How Sage and Rosemary Essential Oils Regulate Postharvest Senescence and Extend the Vase Life of Cut Gladiolus Spikes
Abstract
1. Introduction
2. Materials and Methods
2.1. Flower Materials
2.2. Extraction and Analyses of Sage and Rosemary EOs
2.3. Treatments and Experimental Design
2.4. Vase Life Evaluation
2.5. Number of Opened and Unopened Florets
2.6. Water Uptake
2.7. Physiological and Biochemical Characteristics
2.7.1. Bacterial Counts
2.7.2. Chlorophyll Contents
2.7.3. Hydrogen Peroxide (H2O2)
2.7.4. Lipid Peroxidation
2.7.5. Membrane Stability
2.7.6. Total Phenols
2.7.7. Antioxidant Enzyme Activities
2.8. Statistical Analysis
3. Results
3.1. Essential Oil Components of Sage and Rosemary
3.2. Vase Life
3.3. Water Uptake
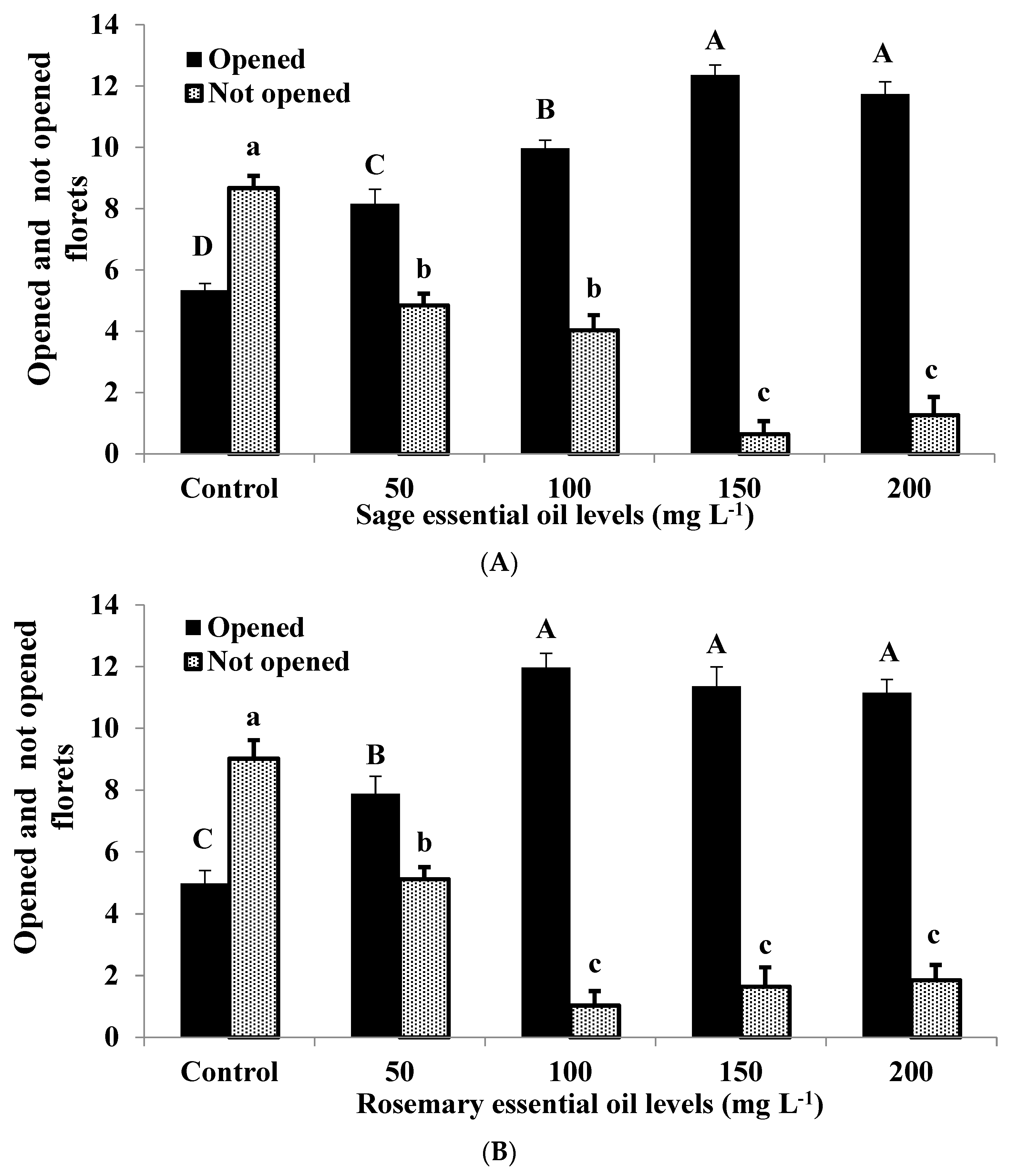
3.4. Opened and Not Opened Floret Number
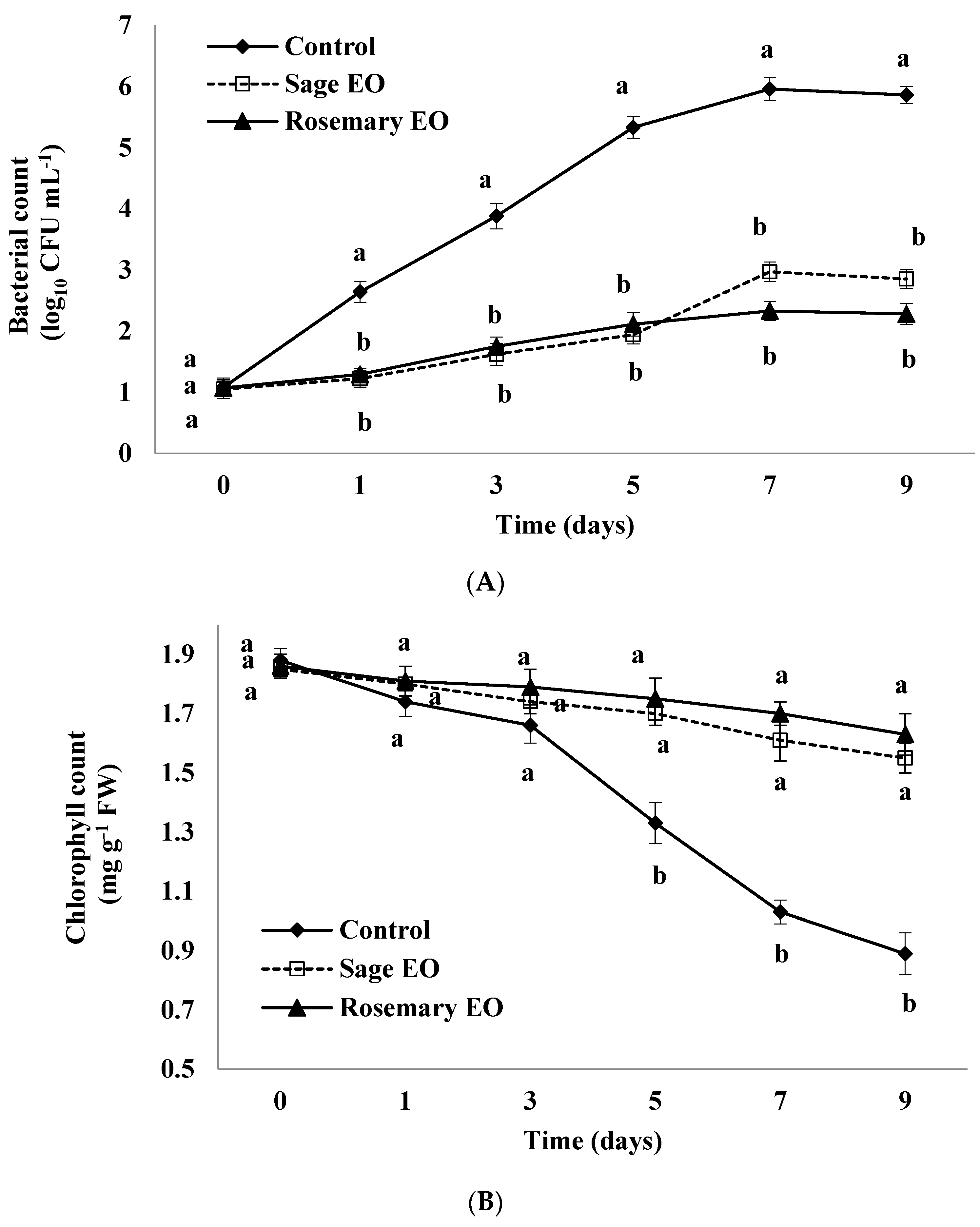
3.5. Bacterial Counts
3.6. Chlorophyll Content
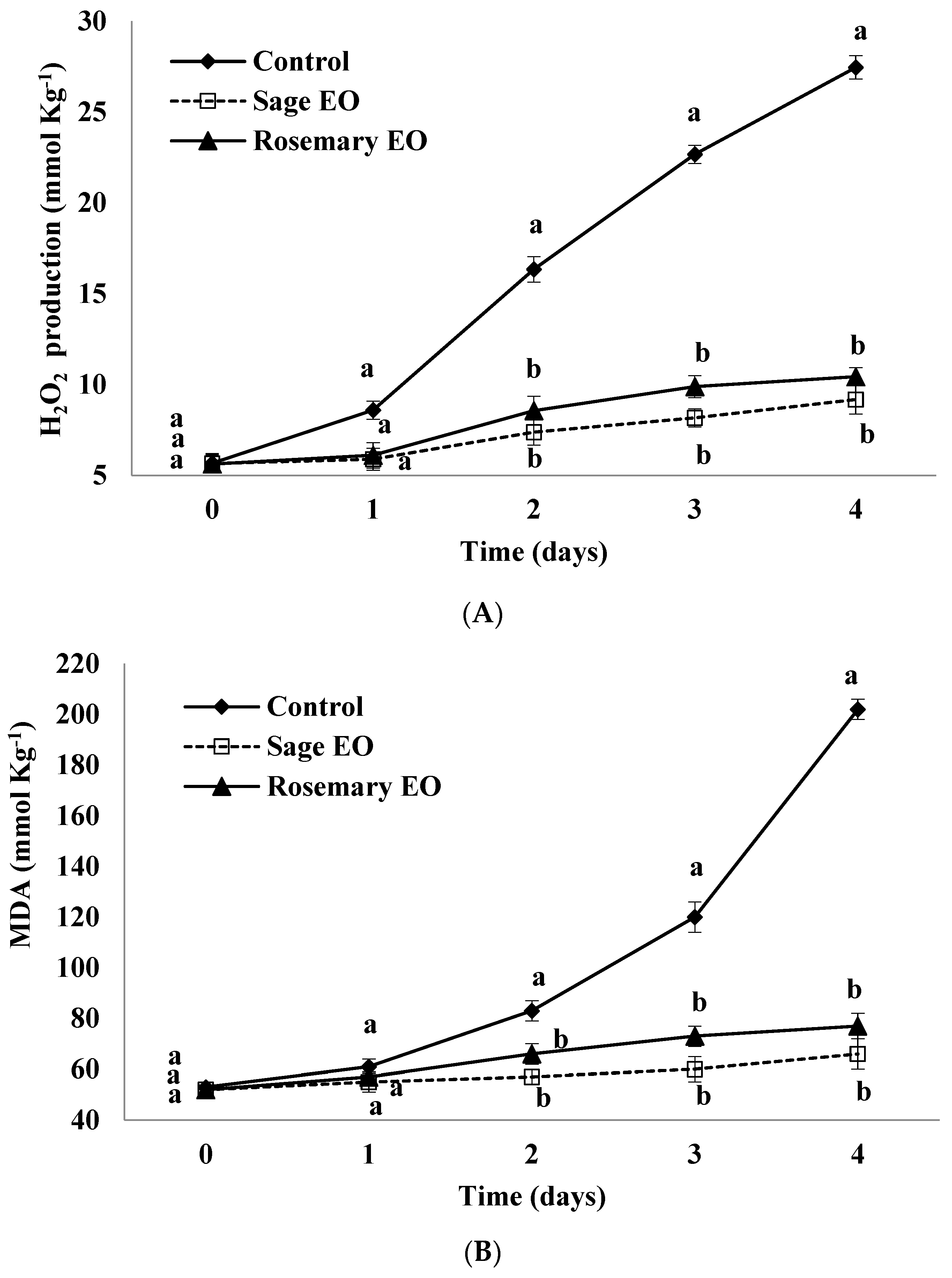
3.7. Hydrogen Peroxide (H2O2) Production
3.8. Malondialdehyde (MDA) Content
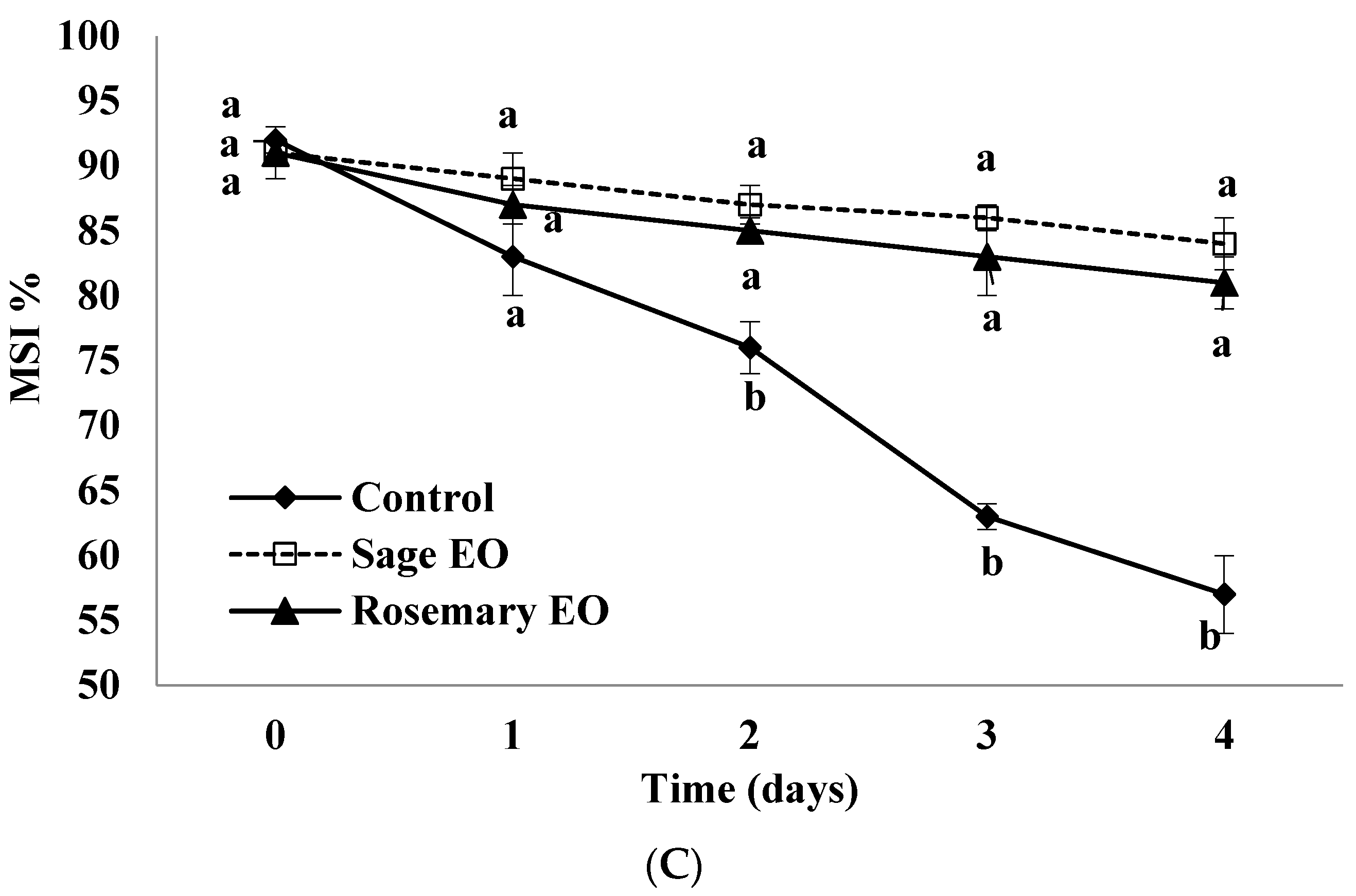
3.9. Membrane Stability Index (MSI)
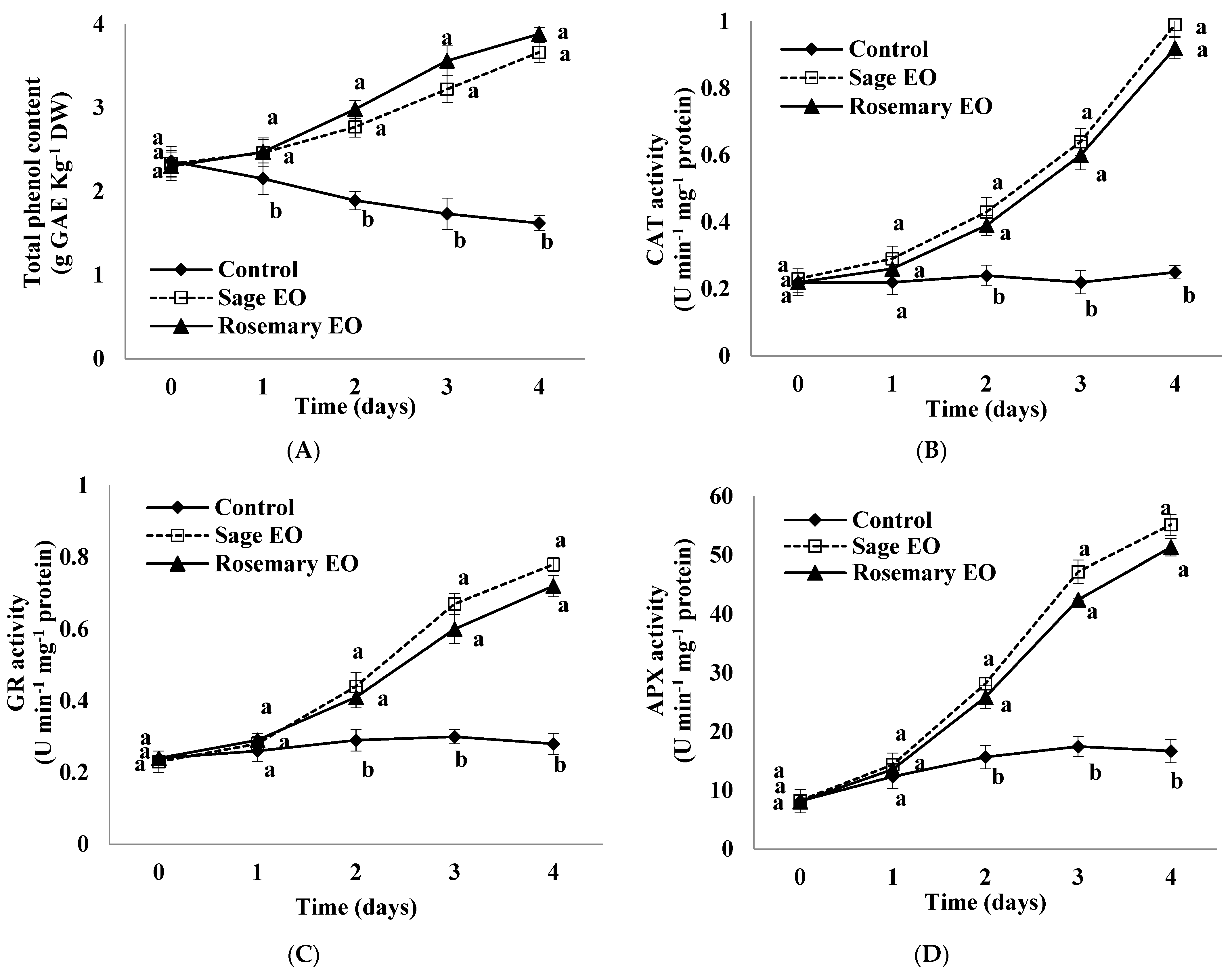
3.10. Total Phenol Content
3.11. Activities of Antioxidant Enzymes
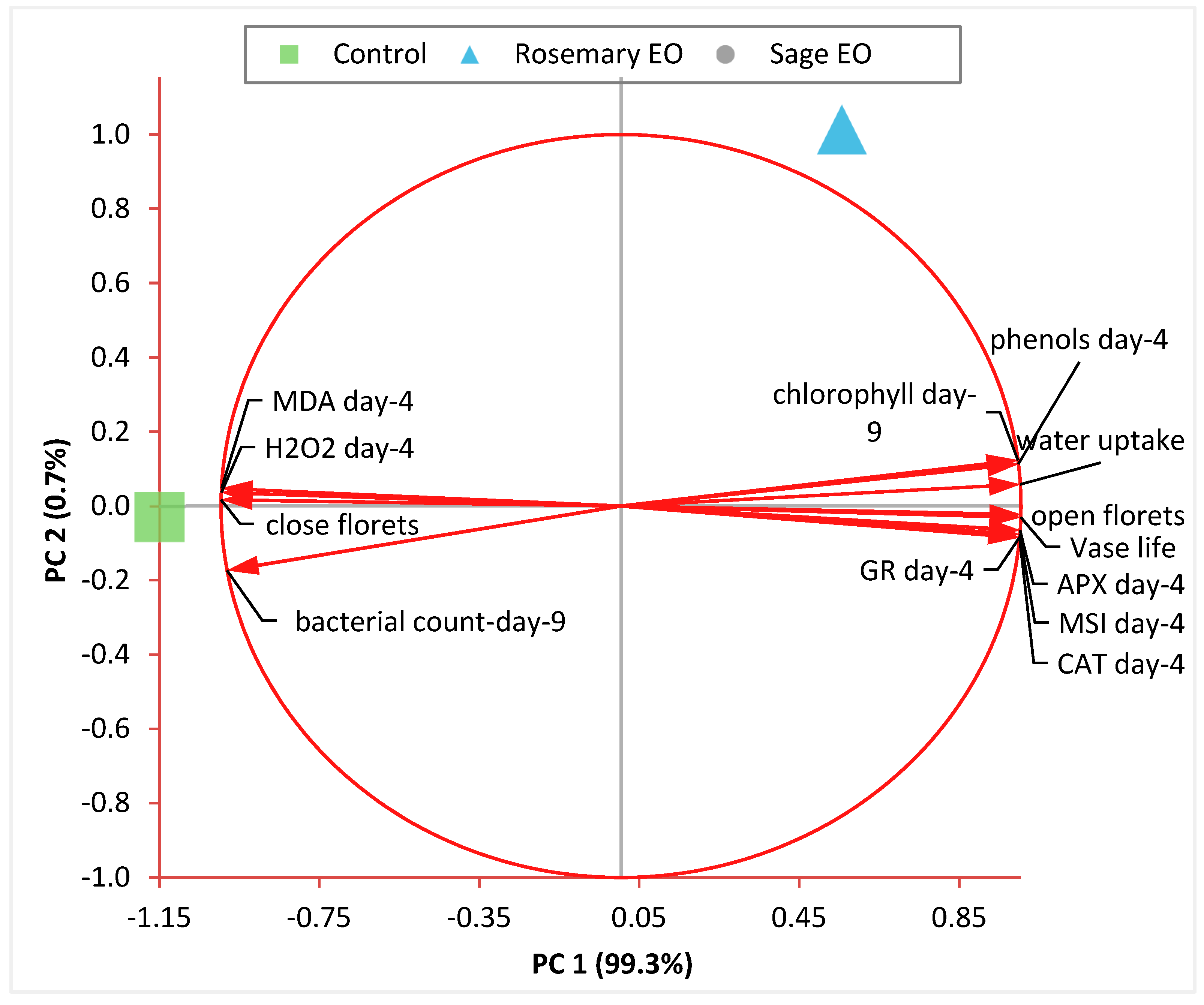
