Abstract
Climatic changes and global warming affect the growth, development, and productivity of crops. In this review, we highlight the possible benefits of using innovative breeding techniques like clustered regularly interspaced short palindromic repeats (CRISPRs), exogenous phytohormone-like strigolactones (SLs), nanomaterials (NMs), and beneficial microbial endophytes to address the challenges in sustainable cultivation of horticultural crops. These applications are evaluated by examining how they affect different metabolic, morphological, and biochemical parameters in diverse crops. Endophytes are symbiotic microorganisms and can be used as nematicides for improving crop yield. With an emphasis on quality control, we examined the impacts of applying NMs, a novel family of phytohormones called SLs, and microbial endophytes on horticultural commodities. Furthermore, we reviewed the benefits of CRISPR for the editing of plant genomes, as well as how it affects gene expression and transcription factors to increase crop tolerance and yield. These innovations hold the potential to improve crop yield, quality, and resilience by acting as safe, natural components in biofertilizers and plant protection solutions. Gradually adopting these methods could decrease reliance on agrochemicals, thereby reducing their negative effects on biodiversity, soil fertility, and human health.
1. Introduction
Horticultural crop cultivation encounters numerous challenges. There are a lot of factors that are responsible for these challenges. Global warming is the major issue, as it leads to infertile soil, several environmental stressors that harm crop yields (salinity, droughts, the presence of pollutants in the soil), and excessive use of pesticides, which also degrades soil quality and affects crop yield and seed germination [1,2,3,4]. To ensure the availability of food for the growing global population, overall production of crops will need to be substantially increased using less agricultural land under extremely harsh environmental conditions. Several approaches have been utilized for increasing crop yield and quality, such as genetic engineering, tissue culture, and breeding [5,6].
Strategies for the improvement of crop production against abiotic stresses include tissue culture, breeding, genetic engineering, grafting, and plant-growth-promoting bacteria, among other strategies. To create a new genotype of a plant species with improved traits, such as the physiological foundation for salinity tolerance, tissue culture techniques have been utilized. Plant tissue culture has been recognized as one of the most effective methods to enhance horticulture plants under various challenging conditions. Genetic engineering is one of the key elements to preventing harm due to water scarcity and irrigation with salinized water [7,8,9,10,11].
The incorporation of endophytic microbes is thought to be an inexpensive, quick, climate-smart, and environmentally friendly alternative strategy for enhancing crop productivity and quality under climate change [12]. Endophytic microbes have the potential to be used as an option for plant health management in horticultural crops [13]. Several product quality characteristics like color, size, shelf life, and firmness may also be positively influenced by endophyte application [14].
Endophytes boost growth and development in plants without any adverse effects. They amplified siderophores synthesis and provided vitamins in nutrient-deficient circumstances [15]. They are microscopic microorganisms that live inside plant tissues, and they do not infect their hosts [16]. Bacterial endophytes colonize a wide range of plants, including numerous horticultural crops, especially fruits, flowers, and medicinal plants. Endophytes can decrease infections by improving host defense, lysis, antibiosis, and siderophore synthesis. Numerous microorganisms synthesize metabolites that have antibacterial activity and become beneficial to prevent plant diseases [17,18]. Endophytic microorganisms are now well acknowledged to be ubiquitous, and there is significant interest in the potential applications of such organisms in crop production due to their ability to promote plant growth and help plants tolerate stressful conditions [14].
The physiological impact of valuable microbial endophytes on the host plant has been connected to several factors such as the formation of phytohormones, several antioxidant and biologically active compounds, and osmo-protective compounds [19,20]. For example, the Bacillus sp. species synthesizes lipopeptides like bacillomycin, iturin, and fengycin, which demonstrate antibacterial and antifungal activities [21]. Messenger molecules also trigger comprehensive defense against biotic and abiotic stresses, photosynthesis, stomatal conductance, water status, and the synthesis of phytohormones and their accumulation in plants [22,23,24]. In the case of abiotic stresses, Burkholderia sp., Arthrobacter sp., and Bacillus sp. elevate proline production and possess heat shock proteins (HSPs) that are synthesized by thermotolerant rhizobacteria and thermophilic bacterial endophytes. In these species, cold-tolerant bacteria in the rhizosphere, rhizoplane, and endo-rhizosphere also synthesize cryoprotective proteins in response to low temperatures and produce of antimicrobial compounds like siderophores, antibiotics, hydrolytic enzymes, and other secondary metabolites against biotic stresses [25,26,27,28].
Endophytic bacteria stimulate plant growth through various mechanisms, including nitrogen fixation, plant growth stimulation via synthesis and modulation of plant hormones, and synthesis of bioactive compounds against phytopathogens [29]. Furthermore, endophytes offer a sustainable alternative to conventional agricultural practices, minimizing the need for synthetic pesticides and fertilizers and, in turn, lowering the risks related to chemical treatments [30].
Strigolactones (SLs) are molecules derived from carotenoids that play an important role in regulating plant development and adaptation. They affect various growth processes, including root development, shoot branching, and leaf senescence. In response to several environmental stresses such as salinity, drought, heat, cold, heavy metals, and nutrient deprivation, SLs build up in plant tissues and improve root architecture. They have the potential to be utilized to develop genetically engineered crops that exhibit increased tolerance to various stresses, potentially aiding in resolving the worldwide scarcity of food grains [31,32].
2. Potential Uses of Microbial Endophytes in Horticulture
2.1. Microbial Endophytes and Their Function
Plant-associated bacteria known as endophytes reside inside the stems, leaves, roots, and different inner cells of plants without producing any disease or harm [33]. A large percentage of soils are rich in microorganisms, some of which are plant-growth-promoting microbes (PGPMs). These microbes are essential partners, supporting several critical tasks that determine the physiological state of hosts, their ability to withstand stress, and the quantity and quality of their crops. These days, PGPMs are receiving a great deal of attention because of their use as bioinoculants [34,35,36].
PGPMs are capable of facilitating growth in plants and stimulating defense mechanisms in the plant against biotic or abiotic stress without causing any harm. Endophytes enhance crop yield, biometric qualities, pathogenic revulsion, plant growth, synthesis of bioactive compounds (mostly from medicinal herbal drugs), and the development of systemic resistance. Endophytes increase the accessibility of intricate environmental micro- and macronutrients [37,38].
Endophytes contain fungi, bacteria, and archaea (the most prevalent and well-researched taxa). Although endophytes live and feed on plants, they cause immunological responses in plants, which help plants to survive a variety of biotic and abiotic challenges. Plants detect the presence of endophytes which are benign, although some are closely linked to plant diseases. In response, the plants produce numerous proteins, chemical compounds, and hormones that provide them with tolerance to pathogenic organisms, herbivores, and environmental stressors [39].
