Different Oligosaccharides Induce Coordination and Promotion of Root Growth and Leaf Senescence during Strawberry and Cucumber Growth
Abstract
1. Introduction
2. Materials and Methods
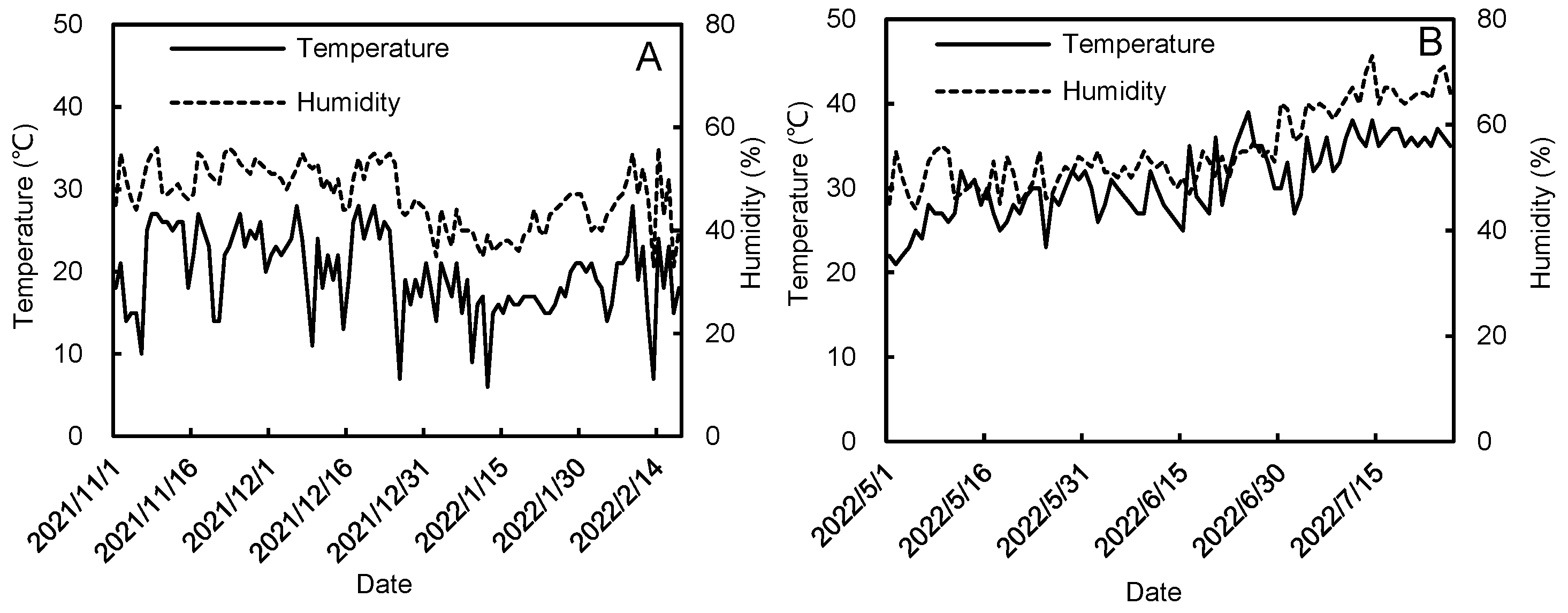
2.1. Experimental Site
2.2. Experimental Materials and Design
2.3. Photosynthetic Physiological Characteristics
2.4. Root Activity and Morphology
2.5. Yield
2.6. Statistical Analysis
3. Results
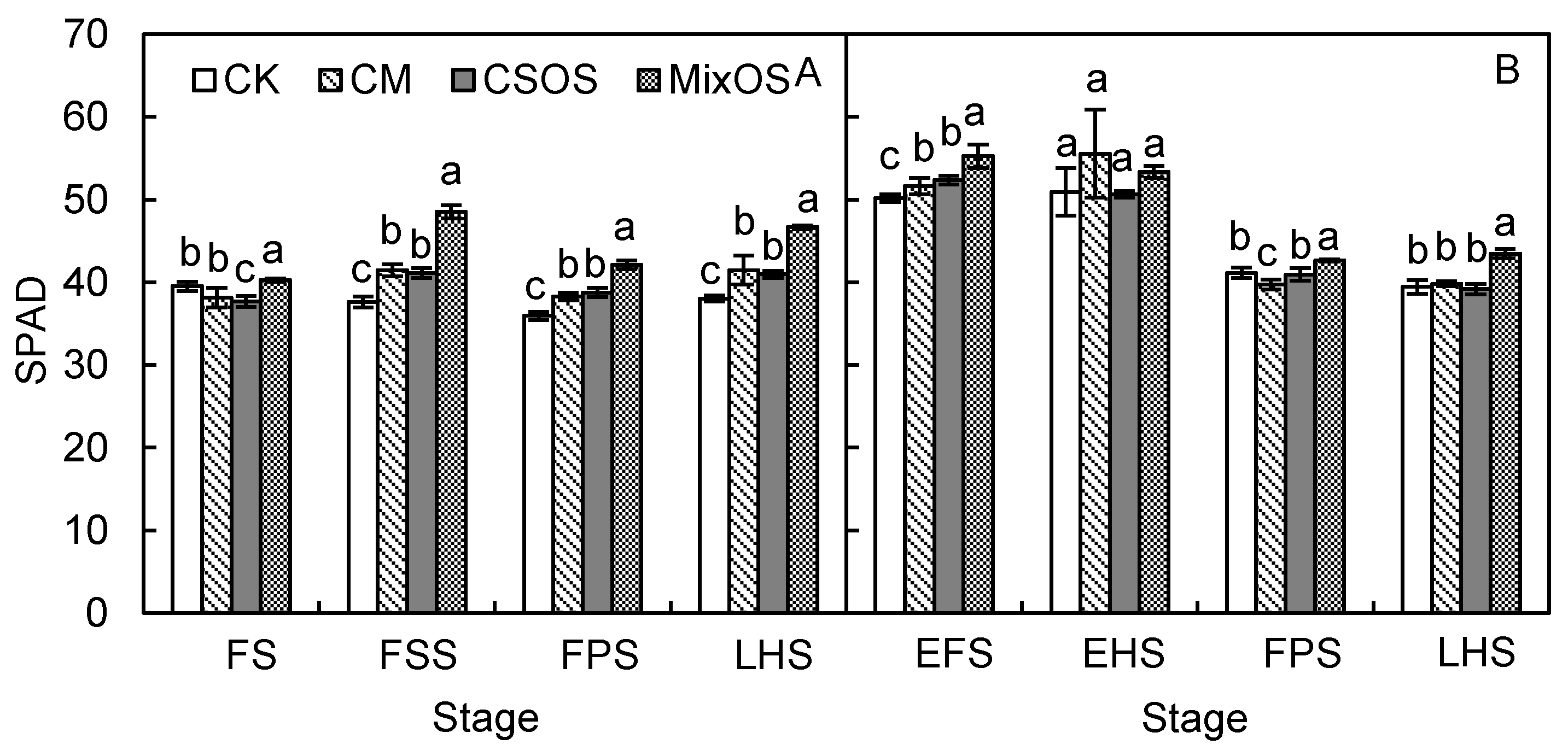
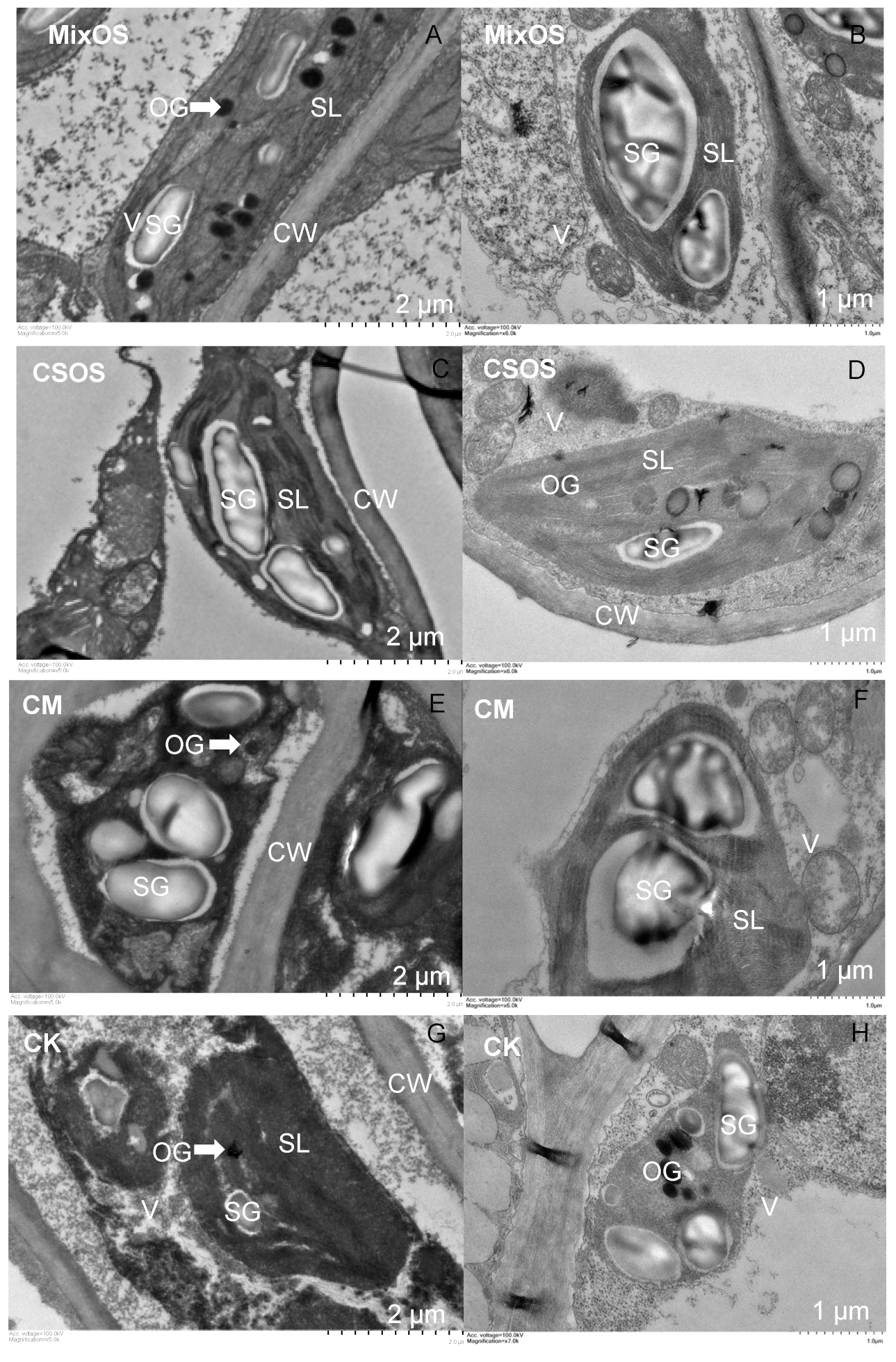
3.1. Dynamic Changes of SPAD and Chloroplast Ultrastructure in Plant Leaves
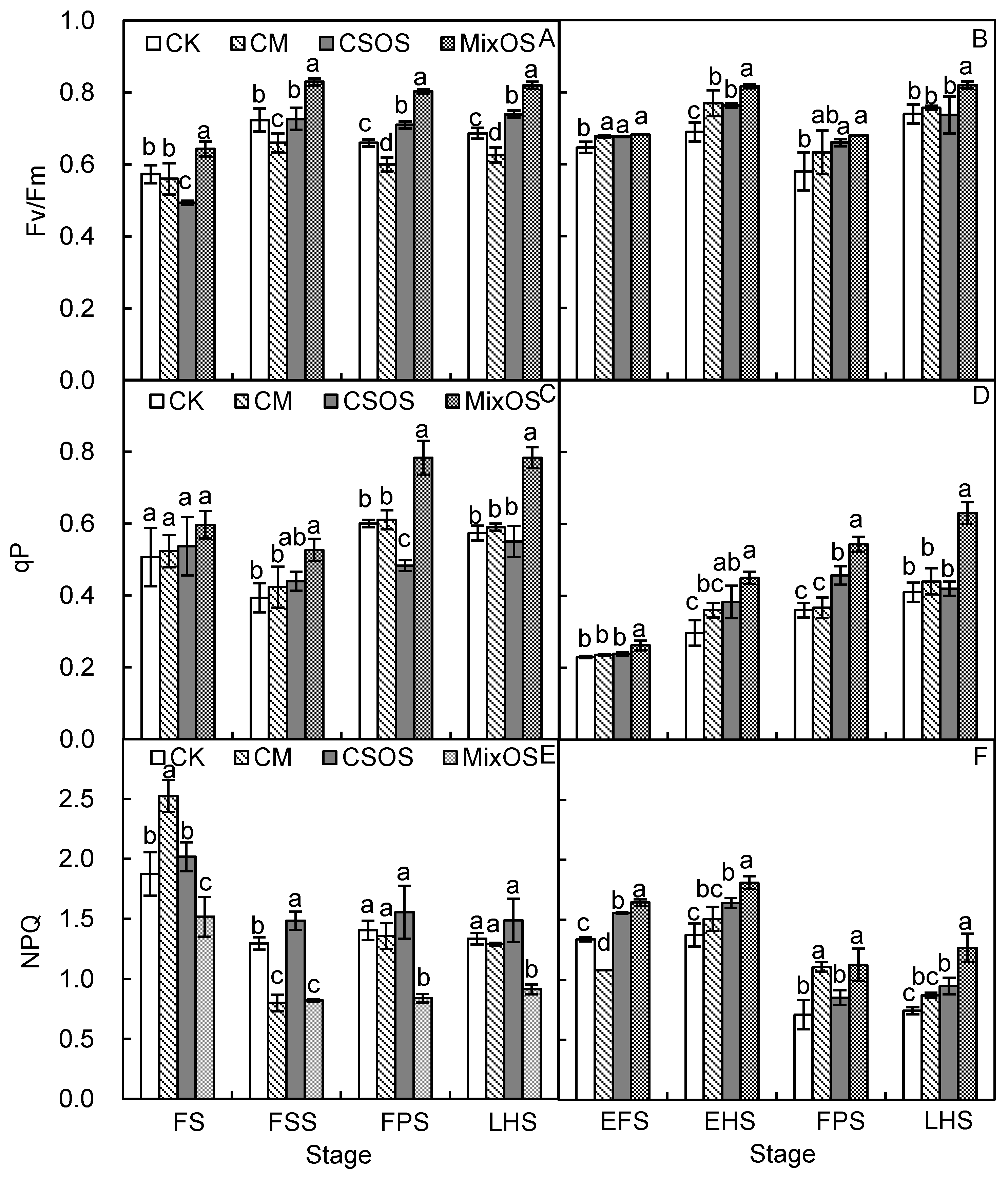
3.2. Dynamic Changes in Leaf Chlorophyll Fluorescence
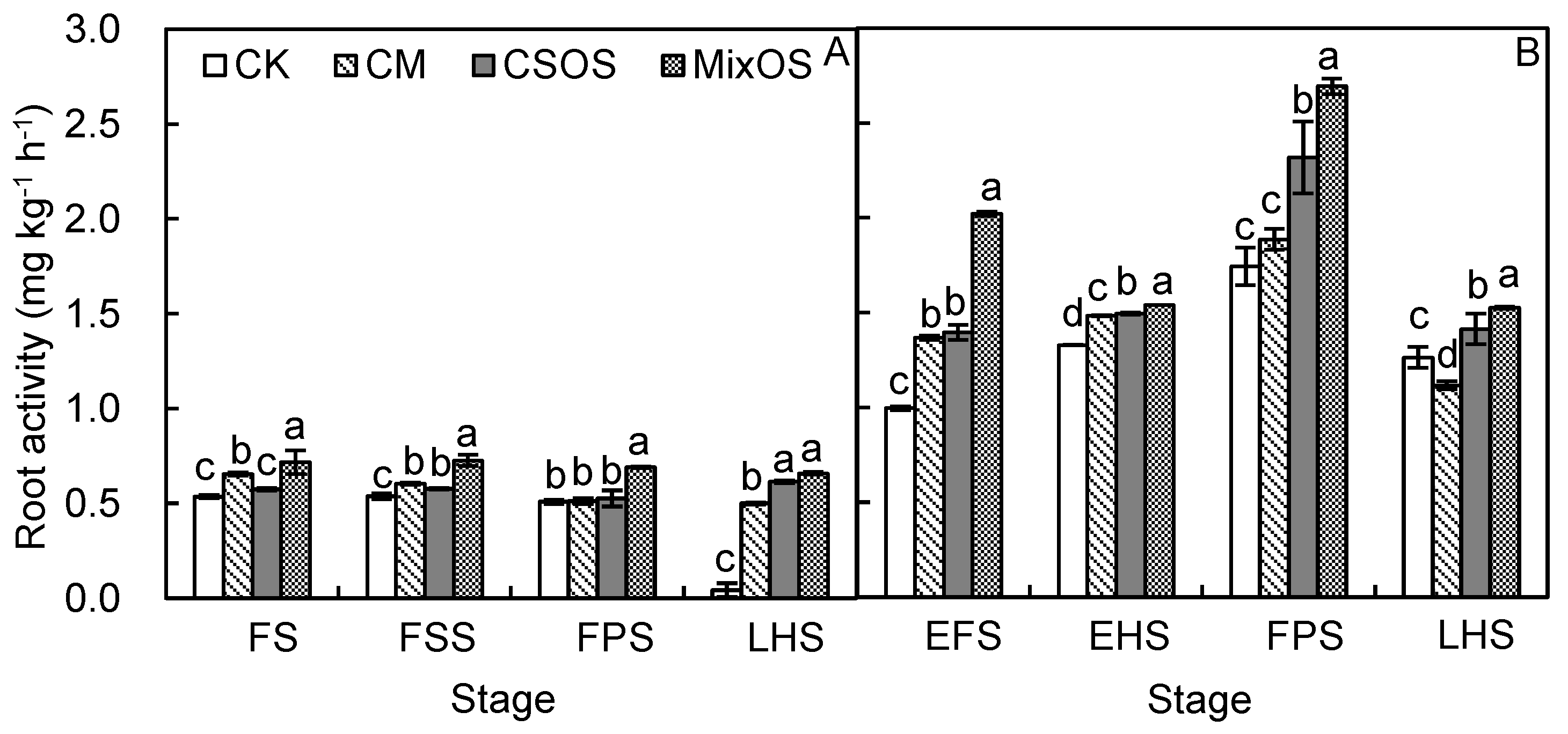
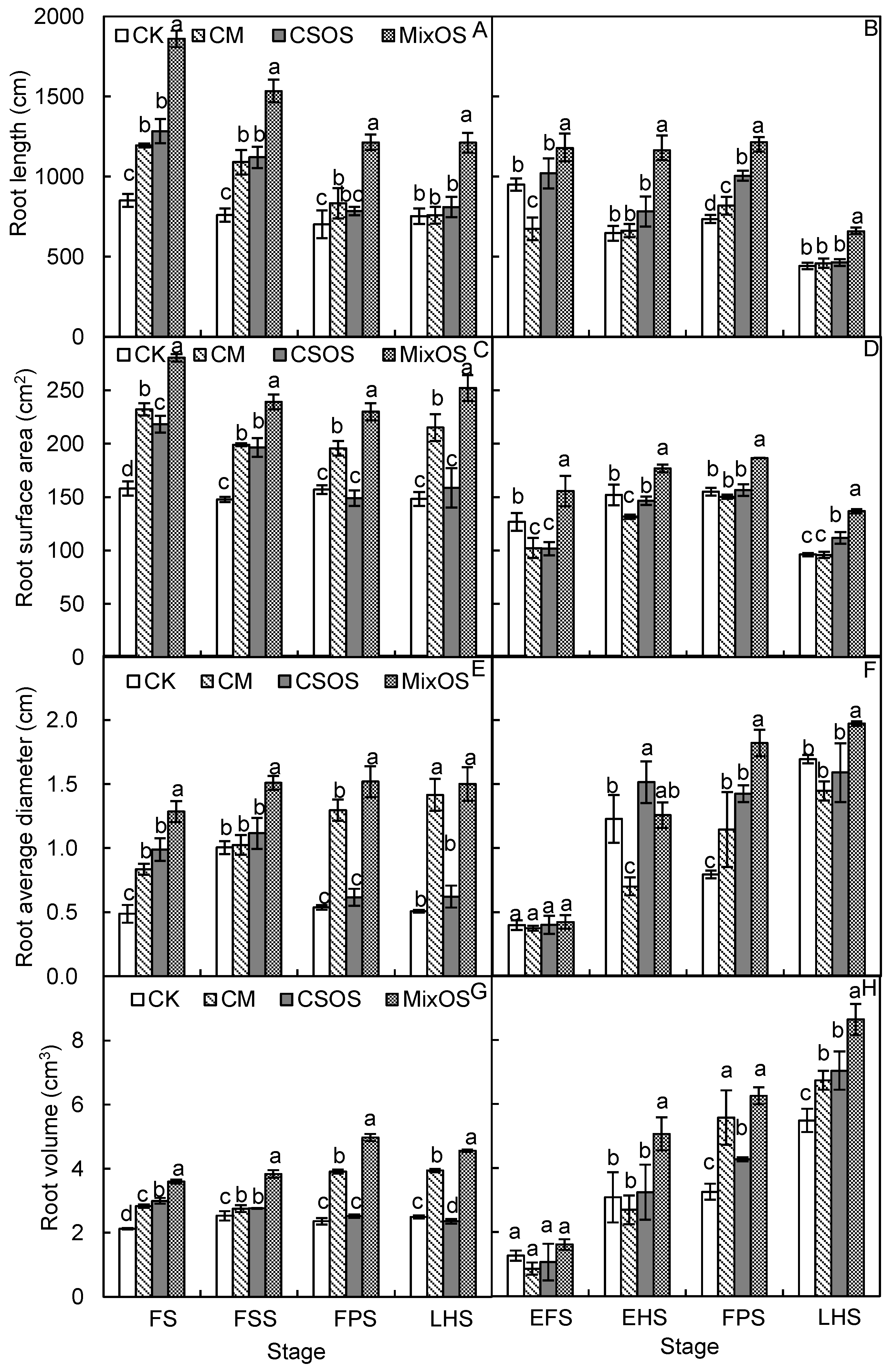
3.3. Dynamic Changes in Root Morphology and Activity
3.4. Regulation of Strawberry and Cucumber Yield by Spraying Oligosaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galli, V.; Da Silva Messias, R.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. Food Sci. Technol. 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Zhang, S.G.; Li, J.Y.; Li, J.F.; Du, N.; Li, D.H.; Li, F.Y.; Man, J. Application status and technical analysis of chitosan-based medical dressings: A review. RSC Adv. 2020, 10, 34308–34322. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.S.; Li, J.; Chankvetadze, B.; Cheng, Y.P.; Xu, J.; Liu, X.G.; Li, Y.B.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral triazole fungicide difenoconazole: Absolute stereochemistry, stereoselective bioactivity, aquatic toxicity, and environmental behavior in vegetables and soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, D.; Hu, M.Q.; Chen, L.Y.; Xu, C.L. Chromatographic analysis and residue degradation of phenamacril and difenoconazole on strawberries. Food Addit. Contam. Part A 2021, 38, 2012–2115. