Abstract
Iris typhifolia Kitag is a perennial herbaceous species with high ornamental and applied value. Elucidating the mechanism of saline–alkali tolerance in Iris is crucial for their promotion in saline–alkali areas. Saline–alkali stress is one of the factors that affects plant growth, which has become a significant global issue. In this study, we measured the physiological and biochemical indexes of I. typhifolia, through germination and potting trials, to evaluate the resistance of I. typhifolia to different levels of artificial saline–alkali stress (0, 50, 100, 150, and 200 mmol·L−1). The results showed that artificial saline–alkali stress negatively impacted germination parameters, cell membrane integrity, and photosynthetic parameters. Different trends in osmoregulatory substances and endogenous hormones were observed. It was shown that I. typhifolia had a potential adaptability to the saline–alkali environment by enhancing its internal defense mechanism. Based on regression analyses, the germination threshold of I. typhifolia was calculated to be 87.15 mmol·L−1, which provided a theoretical basis for the application in soil saline–alkalization areas.
1. Introduction
Saline–alkali soil is the result of a complex interaction between various factors, including rainfall, hydrogeological conditions, irrigation methods, and water conservancy projects [1]. In addition, the application of chloride-based snow-melting agents in the regions where snowfall occurs during the winter season has been identified as a significant contributing factor to the development of saline–alkali soil. The process of snow melting can result in the accumulation of toxic ions within the soil [2]. Excessive use of snow-melting agents results in a rise in pH levels and the accumulation of toxic concentrations in plant tissues, which can lead to saline–alkali stress and an increased osmotic pressure [3]. This factor has a negative impact on the seed germination and photosynthetic response of plants [4]. In order to enhance the promotion of saline–alkali tolerance cultivars, it is crucial to investigate the mechanisms of plant germination and physiological response under saline–alkali stress. This will facilitate the optimization of the utilization of saline–alkali soils [5]. The majority of research conducted under saline–alkali stress has focused on the following areas: saline–alkali-tolerant plant germplasm resources [6], seed germination and seedling photosynthetic characteristics [7], and the relationship between soil salinity and plant growth [8]. Currently, research on the photosynthetic response of plants under saline–alkali stress is primarily focused on NaCl [9]. The main research areas include Na+ metabolism [10], the molecular biology of salt-resistant genes [11,12], and the transmission of salt stress information [13].
Previous studies have investigated the effect of salt and alkali stress on the growth of plants, such as Dichanthium annulatum [10]. It has been demonstrated that saline–alkali stress can impede the hydrolysis and transport of nutrients in the internal storage organs of seeds, disrupt the balance between them and the supply of nutrients in growth tissues, and restrict seed germination [14,15,16]. Germination rate, radicle length, germ length, and fresh weight, as well as average germination time, could all be affected by salinity stress, such as Aquilegia [17]. The accumulation of toxic ions in plant tissue could induce the concentration of reactive oxygen species (ROS), which lead to metabolic disorders. To maintain the membrane stability, the antioxidant enzyme system rapidly eliminates excess ROS [18]. The antioxidant enzyme system mainly comprises superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) [18]. Furthermore, plant hormones, including indole-3-acetic acid (IAA), abscisic acid (ABA), gibberellin (GA), and cytokinins (CKs), play a crucial role in regulating plant growth and development under saline–alkali stress conditions [19]. For instance, IAA has been shown to alleviate the inhibitory effect of abiotic stress on root growth [20]. Salt-alkali tolerance cultivars of Oryza sativa have been observed to accumulate higher levels of GA than the sensitive cultivars [21]. Currently, it is well illustrated that nine plant hormones are involved in the abiotic stress defensive system [22]. Photosynthesis is the most important part of the biosphere material cycle [23] and plays a pivotal role in the absorption, fixation, distribution, and transformation of light energy [24]. Photosynthesis is one of the most sensitive indexes to saline–alkali stress [23]. The photosynthetic parameters of plant leaves include the net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), transpiration rate (Tr), and stomatal conductance (gs), which are important parameters for measuring the photosynthetic function of a plant.
I. typhifolia is an exemplary perennial plant with highly ornamental flowers and foliage, which is endemic to China and is mainly distributed in northeast and Inner Mongolia. Additionally, the special fragrance in the flower of I. typhifolia has a highly commercial value in aroma and medicinal industries [25]. The study of the saline–alkali tolerance for I. typhifolia is conducive to the promotion of the greening of saline–alkali soil in Northeast China. Although research has been conducted on Iris under salt [26] and drought stress [27,28], there is a paucity of knowledge regarding the study of I. typhifolia under saline–alkali conditions. Therefore, the present research was designed to investigate the effects of artificial saline–alkali stress on seed germination and the seedling physiological characteristics of I. typhifolia, to evaluate the growth of I. typhifolia under artificial saline–alkali stress, and to determine the salt tolerance threshold.
2. Materials and Methods
2.1. Plant Materials
The original variant of I. typhifolia seedlings (Figure 1) were obtained by division from the ornamental plant resource nursery at Jilin Agricultural University, Changchun, China (43.49′1.283″ N, 125.240′33.964″ E). The seedlings were subsequently transplanted into pots measuring 12 cm in height and 14 cm in diameter and were then cultivated for 30 days. The substrate for cultivating seedlings was prepared by mixing them with peat and perlite in a ratio of 3:1 [29]. Seeds of I. typhifolia (original variant) were harvested from the nursery, which had a 1000-grain weight of 10.08 g, and were stored at 4 °C before the experiment.

Figure 1.
I. typhifolia seedlings from ornamental plant resources nursery of Jilin Agricultural University.
2.2. Method
2.2.1. Experimental Design
Artificial Salinity−Alkalinity Conditions
The saline–alkaline solution was prepared with NaCl (Yongda, Tianjin, China) and NaHCO3 (Jingshi, Beijing, China), mixed in a molar ratio of 3:1. The treatments were divided into the following four levels: T50 (50 mmol·L−1), T100 (100 mmol·L−1), T150 (150 mmol·L−1), and T200 (200 mmol·L−1) [24].
Seeds Treatment
After disinfecting the seeds with a 10% NaClO (Xinbote, Tianjin, China) solution for 10 min, the seeds were subjected to repeated washing with distilled water. Then, the seeds were immersed in distilled water for 24 h [30,31]. The seeds were sown on double-layered filter paper in Petri dishes (9 cm diameter), with 30 seeds being placed in each replicate. Each Petri dish was filled with 5 mL of the saline–alkaline solution at different concentrations. The control group was filled with the same volume of distilled water. The culture conditions for all treatment groups were set at 25 °C/15 °C [32], a photoperiod of 12 h (light)/12 h (dark) [33], and a light intensity of 2600 lx for 21 days [23] (chamber: RDN-300B, Yanghui, Ningbo, China). To prevent evaporation, water was added daily at a constant weight [34]. Each treatment was replicated three times.
Seedlings Treatment
A total of fifteen seedlings with strong and consistent growth were randomly selected for artificial stress treatment at each concentration. Each treatment level comprised three seedlings. The control group was irrigated with 200 mL distilled water every three days, while the treatment group was irrigated with the same volume of saline–alkali solution. The artificial stress period lasted 21 days, and leaves that were in the same orientation were collected at 0, 7, 14, and 21 days of treatment. Treatment and sampling were processed in the ornamental greenhouse of Jilin Agricultural University, Changchun, China (43.48′59.598″ N, 125.25′40.368″ E). After flash freezing with liquid nitrogen (Ruide, Changchun, China), the samples were stored in a −80 °C freezer (TSE320V-ULTS, Thermofisher, Waltham, MA, USA) for future analysis.
