Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Total Carotenoid Content
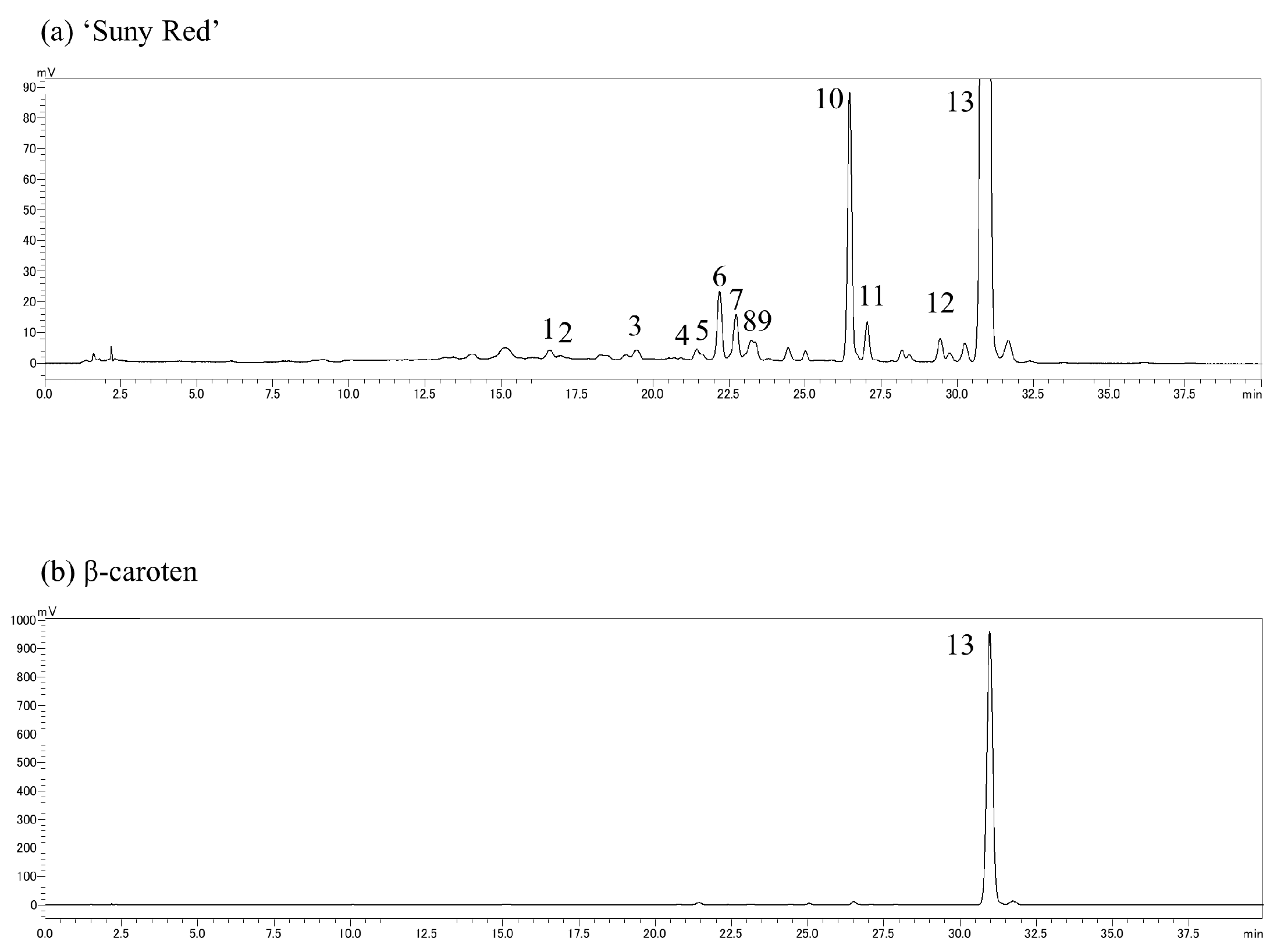
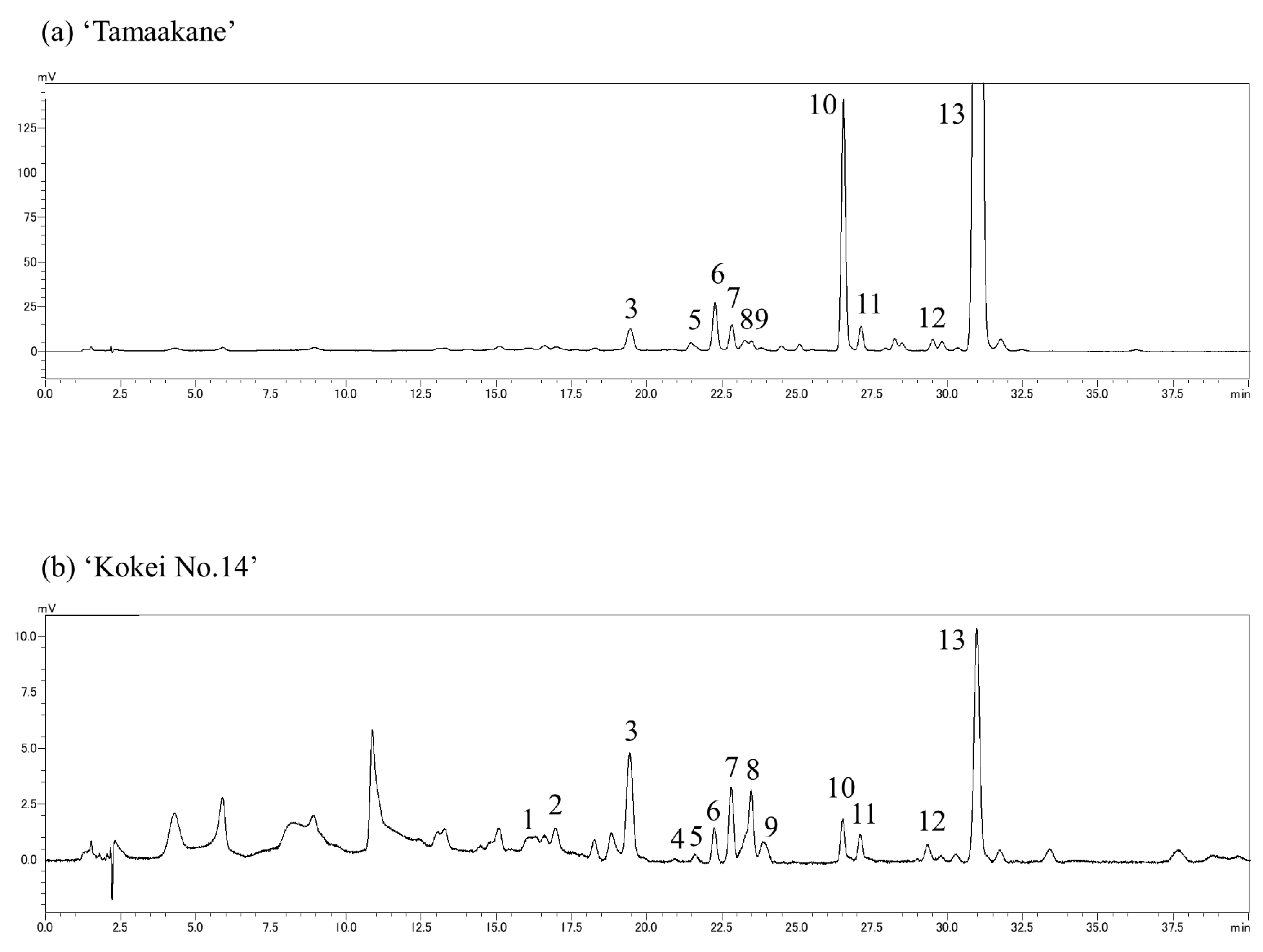
2.3. Compositional Analysis of Carotenoids
2.4. Compositional Analysis of Polyphenols
2.5. Compositional Analysis of Anthocyanin
2.6. Statistical Analysis
3. Results
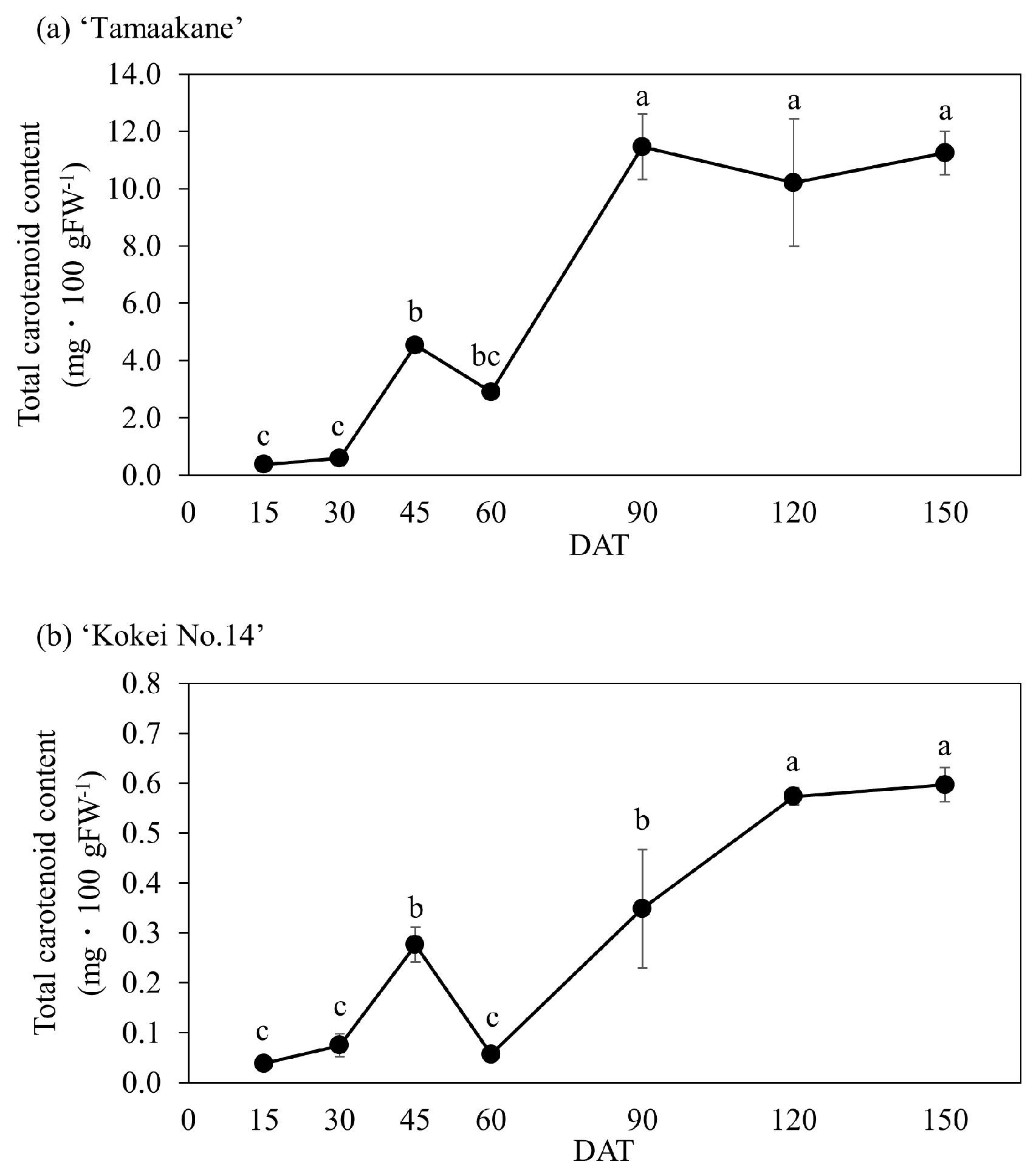
3.1. Total Carotenoid Contents
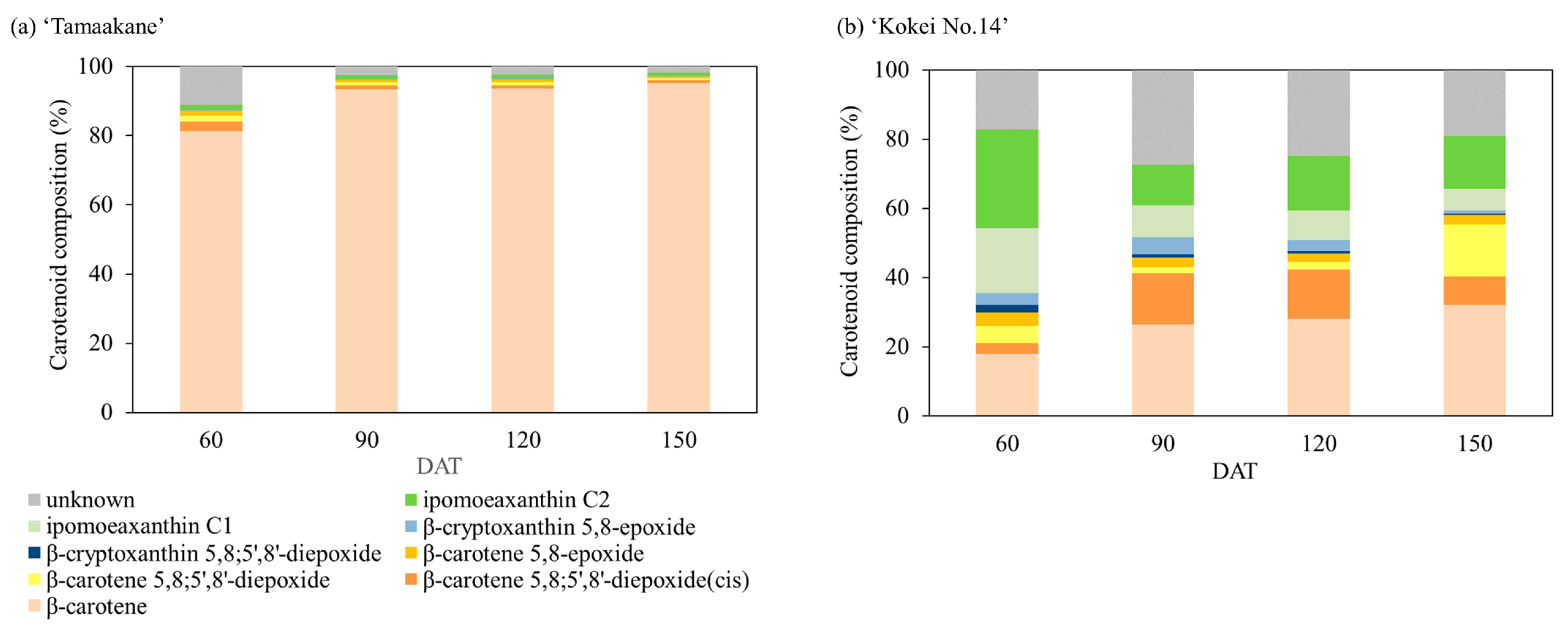
3.2. Carotenoid Composition
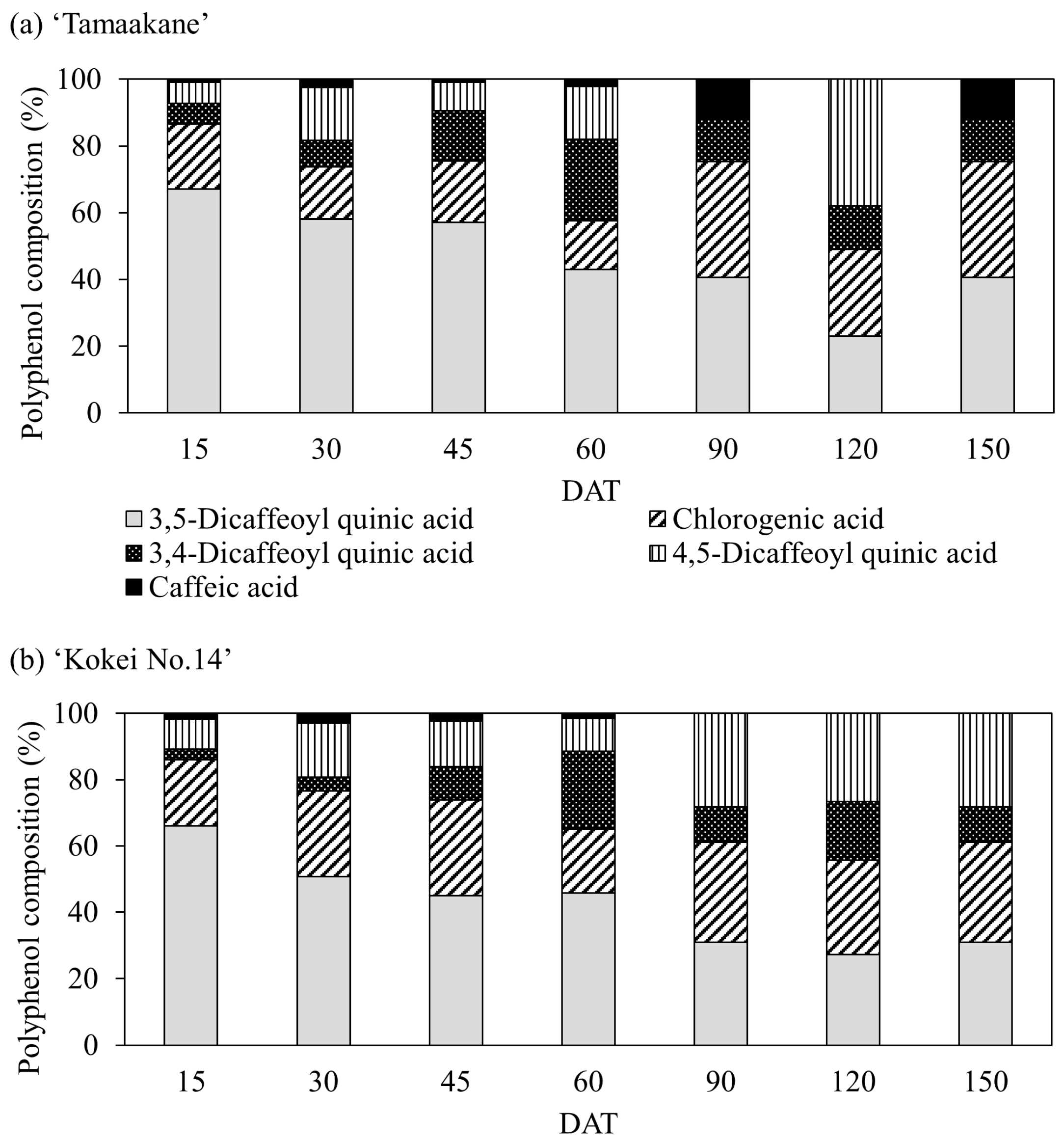
3.3. Polyphenol Content and Composition
3.4. Total Anthocyanin Content
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suematsu, K.; Tanaka, M.; Isobe, S. Identification of a major QTL for root thickness in diploid wild sweetpotato (Ipomoea trifida) using QTL-seq. Plant Product. Sci. 2022, 25, 120–129. [Google Scholar] [CrossRef]
- Park, H.; Abe, T.; Kunitake, H.; Hirano, T. Characterization of a novel mutant with inhibition of storage root formation in sweet potato. Breed. Sci. 2023, 73, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, J.; Sun, L.; Kong, Y.; Chen, J.; Zhu, M.; Xu, T.; Li, Z.; Dong, T. Progress on physiological and molecular mechanisms of storage root formation and development in sweetpotato. Sci. Hortic. 2023, 308, 111588. [Google Scholar] [CrossRef]