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Lee, D.J.; Siddique, K.H.M. Sustainable agricultural practices for food security and ecosystem services. Environ. Sci. Pollut. Res. 2022, 29, 84076–84095. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Metwaly, E.E.; AL-Huqail, A.A.; Farouk, S.; Omar, G.F. Effect of chitosan and micro-carbon-based phosphorus fertilizer on strawberry growth and productivity. Horticulturae 2023, 9, 368. [Google Scholar] [CrossRef]
- Wang, Q.S.; Zhou, X.; Liu, Y.; Han, Y.; Zuo, J.; Deng, J.; Yuan, L.Y.; Gao, L.J.; Bai, W.B. Mixed oligosaccharides-induced changes in bacterial assembly during cucumber (Cucumis Sativus L.) growth. Front Microbiol 2023, 14, 1195096. [Google Scholar] [CrossRef] [PubMed]
- He, J.X.; Han, W.; Wang, J.; Qian, Y.C.; Saito, M.; Bai, W.B.; Song, J.Q.; Lv, G.H. Functions of oligosaccharides in improving tomato seeding growth and chilling resistance. J. Plant Growth Regul. 2022, 41, 1394–1395. [Google Scholar] [CrossRef]
- Ru, L.; Jiang, L.F.; Wills, R.B.H.; Golding, J.B.; Huo, Y.R.; Yang, H.Q.; Li, Y.X. Chitosan oligosaccharides induced chilling resistance in cucumber fruit and associated stimulation of antioxidant and HSP gene expression. Sci. Hortic. 2020, 264, 109187. [Google Scholar] [CrossRef]