2.2.2. Determination Method
Seed Germination Parameters
The germination was observed and recorded at a consistent time each day. The germination rate and germination index after 21 days were calculated with the radicle exposing the seed coat by 1–2 mm as the germination standard [35]. Otherwise, the seeds were evaluated as abnormal germination. Radicle lengths of both normally germinating and abnormally germinating seeds were measured using vernier calipers at 21 days (absolute measurement mode, Greener, Yantai, China).
Germination rate (%) = number of seed germination/total number of seeds × 100
Germination index GI = ∑Gt/Dt
Gt represents the daily germination number during the final stage of the germination test; Dt represents the number of germination days; and Σ represents the sum [36].
Physiological Parameters
A total of 30 germinated seeds of I. typhifolia were collected under different saline–alkali concentrations. The surface residual solution was then dried with absorbent paper and was subsequently ground into powder using a grinder (SV48R, Dinghaoyuan, Tianjin, China).
The measurement of POD activity was conducted following the manual of the assay kit (P8021, Solarbio, Beijing, China). In brief, 1 mL of extraction solution was mixed with 0.1 g of sample powder and was centrifuged at 4 °C for 10 min at 8000× g. After adding the reagents into the sample tube, the absorbance was measured at 470 nm for 30 s and also 1 min 30 s using a visible light spectrophotometer (722N, INESA, Shanghai, China). The POD activity was calculated using the following formula: POD (U/g m) = ΔA × VTr ÷ (W × Vs/Ve)/0.01/T = 7133 × ΔA/W.
SOD activity was measured with an SOD activity assay kit (BC5165, Solarbio), as follows: 1 mL of extraction solution was added into 0.1 g sample powder and was then centrifuged for 10 min at 4 °C at 8000× g. Then, the reagents were added to a 37 °C water bath for 30 min and the absorbance was measured at 450 nm. The formula was SOD (U/g m) = 10 × percentage inhibition/(1 − percentage inhibition)/W × F.
CAT activity was measured with a CAT activity assay kit (C8070, Solarbio), as follows: 0.1 g of sample powder was mixed with 1 mL extraction solution and was centrifuged at 4 °C for 10 min at 8000× g. Then, 10 μL of sample and 190 μL of the working solution were added. The absorbance was measured at 240 nm. The CAT activity was calculated with the following formula: CAT (U/g m) = [ΔA × VTr/(ε × d) × 106]/(Vs/Ve × W)/T = 764.5 × ΔA/W.
The concentration of Pro was measured with a Pro extraction assay kit (P9460, Solarbio). In total, 0.1 g of sample powder was mixed with 1 mL extraction solution and a boiling water bath oscillation extraction was performed for 10 min; then, the sample was centrifugated at room temperature for 10 min at 10,000× g. The reagent was mixed with supernatant and was insulated in a boiling water bath for 30 min. The absorbance was measured at 520 nm. The calculation was conducted with the following formula: Pro (μg/g m) = x × Ve/W = x/W.
SS concentration was measured using the following steps, according to the SS extraction assay kit (BC0030, Solarbio): 0.1 g of sample powder was mixed with 1 mL of reagent one, placed in an 80 °C water bath for 40 min, then shaken 8–10 times at 8000× g, before being centrifugated at 25 °C for 10 min. Then, reagents were mixed with supernatant and were heated in boiling water bath for 5 min. After they were cooled to room temperature, the absorbance was measured at 540 nm. The formula was as follows: SS (μg/g m) = 1000 × y × V1/W = 1000 × y/W.
MDA concentration was measured using an MDA extraction kit (BC0020, Solarbio), as follows: 1 mL of extraction solution was added to 0.1 g of sample powder and was centrifuged at 8000× g at 4 °C for 10 min. After, it was mixed with supernatant and reagent and placed in a 100 °C water bath for 60 min. Then, the sample was centrifuged at 10,000× g at room temperature for 10 min. The absorbance was measured at both 532 nm and 600 nm. The concentration of MDA was calculated with the following formula: MDA (nmol/g m) = [ΔA × VTr/(ε × d) × 109]/(W × Vs/Ve) × F = 32.258 × ΔA/W × F.
In the formulas above, Ve represents the extract volume; W represents sample quality; VTr represents the total volume of the reaction system; Vs represents the sample volume; ε represents the H2O2 molar extinction coefficient; T represents reaction time; 106 represents the unit conversion coefficient, 1 mol = 106 μmol; d represents cuvette light diameter; y represents the concentration of standard tubes; V1 represents that one volume of the reagent was added; ΔA represents light absorption value; x represents the standard solution concentration; y represents the concentration of standard tubes; and F represents the dilution ratio. Distilled water was used as a control for the measurement of POD, SOD, and CAT activity, as well as for the concentration of Pro, SS, and MDA.
An enzyme-linked immunosorbent assay (ELISA) was employed to quantify the concentrations of GA (m1072782, mlbio, Shanghai, China), CTK (m1207361, mlbio), ABA (m1233061, mlbio), and IAA (m1147100, mlbio). And an enzyme marker (Thermo Scientific, Multiskan, GO, Waltham, MA, USA) was used for absorbance measurements. The protocol was conducted manually.
Chlorophyll Concentration
The acetone-ethanol method was employed to extract the chlorophyll concentration. A total of 0.05 g of fresh leaf fragments were immersed in 15 mL of a 1:1 acetone-ethanol solution (acetone: Beijing Chemical Works, Beijing, China; ethanol: Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China). The absorbance value was measured using a visible light spectrophotometer (722N, INESA, Shanghai, China) at both 665 nm and 649 nm. An acetone-ethanol 1:1 solution was used as control [37]. Each treatment was replicated three times.
Chlorophyll a concentration = 13.95 × A665 − 6.88 × A665
Chlorophyll b concentration = 24.96 × A649 − 7.32 × A665
Chlorophyll concentration = color concentration/volume of extract dilution factor fresh weight of the sample
Photosynthetic Parameters
A total of fifteen separate seedlings were selected for photosynthetic parameter measurements. A portable photosynthetic apparatus (CIRAS-2, PP system, Amesbury, MA, USA) was used for measuring photosynthetic parameters from 9:30 am to 11:30 am on a distinctly cloudless and windless day. The photosynthetic parameters measured included intercellular CO2 concentration (Ci), net photosynthetic rate (Pn), water use efficiency (WUE), transpiration rate (Tr), and stomatal conductance (gs). The light intensity of the instrument was set to 1200 μmol·m−2·s−1 [38], the gas flow rate of the sample chamber was set to 500 μmol·s−1, and the carbon dioxide concentration was set to 400 μmol·m−1. Each treatment was replicated three times.
Chlorophyll Fluorescence Parameters
A total of fifteen separate seedlings were relocated to a dark room for 30 min; then, the chlorophyll fluorescence parameters were measured using the chlorophyll fluorescence imaging system (Li-6800, LI-COR, Lincoln, NE, USA). The maximum photochemical efficiency of PSII (Fv/Fm), photochemical quenching coefficient (qP), non-photochemical quenching coefficient (NPQ), and quantum yield of PSII (ΦPSII) were observed. Each treatment was replicated three times.
2.2.3. Statistical Analysis
Regression analysis was employed to ascertain the salt tolerance threshold of a single plant. The salinity tolerance threshold was calculated using the simultaneous equation when the index was 50% of the control treatment. SPSS 29.0 (Version: 29.0, San Marcos, CA, USA) statistical software was used for regression analysis, correlation analysis, significance analysis, and an ANOVA of test data, while Origin2022 was used for data visualization.
