Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture
Abstract
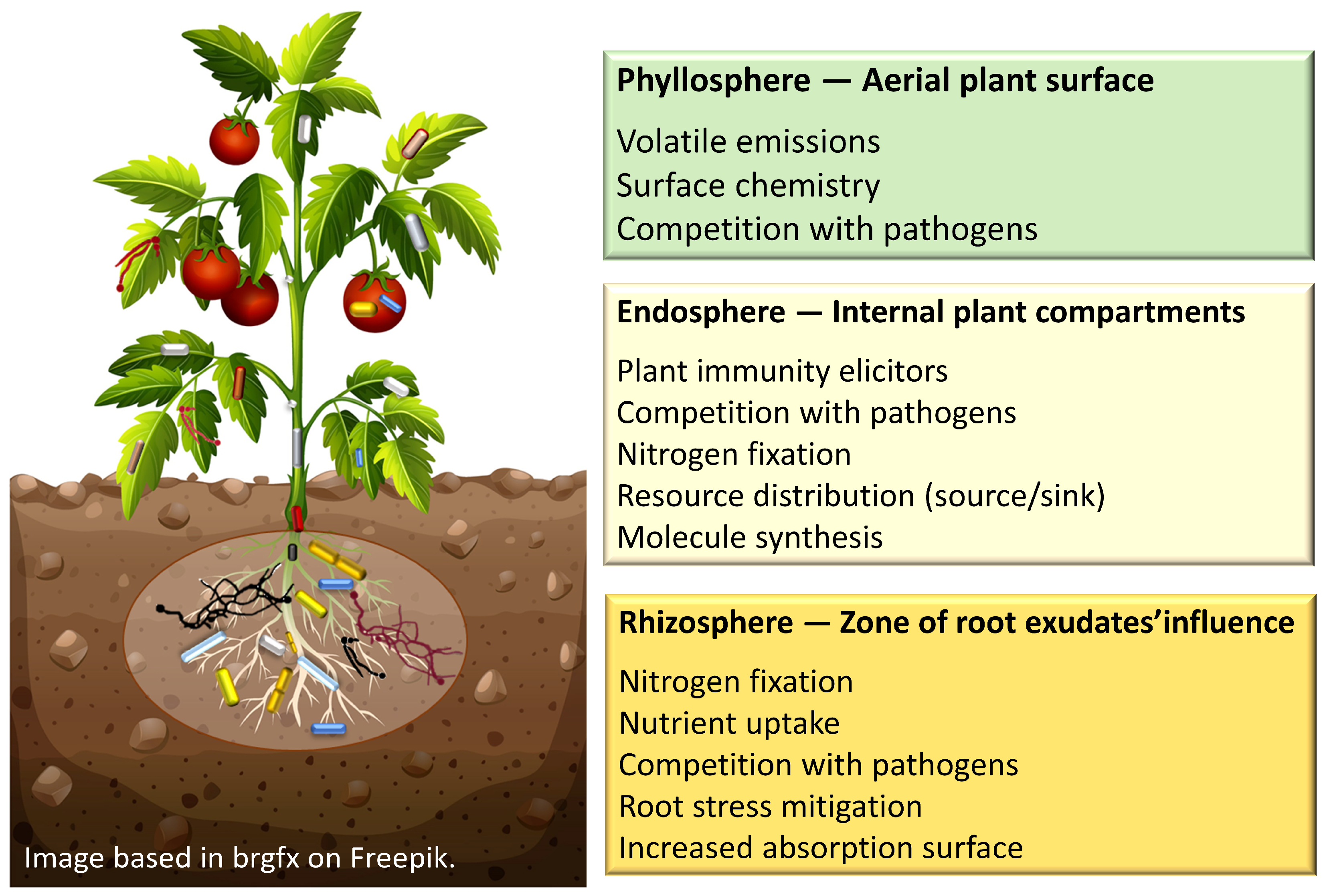
1. Introduction
2. Beneficial Microorganisms
2.1. Bacteria
2.1.1. Nitrogen-Fixing Bacteria
2.1.2. Phosphate- and Potassium-Solubilizing Bacteria
2.2. Fungi
2.2.1. Mycorrhizal Fungi
2.2.2. Endophytic Fungi
3. Benefits of Microorganisms in Agriculture
3.1. Advances in Agriculture
3.2. Limiting Factors
4. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Sachan, A.; Sachan, S.G. Current Aspects and Applications of Biofertilizers for Sustainable Agriculture. In Plant Microbiomes for Sustainable Agriculture; Springer: New York, NY, USA, 2020; pp. 445–473. [Google Scholar]
- Sarkar, D.; Rakshit, A.; Al-Turki, A.I.; Sayyed, R.Z.; Datta, R. Connecting Bio-Priming Approach with Integrated Nutrient Management for Improved Nutrient Use Efficiency in Crop Species. Agriculture 2021, 11, 372. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Monje, D.; Vergara, L.I.; Lopez-Mejia, J.; White, J.F. Plant microbiomes as contributors to agricultural terroir. Front. Agron. 2023, 5, 1216520. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Mandal, A.; Naresh, R.K.; Verma, G. Improving soil micronutrient availability under organic farming. In Advances in Organic Farming: Agronomic Soil Management Practices; Woodhead Publishing: Cambridge, UK, 2021; pp. 93–114. [Google Scholar]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing Causality: Opportunities of Synthetic Communities for Plant Microbiome Research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, A.; González-hernández, A.I.; Llorens, E.; García-agustín, P.; Scalschi, L.; Vicedo, B. The apoplast: A key player in plant survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Eroğlu, G.; Cabral, C.; Ravnskov, S.; Bak Topbjerg, H.; Wollenweber, B. Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol. 2020, 22, 863–871. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Jin, J.; Christensen, M.J.; Gu, L.; Cheng, C.; Wang, J. Epichloë gansuensis increases the tolerance of achnatherum inebrians to low-p stress by modulating amino acids metabolism and phosphorus utilization efficiency. J. Fungi 2021, 7, 390. [Google Scholar] [CrossRef] [PubMed]
- del Orozco-Mosqueda, M.C.; Santoyo, G. Plant-microbial endophytes interactions: Scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2021, 25, 100189. [Google Scholar] [CrossRef]
- Thamer, S.; Schädler, M.; Bonte, D.; Ballhorn, D.J. Dual benefit from a belowground symbiosis: Nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil 2011, 341, 209–219. [Google Scholar] [CrossRef]
- Prakash, J.; Mishra, S. Role of beneficial soil microbes in alleviating climatic stresses in plants. In Microbiome under Changing Climate: Implications and Solutions; Woodhead Publishing: Cambridge, UK, 2022; pp. 29–68. [Google Scholar]
- Soni, S.K.; Manhas, R.; Jakhar, Y.; Sharma, A.; Soni, R. Biofertilizers for Sustainable Agriculture: Current Trends and Future Perspective. In Genomic, Proteomics, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 331–356. [Google Scholar]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A Drought Resistance-Promoting Microbiome Is Selected by Root System under Desert Farming. PLoS ONE 2012, 7, e48479. [Google Scholar] [CrossRef] [PubMed]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum sensing system: Target to control the spread of bacterial infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Baldani, J.I.; Baldani, V.L.D. History on the biological nitrogen fixation research in graminaceous plants: Special emphasis on the Brazilian experience. An. Acad. Bras. Cienc. 2005, 77, 549–579. [Google Scholar] [CrossRef]