- Tan, C.; Li, N.; Wang, Y.D.; Yu, X.J.; Yang, L.; Cao, R.F.; Ye, X.L. Integrated physiological and transcriptomic analyses revealed improved cold tolerance in cucumber (Cucumis sativus L.) by exogenous chitosan oligosaccharide. Int. J. Mol. Sci. 2023, 24, 6202. [Google Scholar] [CrossRef]
- Abd-Elrahman, S.H.; El-Gabry, Y.A.E.G.; Hashem, F.A.; Ibrahim, M.F.M.; El-Hallous, E.I.; Abbas, Z.K.; Darwish, D.B.E.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Taha, N.M. Influence of nano-chitosan loaded with potassium on potassium fractionation in sandy soil and strawberry productivity and quality. Agronomy 2023, 13, 1126. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Del Valle, T.A.; Zenatti, T.F.; Antonio, G.; Campana, M.; Gandra, J.R.; Zilio, E.M.C.; de Mattos, L.F.A.; de Morais, J.G.P. Effect of chitosan on the preservation quality of sugarcane silage. Grass Forage Sci. 2018, 73, 630–638. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, S.Y.; Lan, W.T.; Qin, W. Fabrication and testing of PVA/chitosan bilayer films for strawberry packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- He, Y.Q.; Bose, S.K.; Wang, M.Y.; Liu, T.M.; Wang, W.X.; Lu, H.; Yin, H. Effects of chitosan oligosaccharides postharvest treatment on the quality and ripening related gene expression of cultivated strawberry fruits. J. Berry Res. 2019, 9, 11–25. [Google Scholar] [CrossRef]
- Zhang, T.G.; Shi, Z.F.; Zhang, X.H.; Zheng, S.; Wang, J.; Mo, J.N. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Winkler, A.J.; Dominguez-Nunez, J.A.; Aranaz, I.; Poza-Carrion, C.; Ramonell, K.; Somerville, S.; Berrocal-Lobo, M. Short-chain chitin oligomers: Promoters of plant growth. Mar. Drugs 2017, 15, 40. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, K.C.; Liu, S.; Xing, R.G.; Yu, H.H.; Chen, X.L.; Li, P.C. Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohydr. Polym. 2016, 138, 27–33. [Google Scholar] [CrossRef]