3. Results
3.1. Seed Germination
The germination rate of I. typhifolia seeds was found to be significantly inhibited under artificial saline–alkali stress (Figure 2). The percentage of seeds that germinated decreased with an increase in artificial saline–alkali concentration. The germination rates of I. typhifolia under T50, T100, and T150 were 90%, 80%, and 64.44%, respectively. The lowest germination rate was observed in T200, which was 17.78% of the control group. The results demonstrated that all treatment groups, with the exception of T50, exhibited statistically significant differences compared to the control group.
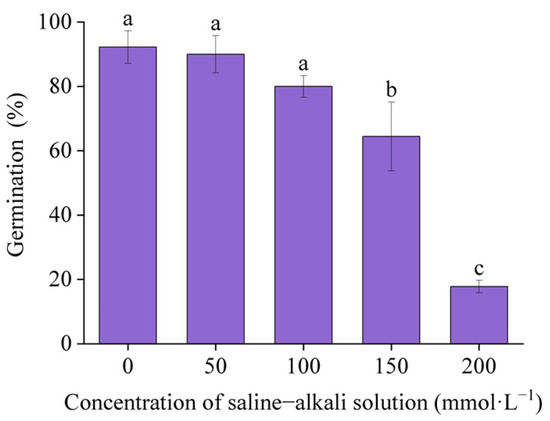
Figure 2.
Germination rate of I. typhifolia under artificial saline–alkali stress. Means with different lowercase letters indicates significant differences among different types.
The value of the germination index and radicle length of I. typhifolia exhibited a significant decline with an increase in the concentration of artificial saline–alkali solution (Figure 3). The germination index of control, T50, T100, T150, and T200 was 23.32, 19.68, 13.44, 9.85, and 2.67, respectively. The germination index in T200 was the lowest, exhibiting an 89% reduction in comparison to the control. The radicle length of the control group was 12.93 mm, while in T50, T100, and T150, the lengths were 9.45, 5.16, and 3.57 mm, respectively. At T200, radicle length was 1.58 mm, which represented an 88% decrease in comparison to the control. The seed germination index and radicle length exhibited significant differences under the artificial saline–alkali stress treatment in comparison to the corresponding control.
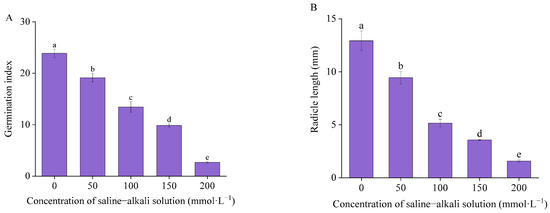
Figure 3.
Germinate situation: (A) seed germination index and (B) radicle length. Means with different lowercase letters indicates significant differences among different types.
3.2. Antioxidant Enzyme Activity and Malondialdehyde Concentration
As the concentration of the artificial saline–alkali solution increased, the activity of CAT and POD were initially increased and then decreased (Figure 4). Compared to the control, the activity of CAT was found to be significantly enhanced by 29.89%, 55.77%, 116.32%, and 76.87% in T50, T100, T150, and T200, respectively. The control group exhibited a POD activity of 8892.47 U·g−1, while the highest POD activity was observed in T100, with a value of 16334.57 U·g−1. Compared to the control group, the POD activity exhibited a notable increase of 65.78% and 83.69% in T50 and T100, respectively. The POD activity of T150 and T200 exhibited a notable decline, with a reduction of 40.11% and 48.4%, respectively.
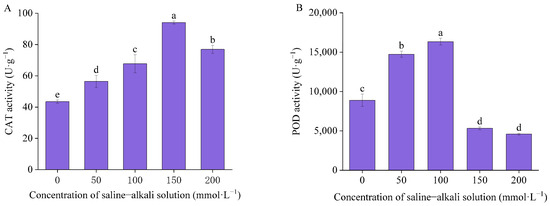
Figure 4.
The antioxidant enzyme activities: (A) catalase (CAT) activities and (B) peroxidase (POD) activities. Means with different lowercase letters indicates significant differences among different types.
The activity of SOD in germinated seeds of I. typhifolia exhibited an initial increase and subsequent decrease with the elevation of the artificial saline–alkali concentration, reaching a peak at T100. In the control group, SOD activity was 18.25 U·g−1. Compared to the control, the SOD activity of the T50, T100, and T150 groups exhibited increases of 66.68%, 71.84%, and 22.91%, respectively. In the control group, the MDA concentration was 20.53 nmol·g−1 (Figure 5). The concentration of MDA in T50, T100, T150, and T200 exhibited a significant increase of 12.04%, 83.77%, 93.19%, and 220.41%, respectively, compared to the control. The highest value, 65.81 nmol·g−1, was observed under T200.
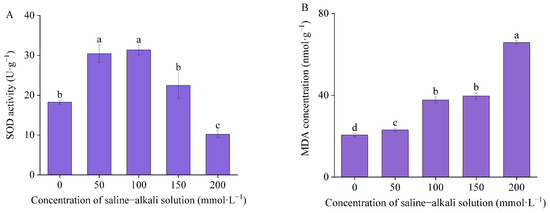
Figure 5.
The antioxidant enzyme activities and membrane lipid peroxidation levels: (A) superoxide dismutase (SOD) activity and (B) malondialdehyde (MDA) concentration. Means with different lowercase letters indicates significant differences among different types.
3.3. Osmotic Adjustment Substances
The maximum concentration of Pro was observed in T200, reaching a value of 60.91 U·g−1. In the control group, Pro concentration was 17.43 U·g−1. The Pro concentration of T50, T100, T150, and T200 exhibited a notable increase of 60.81%, 91.98%, 176.81%, and 249.38%, respectively, in comparison to the control. The concentration of SS initially increased and then decreased, reaching a peak value of 9.58 mg·g−1 at T100 (Figure 6). The concentration of SS in the control was 6.42 mg·g−1. In comparison to the control, the SS concentration exhibited a notable increase of 46.77%, 49.19%, 25.44%, and 24.02% in T50, T100, T150, and T200, respectively.
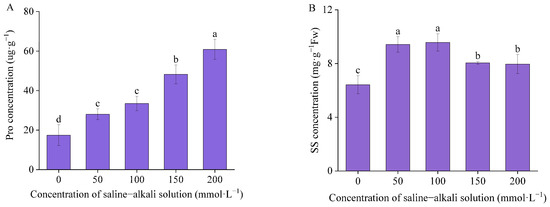
Figure 6.
The concentration of osmotic adjustment substances: (A) proline concentration (Pro) and (B) soluble sugar (SS) concentration. Means with different lowercase letters indicates significant differences among different types.
3.4. Endogenous Hormones Concentration
The concentration of ABA in the germinated seeds of I. typhifolia exhibited a gradual increase with the application of artificial stress treatments, reaching a peak at T200 (Figure 7A). Compared with the control, T50, T100, T150, and T200 exhibited marked increases of 124.95%, 144.55%, 165.18%, and 306.3%, respectively. Nevertheless, no significant difference was observed between T50, T100, and T150. CTK concentration exhibited an initial increase, followed by a subsequent decline, reaching a peak at T50 (Figure 7B). The control group exhibited a CTK concentration of 3.71 pmol·mL−1. Compared with the control, the CTK concentration of T50, T100, and T150 exhibited an increase of 44.97%, 42.62%, and 10.72%, respectively. Conversely, the CTK concentration of T200 exhibited a 9.73% decline. There were significant differences between T50 and T100, with the control, while no significant difference was observed between T150 and T200 with the control.