Several classes, such as Acidobacteria, Deinococcus-thermus, Verrucomicrobia, Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria, etc., are home to endophytic microbes. Bacillus, Burkholderia, Pseudomonas, Streptomyces, and Klebsiella are the most common genera and are effective against plant pathogens and abiotic stresses [40,41,42,43]. Endophytes can be further categorized, as shown in Figure 1 [44].
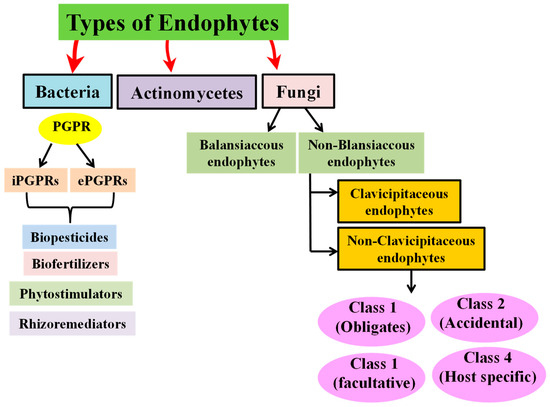
Figure 1.
Types of endophytes based on functions and their hosts.
2.2. Mechanism of Action of Endophytes
The mode of action of endophytes—promoting plant growth and development—is well known and understood. Endophytes improve a plant’s condition by obstructing the growth and development of different plant-parasitic nematode (PPN) stages via many methods. Endophytic microorganisms improve plant fitness through a variety of mechanisms. The mode of action of endophytes includes direct and indirect mechanisms [45,46].
2.2.1. Direct Mechanism
Endophytes activate a defense mechanism against PPNs. Plants produce various secondary metabolites and suppress the growth and development of PPNs. For example, F. oxysporum is isolated from bananas, paralyzes, and kills P. goodeyi [47,48]. Endophytes can directly benefit plants by synthesizing iron chelators, phosphate-solubilizing chemicals, antimicrobial metabolites, and nitrogen-fixing abilities [49]. Additionally, a number of sulfur-oxidizing endophytes have been identified that convert elemental sulfur into sulfate so that plants can use it. They also stimulate the secretion of lytic enzymes, phosphate solubilization, antibiosis, and siderophore and phytohormone production. Some endophytes cause thickening of the endodermal cell wall, which decreases the likelihood of pests penetrating the stele. Endophytes also play a crucial role in synthesis of phytohormones, including auxins and gibberellins, which promotes cell proliferation and elongation [49,50,51,52].
2.2.2. Indirect Mechanism
Systemic resistance is induced by endophytes through the upregulation of genes that produce a variety of phytohormones, phytoalexins, and volatile organic compounds and initiate pathways for acids, such as salicylic acid and ethylene, which safeguard plants against stressful circumstances. For example, the application of Rhizobium etli G12 and Bacillus sphaericus B43 encouraged systemic resistance to Globodera pallida in potato; M. incognita had the same effect in tomato [53,54].
Endophytes stimulate plant growth under stress conditions by producing secondary metabolites that possess antifungal, antiviral, and antibacterial properties [55,56,57,58]. It was noted that the induction of endophytic bacteria B. thuringiensis B-5351 results in a reduction of late blight in potatoes. The use of endophytic B. subtilis-based formulations increased the formation of proteinase inhibitors and minimized illnesses in sugar cane plants, increasing both the quality and quantity of vegetable roots [59,60]. The majority of PGPMs possess the capacity to synthesize substances like polymyxin, circulin, and colistin. These substances restrict the growth of harmful fungi and bacteria, which synthesize substances called bacteriocins and hinder cell functions [61,62].
As a crucial defense against a variety of stressors, endophytes have the ability to both synthesize and regulate phytohormone levels in plants [63]. Root system design could be altered as a consequence of endophyte-induced alterations in endogenous ABA and IAA [64]. Certain microbial endophytes can modify the amount of ethylene present in the soil, which improves the host plant’s resistance to abiotic stressors and diseases. This is known as a stress hormone and can set off a variety of defensive responses [65,66].
2.3. Endophytes as Bionematicides
Plant-parasitic nematodes (PPNs) include cyst nematodes (Globodera spp. and Heterodera spp.), root lesion nematodes (Pratylenchus spp.), and root-knot nematodes (Meloidogyne spp.) are major pests that affect many crops globally and result in major losses in yields [67]. They mostly target the roots by creating feeding sites, including coenocytes, syncytia, solitary giant cells, and non-hypertrophied nurse cells, that offer a protected feeding environment [68].
PPN treatment results in low production, yellowing of leaves, plant stunting, and deformation of roots. There are numerous cases of nematode-suppressing soils where the presence of fungi and bacteria suppresses PPN numbers [69]. These beneficial organisms produce poisons or use specific trapping structures to prevent PPNs from proliferating. Most endophytes synthesize secondary metabolites with pesticidal effects and have the potential to be good candidates for bionematicides [70,71]. Table 1 lists the endophytes as microbial agents for various horticulture crops.

Table 1.
Endophytes are utilized as microbial control agents of PPNs in many horticulture crops.
2.4. Use of Metabolites from Endophytes
These bacteria include various species of Bacillus and Pseudomonas. Notably, lipopeptides produced by non-ribosomal peptide synthetases are essential for rhizosphere bacteria in antibiosis and for inducing plant defense mechanisms [95,96]. Endophytes produce several secondary metabolites that possess biopesticidal activity against several plant parasite nematodes and plant pathogens that cause resistance in plants against numerous abiotic and biotic stressors [97]. Endophytes of Chinese medicinal plants have been found to produce various metabolites, including HKI0595 from the mangrove tree Kandelia candel, antitrypanosomal alkaloids spoxazomicins A-C from the endophytic actinomycete Streptosporangium oxazolinicum in orchids, and several NRPS (non-ribosomal peptide synthetase) and PKS (polyketide synthetase) gene clusters with uncharacterized metabolites. These spoxazomicins share structural similarities with siderophores from Pseudomonas aeruginosa [98,99,100]. Some metabolites possess nematicidal properties, as mentioned in Table 2.

Table 2.
Secondary metabolites in endophytes and their impact on plant-parasitic nematodes.