- Liu, X.W.; Li, X.F.; Bai, Y.X.; Zhou, X.; Chen, L.; Qiu, C.; Lu, C.; Jin, Z.Y.; Long, J.; Xie, Z.J. Natural antimicrobial oligosaccharides in the food industry. Int. J. Food Microbiol. 2023, 386, 110021. [Google Scholar] [CrossRef]
- He, J.X.; Kong, M.; Qian, Y.C.; Gong, M.; Lv, G.H.; Song, J.Q. Cellobiose elicits immunity in lettuce conferring resistance to Botrytis cinerea. J. Exp. Bot. 2023, 74, 1022–1038. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Hu, P.P.; Lin, M.L.; Ye, X.; Chen, L.S.; Huang, Z.R. Photosynthetic characteristics and chloroplast ultrastructure responses of citrus leaves to copper toxicity induced by bordeaux mixture in greenhouse. Int. J. Mol. Sci. 2022, 23, 9835. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhang, Z.L. Experimental Supervision of Plant Physiology; Higher Education Press: Beijing, China, 2016. [Google Scholar]
- Zhang, Y.; Guo, R.Y.; Li, S.H.; Chen, Y.; Li, Z.D.; He, P.Y.; Huang, X.Y.; Huang, K.F. Effects of continuous cropping on soil, senescence, and yield of Tartary buckwheat. Agron. J. 2021, 113, 5102–5113. [Google Scholar] [CrossRef]
- Song, H.X.; Li, Y.L.; Xu, X.Y.; Zhang, J.; Zheng, S.W.; Hou, L.P.; Xing, G.M.; Li, M.L. Analysis of genes related to chlorophyll metabolism under elevated CO2 in cucumber (Cucurnis sativus L.). Sci. Hortic. 2020, 261, 108988. [Google Scholar] [CrossRef]