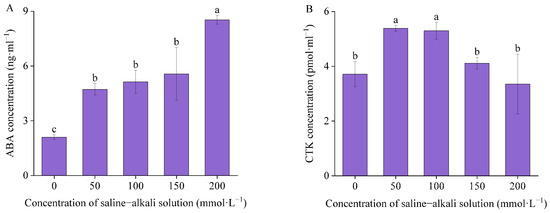
Figure 7.
The concentration of endogenous hormones: (A) abscisic acid (ABA) and (B) cytokinin (CTK). Means with different lowercase letters indicates significant differences among different types.
As the concentration of artificial saline–alkali increased, the concentration of IAA and GA in the germinated seeds of I. typhifolia initially increased and then decreased. The concentration of IAA reached a peak at T50, while the concentration of GA reached a peak at T150 and subsequently declined significantly (Figure 8). Compared with the control, the concentration of IAA exhibited a significant increase of 52.33%, 37.66%, 24.8%, and 23.5% in T50, T100, T150, and T200, respectively. The GA concentration of the control was 6.97 pmol·mL−1. Compared with the control, the T50, T100, T150, and T200 groups exhibited significant increases of 59.11%, 75.48%, 137.9%, and 97.34%, respectively.
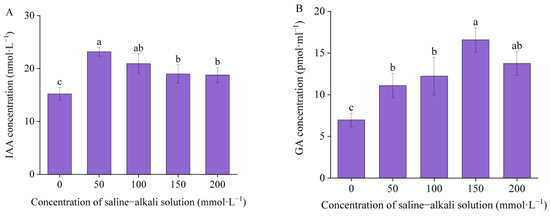
Figure 8.
The concentration of endogenous hormones: (A) indole-3-acetic acid (IAA) and (B) gibberellin (GA). Means with different lowercase letters indicates significant differences among different types.
3.5. Determination of Threshold Value of Seed Germination Period
Through the correlation analysis of 14 indexes, it was revealed that the germination index and Pro concentration exhibited the highest correlation coefficient with the artificial saline–alkali concentration (Table 1). The two indexes were fitted to different levels of artificial saline–alkali concentrations in order to obtain the equations (Table 2). The stress threshold of artificial saline–alkali stress on the germination index and Pro levels of I. typhifolia were calculated to be 120.8 mmol·L−1 and 87.15 mmol·L−1, respectively (Table 2). The lowest value was identified as the threshold for artificial saline–alkali stress during germination, with a value of 87.15 mmol·L−1 (Figure 9).

Table 1.
Correlation coefficient matrix of various indexes seed germination of I. typhifolia under artificial saline–alkali stress.

Table 2.
Regression analysis of related indexes of seed germination of I. typhifolia under artificial saline–alkali stress.
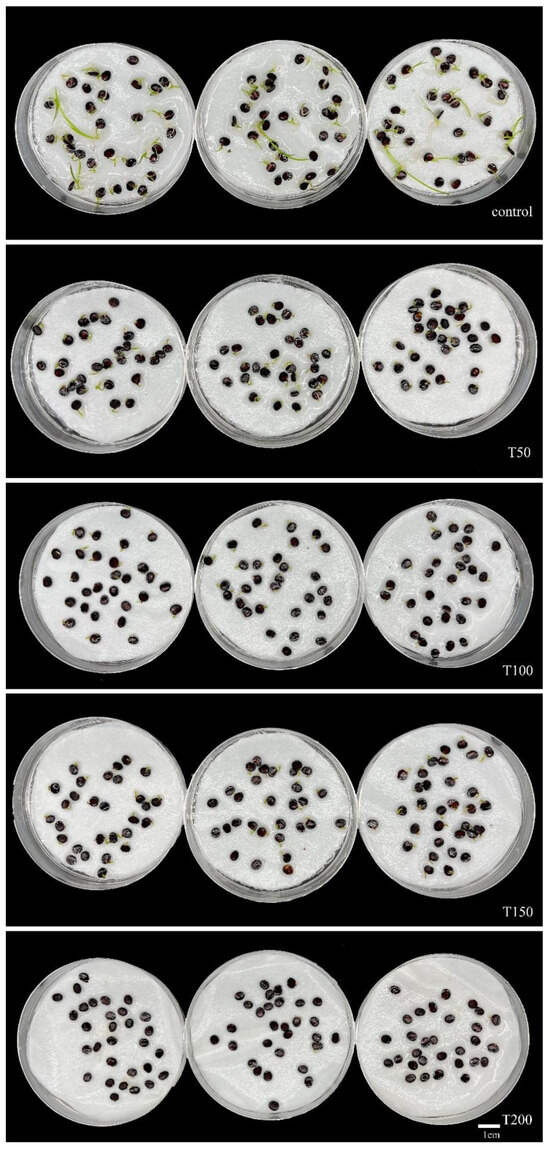
Figure 9.
Seed germination situation of I. typhifolia under 21 days of artificial saline–alkali treatment.
3.6. Chlorophyll Concentration
In the same artificial saline–alkali levels, the concentrations of chlorophyll a, chlorophyll b, and total chlorophyll in I. typhifolia exhibited a downward trend over time (Figure 10A–C), reaching their lowest values at T200. The concentrations of chlorophyll a, chlorophyll b, and total chlorophyll decreased by 67.29%, 78.52%, and 70.55%, respectively, in comparison to the control at 21 days. At 7 days, no significant differences were observed between the treatment groups (T50, T100, and T150) and the control. However, at 14 and 21 days, significant differences were evident between the treatment groups (T100, T150, and T200) and the control.
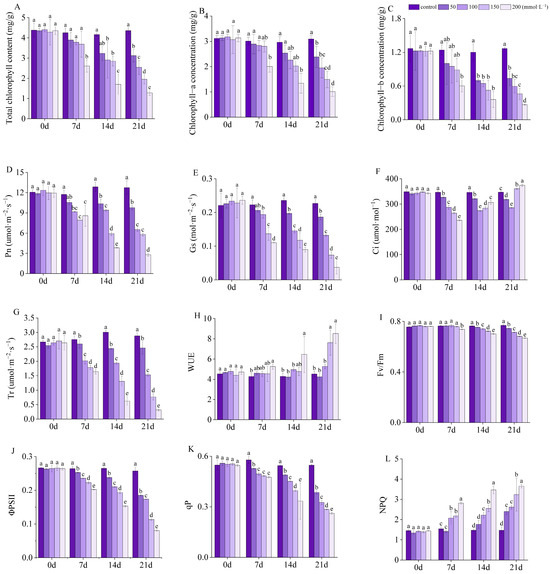
Figure 10.
Photosynthetic and chlorophyll fluorescence parameters: (A) total chlorophyll; (B) chlorophyll a; (C) chlorophyll b; (D) net photosynthetic rate (Pn); (E) stomatal conductance (gs); (F) intercellular CO2 concentration (Ci); (G) transpiration rate (Tr); (H) water use efficiency (WUE); (I) maximum photochemical efficiency of PSII (Fv/Fm); (J) quantum yield of PSII (ΦPSII); (K) photochemical quenching coefficient (qP); (L) non-photochemical quenching coefficient (NPQ). Means with different lowercase letters indicates significant differences among different types.
3.7. Photosynthetic Parameters
In the same artificial saline–alkali concentration, the Pn value of I. typhifolia seedlings exhibited a decline over time, reaching its lowest value at 21 days (Figure 10D). Compared to the control at 7 days, Pn exhibited a significant reduction of 32.38% in the T150 group, reaching a minimum value of 7.94 μmol·m−2·s−1. At 14 and 21 days of treatment, the Pn value decreased significantly and reached its lowest value at T200, with 70.17% and 78.14%, lower than that of the control. Furthermore, the Pn value for T50–200 exhibited a significant decrease in comparison to the control after 14 and 21 days’ treatment.
