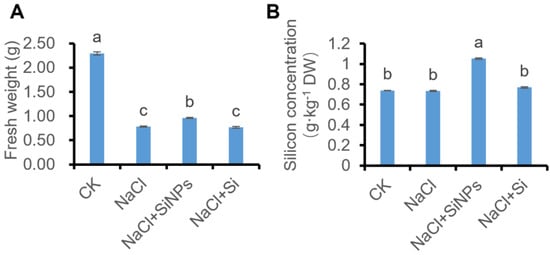
Figure 2.
Effects of Si and SiNPs on (A) fresh weight and (B) silicon concentration of tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
3.3. SiNPs Decreased MDA and Protein in Tomato Seeds under Salt Stress
In tomato seeds, salt stress induced significant accumulation of MDA and proline along with treatment time in contrast with CK treatment (Figure 3A,B). Under salt stress, both the addition of Si and SiNPs decreased MDA concentration in tomato seeds, while SiNPs induced a more significant decline of MDA content than Si treatment (Figure 3A). As for proline content, no obvious regulation was induced by either Si or SiNPs treatment under salt stress (Figure 3B). In addition, a gradual decline of protein content was observed along with germination in tomato seeds under CK, and similar trends of the change in protein content was found under SiNPs addition, which was not in tomato seeds under NaCl and Si treatment (Figure 3C).
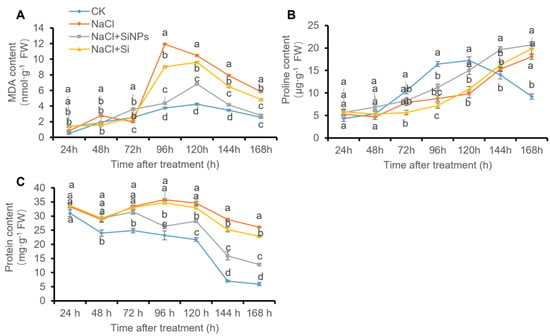
Figure 3.
Effects of Si and SiNPs on (A) MDA, (B) proline, and (C) protein concentration in tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
3.4. SiNPs Decreased ROS Accumulation in Tomato Seeds under Salt Stress
In this study, the concentration of hydrogen peroxide (H2O2) and superoxide anion (O2·−) was measured at different times in the process of tomato seed germination (Figure 4). Along with the treatment time, salt stress induced significant accumulation of ROS including H2O2 and O2·−, and SiNPs treatment dramatically decreased the content of H2O2 and O2·−, while no significant regulation on ROS content was observed under Si addition (Figure 4). In addition, the visualization of O2·− was conducted using nitroblue tetrazolium (NBT) staining (Figure 5). In accordance with the results above, salt stress induced accumulation of O2·− in tomato seeds and inhibited seed germination, while SiNPs promoted seed germination and decreased O2·− content, which was not observed under Si addition (Figure 5).
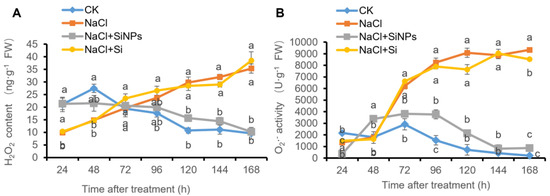
Figure 4.
Effects of Si and SiNPs on (A) H2O2 and (B) O2·− concentration in tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
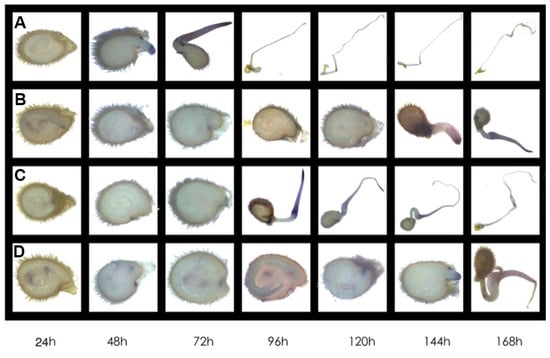
Figure 5.