3. Nanotechnology in Crop Production
There is growing interest in the usage of nanomaterials (NMs), which can lower costs, increase the quantity and quality of horticultural crops, and minimize the harmful effects of conventional pesticides [110,111,112]. In the late 2000s, crop production and nanotechnologies were initially addressed by the new technological revolution that the human race experienced [113]. Sustainable horticultural crops can be achieved by using nanotechnologies as an alternative to traditional technologies. Both biological and chemical processes are used to create NMs [114]. The use of nanomaterials in agriculture appears to be crucial for raising output, improving product quality, and lowering post-harvest fruit and vegetable losses. Up to 30% of horticultural crop products are thrown away, mainly due to biological processes and microbial degradation. The chemical approach is more costly and involves chemical-reducing agents. When compared to chemical nanoparticles (NPs), biological NPs are safer choices. Biogenic nanoparticles possess the ability to self-assemble and possess morphological control systems [115]. For example, ZnO nanoparticles enhance the yield of peanuts (Arachis hypogea). Similarly, the application of SiO2 nanoparticles increases plant biomass and the levels of biomolecules like chlorophyll, proteins, and phenols in maize grains. At low concentrations, carbon nanotubes promote the growth of hexaploidy wheat roots and promote seed growth and seed germination in mustard (Brassica juncea), tobacco (Nicotiana tabacum) (cell growth increase of 16%), black gram (Phaseolus mungo), and rice (Oryza sativa) [14].
In horticulture, nanofertilizers are employed to boost vegetative growth, pollination, and flower fertility, leading to increased yields and improved fruit tree product quality. Similarly, spraying nano-boron on the leaves of mango trees positively affects the overall yield and chemical properties of the fruits. This improvement is likely due to the increased chlorophyll content and essential nutrients, such as nitrogen (N), phosphorus (P), potassium (K), manganese (Mn), magnesium (Mg), boron (B), zinc (Zn), and iron (Fe), in the leaves. Exogenous nano-Ca supplementation on blueberries under saline stress conditions leads to increased vegetative growth and higher chlorophyll content in the leaves [36]. The application of nano-boron and nano-zinc fertilizers enhances fruit quality, increases fruit count, boosts the ratio of total soluble sugars (TSS) and the maturity index, and raises the levels of total sugars and total phenols in pomegranates. Spraying mango trees with nano-zinc increases fruit weight, fruit number, yield, leaf chlorophyll and carotene content, and concentrations of several nutrients, including N, P, K, and Zn [14]. Nanotechnology uses different approaches for improving crop yield, as shown in Figure 2.
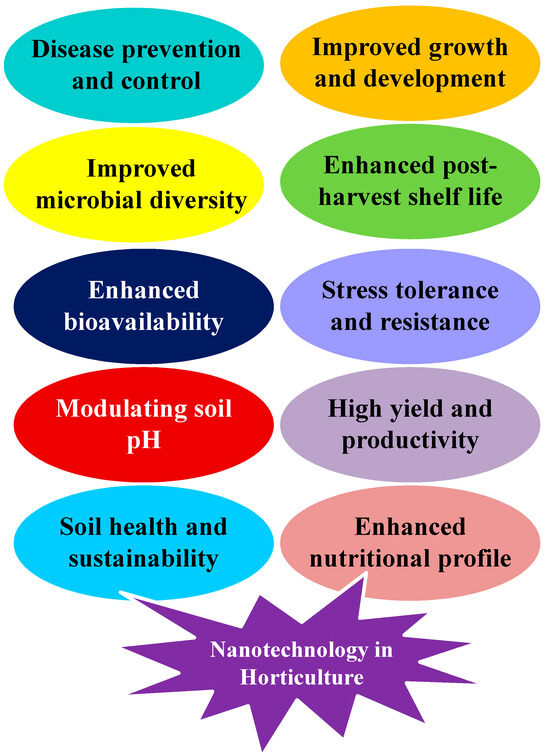
Figure 2.
Several applications of nanotechnology in horticulture.
In horticulture, the use of nanomaterials helps plants to deal with environmental stress in several ways: (i) supplies nutrients an emulsion or nanoparticles of nanoscale measurements; (ii) envelopes the plant in a thin protective layer of polymer; (iii) coats the plants with nanoparticles in the form of nanoporous materials or nanotubes. They also enhance the solubility and coverage of hydrophobic leaf surfaces. Nanoparticles like silver, silicon, and copper are used as nano-biofertilizers. They are used to supply nutrients to the plants. Based on their distribution and function, biofertilizers are categorized into micronutrient nanofertilizers and macronutrient nanofertilizers [113]. Several effects of nanoparticles on crop growth, development, and yield are mentioned in Table 3.

Table 3.
Effect of NPs on growth, development, and yield of horticultural crops.
Nanofertilizers are used in horticulture to improve floral fertility, pollination, and vegetative growth, which increases productivity and improves the quality of the final product. When blueberries are grown under saline stress, external incorporation of nano-Ca increases both vegetative development and the amount of chlorophyll in the leaf [130,131,132].
4. Application of Strigolactone in Horticulture
Plant hormones play a major part in controlling all of the complicated chemical processes that regulate a plant’s growth and development. Over the past few years, a class of novel plant hormones known as strigolactones (SLs) has been identified. In 1996, Strigol, the first SL, was isolated from the root exudates of cotton. The most noticeable biological activity of strigol is its capability germinating seeds of parasitic weeds Orobanche spp. and Striga [133]. This class of phytohormones is involved in several biological processes, such as the beginning of plant–fungal symbiosis and the germination of parasitic plants, which are extremely dangerous for agriculture [134].
Many plant species produce SLs, but angiosperms produce them in large quantities. The pathway of metabolism of abscisic acid (ABA) and the synthesis of SLs are the same. Furthermore, biotic or abiotic stressors can regulate them. It is beneficial to combine them with agricultural food or manufacturing pharmaceuticals using these chemical compounds [135].
It was discovered that SLs impair both shoot and bud branching and act as a branching element of arbuscular mycorrhizal (AM) fungus. In a broader sense, they are essential for the regulation of plant development. Because of this, SLs are currently regarded as a novel family of plant hormones with a promising future. SLs, in addition to cytokines and auxins, can control the amount of chlorophyll, the growth of plant parts, as well as the overall process of photosynthesis. So, in addition to producing the maximum yield, varieties produced through molecular genetic techniques modify the synthesis of SLs because of their soil stability, and they can be used to generate new varieties of plants. As an example, the growth of onions (Allium cepa L.) is significantly facilitated by forming a mixture of synthetic SL along with a macerate of carrots in a combination of surfactants with additional citric acid [136,137,138]. SLs promote plant growth and development in multiple ways (Figure 3).
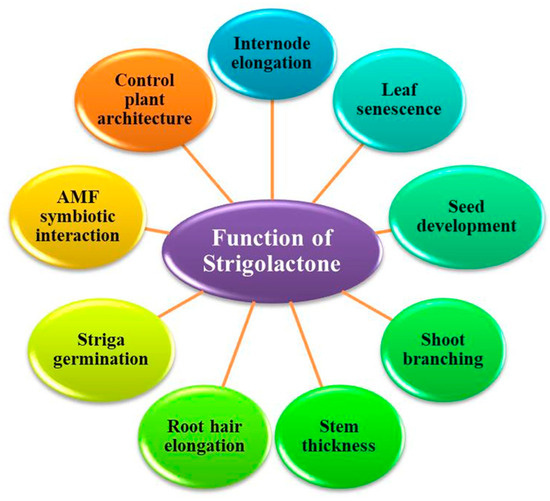
Figure 3.