- Siddique, M.I.; Han, K.; Lee, J.U.; Lee, E.S.; Lee, Y.R.; Lee, H.E.; Lee, S.Y.; Kim, D. QTL Analysis for chlorophyll content in strawberry (Fragaria x ananassa Duch.) leaves. Agriculture 2021, 11, 1163. [Google Scholar] [CrossRef]
- Wang, F.B.; Liu, J.C.; Chen, M.X.; Zhou, L.J.; Li, Z.W.; Zhao, Q.; Pan, G.; Zaidi, S.H.R.; Cheng, F.M. Involvement of abscisic acid in PSII photodamage and D1 protein turnover for light-induced premature senescence of rice flag leaves. PLoS ONE 2016, 11, e0161203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Masclaux-Daubresse, C. Current understanding of leaf senescence in rice. Int. J. Mol. Sci. 2021, 22, 4515. [Google Scholar] [CrossRef] [PubMed]
- Palencia, P.; Martinez, F.; Vazquez, M.A. Oxyfertigation and transplanting conditions of strawberries. Agronomy 2022, 11, 2513. [Google Scholar] [CrossRef]
- Liu, X.S.; An, R.H.; Li, G.F.; Luo, S.F.; Hu, H.L.; Li, P.X. Melatonin delays leaf senescence in pak choi (Brassica rapa subsp. chinensis) by regulating biosynthesis of the second messenger cGMP. Hortic. Plant J. 2024, 10, 145–155. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in finger millet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Chen, Y.Q.; Hu, Z.C.; Ma, S.; Zhang, J.E.; Shen, H. Alginate oligosaccharides alleviate the damage of rice leaves caused by acid rain and high temperature. Agronomy 2021, 11, 500. [Google Scholar] [CrossRef]
- Fu, E.R.; Zhang, Y.Z.; Li, H.L.; Wang, X.Z.; Zhang, H.X.; Xiao, W.; Chen, X.D.; Li, L. Chitosan reduces damages of strawberry seedlings under high-temperature and high-light stress. Agronomy 2023, 13, 517. [Google Scholar] [CrossRef]