- Kim, H.S.; Wang, W.; Kang, L.; Kim, S.E.; Lee, C.J.; Park, S.C.; Park, W.S.; Ahn, M.J.; Kwak, S.S. Metabolic engineering of low-molecular-weight antioxidants in sweetpotato. Plant Biotechnol. Rep. 2020, 14, 193–205. [Google Scholar] [CrossRef]
- Ellong, E.N.; Billard, C.; Adenet, S. Comparison of physicochemical, organoleptic and nutritional abilities of eight sweet potato (Ipomoea batatas) varieties. Food Nutr. Sci. 2014, 5, 42184. [Google Scholar] [CrossRef]
- Nakagawa, S.; Ohmura, R.; Toshima, S.; Park, H.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Changes in polyphenols, anthocyanins, and DPPH radical-scavenging activities in sweetpotato (Ipomoea batatas L.) during tuber growth. Sci. Hortic. 2021, 284, 110100. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yahara, S.; Yoshimoto, M. Changes in polyphenolic content and radical-scavenging activity of sweetpotato (Ipomoea batatas L.) during storage at optimal and low temperatures. J. Agric. Food Chem. 2007, 55, 10773–10778. [Google Scholar] [CrossRef] [PubMed]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, Q.; Chen, Z.; Chen, J.; Geng, S. Evaluation of morphological and phytochemical characteristics of Mesona chinensis populations in southern China. Plant Product. Sci. 2021, 24, 374–387. [Google Scholar] [CrossRef]