- da Silva, K.; Nóbrega, R.S.A.; Lima, A.S.; Barberi, A.; de Moreira, F.M.S. Density and diversity of diazotrophic bacteria isolated from Amazonian soils using N-free semi-solid media. Sci. Agric. 2011, 68, 518–525. [Google Scholar] [CrossRef]
- Kaschuck, G.; Hungria, M. Diversity and importance of diazotrophic bacteria to agricultural sustainability in the tropics. In Diversity and Benefits of Microorganisms from the Tropics; Springer International Publishing: New York, NY, USA, 2017; pp. 269–292. [Google Scholar]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Siqueira, J.O. Inter-Relação Fertilidade, Biologia do solo e Nutrição de Plantas; Sociedade Brasileira de Ciência do Solo: Lavras, Brazil, 1999. [Google Scholar]
- Monteiro, R.A.; Balsanelli, E.; Wassem, R.; Marin, A.M.; Brusamarello-Santos, L.C.C.; Schmidt, M.A.; Tadra-Sfeir, M.Z.; Pankievicz, V.C.S.; Cruz, L.M.; Chubatsu, L.S.; et al. Herbaspirillum-plant interactions: Microscopical, histological and molecular aspects. Plant Soil 2012, 356, 175–196. [Google Scholar] [CrossRef]
- Hurek, T.; Handley, L.L.; Reinhold-Hurek, B.; Piché, Y. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol. Plant-Microbe Interact. 2002, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Meng, A.; Zhang, J.; Xie, M.; Qin, Y.; Faulk, D.C.; Zhang, B.; Yang, S.; Qiu, L. Isolation and molecular identification of endophytic diazotrophs from seeds and stems of three cereal crops. PLoS ONE 2017, 12, e0187383. [Google Scholar] [CrossRef] [PubMed]
- Montañez, A.; Abreu, C.; Gill, P.R.; Hardarson, G.; Sicardi, M. Biological nitrogen fixation in maize (Zea mays L.) by 15N isotope-dilution and identification of associated culturable diazotrophs. Biol. Fertil. Soils 2009, 45, 253–263. [Google Scholar] [CrossRef]
- Rout, M.E.; Chrzanowski, T.H. The invasive Sorghum halepense harbors endophytic N2-fixing bacteria and alters soil biogeochemistry. Plant Soil 2009, 315, 163–172. [Google Scholar] [CrossRef]
- Davis, S.C.; Parton, W.J.; Dohleman, F.G.; Smith, C.M.; Del Grosso, S.; Kent, A.D.; DeLucia, E.H. Comparative biogeochemical cycles of bioenergy crops reveal nitrogen-fixation and low greenhouse gas emissions in a Miscanthus × giganteus agro-ecosystem. Ecosystems 2010, 13, 144–156. [Google Scholar] [CrossRef]
- Videira, S.S.; de Pereira e Silva, M.C.; de Souza Galisa, P.; Dias, A.C.F.; Nissinen, R.; Divan, V.L.B.; van Elsas, J.D.; Baldani, J.I.; Salles, J.F. Culture-independent molecular approaches reveal a mostly unknown high diversity of active nitrogen-fixing bacteria associated with Pennisetum purpureum-a bioenergy crop. Plant Soil 2013, 373, 737–754. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Gundale, M.J.; Nilsson, M.C. Ecosystem feedbacks and nitrogen fixation in boreal forests. Science 2008, 320, 1181. [Google Scholar] [CrossRef] [PubMed]
