Comparison of the Climate Change Tolerance of Native and Non-Native Species Used or Potentially Used as Ornamentals in Mediterranean Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination Assays
2.2. Germination Modelling
2.3. Statistical Analysis
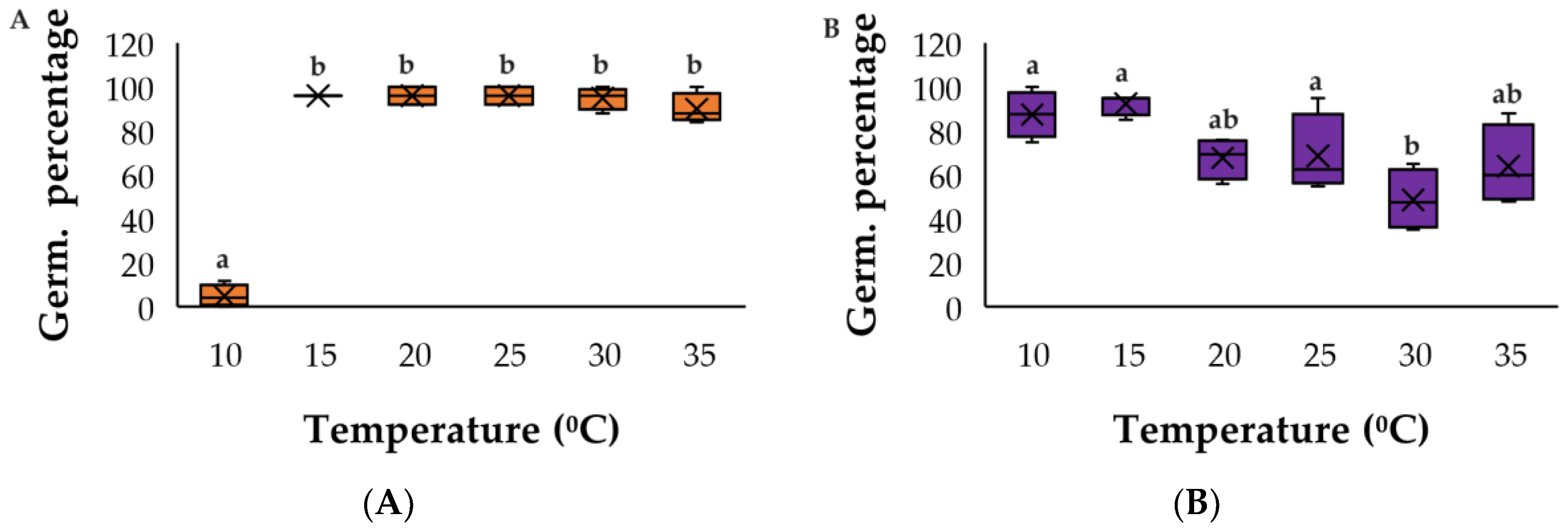
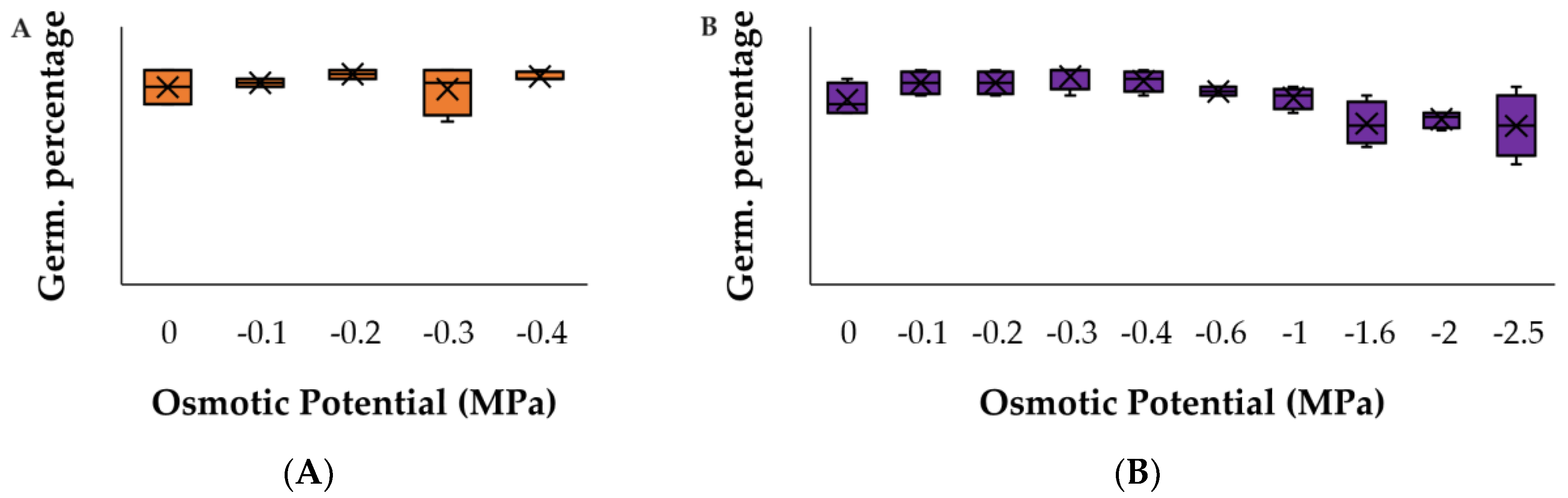
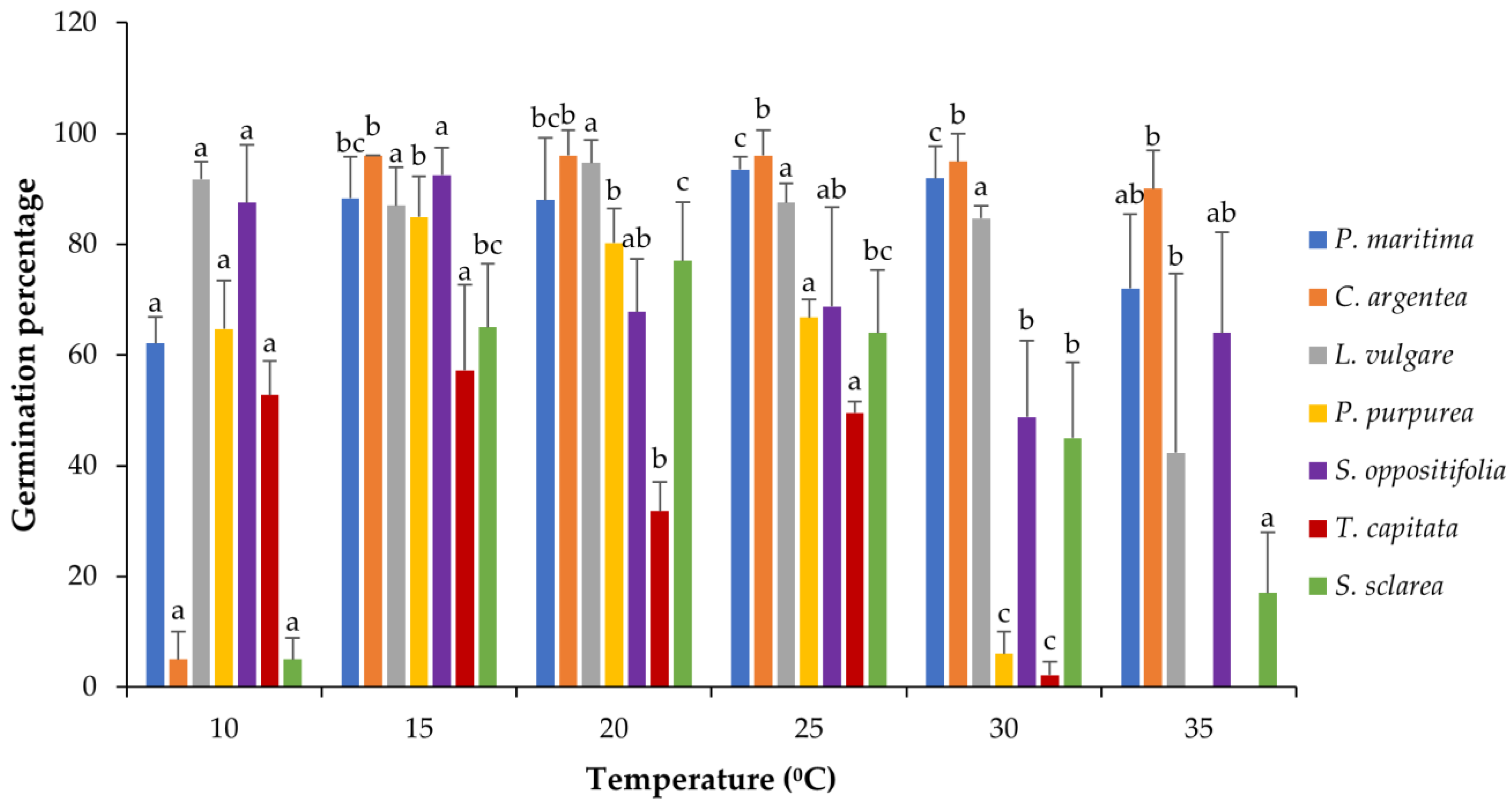
3. Results
3.1. Temperature Assays
3.2. Water Stress Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
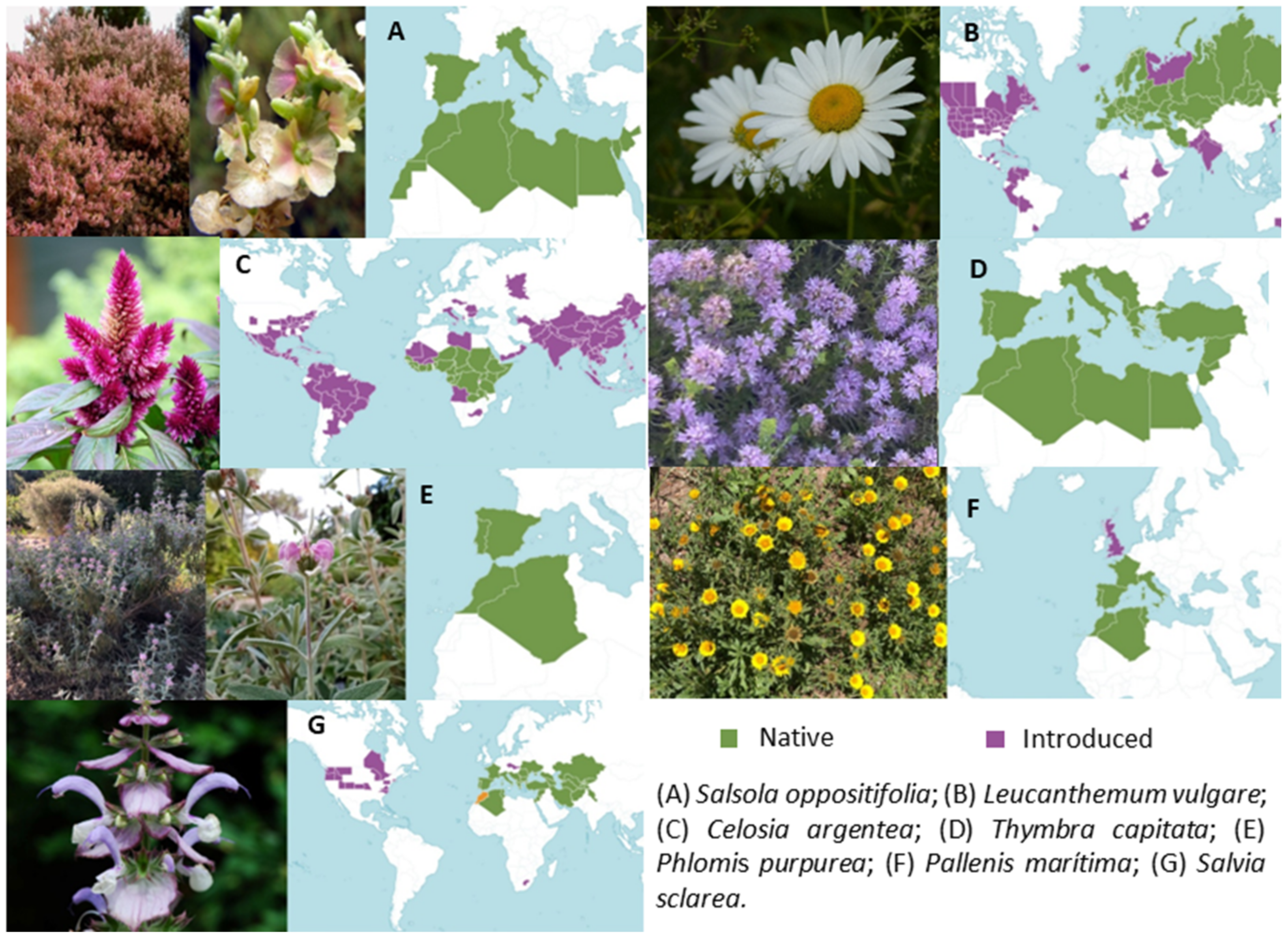
References
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Paris, France, 2023; Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 14 March 2024).
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Chapter 11: Regional Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Foruth Assessment Report of the Intergovernmental Panel on climate Change Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Petit, R.; Hampe, A.; Cheddadi, R. Climate