3.12. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Younis, A.; Akhtar, M.S.; Riaz, A.; Zulfiqar, F.; Qasim, M.; Farooq, A.; Tariq, U.; Ahsan, M.; Bhatti, Z.M. Improved cut flower and corm production by exogenous moringa leaf extract application on gladiolus cultivars. Acta Sci. Pol. Hortorum Cultus 2018, 17, 25–38. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Schmidt, G. Postharvest characteristics of cut carnations as the result of chemical treatments. Acta Agron. Hung. 2004, 52, 125–132. [Google Scholar] [CrossRef]
- Mazrou, R.M.; Hassan, S.; Yang, M.; Hassan, F.A.S. Melatonin preserves the postharvest quality of cut roses through enhancing the antioxidant system. Plants 2022, 11, 2713. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; De, L.C. Post-Harvest Technology of Flowers and Ornamental Plants; Aavishkar Publishers: Jaipur, India, 2005; pp. 11–19. [Google Scholar]
- Darras, A. Overview of the dynamic role of specialty cut flowers in the international cut flower market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Ezhilmathi, K.; Singh, V.P.; Arora, A.; Sairam, R.K. Effect of 5-sulfusalicylic acid on antioxidant activity in relation to vase life of gladiolus cut flowers. Plant Growth Regul. 2007, 51, 99–108. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.F. Protective effects of 1-methylcyclopropene and salicylic acid on senescence regulation of gladiolus cut spikes. Sci. Hortic. 2014, 179, 146–152. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Fetouh, M.I. Does moringa leaf extract have preservative effect improving the longevity and postharvest quality of gladiolus cut spikes? Sci. Hortic. 2019, 250, 287–293. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Loubaud, M.; van Doorn, W.G. Wound induced and bacteria induced xylem blockage in roses, Astilbe, and Viburnum. Postharvest Biol. Technol. 2004, 32, 281–288. [Google Scholar] [CrossRef]
- Hatamzadeh, A.; Hatami, M.; Ghasemnezhad, M. Efficiency of salicylic acid delay petal senescence and extended quality of cut spikes of Gladiolus grandiflora cv ‘wing’s sensation’. Afric. J. Agric. Res. 2012, 7, 540–545. [Google Scholar]
- Hassan, F.A.S.; Mazrou, R.; Gaber, A.; Hassan, M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A. Effect of different levels of pulsing concentrations on vase life of gladiolus (Gladiolus grandiflorus L.). Int. J. Curr. Microbiol. App. Sci. 2018, 7, 330–334. [Google Scholar] [CrossRef]
- Knee, M. Selection of biocides for use in floral preservatives. Postharvest Biol. Technol. 2000, 18, 227–234. [Google Scholar] [CrossRef]
- Saeed, T.; Hassan, I.; Abbasi, N.; Jilani, G. Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul. 2014, 72, 89–95. [Google Scholar] [CrossRef]
- Akhtar, G.; Rajwana, I.A.; Sajjad, Y.; Shehzad, M.A.; Amin, M.; Razzaq, K.; Ullah, S.; Faried, H.N.; Farooq, A.; Samiullah. Do natural leaf extracts involve regulation at physiological and biochemical levels to extend vase life of gladiolus cut flowers? Sci. Hortic. 2021, 282, 110042. [Google Scholar] [CrossRef]