The Gs value of I. typhifolia seedlings exhibited a decline over time (Figure 10E). Compared with the control, the gs value did not exhibit any significant changes under T50. However, a significant decline in gs was observed in T200. Values for gs were 0.11, 0.09, and 0.04 mol·m−2·s−1 at 7, 14, and 21 days, respectively. These values were 50.56%, 61.85%, and 83.63% lower than control, respectively. Significant differences between treatments and the control were observed from 7 days to 21 days, except for T50 at 7 days.
Under T50, there was a decline in Ci value over time, although this change was not statistically significant. Under T100, T150, and T200, Ci showed a trend of decreasing first and then increasing (Figure 10F). Seedlings exposed to T200 for 7 days exhibited a 32.01% reduction in Ci compared to the control, which reached its lowest value at 235.61 umol·mol−1. A significant difference was observed between each treatment and the control. At 14 and 21 days of saline–alkali treatment, Ci decreased at first and reached its lowest value at T100, which was 7.18% and 8.34% lower than the corresponding control, respectively.
With the increase in artificial saline–alkali concentration and treatment time elongation, the Tr value of I. typhifolia seedlings showed a downward trend (Figure 10G), whereas WUE generally demonstrated an upward trend (Figure 10H). After treatment for 7 days, the lowest values of Tr were observed in T200. Compared with the control, Tr was found to have significantly decreased by 40.61%, 79.31%, and 89.08%, at 7, 14, and 21 days, respectively. However, the maximum values of WUE were found in T200 after treatment for 7 days. Compared with the corresponding control, WUE exhibited an increase of 23.08%, 50.76%, and 88.66% at 7, 14, and 21 days, respectively. At 21 days, a statistically significant difference was observed between T150 and T200 compared to the control.
3.8. Chlorophyll Fluorescence Parameters
Fv/Fm and ΦPSII values in I. typhifolia seedlings exhibited a decline with the prolongation of time and the increase in artificial saline–alkali concentration (Figure 10I,J). At 21 days, Fv/Fm and ΦPSII in T200 reduced by 13.1% and 68.64%, respectively, compared to the control. At 7 days, only the T200 showed a significant difference in Fv/Fm compared to the control.
Under stress conditions, qP in T200 exhibited a significant decline of 52.22% at 21 days, compared with control (Figure 10K). Conversely, in T200, the NPQ value increased by 148.47% in 21 days compared with the control. At 14 and 21 days, a notable distinction was observed between each treatment and the control group.
4. Discussion
It is clear that soil saline alkalization has taken hold of over a billion hectares of land across the globe [39]. With the ongoing global climate change and irrational irrigation, saline–alkali stress has become a significant adverse factor that hinders plant growth, development, and reproduction, as well as reducing crop yields [18,40]. In order to reveal the stress adaption of I. typhifolia, a series of measurements were taken on a range of parameters, including germination parameters, antioxidant enzyme activity, osmotic adjustment substances, endogenous hormones, and photosynthetic parameters, under artificial saline–alkali stress; the germination threshold was confirmed based on regression analyses.
4.1. Effects of Artificial Saline–Alkali Stress on Seed Germination of I. typhifolia
Germination represents the initial stage in the growth and development of plants, which is of critical importance in determining the quality of the whole crop production. Saline–alkali stress is known to impede germination, which was confirmed in Chenopodium quinoa [41], Lolium perenne [42], and Sorghum bicolor [43]. In this study, the germination rate, germination index, and radicle length of I. typhifolia seeds were suppressed by an over-T50 artificial saline–alkali stress treatment. This interpreted that a high concentration of Na+ and Cl− results in a reduction in the osmotic potential of I. typhifolia seed cells, which impedes their germination [44]. In addition, the accumulation of ROS has been demonstrated to damage cell structure and macromolecular nutrients, thereby inhibiting seed germination [45].
4.2. Effects of Artificial Saline–Alkali Stress on Antioxidant Enzyme Activity and Malondialdehyde Concentration in Germinated Seeds of I. typhifolia
The inhibition of germination in I. typhifolia seeds can also be attributed to the antioxidant enzyme activity. It is known that the maintenance of ROS homeostasis is a fundamental prerequisite for the germination process [46]. SOD and POD are essential enzymes for ROS detoxification under saline–alkali stress. This study observed an initial increase in POD, SOD, and CAT activities in I. typhifolia under mild artificial stress (50–100 mmol·L−1). Similar results were found in Leymus chinensis [47], Avena sativa [48] and Cotyledon vs. True Leaf [49]. When the concentration of saline–alkali stress exceeds the capacity of the defense system to regulate it, the mechanism for scavenging ROS is inhibited to a certain extent. This results in the inhibition of the synthesis of POD, SOD, and CAT, as well as a decrease in their expression activity. In this study, the POD and SOD activities of I. typhifolia germinated seeds peaked at T100, while the CAT activity peaked at T150. Subsequently, all of these antioxidant enzyme activities exhibited a decline. A comparable pattern was observed in I. germanica, where the activities of SOD and POD decreased when NaCl concentration exceeded 105 mM [26]. A previous study demonstrated that, in Sorbus pohuashanensis [50], POD and SOD were primarily employed to treat excessive ROS in low-concentration saline–alkali environments, while CAT was mainly used to remove excessive reactive oxygen species in high-concentration saline–alkali environments [48]. In this case, SOD, POD, and CAT were employed in unison to scavenge ROS in order to alleviate stress below T150. However, T200 was beyond the scope of the defensive system. The MDA concentration can reflect the level of membrane lipid peroxidation and can thus indicate the degree of membrane damage under stress conditions [51]. It is usually enhanced with stress treatments [52,53]. In this study, the MDA concentration of I. typhifolia seeds exhibited a slight increase at T50, reaching its peak at T200. When combined with the results of the antioxidant enzyme activity, SOD, POD, and CAT effectively alleviated the damage to the membrane under mild stress conditions. However, the damage of the membrane significantly intensified when stress was beyond the defensive system limit.
4.3. Effects of Artificial Saline–Alkali Stress on the Concentration of Osmotic Adjustment Substances in the Germinated Seeds of I. typhifolia
The high salt and alkaline concentration of the soil increases its osmotic potential. In order to maintain their water potential, plants synthesize columns of small molecules to prevent water loss, including Pro, soluble proteins, betaine, and SS [40]. Previous research has illustrated that the SS and Pro concentration in Aquilegia species [17] and Trollius chinensis [54] increased under salt or saline–alkali stress. In this study, the concentration of Pro and SS was found to be elevated in I. typhifolia under artificial saline–alkali stress (T50–T200), which showed that Pro and SS play essential roles in defending against artificial saline–alkali stress. Moreover, excessive stress (T150–T200) resulted in the disruption of intracellular ion homeostasis, the inhibition of seed metabolic activity, affects intracellular enzymes, and reduces SS accumulation. Nevertheless, the Pro concentration continued to increase throughout the stress treatment. Consequently, it can be postulated that Pro is correlated with the defensive mechanisms of I. typhifolia in response to high artificial saline–alkali stress (T150–T200).