Functions of Strigolactone.
It is well established that interaction between SLs and auxins inhibits the development of lateral branches in tomato seedlings, their nutrient consumption, and their production. SLs control secondary growth and enhance biomass in plants, especially those that are grown to obtain wood [139]. GR24, a synthetic SL analog, is frequently used in research on plant hormone regulation. Exogenous GR24 application suppresses the growth of potato tubers and stolon buds, which reduces tuber formation [140]. In contrast, potato plants (Solanum tuberosum L.) exhibit reduced height, increased primary and lateral branching, and improved branch growth when the essential strigolactone biosynthetic gene CCD8 is deleted [141].
Fruits, vegetables, and flowers have a limited shelf life, and it is crucial to monitor their post-harvest handling. SLs can also be used for the storage of fruits and vegetables. They enhance the functioning of antioxidants and phenylpropanoid metabolism to preserve the quality of soft strawberry (Fragaria × ananassa Duch., cv. Akihime) fruits during storage [142]. The administration of exogenous SLs increases the concentration of photosynthetic pigments and photosynthesis while having a beneficial impact on water content and homeostasis of ions [143,144]. In horticultural plants like cucumbers (Cucumis sativus L.) and tomatoes, GR24 treatment enhances the expression of genes and activity of antioxidant enzymes along with the content and effectiveness of non-enzymatic antioxidants [145].
5. CRISPR Technology in Horticultural Crop Production
It is necessary to improve the nutritional quality of horticulture food crops to meet the growing demand for such crops (Figure 4). Through the precise alteration of specific genomes, gene editing methods, including transcription activator-like effector nucleases, zinc finger nucleases, and clustered regularly interspaced short palindromic repeats (CRISPRs/CRISPR-associated 9 (Cas9)), have accelerated the advancement of agriculture [146]. For instance, white button mushrooms (Agaricus bisporus) resistant to browning have been produced, novel waxy corn types have been produced, and rice (Oryza sativa) pyl1/4/6 variants have been produced which showed significant development and increased crop yields using CRISPR technology [147]. CRISPR/SpCas9 is utilized in numerous horticultural plants, including the tomato (Solanum lycopersicum), cucumber (Cucumis sativus), cabbage (Brassica oleracea var. capitata), apple (Malus domestica), grapefruit (Citrus paradisi), and Dendrobium officinale [148,149,150]. According to Zhang et al., the delivery strategy, sgRNA target sequences, Cas9 variant proteins, and promoters driving both sgRNA and Cas9 can all have an impact on the efficacy of CRISPR/SpCas9, which differs among species. The CRISPR/Cas9 systems mediated by Agrobacterium have made the most impressive advancements in horticultural crop quality improvement [144]. Table 4 shows CRISPR technology for the improvement of horticultural crops.
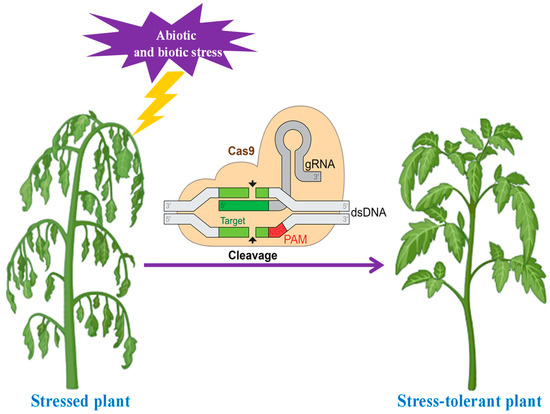
Figure 4.
CRISPR technology showing positive effects for producing plants resistant to various biotic and abiotic stresses.

Table 4.
CRISPR technology is used for horticultural crop improvement.
6. Conclusions
Horticulture is an important sector of the economy that affects many small and large farmers’ livelihoods and is vital to the fight against global poverty. It is still necessary for horticulture to keep improving to maximize safety precautions, boost output, and guarantee food safety and quality, especially in light of changing environmental conditions. This article explores several low-cost, quick, eco-friendly, and effective techniques that are essential for productive horticulture crop production. Notably, the external use of strigolactones, nanoparticles, and beneficial strains of endophytic microorganisms shows great promise in replacing specific agrochemicals. These developments have the potential to improve crop output, overall quality, and plant resilience by acting as safe, natural ingredients in biofertilizers and plant protection solutions. Moreover, CRISPR-based genetic editing has started to be used for the production of horticulture crops. By gradually implementing these methods, the use of agrochemicals in horticulture practices may be reduced, which will lessen their detrimental effects on biodiversity, soil fertility, and human health. These strategies represent a significant step forward in creating a more sustainable and resilient horticultural sector, ensuring a better future for farmers, consumers, and the environment alike.
