Abstract
The commensal/pathogenic Escherichia coli affects humans and animals, being present in diverse environmental niches, possibly surviving due to its adaptation to transient plant hosts like crops, increasing the risk of foodborne diseases. E. coli interaction with the plant host remains unknown, particularly the impacts on photosynthesis. We hypothesize that E. coli influences the tomato transient host’s photosynthetic capacity. To validate this hypothesis, we exposed 57-day-old tomato plants (Solanum lycopersicum) to different inoculation conditions, namely, non-inoculated plants (negative control, C−); plants directly injected with E. coli SL6.1 (107 CFU/mL) (positive control, C+); plants irrigated one time with E. coli SL6.1 (107 CFU/mL); and plants chronically irrigated with E. coli SL6.1 (104 CFU/mL). No significant changes were observed in chlorophyll fluorescence, pigments’ contents, morphological aspects, and fruiting in all conditions. However, irrigated plants (chronically and one-time contaminated) had decreased stomatal conductance (gs, 31.07 and 34.42 mol m−2 s−1, respectively, vs. 53.43 and 48.08 mol m−2 s−1 in C− and C+, respectively), transpiration rate (E, 0.32 and 0.35 mol m−2 s−1 in chronically and one-time contaminated conditions vs. 0.57 and 0.48 mol m−2 s−1 in C− and C+, respectively), and a trend of increased intrinsic carboxylation (Ci, 384 and 361 ppm in chronically and one-time irrigated plants vs. 321 and 313 ppm in C− and C+, respectively). The one-time inoculated plants presented more severe effects than the remaining conditions, with lower net photosynthetic rate (PN, 0.93 vs. 3.94–5.96 μmol (CO2) m−2 s−1 in the other conditions), intrinsic water use efficiency (iWUE, 33.1 vs. 74.51–184.40 μmol (CO2)/mmol (H2O) in the chronically irrigated and the control plants), and intrinsic carboxylation efficiency (iCE, 0.003 vs. 0.012–0.022 μmol (CO2)/ppm in the remaining conditions). Our data support that some observed effects are similar to those associated with phytopathogenic bacteria. Lastly, we propose that the decrease in some parameters of gas exchange requires direct contact with the leaf/stomata, and is mainly observed for high concentrations of E. coli.
1. Introduction
In recent years, the consumption of fresh produce has increased notably due to its health benefits [1]. However, concerns are emerging over foodborne pathogens and antibiotic resistance, and their implications for food safety have garnered significant attention [2]. Among these pathogens, Escherichia coli stands out due to its prevalence in diverse environmental niches, including soil, water, the gastrointestinal tracts of humans and animals, and plants [3]. While commensal E. coli strains live in mutually beneficial relationships with their host and rarely cause disease, certain strains possess virulence factors such as diarrheagenic E. coli (DEC) that include enteroaggregative E. coli (EAEC), enterohemorrhagic/Shiga toxigenic E. coli (EHEC/STEC), or enterotoxigenic E. coli (ETEC), which pose significant risks to human health when introduced into the food chain [4]. Identifying the fresh produce contamination route is complex, as it can occur during production, harvest, and post-harvest stages. The main sources of contamination at the pre-harvest level are irrigation water, soil, and organic fertilizers. In contrast, during the post-harvest stage, contamination can occur at the storage and distribution stages [5,6].
Fresh produce disinfection methods can be applied at post-harvest stages; however, internalized human pathogens that penetrate plant tissue through wounds or natural plant openings can escape sanitization [1,7]. Of particular concern is the colonization of crops by E. coli strains exhibiting antibiotic resistance and virulence traits [8]. Clinically relevant antibiotic–resistant bacteria have been classified into concerning, serious, and urgent threats, with colistin-resistant/mcr-carrying E. coli being classified as urgent threats [9].
E. coli O157:H7 and Salmonella enterica ser. Typhimurium possess a type III secretion system (T3SS) that is related to adhesion or motility, which are important traits in their establishment on the leaf surface and critical for the initiation of the internalization process and eventual suppression of the innate immune response of plants [7,10,11]. However, and contrarily to S. enterica ser. Typhimurium, which can bypass the plant stomatal immune response, keeping the stomata open and entering the plant tissue, the ability of E. coli to overcome stomatal closure remains elusive [12]. Previous studies have reported that E. coli did not cause symptoms on Arabidopsis leaf tissue and, contrarily to the phytopathogen Pseudomonas syringae pv. tomato (Pst), which downregulated photosynthetic–related genes, E. coli inoculation did not lead to a significant genetic regulation [13]. On the other hand, another study reported that E. coli O157:H7 induced plant immunity by triggering stomatal closure and PR1 gene expression in Arabidopsis leaves, suggesting that plants may recognize and respond to some human pathogens more effectively than others [14]. Stomata play critical roles in multiple functions such as photosynthesis, transpiration, and plant innate immunity [15]. However, questions persist regarding whether E. coli colonization is transient or if it elicits physiological responses in the host plant, which can potentially influence host growth, development, or susceptibility to other stressors. Furthermore, its role in the stomata aperture further raises important questions considering the stomatal-immunity responses, namely, how this temporary host–E. coli interaction affects plant photosynthesis.