- Ramezanian, A.; Azadi, M.; Mostowfizadeh-Ghalamfarsa, R.; Saharkhiz, M.J. Effect of Zatariamultiflora Boiss and Thymus vulgaris L. essential oils on black rot of ‘Washington Navel’ orange fruit. Postharvest Biol. Technol. 2016, 112, 152–158. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alferi, M.; Sasso, M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrob. Agents 2008, 31, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Solgi, M.; Ghorbanpour, M. Application of essential oils and their biological effects on extending the shelf-life and quality of horticultural crops. Trakia J. Sci. 2014, 12, 198–210. [Google Scholar]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef]
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef]
- Mallahi, T.; Ramezanian, A.; Saharkhiz, M.J.; Javanmardi, J.; Iraji, A. Antimicrobial activities of Asafoetida and Shirazi thyme essential oils improve the vase life of gerbera cut flowers. Acta Ecol. Sin. 2018, 38, 228–233. [Google Scholar] [CrossRef]
- Basiri, Y.; Zarei, H.; Mashayekhy, K.; Pahlavany, M.H. Effect of Rosemary extract on vase life and some qualitative characteristics of cut Carnation flowers (Dianthus caryophyllus cv. White librity). J. Stored Prod. Postharvest Res. 2011, 14, 261–265. [Google Scholar]
- Salmi, M.S.; Hoseini, M.F.; Heidari, M.; Daneshvar, M.H. Extending vase life of cut rose (Rosa hybrida L.) cv. Bacara by essential oils. Adv. Hortic. Sci. 2018, 32, 61–69. [Google Scholar]