Author Contributions
D.S. and B.R.: writing—original draft preparation; A.D.: data curation, resources; D.J.: writing—review and editing, visualization; D.B. and G.K.: conceptualization, investigation, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Van Meeteren, U. Natural genetic variation in stomatal response can help to increase acclimation of plants to dried environments. Acta Hortic. 2016, 1190, 71–76. [Google Scholar] [CrossRef]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of 7-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Tanveer, M. Salt and Drought Stress Tolerance in Plants Signaling Networks and Adaptive Mechanisms: Signaling Networks and Adaptive Mechanisms; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Athar, H.U.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Helaly, A. Strategies for Improvement of Horticultural Crops against Abiotic Stresses. J. Hortic. 2017, 4, 1–2. [Google Scholar]
- Xiao, B.; Huang, Y.; Tang, N.; Xiong, L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor. Appl. Genet. 2007, 115, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, K.; Takabe, T.; Takabe, T.; Kishitani, S. Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation of their tolerance to abiotic stress. Ann. Bot. 2006, 98, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Venema, J.H.; Dijk, B.; Bax, J.; van Hasselt, P.R.; Elzenga, J.T. Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot. 2008, 63, 359–367. [Google Scholar] [CrossRef]
- Suchoff, D.H.; Perkins-Veazie, P.; Sederoff, H.W.; Schultheis, J.R.; Kleinhenz, M.D.; Louws, F.J.; Gunter, C.C. Grafting the indeterminate tomato cultivar moneymaker onto multifort rootstock improves cold tolerance. HortScience 2018, 53, 1610–1617. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xue, T.T.; Gao, F.F.; Zhang, L.; Han, X.; Wang, Y.; Hui, M.; Wu, D.; Li, H.; Wang, H. Intraspecific recurrent selection in V. vinifera: An effective method for breeding of high quality, disease-, cold-, and drought-resistant grapes. Euphytica 2021, 217, 111. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Bora, L.C.; Begum, M. Eco-friendly management of soil borne diseases in brinjal through application of antagonistic microbial population. J. Biol. Control. 2013, 27, 29–34. [Google Scholar]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world–bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Bora, L. Disease management in horticulture crops through microbial interventions: An overview. Indian J. Agric. Sci. 2020, 90, 1389–1396. [Google Scholar] [CrossRef]
- Garipova, S.R. Prospects of using endophytic bacteria for bioremediation of arable soils polluted by residual amounts of pesticides and xenobiotics. Uspekhi. Sovremennoi. Pleiades Publishing, Ltd. Biol. Bull. Rev. 2014, 4, 300–310. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, T.; Hussain, T.; Ali, F.; Shi, F.; Latef, A.A.H.A.; Ali, O.M.; Hayat, K.; Mehmood, S. Halotolerant-Koccuria rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na+ uptake and elevating production of antioxidants. Agronomy 2021, 11, 1907. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, Y.; Zhang, J.; Wan, S.; Huang, Y.; Yun, T.; Xie, J.; Wang, W. Biocontrol potential of endophytic Streptomyces malaysiensis 8ZJF-21 from medicinal plant against banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense tropical race 4. Front. Plant Sci. 2022, 11, 874819. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Szyma ’nska, S.; Tyburski, J.; Piernik, A.; Sikora, M.; Mazur, J.; Katarzyna, H. Raising beet tolerance to salinity through bioaugmentation with halotolerant endophytes. Agronomy 2020, 10, 1571. [Google Scholar] [CrossRef]
- Neelipally, R.T.K.R.; Anoruo, A.O.; Nelson, S. Effect of Co-inoculation of Bradyrhizobium and Trichoderma on growth, development, and yield of Arachis hypogaea L. (peanut). Agronomy 2020, 10, 1415. [Google Scholar] [CrossRef]
- Kaushal, M. Microbes in cahoots with plants: MIST to hit the jackpot of agricultural productivity during drought. Int. J. Mol. Sci. 2019, 20, 1769. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.; Muleta, D.; Assefa, F.; Kiros, S. Field application of rhizobial inoculants in enhancing faba bean production in acidic soils: An innovative strategy to improve crop productivity. Salt Stress Microbes Plant Interact. Causes Solut. 2019, 1, 147–180. [Google Scholar]
- Maitra, S.; Pramanick, B.; Dey, P.; Bhadra, P.; Shankar, T.; Anand, K. Thermotolerant soil microbes and their role in mitigation of heat stress in plants. Soil Microbiomes Sustain. Agric. Funct. Annot. 2021, 27, 203–242. [Google Scholar]
- Sheikh, A.A.; Rehman, N.Z.; Kumar, R. Diverse adaptations in insects: A review. J. Entomol. Zool. Stud. 2017, 5, 343–350. [Google Scholar]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial Endophytes: The Hidden Actor in Plant Immune Responses against Biotic Stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Watts, D.; Palombo, E.A.; Jaimes Castillo, A.; Zaferanloo, B. Endophytes in Agriculture: Potential to Improve Yields and Tolerances of Agricultural Crops. Microorganisms 2023, 11, 1276. [Google Scholar] [CrossRef]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 28. [Google Scholar] [CrossRef]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.N.; Akhtemova, G.A.; Zhukov, V.A.; Tikhonovich, I.A. Endophytic microorganisms in fundamental research and agriculture. Ecol. Genet. 2019, 17, 19–32. [Google Scholar] [CrossRef]
- Olmo, R.; Wetzels, S.U.; Armanhi, J.S.L.; Arruda, P.; Berg, G.; Cernava, T.; Cotter, P.D.; Araujo, S.C.; de Souza, R.S.C.; Ferrocino, I.; et al. Microbiome research as an effective driver of success stories in agrifood systems—A selection of case studies. Front. Microbiol. 2022, 13, 834622. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–383. [Google Scholar]
- Gamalero, E.; Bona, E.; Glick, B.R. Current techniques to study beneficial plant-microbe interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, A.K. Endophytic Fungi as sources of novel natural compounds. In Plant Mycobiome: Diversity, Interactions and Uses; Rashad, Y.M., Baka, Z.A.M., Moussa, T.A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 339–373. [Google Scholar]
- Sharma, D.; Gahtyari, N.C.; Chhabra, R.; Kumar, D. Role of microbes in improving plant growth and soil health for sustainable agriculture. In Advances in Plant Microbiome and Sustainable Agriculture: Diversity and Biotechnological Applications; Yadav, A.N., Rastegari, A.A., Yadav, N., Kour, D., Eds.; Springer: Singapore, 2020; pp. 207–256. [Google Scholar]
- Sikora, R.A.; Schafer, K.; Dababat, A.A. Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. Aust. Plant Pathol. 2007, 36, 124–134. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of Endophytic Bacillus Subtilis and Salicylic Acid on Postharvest Diseases (Phytophthora infestans, Fusarium oxysporum) Development in Stored Potato Tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Kasnak, C.; Palamutoglu, R.; Shpirnaya, I.; Mardanshin, I.; Maksimov, I. Improving the biocontrol potential of endophytic bacteria Bacillus subtilis with salicylic acid against Phytophthora infestans-caused postharvest potato tuber late blight and impact on stored tubers quality. Horticulturae 2022, 8, 117. [Google Scholar] [CrossRef]