- Nagao, N.; Sakuma, Y.; Funakoshi, T.; Itani, T. Variation in antioxidant capacity of the seven azuki bean (Vigna angularis) varieties with different seed coat color. Plant Product. Sci. 2023, 26, 164–173. [Google Scholar] [CrossRef]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; Serio, M.; Amoresano, A. Quantification of polyphenols and metals in Chinese tea infusions by mass spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, Y.; Nakagawa, S.; Ohmura, R.; Toshima, S.; Park, H.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Effect of Growth Stages on Anthocyanins and Polyphenols in the Root System of Sweet Potato. Plants 2023, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Yoshinaga, M.; Kai, Y.; Maoka, T.; Yoshimoto, M. Composition, content and antioxidative activity of the carotenoids in yellow-fleshed sweetpotato (Ipomoea batatas L.). Breed. Sci. 2010, 60, 324–329. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.J.; Lee, H.S.; Kwak, S.S. Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 2012, 74, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.H.; Ahn, Y.O.; Ahn, M.J.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Downregulation of the lycopene ϵ-cyclase gene increases carotenoid synthesis via the β-branch-specific pathway and enhances salt-stress tolerance in sweetpotato transgenic calli. Physiol. Plant 2013, 147, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.J.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Cloning and characterization of an Orange gene that increases carotenoid accumulation and salt stress tolerance in transgenic sweetpotato cultures. Plant Physiol. Biochem. 2013, 70, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Tanaka, M.; Kurata, R.; Kai, Y. Comparative transcriptome analysis implied a ZEP paralog was a key gene involved in carotenoid accumulation in yellow-fleshed sweetpotato. Sci. Rep. 2020, 10, 20607. [Google Scholar] [CrossRef]
- Kang, L.; Park, S.C.; Ji, C.Y.; Kim, H.S.; Lee, H.S.; Kwak, S.S. Metabolic engineering of carotenoids in transgenic sweetpotato. Breed. Sci. 2017, 67, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Hong, Z.; Wei, C.; He, S.Z.; Liu, Q.C. Cloning and functional analysis of lycopene ɛ-cyclase (IbLCYe) gene from sweetpotato, Ipomoea batatas (L.) Lam. J. Integr. Agric. 2013, 12, 773–780. [Google Scholar] [CrossRef]