- Valkama, E.; Kivimaenpaa, M.; Hartikainen, H.; Wulff, A. The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria × ananassa) and barley (Hordeum vulgare) treated in the field. Agric. For. Meteorol. 2003, 120, 267–278. [Google Scholar] [CrossRef]
- Sang, C.W.; Ren, W.C.; Wang, J.J.; Xu, H.; Zhang, Z.H.; Zhou, M.G.; Chen, C.J.; Wang, K. Detection and fitness comparison of target-based highly fludioxonil-resistant isolates of Botrytis cinerea from strawberry and cucumber in China. Pestic. Biochem. Physiol. 2018, 147, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fanello, D.D.; Kelly, S.J.; Bartoli, C.G.; Cano, M.G.; Alonso, S.M.; Guiamet, J.J. Plasticity of root growth and respiratory activity: Root responses to above-ground senescence, fruit removal or partial root pruning in soybean. Plant Sci. 2020, 290, 110296. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Dalpe, Y.; Fang, C.Q.; Dube, C.; Khanizadeh, S. Influence of arbuscular mycorrhizae on biomass and root morphology of selected strawberry cultivars under salt stress. Botany 2011, 89, 397–403. [Google Scholar] [CrossRef]
- Kaya, C.; Kirnak, H.; Higgs, D. Effects of supplementary potassium and phosphorus on physiological development and mineral nutrition of cucumber and pepper cultivars grown at high salinity (NaCl). J. Plant Nutr. 2001, 24, 1457–1471. [Google Scholar] [CrossRef]
| Treatment | Code | Foliar Operation | Note |
|---|---|---|---|
| Conventional control | CM | No foliar spraying | Completely consistent with farmers’ conventional planting management |