We hypothesize that E. coli, by possibly internalizing through stomata, will influence the tomato–transient host’s photosynthetic capacity. This work aimed to validate this and clarify the knowledge gap of if and how human pathogens like E. coli influence gas exchange parameters (and thus the photosynthetic performance). For that, we exposed tomato (Solanum lycopersicum) plants to different inoculation conditions and analyzed the chlorophyll a fluorescence and stomatal gas exchange parameters, as it represents a major commodity for the food industry worldwide according to the Food and Agriculture Organization (FAO) [16], including in South Europe, which includes top tomato-producing countries.
2. Materials and Methods
2.1. Bacterial Strain and Culture Preparation
Throughout the study, the mcr1-carrying E. coli SL6.1 strain was used in the plant and water inoculation experiments. To prepare the inoculation solutions, frozen bacteria were grown on a Tryptone Bile X-glucuronide agar (TBX) plate. After an incubation period of 24 h at 37 °C, a liquid culture was grown overnight at 37 °C with agitation from a single pure colony in 5 mL of Tryptic Soy Broth (TSB). After vortexing to resuspend the bacterial growth, the optical density at 600 nm (OD600) was determined to achieve a final concentration for each of the inoculation conditions.
2.2. Plant Growth and Inoculation Conditions
The plant assay took place in a growth chamber under greenhouse conditions, namely, under a 16 h:8 h (day:night) light photoperiod, with a 200 µmol m−2 s−1 of photosynthetically active radiation (PAR) (white fluorescent lamps, OSRAM, Munich, Germany), at 25 ± 1 °C. Seeds of S. lycopersicum L. cv ‘MicroTom’ were randomly chosen and disinfected in commercial bleach at a final concentration of 10%, followed by two consecutive washes in sterile distilled water. The seeds were then transferred to Petri dishes (11 × 11 cm) with Hoagland medium (pH = 5.7) with 1% agar (w/v), and then the plates were disposed vertically in the growth chamber. After ten days of germination, the seedlings were transferred to trays with peat–perlite and watered with ½-strength Hoagland solution (pH = 5.7) three times a week until the three-leaf stage was achieved. Once in this stage, the plantlets were transferred to 150 g pots with soil–perlite (140 g soil and 10 g perlite) and watered with ½-strength Hoagland solution (pH = 5.7) three times a week. Throughout the entire study, tomato plants were watered with this solution except when described otherwise.
After 57 days, all plants were synchronized in the flowering production stage, and at this point, plants were randomly divided into four conditions, each including 5 plants (biological replicates) that were analyzed individually (n = 5). Each condition was exposed to a different inoculation of E. coli. The inoculation solutions were prepared with an appropriate amount of OD600-adjusted bacterial suspension added to tap water (for the water contamination conditions) or to phosphate-buffered saline (PBS) (for plant contamination through injection). (Condition 1) Negative control (C−): plants not inoculated with E. coli, being irrigated with ½-strength Hoagland solution every two days until the end of the study (day 98). (Condition 2) Positive control (C+): plants were injected in the stem (to ensure that E. coli internalization occurred) with 100 µL of a suspension of 107 CFU/mL of E. coli, being thereafter irrigated with ½-strength Hoagland solution every two days until the end of the study (day 98). (Condition 3) One-time inoculation by irrigation, in which plants were exposed to one aerial irrigation (approximately 20 cm above the soil) with 60 mL of a suspension of 107 CFU/mL of E. coli, being thereafter irrigated with ½-strength Hoagland solution every two days until the end of the study (day 98). (Condition 4) Chronic inoculation by irrigation, in which plants were also exposed to aerial irrigation (approximately 20 cm above the soil) with 60 mL of contaminated water containing 104 CFU/mL of E. coli once a week until the end of the study. The strain of E. coli carrying mcr1 was used for the experiments since it is found in environments associated with, e.g., intensive farming, with the mcr1 not being associated with virulence and other colonization traits, but rather with resistance. The positive control was injected with a high concentration of bacteria (107 CFU/mL of E. coli) to ensure that the microorganism entered the plant’s vascular system, whereas the different concentrations in the irrigation contaminations are due to our aim to mimic a situation that could occur in the agricultural field, namely, a high concentration, one-off contamination (with 107 CFU/mL of E. coli) and also long-term and chronic contamination, at a lower concentration (with 104 CFU/mL of E. coli). On day 98, samples were collected for the following assessments.