changes and tree phylogeography in the Mediterranean. Taxon 2005, 54, 877–885. [Google Scholar] [CrossRef]
- MedECC. First Mediterranean Assessment Report (MAR1). 2020. Available online: https://climate.copernicus.eu/notes-editors (accessed on 15 March 2024).
- Egerer, M.H.; Lin, B.B.; Threlfall, C.G.; Kendal, D. Temperature variability influences urban garden plant richness and gardener water use behavior, but not planting decisions. Sci. Total Environ. 2019, 646, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Naudiyal, N.; Wang, J.; Gaire, N.P.; Wu, Y.; Wei, Y.; He, J.; Wang, C. Assessing the impact of climate change on potential distribution of Meconopsis punicea and its influence on ecosystem services supply in the southeastern margin of Qinghai-Tibet Plateau. Front. Plant Sci. 2022, 12, 3338. [Google Scholar] [CrossRef]
- Sari, D.; Karaşah, B. Future adaptability of urban trees due to the effects of climate change: The case of Artvin, Turkey. J. Environ. Sci. Manag. 2020, 23, 60–70. [Google Scholar] [CrossRef]
- Marco, A.; Barthelemy, C.; Dutoit, T.; Bertaudière-Montes, V. Bridging human and natural sciences for a better understanding of urban floral patterns: The role of planting practices in Mediterranean gardens. Ecol. Soc. 2010, 15, 1–18. [Google Scholar] [CrossRef]
- Saurí, D. Lights and shadows of urban water demand management: The case of the metropolitan region of Barcelona. Eur. Plan. Stud. 2003, 11, 229–243. [Google Scholar] [CrossRef]
- Wade, G.L.; Midcap, J.T.; Coder, K.D.; Landry, G.W.; Tyson, A.W.; Weatherly, N., Jr. Xeriscape: A Guide to Developing a Water-wise Landscape; University of Georgia: Athens, GA, USA, 2010; Available online: https://esploro.libs.uga.edu/esploro/outputs/report/Xeriscape-a-guide-to-developing-a/9949316545202959#file-0 (accessed on 10 March 2024).