4.4. Effects of Artificial Saline–Alkali Stress on Endogenous Hormones in I. typhifolia Germinated Seeds
It was demonstrated that plant hormones regulate a multitude of physiological and biochemical responses under stress conditions throughout the plant life cycle [55]. These include ABA, IAA, GA, ethylene (ET), and jasmonates (JAs) [56]. Among them, GA and ABA are usually closely related to seed germination [48,56]. The importance of GA in the context of seed germination is clear. A reduction in GA concentration is a contributing factor to the inhibition of seed germination under stress conditions [57]. Previous research has demonstrated that the utilization of exogenous GA effectively alleviates germination inhibition [58,59]. Conversely, ABA is a factor that inhibits seed germination, induces seed dormancy, and prevents vivipary [60]. In this study, although GA promotes germination, under T200 treatment, GA showed a downward trend, while ABA continued to rise, further strengthening its inhibitory effect on seed germination, resulting in the lowest level of seed germination being under the T200 treatment. Our findings demonstrated that IAA and CTK undergo significant changes during artificial saline–alkali stress, which indicated that at least GA, ABA, IAA, and CTK played important roles in I. typhifolia’s defensive system.
4.5. Effects of Artificial Saline–Alkali Stress on Chlorophyll and Photosynthetic Parameters of I. typhifolia Seedlings
Excess ions are toxic to plant cells and are particularly damaging to chloroplasts. Saline–alkali stress can result in the destruction of structures, cell membranes, and nucleic acids within chloroplasts [61]. Chlorophyll synthesis could also be inhibited and subsequently degraded by stress conditions [62]. In this study, I. typhifolia seedlings synthesized less chlorophyll under artificial saline–alkali stress (T50–200 for over 14 d), which was similar to the results of I. japonica [8] and three Iris cultivars ‘Purple Blue Magic’, ‘White Madonna’, and ‘Blue Deep River’ [63] under abiotic stress. However, different results were found in studies related to Karelinia caspia [64] and Arundo donax [65], which indicated different defensive mechanisms.
In response to saline–alkali conditions, plants typically close their stomata, a process that reduces water loss and serves as a survival strategy [66]. A previous study illustrated that gs is the most crucial photosynthesis parameter under abiotic stress [53]. In this study, the gs values of I. typhifolia all declined under artificial saline–alkali stress (T50–200 for over 7 d). Similar results were observed in previous studies related to I. germanica [26] and Aquilegia [14]. With treatment for 7 d, the trends for Pn, gs, and Ci exhibited a consistent decline, indicating that stomatal limitations (SLs) were the main factor to limiting photosynthesis. When treatment was maintained for 14 d–21 d, trends of Ci were in the opposite direction to those of Pn and gs, in the 400–500 mmol·L−1 groups. This indicated that the chloroplast structure had been further damaged and that non-stomatal limitations (NSLs) were the main factor inhibiting photosynthesis [67].
4.6. Effects of Artificial Saline–Alkali Stress on Chlorophyll Fluorescence Parameters of I. typhifolia Seedlings
The chlorophyll fluorescence parameters permit the rapid assessment of the capacity for light absorption and carbon synthesis in leaves without any damage [49,68]. Abiotic stress conditions predominantly impact photosystem II (PSII), which represents the most vulnerable structure within chloroplasts. Fv/Fm is regarded as a reliable indicator for the assessment of PSII damage [69]. In this study, the Fv/Fm values of I. typhifolia showed a downward trend in the T50-200 groups, indicating that the PSII of I. typhifolia was damaged to varying degrees under artificial saline–alkali stress. It appears that the trend of the Fv/Fm values’ changes regarding Lolium perenne [70] and Atractylodes lancea [71] under abiotic stresses are similar. Meanwhile, ΦPSII exhibited a more pronounced decline, indicative of a photoprotective response to saline–alkali stress [69]. The reduction in light energy for carbon assimilation is accompanied by an increase in energy dissipation as heat, due to the damage to PSII. This was well confirmed by the results of NPQ in this study, which increased after 7 days of artificial stress (T50–200). Therefore, it can be observed that when I. typhifolia was exposed to relatively brief periods (7 d) of artificial saline–alkali stress, stomata were enclosed to prevent water loss. Additionally, the photosynthetic efficiency was reduced to protect PSII, which was the adaptation strategy to artificial saline–alkali stress. However, when the ion concentration was elevated to an excessive degree (T50–200) or was prolonged (14–21 d), this contributed to the damage in the PSII and degradation of chlorophyll, resulting in a non-stomatal limitation for photosynthesis.
5. Conclusions
The artificial salt and alkali stress resulted in a decrease in both germination and seedling periods, which was reflected in germination status, degree of membrane peroxidation, and non-stomatal limitation. I. typhifolia employs a suite of physiological adaptation strategies to resist saline–alkali stress, including the adjustment of endogenous osmoregulators, hormone levels, the mobilization of the antioxidant system, and the regulation of stomatal opening and closing. A correlation coefficient matrix and regression analysis were employed to calculate the germination threshold, which was found to be 87.15 mL−1. The concentration of artificial saline–alkali stress used in this study was higher than the level of saline–alkali deposition in green spaces after the application of snow-melting agents, which was within the tolerance range of the seeds and seedlings of I. typhifolia. Consequently, I. typhifolia is a suitable candidate for open-air ornamental planting in areas where snow-melting agents are employed. This study provided a theoretical reference for tolerance cultivars’ breeding by revealing the salt tolerance mechanism of I. typhifolia, which has significant practical implications for enhancing ecological environment construction in saline and alkaline lands. Further research is required to elucidate the molecular mechanism of I. typhifolia under saline–alkali tolerance, which is expected to provide more insights for the tolerance breeding of Iris.
Author Contributions
L.C., J.Y. and X.L.: designed the research methodology, conducted the experiments, processed and analyzed the data collection, wrote the first draft of the paper, and participated in the discussions and revisions together. Q.W.: validation and data organization. S.W. and Y.S.: Conceptualizing the experimental plan. Y.L.: analyzed the data. Y.M. and Y.Z.: edited and reviewed the manuscript, provided laboratory instrumentation and equipment and technical support, designed the experimental protocols, and contacted and involved all authors in major decisions regarding publication together. All authors have read and agreed to the published version of the manuscript.