2.3. Chlorophyll a Fluorescence, Pigment Content, and Gas Exchange
Fully expanded leaves of each plant of each condition were used for chlorophyll a fluorescence and gas exchange determination according to Mendes et al. [17]. Chlorophyll a (Chl a) efficiency was obtained by determining the minimal fluorescence yield (F0) in expanded leaves adapted to the dark for 30 min with a fluorometer (OS30p+, Opti-Sciences Inc., Hudson, New Hampshire, USA). Then, with a saturating pulse of white light, the maximum fluorescence yield of the dark-adapted leaves (Fm) was determined. The minimal (F0′) and also maximal (Fm′) fluorescence values from light-adapted leaves were also measured on the same expanded leaves after an adaptation to light of at least 30 min. The variable fluorescence values, that is, Fv = Fm − F0 and Fv′ = Fm′ − F0′, were estimated to obtain the maximum PSII efficiency (Fv/Fm) and the effective photochemical efficiency of PSII (ФPSII). The fluorescence emission from light-adapted leaves (F′) was also recorded to obtain photochemical quenching [qP = (Fm′ − F′)/(Fm′ − F0′)], non-photochemical quenching [NPQ = (Fm − Fm′)/Fm′] and the effective efficiency of PSII [ФPSII = (Fm′ − F′)/Fm′] [18].
Photosynthetic pigments were quantified according to Sims and Gamon [19]. Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Car) were extracted from leaf powders and homogenized in acetone–50 mM Tris-HCl pH 7.8 buffer (80:20, v/v). Absorbances at 470, 537, 647, and 663 nm were read in a microplate spectrophotometer (FLUOstar Omega, BMG LABTECH, Ortenberg, Germany). Results are expressed as µmol gFM−1.
The stomatal conductance (gs, mol m−2 s−1), transpiration rate (E, mol m−2 s−1), intercellular CO2 concentration (Ci, ppm), and net photosynthetic rate (PN, μmol (CO2) m−2 s−1) were assessed in situ in fully expanded leaves, with an infrared gas analyzer (IRGA) model “LC pro+” (ADC, Hoddesdon, UK). In addition, the intrinsic water use efficiency [iWUE, μmol (CO2)/mmol (H2O)] was calculated as follows, iWUE = PN/gs. The intrinsic carboxylation efficiency (iCE, μmol (CO2)/ppm) was also obtained, as iCE = PN/Ci. The atmospheric CO2 concentration (Ci/Ca ratio), given by the intercellular CO2 concentration (Ci) and the ambient CO2 concentration (Ca), was also determined.
2.4. Cell Membrane Stability
One leaf per plant, all with similar ages and sizes, was collected and submerged in 30 mL of deionized ultrapure water at room temperature with slight agitation for 24 h. After the incubation period, electric conductivity (L1) was measured, and then the samples were autoclaved for 15 min at 120 °C. After this, the electric conductivity of the autoclaved leaves was measured once again (L2), and the results for the cell membrane stability (CMS) were presented as a percentage of membrane damage, calculated by %MD = (L1/L2) × 100 [20].
2.5. Leaf Biomass
One leaf per plant was recovered, labeled, and its fresh weight (FW) was recorded. Following this, the leaves were kept in an incubator at 70 °C for five days, and dry weight (DW) was obtained afterwards. The relative water content (RWC) was calculated using the following formula: RWC = ((FW − DW)/(TW − DW)) × 100.
2.6. Plant Morphology, Fruiting and Fruit Ripening
Plant morphological characteristics, such as shoot length and number of chlorotic and necrotic leaves, were evaluated. In addition, the number of fruits in each plant was quantified and classified taking into account three categories based on their level of development [21], namely, “green”, “yellow” and “red”. Fruits’ size (h/w, mm) and weight (g) were also noted.
2.7. Statistical Analysis
Except when specifically mentioned, 5 plants were used for the overall experiments, which were treated as individual samples (n = 5 biological replicates). Each assay consisted of 3 technical replicates for each biological replicate, except for the chlorophyll a fluorescence and gas exchange analysis. The values are presented as mean ± standard deviation. Comparisons between treatments and control were based on a one-way ANOVA test with Tukey’s multiple comparisons (p < 0.05), using GraphPad Prism.v9 (GraphPad Software, La Jolla, CA, USA). Multivariate analyses for data correlation were also performed on GraphPad Prism.v9 based on principal component analysis (PCA).