Visualization of O2·− accumulation using nitroblue tetrazolium (NBT) staining in tomato seeds under different treatments. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. (A) CK: control, H2O; (B) NaCl: 125 mmol L−1 NaCl; (C) NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); (D) NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate.
3.5. SiNPs Upregulated Antioxidant Enzymes Activities
To evaluate the effects of Si and SiNPs on the antioxidant enzymes, the activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD) were determined in this study. As shown in Figure 6, the activities of SOD, CAT, APX, and POD gradually increased along with germination in tomato seeds under CK treatment. However, salt stress significantly downregulated the activity of antioxidant enzymes. Although no significant change in antioxidant enzyme activity was induced by exogenous Si addition, SiNPs treatment promoted the activities of antioxidant enzymes including SOD, CAT, APX, and POD (Figure 6).
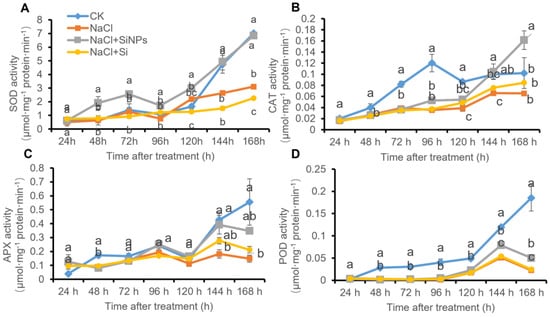
Figure 6.
Effects of Si and SiNPs on (A) SOD, (B) CAT, (C) APX and (D) POD activity in tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
3.6. SiNPs Regulated the Expression of Genes Related to Hormone Metabolism
Considering the pivotal role of plant hormones in seed germination, the expression of genes responsible for the synthesis (including GA20ox1 and GA20ox2) and deactivation (including GA2ox1 and GA2ox2) of GA, and the synthesis (including NCED1, NCED2, and AAO3) and catabolism (CYP707A2) of ABA were evaluated in this study. Under CK treatment, the expression of GA20ox1, GA20ox2 and CYP707A2 increased significantly along with seed germination, while the expression of GA2ox1, GA2ox2, NCED1, NCED2, and AAO3 was maintained at relatively lower levels (Figure 7). In contrast, salt stress upregulated the expression of GA2ox1, GA2ox2, NCED1, NCED2, and AAO3, and downregulated the expression of GA20ox1, GA20ox2 and CYP707A2. Under salt stress, exogenous SiNPs addition modulated the expression of genes responsible for GA and ABA metabolism. The expression of GA20ox1, GA20ox2 and CYP707A2 was promoted, while the expression of GA2ox1, GA2ox2, NCED1, NCED2, and AAO3 was inhibited in comparison with NaCl treatment (Figure 7). However, no obvious regulative effect on these genes was induced by Si treatment in tomato seeds under salt stress (Figure 7).
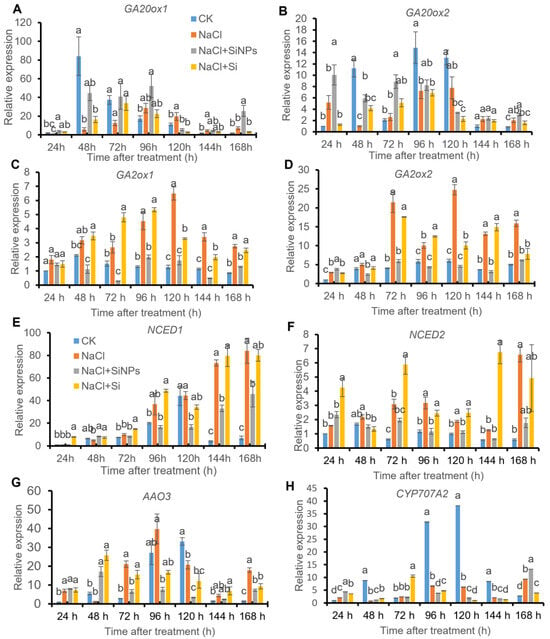
Figure 7.