- Carrell, A.A.; Frank, A.C. Pinus flexilis and Picea engelmannii share a simple and consistent needle endophyte microbiota with a potential role in nitrogen fixation. Front. Microbiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Moyes, A.B.; Kueppers, L.M.; Pett-Ridge, J.; Carper, D.L.; Vandehey, N.; O’Neil, J.; Frank, A.C. Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytol. 2016, 210, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Terakado-Tonooka, J.; Ohwaki, Y.; Yamakawa, H.; Tanaka, F.; Yoneyama, T.; Fujihara, S. Expressed nifH Genes of Endophytic Bacteria Detected in Field-Grown Sweet Potatoes (Ipomoea batatas L.). Microbes Environ. 2008, 23, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Salgado, T.; Fuentes-Ramirez, L.E.; Tapia-Hernandez, A.; Mascarua-Esparza, M.A.; Martinez-Romero, E.; Caballero-Mellado, J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Herschberger, N.; Kim, S.H.; Doty, S.L. Diazotrophic Endophytes of Poplar and Willow for Growth Promotion of Rice Plants in Nitrogen-Limited Conditions. Crop Sci. 2015, 55, 1765–1772. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-ściseł, J. Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the biolog gen iii microplateTM test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Nitrogen-Fixation by Endophytic Bacteria in Agricultural Crops: Recent Advances. In Nitrogen in Agriculture—Updates; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O.; Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Raklami, A.; Benidire, L.; Tahiri, A.I.; Göttfert, M.; Oufdou, K. Effects of PGPR co-inoculation on growth, phosphorus nutrition and phosphatase/phytase activities of faba bean under different phosphorus availability conditions. Pol. J. Environ. Stud. 2020, 29, 1557–1565. [Google Scholar] [CrossRef]
- Thiebaut, F.; de Urquiaga, M.C.O.; Rosman, A.C.; da Silva, M.L.; Hemerly, A.S. The Impact of Non-Nodulating Diazotrophic Bacteria in Agriculture: Understanding the Molecular Mechanisms That Benefit Crops. Int. J. Mol. Sci. 2022, 23, 11301. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Abd-Elsalam, K.A.; Mohamed, H.I. Synthesis and application of bacterial secondary metabolites in agroecosystems: A note from the editors. In Bacterial Secondary Metabolites: Synthesis and Applications in Agroecosystem; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–14. [Google Scholar]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Manter, D.K.; Fonte, S.J.; Vivanco, J.M. Root exudate-derived compounds stimulate the phosphorus solubilizing ability of bacteria. Sci. Rep. 2023, 13, 4050. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Ramirez, M.D.A.; Yamaya, H.; Sekimoto, H.; Yokoyama, T. Isolation and Screening of Indigenous Plant Growth-promoting Rhizobacteria from Different Rice Cultivars in Afghanistan Soils. Microbes Environ. 2019, 34, 347. [Google Scholar] [CrossRef] [PubMed]
- Udaondo, Z.; Duque, E.; Daddaoua, A.; Caselles, C.; Roca, A.; Pizarro-Tobias, P.; Ramos, J.L. Developing robust protein analysis profiles to identify bacterial acid phosphatases in genomes and metagenomic libraries. Environ. Microbiol. 2020, 22, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Lace, B.; Ott, T. Commonalities and Differences in Controlling Multipartite Intracellular Infections of Legume Roots by Symbiotic Microbes. Plant Cell Physiol. 2018, 59, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.F.; Bledsoe, C.; Kahn, M.; O’gara, F.; Simms, E.L.; Thomashow, L.S. Cooperation in the rhizosphere and the “free rider” problem. Ecology 2003, 84, 838–845. [Google Scholar] [CrossRef]