Funding
The Key Research and Development Project of Jilin Provincial Science and Technology Department (20210202081NC); the Natural Science Foundation of Jilin Provincial Science and Technology Department (20240101197JC); the Jilin Provincial Science and Technology Department’s Key Research and Development Projects “breeding and demonstration of good varieties (lines) of Changbai Mountain Iris” (20240303102NC); and the Jilin Provincial Education Department’s scientific research project (effect of saline and alkaline stress on seed germination and physiological characteristics of Iris typhifolia) (JJKH20240448KJ).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bai, J.; Li, Y.; Zhang, J.; Xu, F.; Bo, Q.; Wang, Z.; Yue, S. Straw returning and one-time application of a mixture of controlled release and solid granular urea to reduce carbon footprint of plastic film mulching spring maize. J. Clean. Prod. 2021, 280, 124478. [Google Scholar] [CrossRef]
- Tao, C.S.; Yao, L.J.; Chen, Y.C.; Li, L.Y.; Kong, Y.P. The impact of expressway snowmelt agent usage on the environment in an extreme freezing snow and sleet condition. IOP Conf. Ser. Earth Environ. Sci. 2018, 191, 012073. [Google Scholar] [CrossRef]
- Green, S.M.; Machin, R.; Cresser, M.S. Effect of long-term changes in soil chemistry induced by road salt applications on n-transformations in roadside soils. Environ. Pollut. 2008, 152, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Li, H.; Huang, S.L.; Ren, C.H.; Weng, X.H.; Zhang, S.Z.; Liu, L.Y.; Pei, J.B. Optimal exogenous calcium alleviates the damage of Snow-melting agent to Salix matsudana seedlings. Front. Plant Sci. 2022, 13, 928092. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.P.; Chong, P.F.; Zhao, M. Effect of salt stress on the photosynthetic characteristics and endogenous hormones, and: A comprehensive evaluation of salt tolerance in Reaumuria soongorica seedlings. Plant Signal. Behav. 2022, 17, 2031782. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.Y.; Liang, X.L.; Li, H.M.; Xie, C.J.; He, W.X.; Qin, Y.X. Identification and characterization of wheat germplasm for salt tolerance. Plants 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Rehman, A.; Li, X.W.; Jiang, X.; Tian, C.Y.; Wang, X.Y.; Li, H.G.; Wang, Z.Z.; He, S.P.; Du, X.M. Comprehensive evaluation and transcriptome analysis reveal the salt tolerance mechanism in semi-wild cotton (Gossypium purpurascens). Int. J. Mol. Sci. 2023, 24, 12853. [Google Scholar] [CrossRef]
- Muhammad, N.; Rabail, Z.; Shehzad, K.K.; Noman, Y.; Farhan, Q.M.; Martin, B.; Jiri, H.; Adnan, M. Unveiling the potential of acidified cow dung in combination with plant growth promoting endophytes on growth, physiology, and yield improvement of maize in salt-affected soil. Arab. J. Geosci. 2023, 16, 551. [Google Scholar] [CrossRef]
- Sasan, S.M.; Akbar, K.; Filippo, M. Photosynthesis and chlorophyll fluorescence of Iranian licorice (Glycyrrhiza glabra L.) accessions under salinity stress. Front. Plant Sci. 2022, 13, 984944. [Google Scholar] [CrossRef]
- Mann, A.; Kumar, N.; Lata, C.; Kumar, A.; Meena, B.L.; Kumar, A. Physiological and differential gene expression reveals a trade-off between antioxidant capacity and salt tolerance in Urochondra setulosa and Dichanthium annulatum. Plant Growth Regul. 2024, 102, 555–570. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.C.; Yan, H.K.; Bao, Y.; Gong, M.S.; Wang, W.H.; Li, S.; Ma, S.Y.; Chen, B.H. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: Transcriptional and metabolic profiling. BMC Plant Biol. 2022, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Miao, R.Q.; Zhang, Y.; Yuan, Y.; Pang, Q.Y.; Zhou, Z.Q. Metabolic and molecular basis for the salt and alkali responses of Suaeda corniculata. Environ. Exp. Bot. 2021, 192, 104643. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, J.P.; Guo, Z.P.; Sui, X.; Xu, N.; Kareem, H.A.; Hassan, M.U.; Yan, M.; Zhang, Q.; Cui, J.; et al. Graphene enhances photosynthesis and the antioxidative defense system and alleviates salinity and alka-linity stresses in alfalfa (Medicago sativa L.) by regulating gene expression. Environ. Sci.-Nano 2021, 8, 2731–2748. [Google Scholar] [CrossRef]
- Kha, W.; Prithivira, B.; Smit, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Basra, S.M.A.; Cheema, M.A.; Farooq, M.; Jafar, M.Z.; Shahid, M.; Yasmeen, A. Seed priming: A shotgun approach for alleviation of salt stress in wheat. Int. J. Agric. Biol. 2013, 15, 1199–1203. [Google Scholar] [CrossRef]
- Pereira, I.C.; Catão, H.C.R.M.; Caixeta, F. Seed physiological quality and seedling growth of pea (Pisum sativum) under water and salt stress. Rev. Bras. Eng. Agríc. Ambient. 2020, 24, 95–100. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Jiang, D.; Yang, F.; Zhou, Y. Physio–biochemical responses of three Aquilegia species seedlings to salt stress. Agronomy 2022, 12, 2841. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline–alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Liu, W.; Zhang, X.; Lu, Y. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, A.; Zhang, W. Higher endogenous bioactive gibberellins and α-amylase activity confer greater tolerance of rice seed germination to saline-alkaline stress. Environ. Exp. Bot. 2019, 162, 357–363. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Niu, C.; Yu, Q.; Wang, Q.; Wang, J.; Sun, X.; Wang, Z.; Shan, X. Experimental investigation of the erodibility of soda saline-alkali soil under freeze-thaw cycle from a microscopic view. Catena 2023, 232, 107430. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, R.; Zhu, H.; Cheng, X.; Shutes, B.; Yan, B. Seed germination and early seedling growth of six wetland plant species in saline-alkaline environment. Int. J. Phytoremediation 2020, 22, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Tian, K.; Ban, Z.; Xu, H.; Jia, W.; Zhu, Y.; Chen, H. Analysis of floral fragrance components in different parts of Iris typhifolia. Horticulturae 2023, 9, 1268. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Cheng, Y.; Wang, F.; Zhao, X. Morphological and metabolic responses of four Iris germanica cultivars under salinity stress. Sci. Hortic. 2021, 281, 109960. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cao, P.; Zeng, X.; Xu, B.; Luo, f.; Yang, X. Morphological structure and physiological and biochemical responses to drought stress of Iris japonica. Plants 2023, 12, 3729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Zhao, X.; Zhang, M. Evaluation of drought resistance and transcriptome analysis for the identification of drought-responsive genes in Iris germanica. Sci. Rep. 2021, 11, 16308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Z.; Liu, Q.; Tang, J.; Huang, S.; Dhankher, O.P.; Yuan, H. Growth, physiological adaptation, and NHX gene expression analysis of Iris halophila under salt stress. Environ. Sci. Pollut. Res. 2018, 25, 207–25216. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, M.; Hossain, M.K. Effect of pre-sowing treatments on germination and initial seedling development of Albizia saman in the nursery. J. For. Res. 2005, 16, 200–204. [Google Scholar] [CrossRef]
- Holloway, P.S. Seed germination of Alaska Ifs. Hortscience 1987, 22, 898–899. [Google Scholar] [CrossRef]
- Ryuya, M.; Katsumasa, Y.; Daisuke, H.; Yasuhisa, H. Effects of salinity, temperature, and immersion conditions on seed germination of invasive Spartina alterniflora Loisel (Smooth cordgrass) in Japan. Reg. Stud. Mar. Sci. 2023, 57, 102738. [Google Scholar] [CrossRef]
- Önen, H.; Farooq, S.; Tad, S.; Özaslan, C.; Gunal, H.; Chauhan, B.S. The influence of environmental factors on germination of burcucumber (Sicyos angulatus) seeds: Implications for range expansion and management. Weed Sci. 2018, 66, 494–501. [Google Scholar] [CrossRef]