- Ameen, M.; Mahmood, A.; Sahkoor, A.; Zia, M.A.; Ullah, M.S. The Role of Endophytes to Combat Abiotic Stress in Plants. Plant Stress. 2024, 15, 100435. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Schouten, A. Mechanisms involved in nematode control by endophytic fungi. Annu. Rev. Phytopathol. 2016, 54, 121–142. [Google Scholar] [CrossRef] [PubMed]
- De Lamo, F.J.; Takken, F.L.W. Biocontrol by Fusarium oxysporum Using Endophyte-Mediated Resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, K. Exploring the potential of endophytes in agriculture: A minireview. Adv. Plants Agric. Res. 2017, 6, 00221. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.R.; Yesmin, L. Sulfur–Oxidizing Plant Growth Promoting Rhizobacteria for Enhanced Canola Performance; Google Patents: San Francisco, CA, USA, 2009. [Google Scholar]
- Gwinn, K.D.; Bernard, E.C. Interactions of endophyte infected grasses with the nematodes Meloidogyn marylandi and Pratylenchus scribneri. In Proceedings of the 2nd international symposium Acremonium/grass interact Plenary Papers, Plamerston North, New Zealand, 25–28 March 1993. [Google Scholar]
- Hasky-Gunther, K.; Sikora, R.A. Induced resistance: A mechanism induced systemically throughout the root system by rhizosphere bacteria towards the potato cyst nematode Globodera pallida. Nematologica 1995, 41, 306. [Google Scholar]
- Schafer, K. Dissecting Rhizobacteria-Induced Systemic Resistance in Tomato against Meloidogyne incognita: The First Step Using Molecular Tools. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universitat Bonn, Bonn, Germany, 2007. [Google Scholar]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic analysis and secondary metabolites production of the endophytic Bacillus velezensis Bvel1: A biocontrol agent against Botrytis cinerea causing Bunch Rot in post-harvest table grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, D.; Jin, R.; Li, E.; Li, P. Bioactivities evaluation of an endophytic bacterial strain Bacillus velezensis JRX-YG39 inhabiting wild grape. BMC Microbiol. 2022, 22, 170. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I.V. By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato Virus X and potato Virus Y. BioMolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Abdalla, S.A.; Sleem, M.M. Endophytic Bacillus subtilis SR22 triggers defense responses in tomato against rhizoctonia root rot. Plants 2022, 11, 2051. [Google Scholar] [CrossRef]
- Pusenkova, L.I.; Il’yasova, E.Y.; Lastochkina, O.V.; Maksimov, I.V.; Leonova, S.A. Changes in the species composition of the rhizosphere and phyllosphere of sugar beet under the impact of biological preparations based on endophytic bacteria and their metabolites. Eurasian Soil Sci. 2016, 49, 1136–1144. [Google Scholar] [CrossRef]
- Lastochkina, O.V.; Pusenkova, L.I.; Il’yasova, E.Y.; Aliniaeifard, S. Effect of Bacillus subtilis based biologicals on physiological and biochemical parameters of sugar beet (Beta vulgaris L.) plants infected with Alternaria alternata. Agrobiology 2018, 53, 958–968. [Google Scholar]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef]
- Xu, G.; Yang, S.; Meng, L.; Wang, B.G. The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef]
- Lastochkina, O.V. Adaptation and tolerance of wheat plants to drought mediated by natural growth regulators Bacillus spp.: Mechanisms and practical importance. Agric. Biol. 2021, 56, 843–867. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Lugtenberg, B. Biotechnological applications of bacterial endophytes. Curr. Biotechnol. 2014, 3, 60–75. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Palomares-Rius, J.E.; Escobar, C.; Cabrera, J.; Vovlas, A.; Castillo, P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front. Plant Sci. 2017, 8, 1987. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Aissani, N.; Demurtas, M.; Ntalli, N.; Onnis, V. Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita, and structure–activity considerations. Pest Manag. Sci. 2016, 72, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.-Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N. Endophytic fungi for plant growth promotion and adaptation under abiotic stress conditions. Acta Sci. Agric. 2019, 3, 91–93. [Google Scholar]
- Kumar, K.K.; Dara, S.K. Fungal and Bacterial Endophytes as Microbial Control Agents for Plant-Parasitic Nematodes. Int. J. Environ. Res. Public Health 2021, 18, 4269. [Google Scholar] [CrossRef]
- Niere, B.I. Significance of Non-Pathogenic Isolates of Fusarium oxysporum Schlecht: Fries for the Biological Control of the Burrowing Nematode Radopholus similis (Cobb) Thorne on Tissue Cultured Banana. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2001. [Google Scholar]
- Dubois, T.; Gold, C.S.; Coyne, D.; Paparu, P.; Mukwaba, E.; Athman, S.; Kapindu, S.; Adipala, E. Merging biotechnology with biological control: Banana Musa tissue culture plants enhanced by endophytic fungi. Uganda J. Agric. Sci. 2004, 9, 445–451. [Google Scholar]
- Mendoza, A.R.; Sikora, R.A. Biological control of Radopholus similis in banana by combined application of the mutualistic endophyte Fusarium oxysporum strain 162, the egg pathogen Paecilomyces lilacinus strain 251 and the antagonistic bacteria Bacillus firmus. Biocontrol 2009, 54, 263–272. [Google Scholar] [CrossRef]
- Pocasangre, L. Biological Enhancement of Banana Tissue Culture Plantlets with Endophytic Fungi for the Control of the Burrowing Nematode Radopholus similis and the Panama Disease (Fusarium oxysporum f. sp. cubense). Ph.D. Thesis, University of Bonn, Bonn, Germany, 2000. [Google Scholar]
- Felde, A.Z.; Pocasangre, L.E.; Carnizares Monteros, C.A.; Sikora, R.A.; Rosales, F.E.; Riveros, A.S. Effect of combined inoculations of endophytic fungi on the biocontrol of Radopholus similis. InfoMusa 2006, 15, 12–18. [Google Scholar]
- Mwaura, P.; Dubois, T.; Losenge, T.; Coyne, D.; Kahangi, E. Effect of endophytic Fusarium oxysporum on paralysis and mortality of Pratylenchus goodeyi. Afr. J. Biotechnol. 2010, 9, 1130–1134. [Google Scholar]
- Van Dessel, P.; Coyne, D.; Dubois, T.; Waele, D.D.; Franco, J. In vitro nematicidal effect of endophytic Fusarium oxysporum against Radopholus similis, Pratylenchus goodeyi and Helicotylenchus multicinctus. Nematropica 2011, 41, 154–160. [Google Scholar]
- Su, L.; Shen, Z.; Ruan, Y.; Tao, C.; Chao, Y.; Li, R.; Shen, Q. Isolation of antagonistic endophytes from banana roots against Meloidogyne javanica and their effects on soil nematode community. Front. Microbiol. 2017, 8, 2070. [Google Scholar] [CrossRef]
- Menjivar, R.D.; Hagemann, M.H.; Kranz, J.; Cabrera, J.A.; Dababat, A.A.; Sikora, R.A. Biological control of Meloidogyne incognita on cucurbitaceous crops by the non-pathogenic endophytic fungus Fusarium oxysporum strain 162. Int. J. Pest Manag. 2011, 57, 249–253. [Google Scholar] [CrossRef]
- Muhae-ud-Din, G.; Moosa, A.; Ghummen, U.F.; Jabran, M.; Abbas, A.; Naveed, M.; Jabbar, A.; Ali, M.A. Host status of commonly planted ornamentals to Meloidogyne incognita and management through endophytic bacteria. Pak. J. Zool. 2018, 50, 1393–1402. [Google Scholar] [CrossRef]