- Ujvári, G.; Turrini, A.; Avio, L.; Agnolucci, M. Possible role of arbuscular mycorrhizal fungi and associated bacteria in the recruitment of endophytic bacterial communities by plant roots. Mycorrhiza 2021, 31, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2023, 125, 73–98. [Google Scholar] [CrossRef]
- Frank, B. Über die auf Wurzelsymbiose Beruhende Ernährung Gewisser Bäume durch Unterirdische Pilze. Berichte Der Dtsch. Bot. Ges. 1885, 3, 128–145. [Google Scholar]
- Zuccaro, A.; Lahrmann, U.; Langen, G. Broad compatibility in fungal root symbioses. Curr. Opin. Plant Biol. 2014, 20, 135–145. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Pozo, M.J.; García-Garrido, J.M. Strigolactones: A cry for help in the rhizosphere. Botany 2011, 89, 513–522. [Google Scholar] [CrossRef]
- Poveda, J.; Díaz-González, S.; Díaz-Urbano, M.; Velasco, P.; Sacristán, S. Fungal endophytes of Brassicaceae: Molecular interactions and crop benefits. Front. Plant Sci. 2022, 13, 932288. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef]
- Antoine, S.; Hériché, M.; Boussageon, R.; Noceto, P.A.; van Tuinen, D.; Wipf, D.; Courty, P.E. A historical perspective on mycorrhizal mutualism emphasizing arbuscular mycorrhizas and their emerging challenges. Mycorrhiza 2021, 31, 637–653. [Google Scholar]
- Paszkowski, U. Mutualism and parasitism: The yin and yang of plant symbioses. Curr. Opin. Plant Biol. 2006, 9, 364–370. [Google Scholar] [CrossRef]
- Chang, X.; Young, B.; Vaccaro, N.; Strickland, R.; Goldstein, W.; Struwe, L.; White, J.F.; Chang, X.; Young, B.; Vaccaro, N.; et al. Endophyte symbiosis evolutionary development, and impacts of plant agriculture. Grass Res. 2023, 3, 18. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 408113. [Google Scholar] [CrossRef]
- Salvioli, A.; Bonfante, P. Systems biology and “omics” tools: A cooperation for next-generation mycorrhizal studies. Plant Sci. 2013, 203–204, 107–114. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1604–1638. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Grassi, A.; Pagliarani, I.; Avio, L.; Cristani, C.; Rossi, F.; Turrini, A.; Giovannetti, M.; Agnolucci, M. Bioprospecting for plant resilience to climate change: Mycorrhizal symbionts of European and American beachgrass (Ammophila arenaria and Ammophila breviligulata) from maritime sand dunes. Mycorrhiza 2024, 1–13. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Vyn, T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crops Res. 2008, 108, 14–31. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; Ali, M.A.; Hussain, S.; El Enshasy, H.A.; Hussain, S.; Ahmed, N.; Gafur, A.; Sayyed, R.Z.; Fahad, S.; Danish, S.; et al. Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi J. Biol. Sci. 2021, 28, 6339–6351. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque da Silva Campos, M. Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: A sustainable alternative. Crop Prot. 2020, 135, 105203. [Google Scholar] [CrossRef]
- Guerrero-Galán, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Thoms, D.; Liang, Y.; Haney, C.H. Maintaining symbiotic homeostasis: How do plants engage with beneficial microorganisms while at the same time restricting pathogens? Mol. Plant-Microbe Interact. 2021, 34, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, M.; MacLean, A.M. Home sweet home: How mutualistic microbes modify root development to promote symbiosis. J. Exp. Bot. 2021, 72, 2275–2287. [Google Scholar] [CrossRef]