- Jianting, L.; Mengjie, D.; Chuanqi, W.; Yanjun, M. Effects of drought and salt stress on seed germination and seedling growth of Elymus nutans. PeerJ 2023, 11, e15968. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Dong, H.; Deng, Z. Physiological mechanisms of Bretschneidera sinensis hemsl. seed dormancy release and germination. Forests 2023, 14, 2430. [Google Scholar] [CrossRef]
- Wang, X.D.; Shen, H.L.; Yang, L. The Response of hormones, reactive oxygen species and nitric oxide in the Polyethylene-Glycol-Promoted, salt–alkali-stress-induced embryo germination of Sorbus pohuashanensis. Int. J. Mol. Sci. 2024, 25, 5128. [Google Scholar] [CrossRef]
- Sifiso, N.; Oluwaseyi, S.B.; Magnus, A.U.; Ida, R. Optimal chlorophyll extraction conditions and postharvest stability in Moringa (M. Oleifera) leaves. J. Food Meas. Charact. 2023, 18, 1611–1626. [Google Scholar] [CrossRef]
- Kochaphan, V.; Piyada, T.; Supranee, S.; Watanachai, L.; Anoma, D. Photosynthesis performance at different growth stages, growth, and yield of rice in saline fields. Plants 2023, 12, 1903. [Google Scholar] [CrossRef]
- Nirmalendu, B.; Kumar, A.R.; Parul, S. Assessing soil quality for rehabilitation of salt-affected agroecosystem: A comprehensive review. Front. Environ. Sci. 2022, 10, 935785. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Stoleru, V.; Slabu, C.; Vitanescu, M.; Peres, C.; Cojocaru, A.; Covasa, M.; Mihalache, G. Tolerance of Three quinoa cultivars (Chenopodium quinoa Willd.) to salinity and alkalinity stress during germination stage. Agronomy 2019, 9, 287. [Google Scholar] [CrossRef]
- Javaid, M.M.; Mahmood, A.; Alshaya, D.S.; Alkahtani, M.D.F.; Waheed, H.; Wasaya, A.; Khan, S.A.; Naqve, M.; Haider, I.; Shahid, M.A.; et al. Influence of environmental factors on seed germination and seedling characteristics of perennial ryegrass (Lolium perenne L.). Sci. Rep. 2022, 12, 9522. [Google Scholar] [CrossRef]
- He, L.; Zhao, Y.Y.; Lu, Z.H. Effects of saline-alkaline stress on seed germination and seedling growth of Sorghum bicolor (L.) Moench. Appl. Biochem. Biotechnol. 2014, 173, 1680–1691. [Google Scholar] [CrossRef]
- Shah, F.A.; Wei, X.; Wang, Q.J.; Liu, W.B.; Wang, D.D.; Yao, Y.Y.; Hu, H.; Chen, X.; Huang, S.; Hou, J.; et al. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Chen, H.; Shi, J.; Tao, L.; Han, X.; Lin, G.; Chen, X. Exogenous spermidine priming mitigates the osmotic damage in germinating seeds of Leymus chinensis under salt–alkali stress. Front. Plant Sci. 2021, 12, 701538. [Google Scholar] [CrossRef]
- Baily, C. The signaling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Chen, H.; Tao, L.; Shi, J.; Han, X.; Cheng, X. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt–alkali stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Ren, C.; Zhan, C.; Wang, C.; Li, J.; Ren, Q.; Liang, X.; Wei, L.; Xiang, D.; et al. Physiological and biochemical mechanisms of exogenous melatonin regulation of saline–alkali tolerance in oats. Agronomy 2023, 13, 1327. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, W.; Peng, X.; Hua, X.; Yan, X.; Zhou, Z.; Lin, J. Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (Cotyledon vs. True Leaf) under salt and alkali stresses: From the growth, photosynthesis and chlorophyll fluorescence. Front. Plant Sci. 2019, 9, 01939. [Google Scholar] [CrossRef]
- Shen, H.L.; Wang, Y.T.; Wang, X.D.; Yang, L.Z.; Cai, H. Exogenous ethylene alleviates the inhibition of Sorbus pohuashanensis embryo germination in a saline-alkali environment (NaHCO3). Int. J. Mol. Sci. 2023, 24, 4244. [Google Scholar] [CrossRef]
- Pavla, V.; Helena, H.; Frantisek, H.; Kamil, K. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca Oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Shu, Y.J.; An, Y.M.; Guo, C.H.; Liu, Y.R.; Song, L.L. De novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef]
- Feng, Z.H.; Lu, G.R.; Sun, M.; Jin, Y.Y.; Xu, Y.; Liu, X.L.; Wang, M.M.; Liu, M.; Yang, H.Y.; Guan, Y.; et al. Comparative study of the priming effect of abscisic acid on tolerance to saline and alkaline stresses in rice seedlings. Agronomy 2023, 13, 2698. [Google Scholar] [CrossRef]
- Hou, R.; Yang, L.; Wuyun, T.; Chen, S.; Zhang, L. Genes related to osmoregulation and antioxidation play important roles in the response of Trollius chinensis seedlings to saline alkali stress. Front. Plant Sci. 2023, 14, 1080504. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, M.; Khan, R.; Masood, A.; Khan, N.A. Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu. Rev. Res. Biol. 2013, 127, 543. [Google Scholar]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Andrade, A.; Masciarelli, O.; Alemano, S.; Luna, V. Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci. Res. 2016, 26, 1–13. [Google Scholar] [CrossRef]
- Nasri, N.; Mahmoudi, H.; Baatour, O.; M’rah, S.; Kaddour, R.; Lachâal, M. Effect of exogenous gibberellic acid on germination, seedling growth and phosphatase activities in Lettuce under salt stress. Afr. J. Biotechnol. 2012, 11, 11967–11971. [Google Scholar] [CrossRef]
- Jamil, M.; Rha, E.S. Gibberellic acid (GA3) enhance seed water uptake, germination and early seedling growth in sugar beet under salt stress. Pak. J. Biol. Sci. 2007, 10, 654–658. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt stress inhibits photosynthesis and destroys chloroplast structure by downregulating chloroplast development–related genes in Robinia pseudoacacia seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; He, G.; Che, Y.; Teng, Z.; Shao, J.; Xu, N.; Sun, G. Chlorophyll synthesis and the photoprotective mechanism in leaves of mulberry (Morus alba L.) seedlings under NaCl and NaHCO3 stress revealed by TMT–based proteomics analyses. Ecotoxicol. Environ. Safe 2020, 190, 110164. [Google Scholar] [CrossRef]
- Sarvandi, S.; Nia, A.E.; Nejad, A.R.; Azimi, M.H. Morpho–physiological responses of some Iris cultivars under drought and salinity stresses. J. Agric. Sci. Technol. 2020, 22, 535–546. [Google Scholar]
- Li, C.; Mur, L.A.J.; Wang, Q.; Hou, X.; Zhao, C.; Chen, Z.; Wu, J.; Guo, Q. ROS scavenging and ion homeostasis is required for the adaptation of halophyte Karelinia caspia to high salinity. Front. Plant Sci. 2022, 13, 979956. [Google Scholar] [CrossRef]
- Müller, B.; Arcoverde Cerveira Sterner, V.; Papp, L.; May, Z.; Orlóci, L.; Gyuricza, C.; Sági, L.; Solti, Á.; Fodor, F. Alkaline salt tolerance of the biomass plant Arundo donax. Agronomy 2022, 12, 1589. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, C.; Liang, S.; Yuan, Y.; Liu, C.; Liu, J.; Feng, B. The alkali tolerance of broomcorn millet (Panicum miliaceum L.) at the germination and seedling stage: The case of 296 broomcorn millet genotypes. Front. Plant Sci. 2021, 12, 711429. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Stomatal limitations to photosynthesis and their critical water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Shi, C.; Yang, F.; Liu, Z.; Li, Y.; Di, X.; Wang, J.; Lin, J. Uniform water potential induced by salt, alkali, and drought stresses has different impacts on the seedling of Hordeum jubatum: From growth, photosynthesis, and chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 733236. [Google Scholar] [CrossRef]
- Guidi, L.; Piccolo, E.L.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 00174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Jiang, Y.; Li, H.; Zhang, Z.; Xu, Z.; Xu, B.; Huang, B. Natural variation of physiological traits, molecular markers, and chlorophyll catabolic genes associated with heat tolerance in perennial ryegrass accessions. BMC Plant Biol. 2020, 20, 520. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).