- Thakur, M.; Verma, V.; Chandel, A.; Kumar, R.; Sharma, T.; Kumar, A.; Bhardwaj, S.; Kumar, R.; Bhargava, B. Lemon grass essential oil improve Gladiolus grandiforus postharvest life by modulating water relations microbial growth, biochemical activity, and gene expression. Sci. Rep. 2023, 13, 2630. [Google Scholar] [CrossRef]
- Balestra, G.M.; Agostini, R.; Bellincontro, A.; Mencarelli, F.; Varvaro, L. Bacterial populations related to gerbera (Gerbera jamesonii L.) stem break. Phytopathol. Mediterr. 2005, 44, 291–299. [Google Scholar]
- Metzner, H.; Rau, H.; Senger, H. Unter suchungen zur synchronisier barteit einzelner pigmentan angel mutanten von chlorela. Planta 1965, 65, 186. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Chem. 1984, 134, 487–492. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acidreactive-substances assay for estimating lipid peroxidation in plant tissue containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–177. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of micro quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theor. Appl. Genet. 1984, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V. Cellular detoxifying mechanisms determine the age dependent injury in tropical trees exposed to SO2. J. Plant Physiol. 1992, 140, 733–740. [Google Scholar] [CrossRef]
- Solgi, M.; Kafi, M.; Taghavi, T.S.; Naderi, R. Essential oils and silver nanoparticles (SNP) as novel agents to extend vase-life of gerbera (Gerbera jamesonii cv. ‘Dune’) flowers. Postharvest Biol. Technol. 2009, 53, 155–158. [Google Scholar] [CrossRef]