3. Results and Discussion
3.1. Chlorophyll Fluorescence, Pigments and Gas Exchange
Forty-one days after inoculations started, it was observed that E. coli did not significantly (p > 0.05) affect any of the most relevant parameters related to the photophosphorylation in dark-adapted leaves (F0, Fv, and Fm) for every condition, and no significant changes were observed between the influence of the irrigation treatments and the C+ and C− (Figure 1A–C). However, a slight increase in F0 was observed in plants exposed one time to E. coli (Figure 1A). Considering the Fv/Fm ratio (Figure 1D), all plants showed a ratio ranging between 0.77 (C−) and 0.67 (one-time irrigation), with no significant changes between each condition (p > 0.05), while the C+ had a value of 0.76, close to the values of C−. The typical ratio of Fv/Fm between 0.75 and 0.83 is observed in healthy plants [22,23], and when values fall progressively below that interval, it indicates that damage to PSII reaction centers might have increased [24], hinting some sort of stress, resulting in photoinhibition and/or increased quenching [18]. In the present work, the tomato plants inoculated with E. coli, despite not having changes with statistical significance (p > 0.05), showed a decrease in the Fv/Fm, particularly in one-time irrigation, which might be justified by the increased value of F0, not paralleled by increases in Fm (Figure 1A,C,D). Similarly, the parameters regarding light-adapted leaves (Fv′/Fm′), qP, ФPSII, and NPQ were not significantly affected by the presence of E. coli in plants, including C+ and C− (Figure 1E–H). These data suggest that the injection of E. coli in the vascular system did not affect the thylakoids’ structure and their photochemical function. Also, the exposure of the plant to contaminated irrigation (one-time vs. chronical) was not detrimental to this photophosphorylation stage of the host.
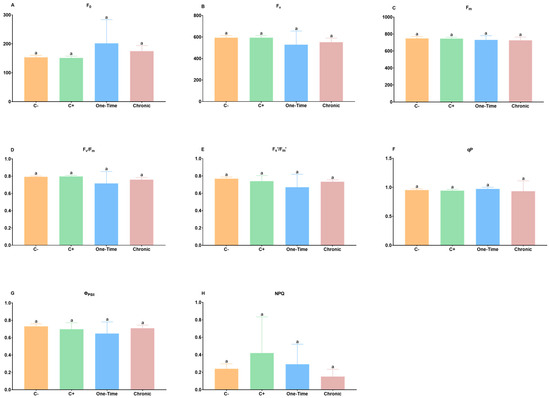
Figure 1.
Effects of inoculation of Escherichia coli in the (A) minimal fluorescence yield (F0); (B) variable fluorescence (Fv); (C) maximum fluorescence yield of the dark-adapted leaves (Fm); (D) maximum PSII efficiency (Fv/Fm); (E) maximum efficiency of PSII photochemistry (Fv′/Fm′); (F) photochemical quenching (qP); (G) effective photochemical efficiency of PSII (ФPSII); and (H) non-photochemical quenching (NPQ). Negative control (C−): plants not inoculated with E. coli; positive control (C+): plants stem-injected with 100 µL of 107 CFU/mL of E. coli; one-time: inoculation by irrigation with only one aerial irrigation (approximately 20 cm above the soil) with 60 mL of 107 CFU/mL of E. coli; and chronic: inoculation by chronic irrigation with 60 mL of water containing 104 CFU/mL of E. coli once a week until the end of the study. Vertical bars: mean value with standard deviation (n = 5). Different letters mean statistically significant differences, p < 0.05.
Additionally, the exposure to E. coli (both C+ and the two irrigation conditions) did not induce significant changes in the chlorophyll levels (p > 0.05), with chl a values ranging from 0.33 to 0.44 µmol gFM−1, and those of chl b from 0.17 to 0.21 µmol gFM−1 (Figure 2A,B). Similarly, the carotenoid levels were not significantly affected (p > 0.05) by the E. coli presence in plants. Since chlorophylls and carotenoids are structural components of the photosystems II and I light-harvesting complexes (LHC–PSII and LHC–PSI), changes in their levels may compromise the structure of the thylakoid and associated fluorescence data [25]. Our data indicate that these non-phytopathogenic bacteria do not significantly affect the structure of the thylakoids, including the levels of their associated pigments (LHC–PSII and LHC–PSI).
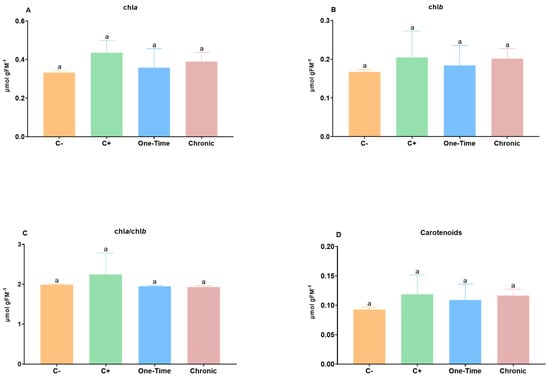
Figure 2.
Effects of the inoculation of Escherichia coli chlorophylls (A–C) and carotenoid contents (D) in tomato plants. Negative control (C−): plants not inoculated with E. coli; positive control (C+): plants stem-injected with 100 µL of 107 CFU/mL of E. coli; one-time: inoculation by irrigation with only one aerial irrigation (approximately 20 cm above the soil) with 60 mL of 107 CFU/mL of E. coli; and chronic: inoculation by chronic irrigation with 60 mL of water containing 104 CFU/mL of E. coli once a week until the end of the study. Vertical bars: mean value with standard deviation (n = 5). Different letters mean significant differences, p < 0.05.