- Zhou, W.; Starr, J.L.; Krumm, J.L.; Sword, G.A. The fungal endophyte Chaetomium globosum negatively affects both above-and belowground herbivores in cotton. FEMS Microbiol. Ecol. 2016, 92, fiw158. [Google Scholar] [CrossRef] [PubMed]
- Padgham, J.L.; Sikora, R.A. Biological control potential and modes of action of Bacillus megaterium against Meloidogyne graminicola on rice. Crop Protect. 2007, 26, 971–977. [Google Scholar] [CrossRef]
- Le, H.T.T.; Padgham, J.L.; Sikora, R.A. Biological control of the rice rootknot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. Int. J. Pest Manag. 2009, 55, 31–36. [Google Scholar] [CrossRef]
- Le, H.T.T.; Padgham, J.L.; Hagemann, M.H.; Sikora, R.A.; Schouten, A. Developmental and behavioural effects of the endophytic Fusarium moniliforme Fe14 towards Meloidogyne graminicola in rice. Ann. Appl. Biol. 2016, 169, 134–143. [Google Scholar] [CrossRef]
- Trifonova, Z.; Tsvetkov, I.; Bogatzevska, N.; Batchvarova, R. Efficiency of Pseudomonas spp. for biocontrol of the potato cyst nematode Globodera rostochiensis (Woll.). Bulg. J. Agric. Sci. 2014, 20, 666–669. [Google Scholar]
- Hallmann, J.; Quadt-Hallmann, A.; Miller, W.G.; Sikora, R.A.; Lindow, S.E. Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology 2001, 91, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vetrivelkalai, P.; Sivakumar, M.; Jonathan, E.I. Biocontrol potential of endophytic bacteria on Meloidogyne incognita and its effect on plant growth in bhendi. J. Biopestic. 2010, 3, 452–457. [Google Scholar] [CrossRef]
- Dababat, A.E.F.A.; Sikora, R.A. Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion. Nematology 2007, 9, 771–776. [Google Scholar] [CrossRef]
- Martinuz, A.; Schouten, A.; Sikora, R.A. Post-infection development of Meloidogyne incognita on tomato treated with the endophytes Fusarium oxysporum strain Fo162 and Rhizobium etli strain G12. BioControl 2013, 58, 95–104. [Google Scholar] [CrossRef]
- Bogner, C.W.; Kariuki, G.M.; Elashry, A.; Sichtermann, G.; Buch, A.K.; Mishra, B.; Thines, M.; Grundler, F.M.W.; Schouten, A. Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycol. Progress 2016, 15, 30. [Google Scholar] [CrossRef]
- Hu, H.; Chen, Y.; Wang, Y.; Tang, Y.; Chen, S.; and Yan, S. Endophytic Bacillus cereus effectively controls Meloidogyne incognita on tomato plants through rapid rhizosphere occupation and repellent action. Plant Dis. 2017, 101, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Amin, N. The use of fungal endophytes Gliocladium spp. in different concentration to control of root-knot nematode Meloidogyne spp. Acad. Res. Int. 2014, 5, 91–95. [Google Scholar]
- Munif, A.; Hallmann, J.; Sikora, R.A. Evaluation of the biocontrol activity of endophytic bacteria from tomato against Meloidogyne incognita. Meded. Fac. Landbouwkund. EnToegepaste Biol. Wet. Univ. Gent. 2000, 65, 471–480. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- Ding, L.; Maier, A.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org. Biomol. Chem. 2011, 9, 4029–4031. [Google Scholar] [CrossRef]
- Inahashi, Y.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Matsumoto, A.; Shiomi, K. Spoxazomicins A–C, novel antitrypanosomal alkaloids produced by an endophytic actinomycete, Streptosporangium oxazolinicum K07-0460T. J. Antibiot. 2011, 64, 303–307. [Google Scholar] [CrossRef]
- Miller, K.I.; Qing, C.; Sze, D.M.; Neilan, B.A. Investigation of the biosynthetic potential of endophytes in traditional Chinese anticancer herbs. PLoS ONE 2012, 7, e35953. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. Nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Köpcke, B.; Weber, R.W.; Sterner, O.; Anke, H. 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry 2004, 65, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.R.; Son, S.W.; Han, H.R.; Choi, G.J.; Jang, K.S.; Choi, Y.H.; Lee, S.; Sung, N.-D.; Kim, J.C. Nematicidal activity of bikaverin and fusaric acid isolated from Fusarium oxysporum against pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol. J. 2007, 23, 318–321. [Google Scholar] [CrossRef]
- Koepcke, B.; Johansson, M.; Sterner, O.; Anke, H. Biologically active secondary metabolites from the ascomycete A111-95 1. Production, isolation and biological activities. J. Antibiot. 2002, 55, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, G.; Wang, X.; Pan, W.; Li, L.; Hua, L.; Liu, F.; Dang, L.; Mo, M.; Zhang, K. Nematicidal endophytic bacteria obtained from plants. Ann. Microbiol. 2008, 58, 569–572. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Rajput, N.A.; Ye, Y. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol. Lett. 2019, 366, 169. [Google Scholar] [CrossRef]
- Li, G.H.; Yu, Z.F.; Li, X.; Wang, X.B.; Zheng, L.J.; Zhang, K.Q. Nematicidal metabolites produced by the endophytic fungus Geotrichum sp. AL4. Chem. Biodivers. 2007, 4, 1520–1524. [Google Scholar] [CrossRef]
- Bogner, C.W.; Kamdem, R.S.T.; Sichtermann, G.; Matthäus, C.; Hölscher, D.; Popp, J.; Proksch, P.; Grundler, F.M.W.; Schouten, A. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb. Biotechnol. 2017, 10, 175–188. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. BB Rep. 2020, 24, 100821. [Google Scholar]
- Liu, C.; Zhou, H.; Zhou, J. The applications of nanotechnology in crop production. Molecules 2021, 26, 7070. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2017, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.A.; Siddiqui, M.N.; Skalicky, M.; Brestic, M.; Hossain, A.; Kayesh, E.; Popov, M.; Hejnak, V.; Gupta, D.R.; Mahmud, N.U.; et al. Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae 2021, 7, 332. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Parihar, P.; Singh, R.; Prasad, S.M. Carbon nanotubes as plant growth regulators: Impacts on growth, reproductive system, and soil microbial community. In Nanomaterials in Plants, Algae and Microorganism; Academic Press: Cambridge, MA, USA, 2019; pp. 23–42. [Google Scholar]
- Singh, N.B.; Jain, P.; De, A.; Tomar, R. Green synthesis and applications of nanomaterials. Curr. Pharm. Biotechnol. 2021, 22, 1705–1747. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Orre-Roche, R.D.L.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; White, J.C. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ. Sci. Technol. 2013, 47, 12539–12547. [Google Scholar] [CrossRef] [PubMed]
- Cańas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; da Silva, J.A.T. The effect of carbon nanotubes on the seed germination and seedling growth of four vegetable species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Yang, X.; Zhang, Y.; Hui, H.; Zhang, D.; Shu, J. Multi-walled carbon nanotubes enhanced the antioxidative system and alleviated salt stress in grape seedlings. Sci. Hortic. 2022, 293, 110698. [Google Scholar] [CrossRef]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Jhou, Z.; Yang, P. Influences of nanoTiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of nano anatase TiO2 on nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, M.H.; Nafady, N.A.; Khalaf, D.M. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agric. Ecosyst. Environ. 2016, 218, 163–177. [Google Scholar]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Plant. Sci. 2016, 7, 90. [Google Scholar] [CrossRef]