- Kattamanchi, K.; Thaneshwari, T.; Kumari, P. Xeriscapping–water efficient gardening. Int. J. All Res. Educ. Sci. Methods 2020, 8, 830–836. [Google Scholar]
- Çetin, N.; Mansuroğlu, S.; Önaç, A.K. Xeriscaping feasibility as an urban adaptation method for global warming: A case study from Turkey. Pol. J. Environ. Stud. 2018, 27, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Lumbreras, E.L.; Ruiz, S.R.; Casas, J. Ornamental potential of the coastal plant Lapiedra martinezii Lag. (Amaryllidaceae): The role of its revalorization in xero-gardening and ex-situ conservation. Nereis Rev. Iberoam. Interdiscip. Métodos Model. Simul. 2021, 13, 211–226. [Google Scholar] [CrossRef]
- Leotta, L.; Toscano, S.; Ferrante, A.; Romano, D.; Francini, A. New strategies to increase the abiotic stress tolerance in woody ornamental plants in Mediterranean climate. Plants 2023, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Soltani, A. Meta-analysis of seed priming effects on seed germination, seedling emergence and crop yield: Iranian studies. Int. J. Plant Prod. 2015, 9, 413–432. [Google Scholar]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Postgermination, Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- El-Madidi, S.A.I.D.; El-Baroudi, B.R.A.H.I.M.; Aameur, F.B. Effects of salinity on germination and early growth of barley (Hordeum vulgare L.) cultivars. Int. J. Agric. Biol. 2004, 6, 767–770. [Google Scholar]
- García-Huidobro, J.; Monteith, J.L.; Squire, G.R. Time, temperature and germination of pearl millet. J. Exp. Bot. 1982, 33, 288–296. [Google Scholar] [CrossRef]
- Trudgill, D.L. Why do tropical poikilothermic organisms tend to have higher threshold temperatures for development than temperate ones? Funct. Ecol. 1995, 9, 136–137. [Google Scholar]
- Gummerson, R.J. The effect of constant temperatures and osmotic potentials on the germination of sugar beet. J. Exp. Bot. 1986, 37, 729–741. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A.J. Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 1999, 50, 655–664. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rayner, G.D. Robustness to non-normality of common tests for the many-sample location problem. J. Appl. Math. Decis. Sci. 2003, 7, 187–206. [Google Scholar] [CrossRef]
- Alpert, P.T.; Ben-Gai, A.; Baharad, Y.; Benjamini, D.; Yekutieli, M.; Colacino, L.; Diodato, C.; Ramis Homar, V.; Romero, R.; Michaelides, S.; et al. The paradoxical increase of Mediterranean extreme daily rainfall in spite of decrease in total values. Geophys. Res. Lett. 2002, 29, 31-1–31-4. [Google Scholar] [CrossRef]