The gas-exchange parameters account for the events that occur inside the stomata and thus are highly dependent on the stomatal aperture. All inoculation conditions decreased the stomatal conductance (gs) and transpiration (E) (Figure 3A,B), with significant differences (p < 0.05) in the one-time and chronical inoculations, meaning that these plants closed their stomata and reduced transpiration rates. The decrease observed in gs in the presence of E. coli supports that stomatal closure integrates a mechanism of plant defense to this non-phytopathogenic bacteria, limiting the growth of bacteria by stranding them on the harsh leaf surface [15]. This is similar to what occurs with phytopathogenic bacteria and fungi that are capable of triggering stomatal closure through pathogen-associated molecular patterns (PAMPs), which prevents penetration through these pores [26,27]. Thus, we propose here that, despite not being phytopathogenic, E. coli also triggers some plant stomatal defenses similar to those observed for phytopathogens, supporting what was proposed for the stomatal responses in Arabidopsis spp. leaves exposed to E. coli O157:H7 [14]. The decrease in stomatal conductance (gs) might lead to a decrease in the intercellular CO2 concentration (Ci), representing a limitation to photosynthesis. However, in E. coli-irrigated plants, no depletion of Ci content was observed (Figure 3C); on the contrary, a slight increase was noted, together with a similar increase in the ratio Ci with atmospheric CO2 values (Ci/Ca) (Figure 3D), which showed to be higher in plants exposed to both irrigation conditions compared with the C+ and C−. To explain this increased Ci, one must also consider the CO2 generated internally due to photorespiration and respiration processes. The photorespiration rates in response to phytopathogens were recently revised [28], and the authors showed a plethora of responses, mostly attributable to different plant–pathogen systems and the complex multi-organellar photorespiratory process. Nevertheless, considering its relation to reactive oxygen species (ROS) (e.g., H2O2) and plant immunity, this process also deserves further studies in plants contaminated with E. coli.
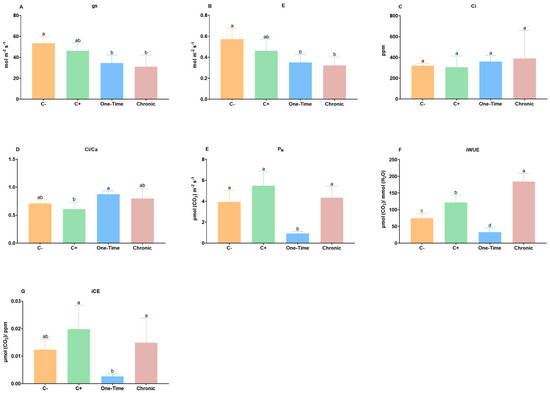
Figure 3.
Effects of inoculation of Escherichia coli on (A) stomatal conductance (gs); (B) transpiration rate (E); (C) intercellular CO2 concentration (Ci); (D) intercellular and atmospheric CO2 concentration ratio (Ci/Ca); (E) net photosynthetic rate (PN); (F) intrinsic water-use efficiency (iWUE); and (G) intrinsic carboxylation efficiency (iCE). Negative control (C−): plants not inoculated with E. coli; positive control (C+): plants stem-injected with 100 µL of 107 CFU/mL of E. coli; one-time: inoculation by irrigation with only one aerial irrigation (approximately 20 cm above the soil) with 60 mL of 107 CFU/mL of E. coli; and chronic: inoculation by chronic irrigation with 60 mL of water containing 104 CFU/mL of E. coli once a week until the end of the study. Vertical bars: mean value with standard deviation (n = 5). Different letters mean significant differences, p < 0.05.