Effects of Si and SiNPs on the expression of (A) GA20ox1, (B) GA20ox2, (C) GA2ox1, (D) GA2ox2, (E) NCED1, (F) NCED2, (G) AAO3, and (H) CYP707A2 in tomato seeds under salt stress. Tomato seeds (cv. Hezuo 903) were sown on germination box under different treatments for 7 d. CK: control, H2O; NaCl: 125 mmol L−1 NaCl; NaCl + SiNPs: 125 mmol L−1 NaCl and 1 mmol L−1 silicate nanoparticles (average diameter 40 nm); NaCl + Si: 125 mmol L−1 NaCl and 1 mmol L−1 sodium silicate. Different letters indicate significant differences (p < 0.05).
4. Discussion
Salt stress limits seed germination and plant growth severely, thereby negatively affecting crop yield and food safety in agricultural practices [1,3]. As a widely recognized beneficial element in plants, Si can promote plant resistance to various biotic and abiotic stresses including salt stress [11,51]. However, it should be noted that, the ability of Si uptake and accumulation differs among plant species significantly [10]. Based on the Si accumulation content in shoots, plants can be classified into three categories including high Si accumulation plant, medium Si accumulation plant, and low Si accumulation plant [27]. Therefore, the beneficial effects induced by exogenous Si addition differ with plant species. With the emergence of nanotechnology in agricultural production, SiNPs, as a novel source of Si, are proven to be an effective and promising tool, which is suitable for sustainable agriculture [33]. Due to the distinct mechanisms of SiNPs uptake and their accumulation in plants in contrast with that of Si, SiNPs are more suitable for application to low Si accumulation plants such as tomato [37,52,53].
In this study, the effects and related mechanisms of Si and SiNPs on tomato (a typical low Si accumulator) seed germination under salt stress were comparatively investigated. The results indicate that salt stress severely inhibited the germination of tomato seeds, while SiNPs were more effective than conventional Si in promoting the germination percentage, germination index, and total fresh weight of tomato seeds under salt stress. More importantly, significant Si accumulation was observed under SiNPs treatment, but not under Si addition. It has been repeatedly documented that Si and SiNPs differ significantly in regulating plant stress resistance, especially when they were applied via different patterns such as seed priming, root application, and foliar spray [32,35,54]. The results of this study indicate that the different regulation of Si and SiNPs on tomato seed germination under salt stress could be based on the distinct uptake and accumulation of Si in tomato seeds exposed to Si and SiNPs.
In the process of plant seed germination, ROS play dual roles including facilitating seed germination and causing oxidative damage, which is based on the concentration of ROS [55,56,57]. Therefore, the sophisticated modulation of ROS accumulation plays a pivotal role in seed germination, especially under stressful conditions [58]. Under salt stress, the excess accumulation of ROS is the one the major toxic symptoms, which would induce oxidative damage and successively impair normal metabolism and limit seed germination [59]. The results of this study show that salt stress induced significant accumulation of ROS and MDA in tomato seeds, which could be responsible, at least partially, for the salt stress-induced inhibition of seed germination. In contrast to the NaCl treatment, SiNPs addition dramatically decreased the contents of H2O2, O2·−, and MDA in tomato seeds. However, the exogenous addition of Si induced no significant regulation of ROS and MDA accumulation, which was in accordance with the distinct effects of Si and SiNPs on tomato seed germination under salt stress. In plant seeds, the cooperation of antioxidant enzymes plays an important role in modulating the accumulation of ROS [59]. In this study, the activities of antioxidant enzymes were determined, and the results showed that SiNPs treatment enhanced the activity of SOD, CAT, APX, and POD, which could be responsible for the SiNPs-induced decrement of ROS accumulation and the alleviation of oxidative damage in tomato seeds under salt stress.