- Gun, S.; Uzun, L.; Tuysuz, M.; Erturk, O.; Ilhan, H.; Acıkgoz, M.; Ozturk, B. Nanofiber mats containing lavender oil and methyl jasmonate as an innovative treatment to extend vase life in cut rose flowers. Postharvest Biol. Technol. 2023, 201, 112343. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Özcan, B.; Birgul, E.M.; Coleri, A.; Yolcu, H.; Caliskan, M. In vitro antimicrobial and antioxidant activities of various extracts of Salvia microstegia (Boiss.) et. Bal. from Antakya, Turkey. Fresenius Environ. Bull. 2009, 18, 658–662. [Google Scholar]
- Günther, M.; Karygianni, L.; Argyropoulou, A.; Anderson, A.; Hellwig, E.; Skaltsounis, A.; Wittmer, A.; Vach, K.; Al-Ahmad, A. The antimicrobial effect of Rosmarinus officinalis extracts on oral initial adhesion ex vivo. Clin. Oral. Investig. 2022, 26, 4369–4380. [Google Scholar] [CrossRef]
- Rong-Hua, L.; Pei-Guo, G.; Baum, M.; Grando, S.; Ceccarelli, S. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Teerarak, M.; Laosinwattana, C. Essential oil from ginger as a novel agent in delaying senescence of cut fronds of the fern (Davallia solida (G. Forst.) Sw.). Postharvest Biol. Technol. 2019, 156, 110927. [Google Scholar] [CrossRef]
- Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The efficacies of 1-methylcyclopropene and chitosan nanoparticles in preserving the postharvest quality of Damask rose and their underlying biochemical and physiological mechanisms. Biology 