- Kuglitsch, F.G.; Toreti, A.; Xoplaki, E.; Della-Marta, P.M.; Zerefos, C.S.; Türkeş, M.; Luterbacher, J. Heat wave changes in the eastern Mediterranean since 1960. Geophys. Res. Lett. 2010, 37, L04802. [Google Scholar] [CrossRef]
- Paz, S.; Negev, M.; Clermont, A.; Green, M.S. Health aspects of climate change in cities with Mediterranean climate, and local adaptation plans. Int. J. Environ. Res. Public Health 2016, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Darras, A.I. Implementation of sustainable practices to ornamental plant cultivation worldwide: A critical review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Ruíz-Pérez, M.R.; Alba-Rodríguez, M.D.; Marrero, M. The water footprint of city naturalisation. Evaluation of the water balance of city gardens. Ecol. Model. 2020, 424, 109031. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Maleki, K.; Soltani, E.; Arabhosseini, A.; Aghili Lakeh, M. A quantitative analysis of primary dormancy and dormancy changes during burial in seeds of Brassica napus. Nord. J. Bot. 2021, 39, e03281. [Google Scholar] [CrossRef]
- Mason, J.; Hopkins, A.; Hopper, J. The best environmental conditions for the germination of Celosia argentea L. CCAMLR Sci. 2019, 26, 416–423. [Google Scholar]
- Gómez, F.; Giménez, E.; Delgado, I.; de Haro, S.; del Moral, F. Estimación de los rangos de tolerancia a los factores de diversas especies mediterráneas de interés ecológico-forestal. Lazaroa 2009, 30, 145–159. [Google Scholar]
- Collins, A.; Stock, M.; Lewis, M.A.; Hansen, S.M. Celosia Cut Flower Production; USU Extension Publications, Utah State University: Logan, UT, USA, 2022. [Google Scholar]
- Okusanya, O.T. Germination and growth of Celosia cristata L., under various light and temperature regimes. Am. J. Bot. 1980, 67, 854–858. [Google Scholar] [CrossRef]
- Constantin, M.F.; Dobrin, A.; Constantin, C.G.; Toma, F. Research on the behavior of ornamental species in saline soils conditions. Sci. Pap. Ser. B Hortic. 2023, 67, 356–366. [Google Scholar]
- Moruno, F.; Soriano, P.; Vicente, O.; Boscaiu, M.; Estrelles, E. Opportunistic germination behaviour of Gypsophila (Caryophyllaceae) in two priority habitats from semi-arid Mediterranean steppes. Not. Bot. Horti Agrobot. 2011, 39, 18–23. [Google Scholar] [CrossRef]