- Choi, J.; Summers, W.; Paszkowski, U. Mechanisms Underlying Establishment of Arbuscular Mycorrhizal Symbioses. Annu. Rev. Phytopathol. 2018, 56, 135–160. [Google Scholar] [CrossRef]
- Duc, N.H.; Vo, H.T.N.; van Doan, C.; Hamow, K.Á.; Le, K.H.; Posta, K. Volatile organic compounds shape belowground plant–fungi interactions. Front. Plant Sci. 2022, 13, 1046685. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Bécard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef]
- Rengel, Z.; Marschner, P. Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 2005, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; López-Moral, A.; Agustí-Brisach, C. Root Exudates Metabolic Profiling Confirms Distinct Defense Mechanisms Between Cultivars and Treatments with Beneficial Microorganisms and Phosphonate Salts Against Verticillium Wilt in Olive Trees. Phytopathology 2024. [Google Scholar] [CrossRef] [PubMed]
- Liu-Xu, L.; Vicedo, B.; García-Agustín, P.; Llorens, E. Advances in endophytic fungi research: A data analysis of 25 years of achievements and challenges. J. Plant Interact. 2022, 17, 244–266. [Google Scholar] [CrossRef]
- Mandyam, K.G.; Jumpponen, A. Mutualism-parasitism paradigm synthesized from results of root-endophyte models. Front. Microbiol. 2014, 5, 123328. [Google Scholar]
- Hyde, K.; Soytong, K. the fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Leuchtmann, A.; Spiering, M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004, 55, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef]
- Belesky, D.P.; Bacon, C.W. Tall fescue and associated mutualistic toxic fungal endophytes in agroecosystems. Toxin Rev. 2009, 28, 102–117. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Meon, S.; Kadir, J.; Radu, S.; Singh, G. Endophytic microorganisms as potential growth promoters of banana. BioControl 2008, 53, 541–553. [Google Scholar] [CrossRef]
- Hamayun, M.; Afzal Khan, S.; Ahmad, N.; Tang, D.S.; Kang, S.M.; Na, C.I.; Sohn, E.Y.; Hwang, Y.H.; Shin, D.H.; Lee, B.H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Khan, A.L.; Lee, I.J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022, 21, 312–326. [Google Scholar] [CrossRef]
- Hagag, A.; Abdelwahab, M.F.; Abd El-kader, A.M.; Fouad, M.A. The endophytic Aspergillus strains: A bountiful source of natural products. J. Appl. Microbiol. 2022, 132, 4150–4169. [Google Scholar] [CrossRef]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. Plant-Microbe Interact. Agro-Ecol. Perspect. 2017, 2, 135–191. [Google Scholar]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant Growth-Promoting Fungi as Biocontrol Tool against Fusarium Wilt Disease of Tomato Plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles: Tansley review. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Thilini Chethana, K.W.; Jayawardena, R.S.; Chen, Y.J.; Konta, S.; Tibpromma, S.; Abeywickrama, P.D.; Gomdola, D.; Balasuriya, A.; Xu, J.; Lumyong, S.; et al. Diversity and Function of Appressoria. Pathogens 2021, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Di Pietro, A. Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Solís, J.M.; Fernández, F.J. Endophytic Fungal Terpenoids: Natural Role and Bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Varma, A.; Bakshi, M.; Lou, B.; Hartmann, A.; Oelmueller, R. Piriformospora indica: A Novel Plant Growth-Promoting Mycorrhizal Fungus. Agric. Res. 2012, 1, 117–131. [Google Scholar] [CrossRef]