- Li, R.; Zhai, H.; Kang, C.; Liu, D.; He, S.; Liu, Q. De novo transcriptome sequencing of the orange-fleshed sweet potato and analysis of differentially expressed genes related to carotenoid biosynthesis. Int. J. Gen. Eng. Technol. 2015, 2015, 843802. [Google Scholar] [CrossRef]
- Khan, M.Z.; Takemura, M.; Maoka, T.; Otani, M.; Misawa, N. Carotenoid analysis of sweetpotato Ipomoea batatas and functional identification of its lycopene β-and ε-cyclase genes. Z. Naturforsch. C 2016, 71, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Kang, L.; Kim, H.S.; Xie, T.; Liu, C.; Ji, C.Y.; Kim, S.H.; Park, W.S.; Ahn, M.J.; Wang, S.; et al. Down-regulation of lycopene ε-cyclase expression in transgenic sweetpotato plants increases the carotenoid content and tolerance to abiotic stress. Plant Sci. 2019, 281, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Kobayashi, A.; Sakai, T.; Kuranouchi, T.; Kai, Y. Recent progress in sweetpotato breeding and cultivars for diverse applications in Japan. Breed. Sci. 2017, 67, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, D.; Xiao, S.; Zhang, A.; Deng, Y.; Dai, X.; Zhou, Z.; Ji, Z.; Cao, Q. Comparative