The behavior of the net photosynthetic rate (PN) was highly influenced by the inoculation condition (Figure 3E), with a significant decrease (p < 0.05) in the one-time inoculation through irrigation compared to every condition. The C+ and chronically inoculated plants showed an increase in PN, yet without significant differences (p > 0.05) with the negative control (C−). The iWUE followed a similar result in all conditions to the PN (Figure 3F), with chronically exposed plants being significantly stimulated (p < 0.05) when compared to both unexposed (C−) and directly inoculated (C+) plants, while one-time-inoculated plants showed significant decreases (p < 0.05). Similarly, an increase in the intrinsic carboxylation efficiency (iCE) in chronically exposed plants was observed, yet this increase was not significant (p > 0.05) (Figure 3G), while those exposed to the one-time inoculation showed a significantly decreased iCE (p < 0.05). These results support that, despite plants exposed to acute inoculation (107 CFU/mL) and chronically inoculated with a lower concentration of bacteria (104 CFU/mL) showing similar responses of closing stomata and decreasing transpiration, the impacts on iWUE, iCE, and ultimately on PN are drastically different. The impact of E. coli on the transient host may thus influence the gas-exchange performance in a way similar to phytopathogenic agents, but the effects depend on the exposure conditions. Our data show that plants with injected bacteria (C+) were less affected on the gas exchange parameters than those irrigated with a one-time inoculation with a similar dose, so the observed effects on photosynthetic gas exchange parameters might not be due to direct effects exerted by the internalized bacteria (e.g., toxins), but to other effects requiring the signaling pathways related to the adhesion of the bacteria to the leaf surface, and biofilm formation that might lead to bacterial aggregation and trigger stomata responses [29]. The irrigation method used (simulating aerial irrigation in a greenhouse) allowed the contact (e.g., through drops and aerosols) of the bacteria with the stems and leaves of the irrigated plants. Previous authors [10,30,31] reported that STEC and enteroaggregative E. coli adhered to the leaf epidermis, including on and around stomata (an ideal transient niche for nutrition and replication). In other pathosystems, there were decreases in CO2 assimilation rate and/or photosynthetic rate associated with the reductions in stomatal conductance [15], which, in this case, was only observed for the single exposure (107 CFU/mL), compared with the chronic inoculation with a lower concentration of bacteria (104 CFU/mL). These results can be explained by previously reported findings [32], which showed that only high concentrations of bacteria might trigger stomatal closure (107–108 CFU/mL). These findings are particularly important in also explaining why the normal microbiota living in/on the phyllosphere does not promote stomatal closure. Thus, our results lead us to suggest that plants chronically inoculated with 104 CFU/mL show less detrimental effects than plants inoculated once with a higher dose of the bacterium.
3.2. Cell Membrane Stability, Leaf Biomass, Plant Morphology, and Fruiting
Independently of the type of irrigation, the CMS of the plants was not significantly affected (p > 0.05) in every condition (Table 1). Equally, the leaves’ biomass was also not affected by the E. coli inoculation, with no changes detected in the fresh and dry weights, as well as in the RWC. However, one-time inoculated plants demonstrated a lower FW.

Table 1.
Effects of the inoculation of Escherichia coli on cell membrane stability (CMS), leaves’ biomass [fresh weight (FW), dry weight (DW), and relative water content (RWC)], as well as on plant morphology (shoot length, number of necrotic and chlorotic leaves). Different letters mean significant differences p < 0.05 (n = 5).
Regarding shoot length, no significant differences (p > 0.05) were observed between each condition inoculated with E. coli. Previous studies stated that this bacterium may have plant growth-promoting properties and can even be endophytic [33,34]; however, such capacity was not observed in the present study, thus supporting previous data obtained in tomato plants contaminated with E. coli [35].
Despite not having evident differences in leaf morphology, one-time-exposed plants showed more necrotic and chlorotic leaves (not significant, p > 0.05) than the plants from other conditions (Table 1).
E. coli O157:H7 has been previously reported to be associated with a reduction in fruit production in different cultivars of tomato plants, except the Micro-Tom cultivar [36], which corroborates our results, as all conditions showed fruit production and a similar number of green, yellow and red tomatoes (Table 2).

Table 2.
Effects of the inoculation of Escherichia coli on production and maturation of fruits. The number of fruits in each plant of each condition was distributed by three different developmental stages (green, yellow, and red). Different letters mean significant differences p < 0.05.
3.3. PCA Analysis
PCA showed a clear separation between the C− and the E. coli exposed conditions, as well as a separation between each condition (Figure 4). PC1 explained 50.23% of the variance and PC2 30.61%. Both controls are located on the right and both irrigation conditions are to the left of the plot. The C− is positioned in the upper-right quadrant scoring part of the gas exchange indicators, such as E and gs, as well as one indicator of chl a fluorescence (Fm). The positive control (C+) is located in the lower-right quadrant, and it mostly scores for photosynthetic pigments, as well as some gas-exchange and chl a fluorescence indicators. Carotenoids, shoot length, and Ci were scored in the left lower quadrant, by the chronic condition, while the one-time exposure scored the Ci/Ca ratio in the upper-left quadrant, positively correlating with F0, and Ci, and negatively correlating with most of the other parameters (e.g., iCE, PN), supporting the data and explanations of this work. The negative correlation of the E. coli inoculation through irrigation with transpiration and stomatal conductance shows that E. coli is capable of triggering stomatal responses in tomato plants. In conclusion, the behaviors of both controls are closer than the behaviors of the chronic and the one-time irrigation.