- Lahrmann, U.; Zuccaro, A. Opprimo ergo sum--evasion and suppression in the root endophytic fungus Piriformospora indica. Mol. Plant. Microbe. Interact. 2012, 25, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant-fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Rai, N.; Gupta, P.; Keshri, P.K.; Verma, A.; Mishra, P.; Kumar, D.; Kumar, A.; Santosh, S.K.; Singh, K.; Gautam, V.; et al. Fungal Endophytes: An Accessible Source of Bioactive Compounds with Potential Anticancer Activity. Appl. Biochem. Biotechnol. 2010, 194, 3296–3319. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Elvira Sánchez-Fernández, R.; Lorena Sánchez-Ortiz, B.; Monserrat Sandoval-Espinosa, Y.K.; Ulloa-Benítez, Á.; Armendáriz-Guillén, B.; Claudia García-Méndez, M.; Lydia Macías-Rubalcava, M. Hongos endófitos: Fuente potencial de metabolitos secundarios bioactivos con utilidad en agricultura y medicina. Tip 2013, 16, 132–146. [Google Scholar] [CrossRef]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef] [PubMed]
- Serna-Cock, L.; Arias-García, C.; Valencia Hernandez, L.J. Effect of Biofertilization on the growth of potted sugarcane plants (Saccharum officinarum). Biotecnol. Sect. Agropecu. Agroindustrial 2011, 9, 85–95. [Google Scholar]
- Antunes, J.E.L.; De Freitas, A.D.S.; Oliveira, L.M.S.; De Lyra, M.D.C.C.P.; Fonseca, M.A.C.; Santos, C.E.R.S.; De Oliveira, J.P.; De Araújo, A.S.F.; Figueiredo, M.V.B. Sugarcane inoculated with endophytic diazotrophic bacteria: Effects on yield, biological nitrogen fixation and industrial characteristics. An. Acad. Bras. Cienc. 2019, 91, e20180990. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Bisht, S.; Singh, B.; Gulati, A.; Tewari, R. Enhanced biomass and steviol glycosides in Stevia rebaudiana treated with phosphate-solubilizing bacteria and rock phosphate. Plant Growth Regul. 2011, 65, 449–457. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Qamar, R.; Ur Rehman, A.; Ali, M.; Rehman, A.; Farooq, M.; Zamir, S.I.; Nadeem, M.; Cheema, M.A.; Shehzad, M.; et al. Improvement in soil characteristics of sandy loam soil and grain quality of spring maize by using phosphorus solublizing bacteria. Sustainability 2019, 11, 7049. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, E.; Alikhani, H.A.; Pourbabaei, A.A.; Etesami, H. Improved Phosphorus (P) Uptake and Yield of Rainfed Wheat Fed with P Fertilizer by Drought-Tolerant Phosphate-Solubilizing Fluorescent Pseudomonads Strains: A Field Study in Drylands. J. Soil Sci. Plant Nutr. 2020, 20, 2195–2211. [Google Scholar] [CrossRef]
- Duarah, I.; Deka, M.; Saikia, N.; Deka Boruah, H.P. Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech 2011, 1, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Barra, P.J.; Pontigo, S.; Delgado, M.; Parra–Almuna, L.; Duran, P.; Valentine, A.J.; Jorquera, M.A.; de la Mora, M.L. Phosphobacteria inoculation enhances the benefit of P–fertilization on Lolium perenne in soils contrasting in P–availability. Soil Biol. Biochem. 2019, 136, 107516. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, X.; Feng, G.; Li, Z.; Zhang, Y.; Cao, N. Arbuscular mycorrhizal enhancement of phosphorus uptake and yields of maize under high planting density in the black soil region of China. Sci. Rep. 2021, 11, 1100. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Bouhraoua, D.; Aarab, S.; Laglaoui, A.; Bakkali, M.; Arakrak, A. Phosphate Solubilizing Bacteria Efficiency on Mycorrhization and Growth of Peanut in the Northwest of Morocco. Am. J. Microbiol. Res. 2015, 3, 176–180. [Google Scholar]