- Estrelles, E.; Biondi, E.; Galiè, M.; Mainardi, F.; Hurtado, A.; Soriano, P. Aridity level, rainfall pattern and soil features as key factors in germination strategies in salt-affected plant communities. J. Arid. Environ. 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Tribulato, A.; Patanè, C. Effects of drought stress on seed germination of ornamental sunflowers. Acta Physiol. Plant. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Gairola, S.; Hameed, A.; Rasheed, A.; AlKetbi, A.; Aljasmi, M.; El-Keblawy, A. Seed germination and salinity tolerance of habitat-indifferent halophytes as associated with geographical distribution. Seed Sci. Technol. 2022, 50, 125–140. [Google Scholar] [CrossRef]
- Maleki, K.; Soltani, E.; Seal, C.E.; Colville, L.; Pritchard, H.W.; Lamichhane, J.R. The seed germination spectrum of 486 plant species: A global meta-regression and phylogenetic pattern in relation to temperature and water potential. Agric. For. Meteorol. 2024, 346, 109865. [Google Scholar] [CrossRef]
- Vicente, M.J.; Martínez-Díaz, E.; Martínez-Sánchez, J.J.; Franco, J.A.; Bañón, S.; Conesa, E. Effect of light, temperature, and salinity and drought stresses on seed germination of Hypericum ericoides, a wild plant with ornamental potential. Sci. Hortic. 2020, 270, 109433. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, R.; Ashraf, M.Y.; Ashraf, M.; Waraich, E.A. Sunflower (Helianthus annuus L.) response to drought stress at germination and seedling growth stages. Pak. J. Bot. 2009, 41, 647–654. [Google Scholar]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; ElAzazi, E.S.M.; Ahmed, T.A. Salt tolerance of selected halophytes at the two initial growth stages for future management options. Sci. Rep. 2021, 11, 10194. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Halophyte seed germination. In Ecophysiology of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 2006; pp. 11–30. [Google Scholar]
- Zaman, S.; Padmesh, S.; Tawfiq, H. Seed germination and viability of Salsola imbricata Forssk. Int. J. Biodivers. Conserv. 2010, 2, 388–394. [Google Scholar]
- Ramírez-Tobías, H.; Peña-Valdivia, C.; Trejo, C.; Aguirre, J.; Vaquera, H. Seed germination of Agave species as influenced by substrate water potential. Biol. Res. 2014, 47, 11. [Google Scholar] [CrossRef] [PubMed]
- Kotzen, B. Plant use in desert climates-looking forward to sustainable planting in the Negev and other world deserts. Acta Hortic. 2004, 643, 39–49. [Google Scholar] [CrossRef]
- Mircea, D.M.; Calone, R.; Estrelles, E.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Responses of different invasive and non-invasive ornamental plants to water stress during seed germination and vegetative growth. Sci. Rep. 2023, 13, 13281. [Google Scholar] [CrossRef] [PubMed]