| Black control | CK | Strawberry experiment: foliar spraying with different solutions at 30 d, 37 d, 44 d, 51 d, and 58 d after transplanting Cucumber experiment: foliar spraying with different solutions at 7 d, 14 d, 21 d, 28 d, and 35 d after transplanting | Foliar spraying with the equal amount of tap water |
| Chitosan oligosaccharide | CSOS | Foliar spraying with the single source of chitosan oligosaccharide | |
| Mixed oligosaccharide | MixOS | Foliar spraying with the mixed oligosaccharides from multiple sources containing chitosan oligosaccharide |
| Treatment | Strawberry Experiment | Cucumber Experiment | ||||
|---|---|---|---|---|---|---|
| Single Fruit Weight (g) | Total Fruit Number per Plant | Total Yield (103 kg·ha−1) | Single Fruit Weight (g) | Total Fruit Weight per Plant (kg) | Total Yield (103 kg·ha−1) | |
| CK | 10.0 ± 0.33 c | 4.4 ± 0.58 c | 10.9 ± 0.15 c | 105.8 ± 8.38 b | 6.49 ± 0.36 b | 30.5 ± 2.74 b |
| CM | 12.3 ± 0.09 b | 5.0 ± 1.00 bc | 11.8 ± 0.48 b | 112.1 ± 4.44 b | 6.56 ± 0.23 b | 30.8 ± 0.73 b |
| CSOS | 12.2 ± 0.09 b | 7.3 ± 0.58 ab | 12.1 ± 0.96 b | 110.1 ± 7.32 b | 7.10 ± 0.25 b | 33.7 ± 2.06 b |
| MixOS | 14.0 ± 0.18 a | 8.3 ± 0.58 a | 13.6 ± 0.27 a | 137.8 ± 29.66 a | 8.11 ± 0.42 a | 38.3 ± 1.95 a |
| Index | Strawberry Experiment | Cucumber Experiment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPAD | qP | RA | RL | RSA | RAD | IFW | SPAD | Fv/Fm | qP | RA | RL | RV | TFW | |
| NPQ | −0.863 | −0.986 * | 0.867 | 0.946 | 0.986 * | |||||||||
| RA | 0.932 | 0.750 | 0.840 | 0.932 | 0.980 * | |||||||||
| RL | 0.997 ** | 0.914 | 0.942 | 0.795 | 0.799 | 0.961 * | 0.953 * | |||||||
| RAD | 0.930 | 0.866 | 0.913 | 0.909 | 0.998 ** | 0.581 | 0.670 | 0.851 | 0.893 | 0.953 * | ||||