- de Novais, C.B.; Sbrana, C.; da Conceição Jesus, E.; Rouws, L.F.M.; Giovannetti, M.; Avio, L.; Siqueira, J.O.; Saggin Júnior, O.J.; da Silva, E.M.R.; de Faria, S.M. Mycorrhizal networks facilitate the colonization of legume roots by a symbiotic nitrogen-fixing bacterium. Mycorrhiza 2020, 30, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Renault, S.; Markham, J. The effect of Frankia and multiple ectomycorrhizal fungil species on Alnus growing in low fertility soil. Symbiosis 2020, 80, 207–215. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef] [PubMed]
- Shternshis, M.V.; Belyaev, A.A.; Shpatova, T.V.; Lelyak, A.A. Influence of Bacillus spp. on strawberry gray-mold causing agent and host plant resistance to disease. Contemp. Probl. Ecol. 2015, 8, 390–396. [Google Scholar] [CrossRef]
- Jayakumar, A.; Krishna, A.; Mohan, M.; Nair, I.C.; Radhakrishnan, E.K. Plant Growth Enhancement, Disease Resistance, and Elemental Modulatory Effects of Plant Probiotic Endophytic Bacillus sp. Fcl1. Probiotics Antimicrob. Proteins 2019, 11, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, M.S.; Tonelli, M.L.; Ibáñez, F.; Morla, F.; Cerioni, G.; del Carmen Tordable, M.; Fabra, A. Induced systemic resistance and symbiotic performance of peanut plants challenged with fungal pathogens and co-inoculated with the biocontrol agent Bacillus sp. CHEP5 and Bradyrhizobium sp. SEMIA6144. Microbiol. Res. 2017, 197, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Kim, Y.C. Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Prot. 2018, 105, 62–69. [Google Scholar] [CrossRef]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. The Plant Pathology Journal Hydrogen Cyanide Produced by Pseudomonas chlororaphis O6 Exhibits Nematicidal Activity against Meloidogyne hapla. Plant Pathol. J. 2018, 34, 35–43. [Google Scholar] [CrossRef]
- Chutulo, E.C.; Chalannavar, R.K. Endophytic Mycoflora and Their Bioactive Compounds from Azadirachta Indica: A Comprehensive Review. J. Fungi 2018, 4, 42. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: An interdisciplinary perspective. Front. Microbiol. 2013, 4, 44840. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Abdulhadi, S.Y.; Hasan, G.Q.; Gergees, R.N. Molecular detection and antimicrobial activity of Endophytic fungi isolated from a medical plant Rosmarinus officinalis. Ann. Trop. Med. Public Health 2020, 23. [Google Scholar] [CrossRef]
- Rashid, T.S. Bioactive metabolites from tomato endophytic fungi with antibacterial activity against tomato bacterial spot disease. Rhizosphere 2021, 17, 100292. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest management via endophytic colonization of tobacco seedlings by the insect fungal pathogen Beauveria bassiana. Pest Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Eschen, R.; Horwood, J.M.; Gange, A.C.; Hill, E.M. Infection by a foliar endophyte elicits novel arabidopside-based plant defence reactions in its host, C irsium arvense. New Phytol. 