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Dehestani-Ardakani, M.; Gholamnezhada, J.; Alizadeha, S.; Meftahizadeha, H.; Ghorbanpour, M. Salicylic acid and herbal extracts prolong vase life and improve quality of carnation (Dianthus caryophyllus L.) flowers. S. Afr. J. Bot. 2022, 150, 1192–1204. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, H.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J. Food Qual. 2017, 3, 3185945. [Google Scholar] [CrossRef]
- Soliman, D.; El-Sayed, I. Study postharvest characteristics, chemical composition and antimicrobial activity of Dianthus caryophyllus L.; cut flowers using some essential oils. Ornam. Hortic. 2023, 29, 37–47. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]

| Salvia Essential Oil | Rosemary Essential Oil | ||||
|---|---|---|---|---|---|
| Oil Constituents | RI * | Percentage | Oil Constituents | RI | Percentage |
| α-Pinene | 952 | 2.38 | α-Pinene | 1028 | 10.64 |
| Camphene | 963 | 2.66 | Camphene | 1050 | 6.82 |
| β-Pinene | 986 | 1.42 | β-Pinene | 1116 | 3.44 |
| β-Myrcene | 992 | 0.80 | β-Myrcene | 1170 | 1.63 |
| p-Cymene | 1028 | 1.20 | α-Phellandrene | 1202 | 0.22 |
| Limonene | 1036 | 1.38 | α-Terpinene | 1244 | 0.67 |
| 1.8-Cineole | 1044 | 7.88 | p-Cymene | 1278 | 2.85 |
| α-Thujone | 1108 | 28.31 | Limonene | 1286 | 1.88 |
| β-Thujone | 1110 | 12.44 | γ-Terpinene | 1322 | 1.22 |
| Camphor | 1149 | 21.68 | 1.8-Cineole | 13.26 | 37.54 |