Moreover, plant endogenous hormones (mainly ABA and GA) are master regulators of seed germination. In plant seeds, ABA and GA play contrary roles in modulating germination. The accumulation of ABA would induce seed dormancy, while the accumulation of GA would break seed dormancy and facilitate seed germination [56,60,61,62]. In this study, the effects of Si and SiNPs on the expression of genes responsible for ABA and GA metabolism were evaluated. Under control treatment, the expression of genes responsible for GA synthesis (GA20ox1, GA20ox2) and ABA catabolism (CYP707A2) increased significantly with time, while the expression of genes related to GA deactivation (GA2ox1, GA2ox2) and ABA synthesis (NCED1, NCED2, and AAO3) was maintained at relatively lower levels. In contrast, salt stress upregulated the expression of GA2ox1, GA2ox2, NCED1, NCED2, and AAO3, and downregulated the expression of GA20ox1, GA20ox2 and CYP707A2, thereby inhibiting seed germination. The exogenous addition of SiNPs promoted the expression of genes of GA synthesis and ABA catabolism, while it decreased the expression of genes related to GA deactivation and ABA synthesis, which would facilitate tomato seed germination under salt stress through regulating GA and ABA homeostasis. However, no significant regulative effect was observed under Si addition, which was in accordance with the regulation of Si and SiNPs on tomato seed germination under salt stress.
Overall, the effects of conventional Si (magnesium silicate) and SiNPs (magnesium silicate nanoparticles) on tomato seed germination, Si content, oxidative damage, ROS accumulation, antioxidant enzymes activities, and the expression of genes responsible for ABA and GA metabolism under salt stress were comparatively investigated in this study. The results indicate that SiNPs were more effective than conventional Si in promoting tomato seed germination, alleviating oxidative damage, modulating antioxidant enzymes and regulating endogenous hormones metabolism, which could be due to the distinct uptake and accumulation of Si and SiNPs in tomato seeds. Seed priming with SiNPs could be a promising agronomic approach in promoting seed germination and seedling growth in agricultural production affected by salt stress.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10080785/s1, Figure S1: Scanning electron microscope image of SiNPs used in this study.
Author Contributions
Conceptualization, G.Y., Y.L. and Z.Z.; investigation and formal analysis, T.W., H.L., S.M. and Z.J.; writing—original draft preparation, T.W. and Y.L.; writing—review and editing, Y.H., Z.Z. and G.Y., project administration, funding acquisition, Z.Z. and G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Zhejiang Province, grant number LQ22C150006 and LZ20C150001, the National Natural Science Foundation of China, grant number 32202583, the National Undergraduate Training Program for Innovation and Entrepreneurship of Zhejiang Agriculture and Forestry University, grant number 202410341033.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Munns, R. Living with salinity. New Phytol. 2008, 179, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Thabet, S.G.; Alqudah, A.M. New genetic insights into improving barley cope with salt stress via regulating mineral accumulation, cellular ion homeostasis, and membrane trafficking. Environ. Exp. Bot. 2023, 208, 105252. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Yan, G.C.; Nikolic, M.; Ye, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Interg. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Liang, Y.C.; Nikolic, M.; Belanger, R.; Gong, H.J.; Song, A.L. Silicon in Agriculture: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J.M. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Gong, H.J. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Yan, G.C.; Fan, X.P.; Peng, M.; Yin, C.; Xiao, Z.X.; Liang, Y.C. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Yan, G.C.; Fan, X.P.; Tan, L.; Yin, C.; Li, T.Q.; Liang, Y.C. Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiol. Biochem. 2021, 158, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bosnic, P.; Bosnic, D.; Jasnic, J.; Nikolic, M. Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ. Exp. Bot. 2018, 155, 681–687. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Saqib, M.; Zorb, C.; Schubert, S. Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Funct. Plant Biol. 2008, 35, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, E.; Egan, T.P. Silicon priming benefits germination, ion balance, and root structure in salt-stressed durum wheat (Triticum durum desf.). J. Plant Nutr. 2018, 41, 2560–2571. [Google Scholar] [CrossRef]