2015, 205, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.O.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arias, C.; Sobrino-Plata, J.; Gil, L.; Rodríguez-Calcerrada, J.; Martín, J.A. Priming of plant defenses against Ophiostoma novo-ulmi by Elm (Ulmus minor Mill.) fungal endophytes. J. Fungi 2021, 7, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-M.; He, W.; Wu, C.-Y.; Sun, K.; Zhang, W.; Dai, C.-C. Phomopsis liquidambaris inoculation induces resistance in peanut to leaf spot and root rot. BioControl 2020, 65, 475–488. [Google Scholar] [CrossRef]
- Llorens, E.; Scalschi, L.; Sharon, O.; Vicedo, B.; Sharon, A.; García-Agustín, P. Jasmonic acid pathway is required in the resistance induced by Acremonium sclerotigenum in tomato against Pseudomonas syringae. Plant Sci. 2022, 318, 111210. [Google Scholar] [CrossRef]
- Sallam, N.; Ali, E.F.; Seleim, M.A.A.; Khalil Bagy, H.M.M. Endophytic fungi associated with soybean plants and their antagonistic activity against Rhizoctonia solani. Egypt. J. Biol. Pest Control 2021, 31, 54. [Google Scholar] [CrossRef]
- Abdou, R.; Alqahtani, A.M.; Attia, G.H. Bioactive Metabolites of Aspergillus neoniger, an Endophyte of the Medicinal Plant Ficus carica. Indian J. Pharm. Sci. 2021, 83, 101–109. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Sayer, J.A.; Gadd, G.M. Microbial solubilization and immobilization of toxic metals: Key biogeochemical processes for treatment of contamination. FEMS Microbiol. Rev. 1997, 20, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Amoozegar, A.; Hesterberg, D.; Osmond, D.L. Phosphorus leaching in a sandy soil as affected by organic and inorganic fertilizer sources. Geoderma 2011, 161, 194–201. [Google Scholar] [CrossRef]
- Rosado, A.S.; De Azevedo, F.S.; Da Cruz, D.W.; Van Elsas, J.D.; Seldin, L. Phenotypic and genetic diversity of Paenibacillus azotofixans strains isolated from the rhizoplane or rhizosphere soil of different grasses. J. Appl. Microbiol. 1998, 84, 216–226. [Google Scholar] [CrossRef]
- Johri, J.K.; Surange, S.; Nautiyal, C.S. Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils. Curr. Microbiol. 1999, 39, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, M.; Singh, H. Fortification of Fertilizers with Organic Amendments and Bio-Inoculants for Augmenting Yield and Quality in Papaya. Agric. Res. J. 2021, 58, 1065–1070. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu-Xu, L.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Scalschi, L.; Llorens, E. Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae 2024, 10, 621. https://doi.org/10.3390/horticulturae10060621
Liu-Xu L, González-Hernández AI, Camañes G, Vicedo B, Scalschi L, Llorens E. Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae. 2024; 10(6):621. https://doi.org/10.3390/horticulturae10060621
Chicago/Turabian StyleLiu-Xu, Luisa, Ana Isabel González-Hernández, Gemma Camañes, Begonya Vicedo, Loredana Scalschi, and Eugenio Llorens. 2024. "Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture" Horticulturae 10, no. 6: 621. https://doi.org/10.3390/horticulturae10060621
APA StyleLiu-Xu, L., González-Hernández, A. I., Camañes, G., Vicedo, B., Scalschi, L., & Llorens, E. (2024). Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae, 10(6), 621. https://doi.org/10.3390/horticulturae10060621