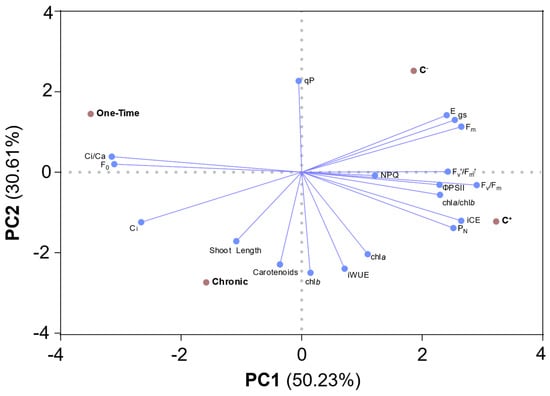
Figure 4.
PCA biplot of the effects on photosynthetic parameters of one-time and chronic exposure through irrigation, as well as stem inoculation (C+) of plants with Escherichia coli on tomato plants. Loading plot for the first axis, PC1, explained 50.23% of the variance, and the second axis, PC2, explained 30.61%.
4. Conclusions
E. coli ranks as one of the most important causes of foodborne diseases in fresh produce, mostly caused by contaminated crops. Previous studies have focused on the survival of this human commensal/pathogenic bacteria in their transient plant hosts, but less is known on how they interact and affect the plant, namely, its photosynthesis. We validated our hypothesis that E. coli influenced the tomato transient host’s photosynthetic capacity. This was especially evident in aerially irrigated ones (one acute inoculation with 107 CFU/mL, and one chronical inoculation with 104 CFU/mL). Also, the main effects observed in the gas-exchange parameters are mostly related to a decrease in stomatal conductance, and transpiration, and with a trend of increased internal CO2. This supports that the external contact of the bacteria with the external surface of the leaf and stomata triggers responses. Also, by comparing the irrigation groups, we demonstrate that one-time exposure with a higher concentration of bacteria leads to a higher decrease in PN, iWUE, and iCE, than the chronical exposure, supporting what was reported for some phytopathogens, in which only high concentrations can induce some plant responses. The present study is, to the best of our knowledge, the first study reporting that E. coli influences the tomato–transient host’s photosynthetic capacity, and thus our data open new perspectives on how non-phytopathogenic bacteria may hamper the photosynthetic pathways of transient hosts.
Author Contributions
Conceptualization, A.G., C.S. and R.J.M.; methodology, A.G., C.S. and R.J.M.; validation, A.G. and R.J.M.; formal analysis, A.G. and R.J.M.; investigation, A.G., L.-T.D. and R.J.M.; data curation, A.G.; writing—original draft preparation, AG.; writing—review and editing, A.G., C.S., L.-T.D. and R.J.M.; visualization, A.G.; supervision, C.S. and R.J.M.; funding acquisition, A.G. and C.S.; All authors have read and agreed to the published version of the manuscript.
Funding
A.G. was supported by a PhD fellowship from Fundação para a Ciência e Tecnologia (FCT) (SFRH/BD/145711/2019). This work received financial support from FCT/MCTES (UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020) through national funds.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work received support and help from FCT/MCTES (LA/P/0008/2020 DOI 10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI 10.54499/UIDP/50006/2020 and UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020), through national funds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Esmael, A.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Filimban, A.A.R.; Alseghayer, M.S.; Almaneea, A.M.; Alhadlaq, M.A.; Ayubu, J.; Teklemariam, A.D.A. Fresh Produce as a Potential Vector and Reservoir for Human Bacterial Pathogens: Revealing the Ambiguity of Interaction and Transmission. Microorganisms 2023, 11, 753. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported Foodborne Outbreaks Due to Fresh Produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Vassallo, A.; Amoriello, R.; Guri, P.; Casbarra, L.; Ramazzotti, M.; Zaccaroni, M.; Ballerini, C.; Cavalieri, D.; Marvasi, M. Adaptation of Commensal Escherichia coli in Tomato Fruits: Motility, Stress, Virulence. Biology 2023, 12, 633. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; Martínez De La Peña, C.; Silva, J.L.; Luna-Guevara, M.L. The Role of Pathogenic E. coli in Fresh Vegetables: Behavior, Contamination Factors, and Preventive Measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Gekenidis, M.T.; Rigotti, S.; Hummerjohann, J.; Walsh, F.; Drissner, D. Long-Term Persistence of blaCTX-M-15 in Soil and Lettuce after Introducing Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli via Manure or Water. Microorganisms 2020, 8, 1646. [Google Scholar] [CrossRef]
- Melotto, M.; Panchal, S.; Roy, D. Plant innate immunity against human bacterial pathogens. Front. Microbiol. 2014, 5, 411. [Google Scholar] [CrossRef]
- Chelaghma, W.; Loucif, L.; Bendahou, M.; Rolain, J.-M. Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review. Microorganisms 2021, 9, 2534. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef]
- Saldaña, Z.; Sánchez, E.; Xicohtencatl-Cortes, J.; Puente, J.L.; Girón, J.A. Surface structures involved in plant stomata and leaf colonization by Shiga-toxigenic Escherichia coli O157: H7. Front. Microbiol. 2011, 2, 119. [Google Scholar] [CrossRef]
- Grivokostopoulos, N.C.; Makariti, I.P.; Tsadaris, S.; Skandamis, P.N. Impact of Salmonella in Leafy greens and Impact on Acid Tolerance. Food Microbiol. 2022, 88, e02249-21. [Google Scholar] [CrossRef]
- Thilmony, R.; Underwood, W.; He, S.Y. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006, 46, 34–53. [Google Scholar] [CrossRef]
- Roy, D.; Panchal, S.; Rosa, B.A.; Melotto, M. Escherichia coli O157:H7 Induces Stronger Plant Immunity than Salmonella enterica Typhimurium SL1344. Phytopathology 2013, 103, 326–332. [Google Scholar] [CrossRef]
- Meddya, S.; Meshram, S.; Sarkar, D.; Rakesh, S.; Datta, R.; Singh, S.; Avinash, G.; Kondeti, A.K.; Savani, A.K.; Thulasinathan, T. Plant Stomata: An Unrealized Possibility in Plant Defense against Invading Pathogens and Stress Tolerance. Plants 2023, 12, 3380. [Google Scholar] [CrossRef]
- Production/Crops and Livestock Products—Metadata. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 6 May 2024).