- Liang, Y.C.; Zhang, W.H.; Chen, Q.; Ding, R.X. Effects of silicon on H+-ATPase and H+-PPase activity, fatty acid composition and fluidity of tonoplast vesicles from roots of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.H.; Ding, R.X. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Yunus, Q.; Zari, M. Effect of exogenous silicon on ion distribution of tomato plants under salt stress. Commun. Soil Sci. Plant Anal. 2017, 48, 1843–1851. [Google Scholar] [CrossRef]
- Gou, T.; Su, Y.; Han, R.; Jia, J.; Zhu, Y.; Huo, H.; Liu, H.; Gong, H. Silicon delays salt stress-induced senescence by increasing cytokinin synthesis in tomato. Sci. Hortic. 2022, 293, 110750. [Google Scholar] [CrossRef]
- Gou, T.Y.; Chen, X.H.; Han, R.; Liu, J.Q.; Zhu, Y.X.; Gong, H.J. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol. Plant. 2020, 42, 12. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Jiang, X.C.; Zhang, J.; He, Y.; Zhu, X.M.; Zhou, X.K.; Gong, H.J.; Yin, J.L.; Liu, Y.Q. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef] [PubMed]
- Lombi, E.; Donner, E.; Dusinska, M.; Wickson, F. A One Health approach to managing the applications and implications of nanotechnologies in agriculture. Nat. Nanotechnol. 2019, 14, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kothalawala, S.; Yu, C.Z. Engineered silica nanomaterials in pesticide delivery: Challenges and perspectives. Environ. Pollut. 2023, 320, 14. [Google Scholar] [CrossRef]
- Rai, P.K.; Song, H.; Kim, K.-H. Nanoparticles modulate heavy-metal and arsenic stress in food crops: Hormesis for food security/safety and public health. Sci. Total Environ. 2023, 902, 166064. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.C.; Huang, Q.Y.; Zhao, S.J.; Xu, Y.M.; He, Y.; Nikolic, M.; Nikolic, N.; Liang, Y.C.; Zhu, Z.J. Silicon nanoparticles in sustainable agriculture: Synthesis, absorption, and plant stress alleviation. Front. Plant Sci. 2024, 15, 1393458. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; George, N.; Dwibedi, V. Emergence of toxic trace elements in plant environment: Insights into potential of silica nanoparticles for mitigation of metal toxicity in plants. Environ. Pollut. 2023, 333, 122112. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Bansal, K.; Hooda, V.; Verma, N.; Kharewal, T.; Tehri, N.; Dhull, V.; Gahlaut, A. Stress alleviation and crop improvement using silicon nanoparticles in agriculture: A review. Silicon 2022, 14, 10173–10186. [Google Scholar] [CrossRef]
- Yan, G.C.; Jin, H.; Yin, C.; Hua, Y.C.; Huang, Q.Y.; Zhou, G.F.; Xu, Y.M.; He, Y.; Liang, Y.C.; Zhu, Z.J. Comparative effects of silicon and silicon nanoparticles on the antioxidant system and cadmium uptake in tomato under cadmium stress. Sci. Total Environ. 2023, 904, 166819. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Atteya, A.K.G.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Heath, R.L.; Lester, P. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascrobate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide scanvenged by ascorbated specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Yan, G.C.; Hua, Y.C.; Jin, H.; Huang, Q.Y.; Zhou, G.F.; Xu, Y.M.; He, Y.; Zhu, Z.J. Sly-miR398 participates in cadmium stress acclimation by regulating antioxidant system and cadmium transport in tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2023, 24, 1953. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Bueno, V.; Gao, X.; Abdul Rahim, A.; Wang, P.; Bayen, S.; Ghoshal, S. Uptake and translocation of a silica nanocarrier and an encapsulated organic pesticide following foliar application in tomato plants. Environ. Sci. Technol. 2022, 56, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Okeke, E.S.; Nweze, E.J.; Ezike, T.C.; Nwuche, C.O.; Ezeorba, T.P.C.; Nwankwo, C.E.I. Silicon-based nanoparticles for mitigating the effect of potentially toxic elements and plant stress in agroecosystems: A sustainable pathway towards food security. Sci. Total Environ. 2023, 898, 165446. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Lenser, T.; Theißen, G. Molecular mechanisms involved in convergent crop domestication. Trends Plant Sci. 2013, 18, 704–714. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).