| Borneol | 1164 | 3.83 | Linalool | 1362 | 1.67 |
| Terpinen-4-ol | 1178 | 0.81 | β-Caryophyllene | 1618 | 2.85 |
| Bornyl acetate | 1276 | 3.66 | Camphor | 1686 | 13.59 |
| Β-Caryophyllene | 1388 | 0.68 | Borneol | 1788 | 7.43 |
| α-Humulene | 1448 | 2.34 | α-Terpineol | 1812 | 4.87 |
| Viridiflorol | 1586 | 7.75 | Bornyl acetate | 1826 | 1.48 |
| Total identified compounds | 99.22 | Total identified compounds | 98.80 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, M.M.; Mazrou, R.M.; Hassan, F.A.S. How Sage and Rosemary Essential Oils Regulate Postharvest Senescence and Extend the Vase Life of Cut Gladiolus Spikes. Horticulturae 2024, 10, 638. https://doi.org/10.3390/horticulturae10060638
Moussa MM, Mazrou RM, Hassan FAS. How Sage and Rosemary Essential Oils Regulate Postharvest Senescence and Extend the Vase Life of Cut Gladiolus Spikes. Horticulturae. 2024; 10(6):638. https://doi.org/10.3390/horticulturae10060638
Chicago/Turabian StyleMoussa, Mohamed M., Ragia M. Mazrou, and Fahmy A. S. Hassan. 2024. "How Sage and Rosemary Essential Oils Regulate Postharvest Senescence and Extend the Vase Life of Cut Gladiolus Spikes" Horticulturae 10, no. 6: 638. https://doi.org/10.3390/horticulturae10060638
APA StyleMoussa, M. M., Mazrou, R. M., & Hassan, F. A. S. (2024). How Sage and Rosemary Essential Oils Regulate Postharvest Senescence and Extend the Vase Life of Cut Gladiolus Spikes. Horticulturae, 10(6), 638. https://doi.org/10.3390/horticulturae10060638