- Süle, G.; Miholcsa, Z.; Molnár, C.; Kovács-Hostyánszki, A.; Fenesi, A.; Bauer, N.; Szigeti, V. Escape from the garden: Spreading, effects and traits of a new risky invasive ornamental plant (Gaillardia aristata Pursh). NeoBiota 2023, 83, 43–69. [Google Scholar] [CrossRef]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
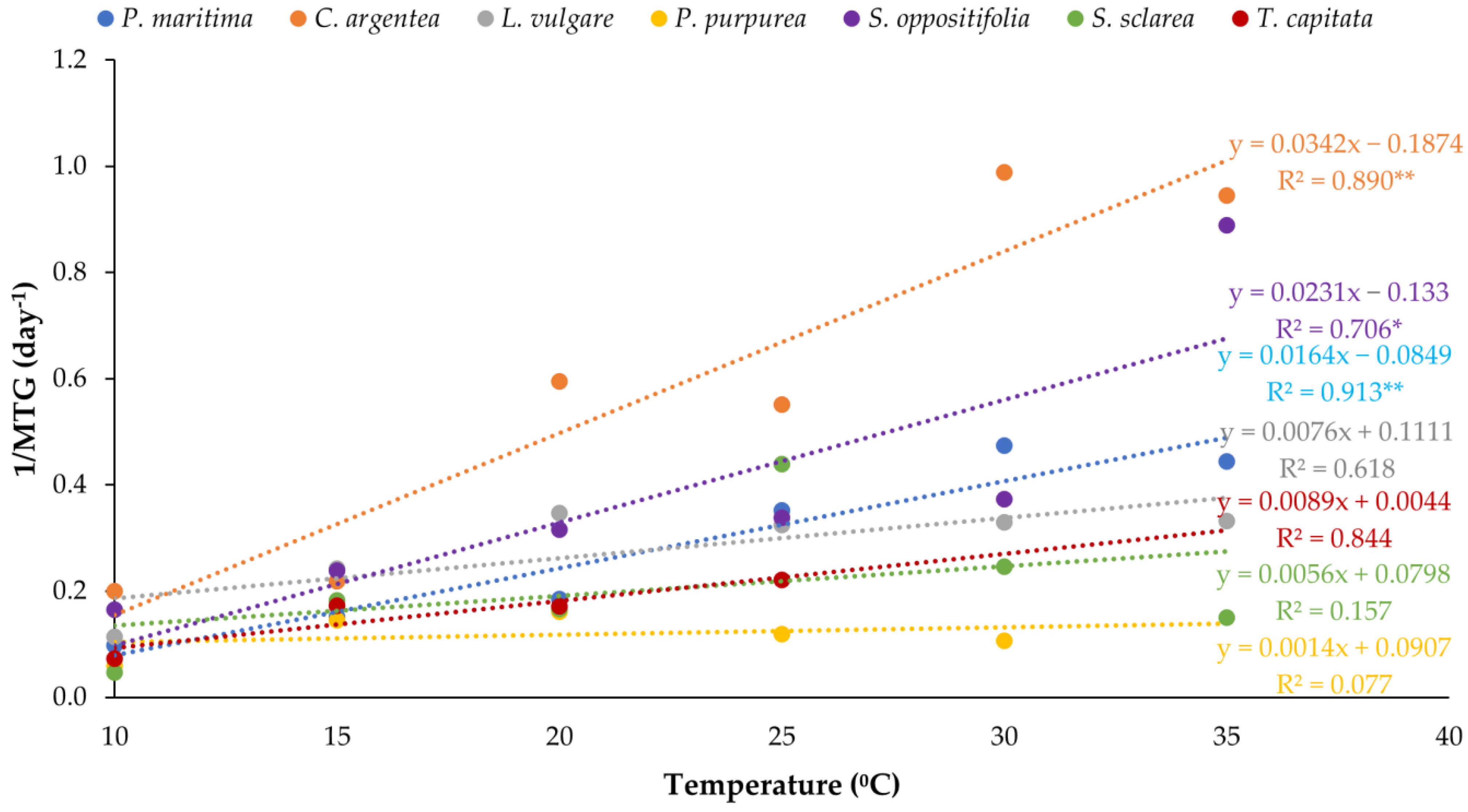
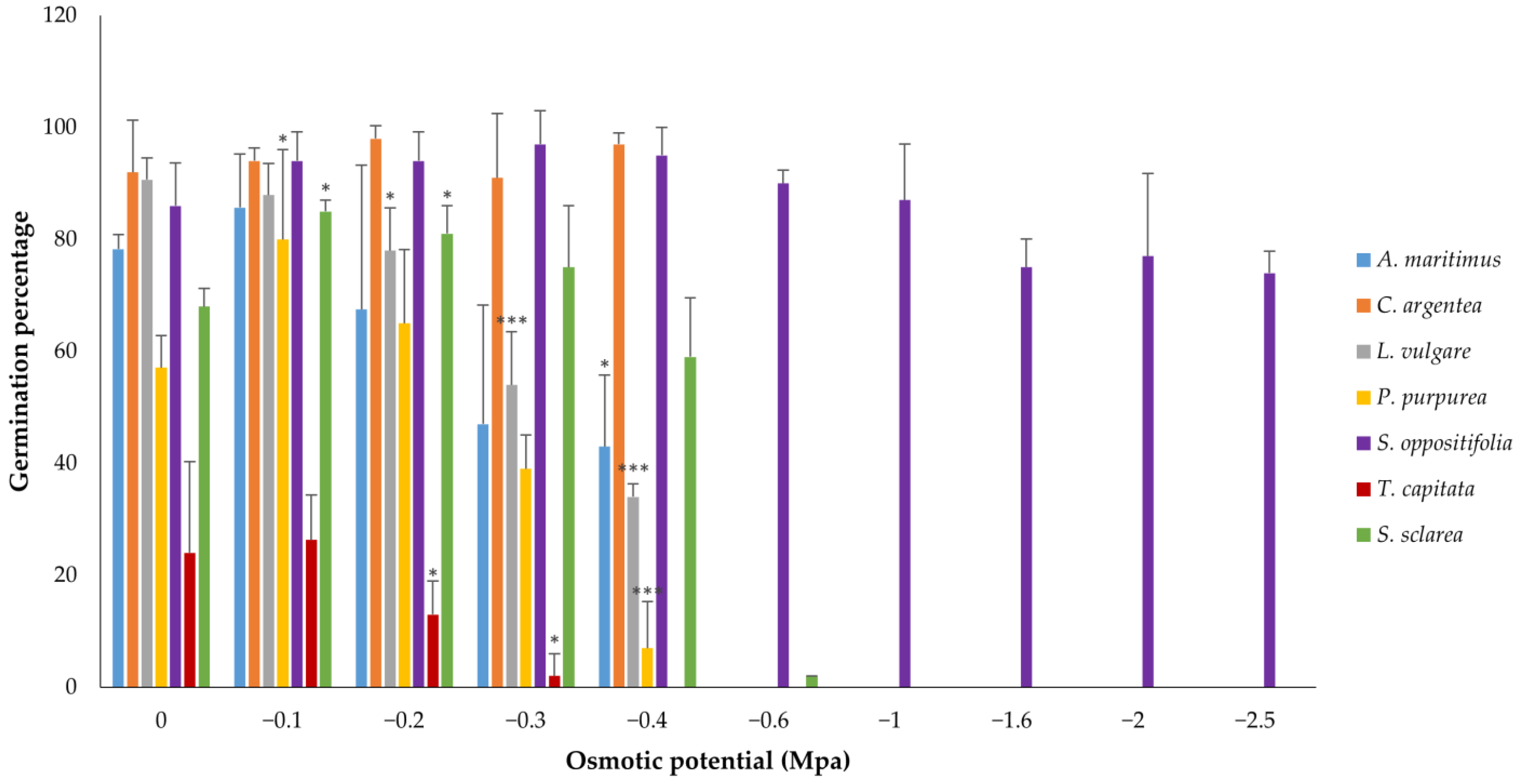
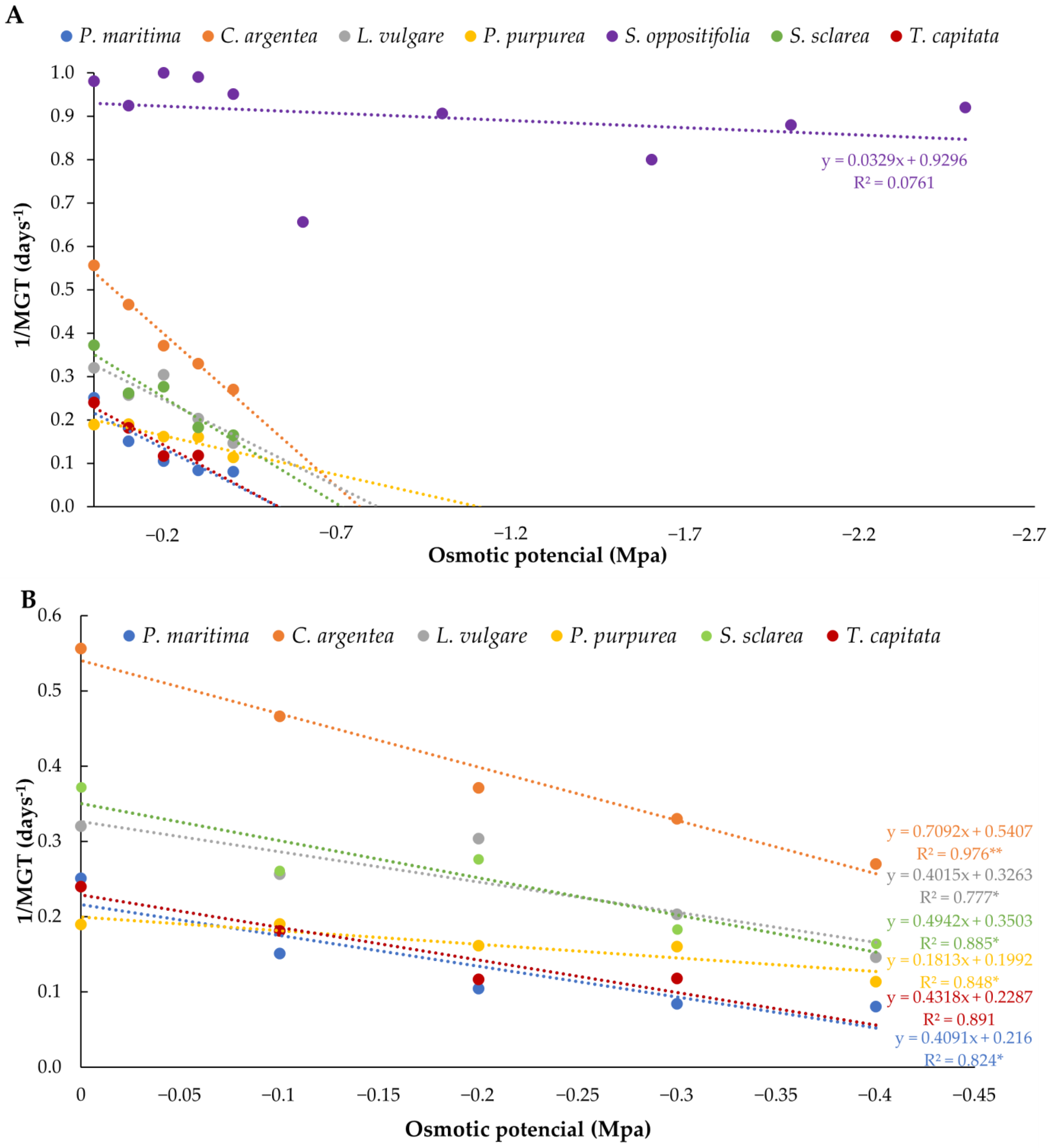
| T (°C) | P. maritima | C. argentea | L. vulgare | P. purpurea | S. oppositifolia | S. sclarea | T. capitata |
|---|---|---|---|---|---|---|---|
| 10 | 10.349 (±0.912) a | 5.000 (±0) | 8.888 (±1.104) a | 16.960 (±1.292) a | 6.191 (±1.134) a | 21.250 (±1.061) a | 14.038 (±2.457) a |
| 15 | 6.696 (±0.840) b | 4.583 (±0.223) a | 4.130 (±0.243) b | 6.896 (±0.470) bc | 4.382 (±1.181) b | 5.504 (±0.458) bc | 6.321 (±2.228) b |
| 20 | 5.600 (±1.275) b | 1.693 (±0.155) b | 2.907 (±0.340) b | 6.276 (±0.788) b | 3.207 (±0.424) bc | 6.095 (±0.893) bc | 6.118 (±1.333) b |
| 25 | 2.914 (±0.504) c | 1.857 (±0.319) b | 3.215 (±0.783) b | 8.548 (±1.228) c | 3.010 (±0.487) bc | 2.295 (±0.248) d | 4.578 (±0.576) b |
| 30 | 2.422 (±1.017) c | 1.011 (±0.023) c | 3.149 (±0.656) b | 9.333 (±0) | 2.706 (±0.281) cd | 4.256 (±1.021) cd | |
| 35 | 2.274 (±0.247) c | 1.063 (±0.078) c | 3.610 (±1.699) b | 1.129 (±0.084) d | 7.119 (±2.044) b |
| P. maritima | C. argentea | L. vulgare | P. purpurea | S. oppositifolia | S. sclarea | T. capitata |
|---|---|---|---|---|---|---|
| Tb (°C) | ||||||
| −0.04 | −0.09 | −1.10 | −64.79 | −0.15 | −3.86 | −1.10 |
| S (°C × day) | ||||||
| 12.20 | 5.84 | 22.57 | 714.29 | 8.66 | 35.84 | 22.57 |
| Ψ (MPa) | P. maritima | C. argentea | L. vulgare | P. purpurea | S. oppositifolia | S. sclarea | T. capitata |
|---|---|---|---|---|---|---|---|
| −0.1 | 9.53 (±−12.23) | 2.17 (±−2.51) | −3.03 (±−6.16) | 40.18 (±−28.04) | 9.3 (±−6) | 25 (±−2.94) | 9.72 (±−33.45) |
| −0.2 | −13.75 (±−32.88) | 6.52 (±−2.51) | −13.96 (±−8.45) | 13.89 (±−23.16) | 9.3 (±−6) | 19.12 (±−7.4) | −45.83 (±−25) |
| −0.3 | −39.93 (±−27.17) | −1.09 (±−12.49) | −40.44 (±−10.5) | −31.66 (±−10.51) | 12.79 (±−6.98) | 10.29 (±−16.2) | −91.67 (±−16.67) |
| −0.4 | −45.04 (±−16.37) | 5.43 (±−2.17) | −62.5 (±−2.55) | −87.73 (±−14.45) | 10.47 (±−5.85) | −13.24 (±−15.47) | |
| −0.6 | 4.65 (±−2.69) | −97.06 (±−0) | |||||