Metabolomic and Transcriptomic Analyses of Phytochemicals in Two Elite Sweet Potato Cultivars for Table Use. Molecules 2022, 27, 8939. [Google Scholar] [CrossRef] [PubMed]
- Historical Weather Data Search/2021 Miyazaki/Japan Meteorological Agency. Available online: https://www.data.jma.go.jp/obd/stats/etrn/view/monthly_s1.php?prec_no=87&block_no=47830&year=2021&month=&day=&view=p1 (accessed on 4 June 2024).
- Wrolstad, R.E. Color and Pigment Analyses in Fruit Products. Ph.D. Thesis, Agricultural Experiment Station, Oregon State University, Corvallis, OR, USA, 1976; pp. 1–17. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Schmidt, É.C.; Felix, M.R.D.L.; Kreusch, M.G.; Pereira, D.T.; Costa, G.B.; Simioni, C.; Ouriques, L.C.; Steiner, N.; Chow, F.; Floh, E.S.L.; et al. Profiles of carotenoids and amino acids and total phenolic compounds of the red alga Pterocladiella capillacea exposed to cadmium and different salinities. J. Appl. Phycol. 2016, 28, 1955–1963. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant 1999, 107, 142–149. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Li, R.; Kang, C.; Song, X.; Yu, L.; Liu, D.; He, S.; Zhai, H.; Liu, Q. A ζ-carotene desaturase gene, IbZDS, increases β-carotene and lutein contents and enhances salt tolerance in transgenic sweetpotato. Plant Sci. 2017, 262, 39–51. [Google Scholar] [CrossRef]
- Aghaei, K.; Komatsu, S. Crop and medicinal plants proteomics in response to salt stress. Front. Plant Sci. 2013, 4, 41038. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant 2016, 38, 72. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setoguchi, Y.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.). Horticulturae 2024, 10, 629. https://doi.org/10.3390/horticulturae10060629
Setoguchi Y, Narasako Y, Hirano T, Otani M, Kunitake H. Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.). Horticulturae. 2024; 10(6):629. https://doi.org/10.3390/horticulturae10060629
Chicago/Turabian StyleSetoguchi, Yuno, Yosuke Narasako, Tomonari Hirano, Motoyasu Otani, and Hisato Kunitake. 2024. "Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.)" Horticulturae 10, no. 6: 629. https://doi.org/10.3390/horticulturae10060629
APA StyleSetoguchi, Y., Narasako, Y., Hirano, T., Otani, M., & Kunitake, H. (2024). Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.). Horticulturae, 10(6), 629. https://doi.org/10.3390/horticulturae10060629