- Elatafi, E.; Fang, J. Effect of silver nitrate (AgNO3) and nano-silver (Ag-NPs) on Physiological characteristics of grapes and quality during storage period. Horticulturae 2022, 8, 419. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romany, S. Effects of chitosan-based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Silva, W.B.; Silva, G.M.C.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Chiralt, A.; Menzel, C.; Hernandez-García, E.; Collazo, S.; Gonzalez-Martinez, C. Use of by-products in edible coatings and biodegradable packaging materials for food preservation. In Sustainability of the Food System: Sovereignty, Waste, and Nutrients Bioavailability; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 101–127. [Google Scholar]
- Zagzog, O.A.; Gad, M.M.; Hafez, N.K. Effect of nano-chitosan on vegetative growth, fruiting and resistance of malformation of mango. Trends Hortic Res. 2017, 7, 11–18. [Google Scholar]
- Zahedi, S.M.; Karimi, M.; da Silva, J.A.T. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hort. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhadra, P. Mini Review on Strigolactones: Newly Discovered Plant Hormones. Biosc. Biotech. Res. Comm. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. The Many Models of Strigolactone Signaling. Trends Plant Sci. 2020, 25, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Aliniaeifard, S.; SeifiKalhor, M.; Bosacchi, M.; Maslennikova, D.; Lubyanova, A. Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects. Horticulturae 2022, 8, 910. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T. The tomato Carotenoid Cleavage Dioxygenase 8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef]
- Xu, M.; Xue, Z. Enhancement of the photosynthetic and removal performance for microalgae-based technologies by co-culture strategy and strigolactone induction. Bioresour. Technol. 2021, 339, 125579. [Google Scholar] [CrossRef] [PubMed]
- Kopta, T.; Antal, M. The influence of synthetic strigolactones and plant extracts on the morphological parameters of onion (Allium cepa). Adv. Hort. Sci. 2017, 31, 235–240. [Google Scholar]
- Kang, S.W. Developmental control of horticultural plants using strigolactone to improve marketability. Tsukuba J. Agric. For. 2017, 5, 1–8. [Google Scholar]
- Roumeliotis, E.; Kloosterman, B. The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 2012, 63, 4539–4548. [Google Scholar] [CrossRef]
- Pasare, S.A.; Ducreux, L.J.M. The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, Y. Strigolactone maintains strawberry quality by regulating phenylpropanoid, NO, and H2S metabolism during storage. Postharvest Biol. Technol. 2021, 178, 111546. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Wang, W.N.; Min, Z. Physiological and transcriptomic analysis of Cabernet Sauvginon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef] [PubMed]
- De Cuyper, C.; Fromentin, J. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2015, 66, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Pandey, D.K.; Goutam, U.; Kumar, V. CRISPR/Cas9-mediated genome editing is revolutionizing the improvement of horticultural crops: Recent advances and future prospects. Sci. Hortic. 2021, 289, 110476. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Li, Y. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol. Plant 2017, 10, 1575–1578. [Google Scholar] [CrossRef]
- Nishitani, C.; Hirai, N.; Komori, S.; Wada, M.; Okada, K.; Osakabe, K.; Yamamoto, T.; Osakabe, Y. Efficient genome editing in apple using a CRISPR/Cas9 system. Sci. Rep. 2016, 6, 31481. [Google Scholar] [CrossRef]
- Kui, L.; Chen, H.; Zhang, W.; He, S.; Xiong, Z.; Zhang, Y.; Yan, L.; Zhong, C.; He, F.; Chen, J.; et al. Building a genetic manipulation tool box for orchid biology: Identification of constitutive promoters and application of CRISPR/Cas9 in the orchid, Dendrobium officinale. Front. Plant Sci. 2017, 7, 2036. [Google Scholar] [CrossRef]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-frequency, precise modification of the tomato genome. Genome boil 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Filler Hayut, S.; Melamed Bessudo, C.; Levy, A.A. Targeted recombination between homologous chromosomes for precise breeding in tomato. Nat. Comm. 2017, 8, 1–9. [Google Scholar]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.B.; Li, C. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant 2020, 13, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, H.; Sun, C.; Li, Q.; Jiang, H.; Du, M.; Li, C.B.; Li, C. Efficient generation of pink-fruited tomatoes using CRISPR/Cas9 system. J. Genet Genom. 2018, 45, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.G.; Qi, Y.; Baranski, R. Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Res. 2018, 27, 25–38. [Google Scholar] [CrossRef]
- Charrier, A.; Vergne, E.; Dousset, N.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient targeted mutagenesis in apple and first time edition of pear using the CRISPR-Cas9 system. Front Plant Sci. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.; Gleave, A.P.; Allan, A.C. Mutagenesis of kiwifruit CENTRORADIALIS-like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Kwon, C.T.; Heo, J.; Lemmon, Z.H.; Capua, Y.; Hutton, S.F.; Van Eck, J.; Park, S.J.; Lippman, Z.B. Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 2020, 38, 182–188. [Google Scholar] [CrossRef]
- Wang, R.; Tavano, E.C.D.R.; Lammers, M.; Martinelli, A.P.; Angenent, G.C.; de Maagd, R.A. Re-evaluation of transcription factor function in tomato fruit development and ripening with CRISPR/Cas9-mutagenesis. Sci. Rep. 2019, 9, 1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Samsulrizal, N.H.; Yan, C.; Allcock, N.S.; Craigon, J.; Blanco-Ulate, B.; Ortega-Salazar, I.; Marcus, S.E.; Bagheri, H.M.; Perez Fons, L.; et al. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019, 179, 544–557. [Google Scholar] [PubMed]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.H.; Huang, S.; Yang, X. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Dai, C.; Luo, H.; Han, Y.; Liu, Z.; Kang, C. Reporter gene expression reveals precise auxin synthesis sites during fruit and root development in wild strawberry. J. Exp. Bot. 2019, 70, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kang, B.C.; Naing, A.H.; Bae, S.J.; Kim, J.S.; Kim, H.; Kim, C.K. CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 2020, 18, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Chen, K.; Zhu, H.; Zhang, R.; Zhang, H.; Li, B.; Gao, C. Fine-tuning sugar content in strawberry. Genome Biol. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef]
- Kusano, H.; Ohnuma, M.; Mutsuro-Aoki, H.; Asahi, T.; Ichinosawa, D.; Onodera, H.; Asano, K.; Noda, T.; Horie, T.; Fukumoto, K.; et al. Establishment of a modified CRISPR/Cas9 system with increased mutagenesis frequency using the translational enhancer dMac3 and multiple guide RNAs in potato. Sci. Rep. 2018, 8, 13753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).