- Mendes, R.J.; Mariz-Ponte, N.; Correia, C.V.; Dias, M.C.; De Sousa, M.L.; Tavares, F.; Santos, C. Fire Blight Management: Physiological Assessment of Cultural Control by Pruning in Pear Orchards. Agriculture 2020, 66, 128–136. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Ferreira de Oliveira, J.M.P.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2017, 221, 32–42. [Google Scholar] [CrossRef]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv.‘Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2009, 61, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Ritchie, G.A. Chlorophyll fluorescence: What is it and what do the numbers mean? In USDA Forest Service Proceeding RMRS; Rocky Mount Research Station: Fort Collins, CO, USA, 2006; pp. 34–42. [Google Scholar]
- Tatagiba, S.D.; DaMatta, F.M.; Rodrigues, F.Á. Leaf gas exchange and chlorophyll a fluorescence imaging of rice leaves infected with Monographella albescens. Phytopathology 2015, 105, 180–188. [Google Scholar] [CrossRef]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef]
- Gahir, S.; Bharath, P.; Raghavendra, A.S. Stomatal Closure Sets in Motion Long-Term Strategies of Plant Defense Against Microbial Pathogens. Front. Plant Sci. 2021, 12, 761952. [Google Scholar] [CrossRef]
- Sakata, N.; Ishiga, Y. Prevention of Stomatal Entry as a Strategy for Plant Disease Control against Foliar Pathogenic Pseudomonas Species. Plants 2023, 12, 590. [Google Scholar] [CrossRef]
- Jiang, X.; Walker, B.J.; He, S.Y.; Hu, J. The role of photorespiration in plant immunity. Front. Plant Sci. 2023, 14, 1125945. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Stomata and pathogens: Warfare at the gates. Plant Signal. Behav. 2009, 4, 1114–1116. [Google Scholar] [CrossRef]
- Xicohtencatl-Cortes, J.; Chacón, E.S.; Saldaña, Z.; Freer, E.; Girón, J.A. Interaction of Escherichia coli O157:H7 with leafy green produce. J. Food Prot. 2009, 72, 1531–1537. [Google Scholar] [CrossRef]
- Berger, C.N.; Shaw, R.K.; Ruiz-Perez, F.; Nataro, J.P.; Henderson, I.R.; Pallen, M.J.; Frankel, G. Interaction of enteroaggregative Escherichia coli with salad leaves. Environ. Microbiol. Rep. 2009, 1, 234–239. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Rehman, A.; Chauhan, P.S. Environmental Escherichia coli occur as natural plant growth-promoting soil bacterium. Arch. Microbiol. 2010, 192, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tharek, M.; Sim, K.S.; Khairuddin, D.; Ghazali, A.H.; Najimudin, N. Whole-Genome Sequence of Endophytic Plant Growth-Promoting Escherichia coli USML2. Genome Announc. 2017, 5, e00305-17. [Google Scholar] [CrossRef]
- Verma, V.P.; Saharan, V.V.; Nimesh, S.; Singh, A.P. Phenotypic and virulence traits of Escherichia coli and Salmonella strains isolated from vegetables and fruits from India. J. Appl. Microbiol. 2018, 125, 270–281. [Google Scholar] [CrossRef]
- Deering, A.J.; Jack, D.R.; Pruitt, R.E.; Mauer, L.J. Movement of Salmonella serovar Typhimurium and E. coli O157:H7 to Ripe Tomato Fruit Following Various Routes of Contamination. Microorganisms 2015, 3, 809–825. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).