| −1 | 0 (±−5.79) | ||||||
| −1.6 | −13.79 (±−11.49) | ||||||
| −2 | −11.49 (±−4.4) | ||||||
| −2.5 | 9.3 (±−6) |
| Ψ (MPa) | P. maritima | C. argentea | L. vulgare | P. purpurea | S. oppositifolia | S. sclarea | T. capitata | |
|---|---|---|---|---|---|---|---|---|
| 0 | 4.08 (±0.78) | 1.85 (±0.40) | 3.13 (±0.199) | 5.38 (±0.82) | 1.02 (±0.04) | 2.86 (±0.89) | 4.26 | (±0.70) |
| −0.1 | 6.70 * (±0.81) | 2.17 (±0.30) | 3.94 (±0.499) | 5.28 (±0.46) | 1.09 (±0.14) | 3.94 (±0.80) | 5.66 | (±1.01) |
| −0.2 | 9.66 *** (±1.10) | 2.70 * (±0.17) | 3.33 (±0.419) | 6.48 (±1.67) | 1.00 (±0.00) | 3.62 (±0.17) | 8.68 *** | (±1.09) |
| −0.3 | 12.15 *** (±1.91) | 3.08 ** (±0.40) | 5.34 (±1.719) | 6.28 (±0.57) | 1.01 (±0.02) | 5.52 *** (±0.68) | 8.50 | (±0.00) |
| −0.4 | 12.51 *** (±0.68) | 3.77 *** (±0.58) | 8.83 * (±4.939) | 8.88 ** (±1.24) | 1.05 (±0.04) | 6.11 *** (±0.39) | ||
| −0.6 | 1.61 *** (±0.39) | |||||||
| −1 | 1.10 (±0.05) | |||||||
| −1.6 | 1.26 (±0.10) | |||||||
| −2 | 1.15 (±0.17) | |||||||
| −2.5 | 1.09 (±0.11) | |||||||
| P. maritima | C. argentea | L. vulgare | P. purpurea | S. oppositifolia | S. sclarea | T. capitata |
|---|---|---|---|---|---|---|
| Ψb (Mpa) | ||||||
| −0.53 | −0.76 | −0.81 | −1.10 | −28.26 | −0.85 | −0.53 |
| ƟH (Mpa × day) | ||||||
| 2.44 | 1.41 | 2.49 | 5.52 | 30.40 | 2.55 | 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, P.; Mora, R.; Estrelles, E.; Martínez-Nieto, M.I. Comparison of the Climate Change Tolerance of Native and Non-Native Species Used or Potentially Used as Ornamentals in Mediterranean Areas. Horticulturae 2024, 10, 620. https://doi.org/10.3390/horticulturae10060620
Soriano P, Mora R, Estrelles E, Martínez-Nieto MI. Comparison of the Climate Change Tolerance of Native and Non-Native Species Used or Potentially Used as Ornamentals in Mediterranean Areas. Horticulturae. 2024; 10(6):620. https://doi.org/10.3390/horticulturae10060620
Chicago/Turabian StyleSoriano, Pilar, Reyes Mora, Elena Estrelles, and M. Isabel Martínez-Nieto. 2024. "Comparison of the Climate Change Tolerance of Native and Non-Native Species Used or Potentially Used as Ornamentals in Mediterranean Areas" Horticulturae 10, no. 6: 620. https://doi.org/10.3390/horticulturae10060620
APA StyleSoriano, P., Mora, R., Estrelles, E., & Martínez-Nieto, M. I. (2024). Comparison of the Climate Change Tolerance of Native and Non-Native Species Used or Potentially Used as Ornamentals in Mediterranean Areas. Horticulturae, 10(6), 620. https://doi.org/10.3390/horticulturae10060620