| RV | 0.946 | 0.918 | 0.886 | 0.923 | 0.990 ** | 0.993 ** | 0.972 * | 0.956 * | 0.981 * | 0.944 | 0.891 | |||
| IFW | 0.933 | 0.759 | 0.999 ** | 0.940 | 0.949 | 0.930 | 0.976 * | 0.886 | 0.971 * | 0.905 | 0.904 | 0.983 * | ||
| TFW | 0.850 | 0.869 | 0.987 * | 0.980 * | 0.992 ** | 0.938 | ||||||||
| TY | 0.983 * | 0.860 | 0.966 * | 0.993 ** | 0.917 | 0.891 | 0.962 * | 0.832 | 0.866 | 0.983 * | 0.983 * | 0.992 ** | 0.929 | 0.999 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Han, Y.; Han, W.; Yang, Y.; Saito, M.; Lv, G.; Song, J.; Bai, W. Different Oligosaccharides Induce Coordination and Promotion of Root Growth and Leaf Senescence during Strawberry and Cucumber Growth. Horticulturae 2024, 10, 627. https://doi.org/10.3390/horticulturae10060627
Xu Y, Han Y, Han W, Yang Y, Saito M, Lv G, Song J, Bai W. Different Oligosaccharides Induce Coordination and Promotion of Root Growth and Leaf Senescence during Strawberry and Cucumber Growth. Horticulturae. 2024; 10(6):627. https://doi.org/10.3390/horticulturae10060627
Chicago/Turabian StyleXu, Yanan, Yan Han, Wei Han, Yigang Yang, Makoto Saito, Guohua Lv, Jiqing Song, and Wenbo Bai. 2024. "Different Oligosaccharides Induce Coordination and Promotion of Root Growth and Leaf Senescence during Strawberry and Cucumber Growth" Horticulturae 10, no. 6: 627. https://doi.org/10.3390/horticulturae10060627
APA StyleXu, Y., Han, Y., Han, W., Yang, Y., Saito, M., Lv, G., Song, J., & Bai, W. (2024). Different Oligosaccharides Induce Coordination and Promotion of Root Growth and Leaf Senescence during Strawberry and Cucumber Growth. Horticulturae, 10(6), 627. https://doi.org/10.3390/horticulturae10060627






