Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Methods
2.2.1. Explant Disinfection and Induction
2.2.2. Successional Proliferation
2.2.3. In Vitro Rooting and Acclimatization
2.2.4. Quantitative Detection of the Active Ingredient of T. arguta
2.3. Statistical Analysis
3. Results
3.1. Explant Disinfection and Induction
3.2. Successional Proliferation
3.2.1. Effect of the Culture Media on the Proliferation of Tissue Culture Plantlets
3.2.2. Effect of Different Hormone Combinations on the Subproliferation of Tissue Culture Plantlets
3.3. In Vitro Rooting and Acclimatization
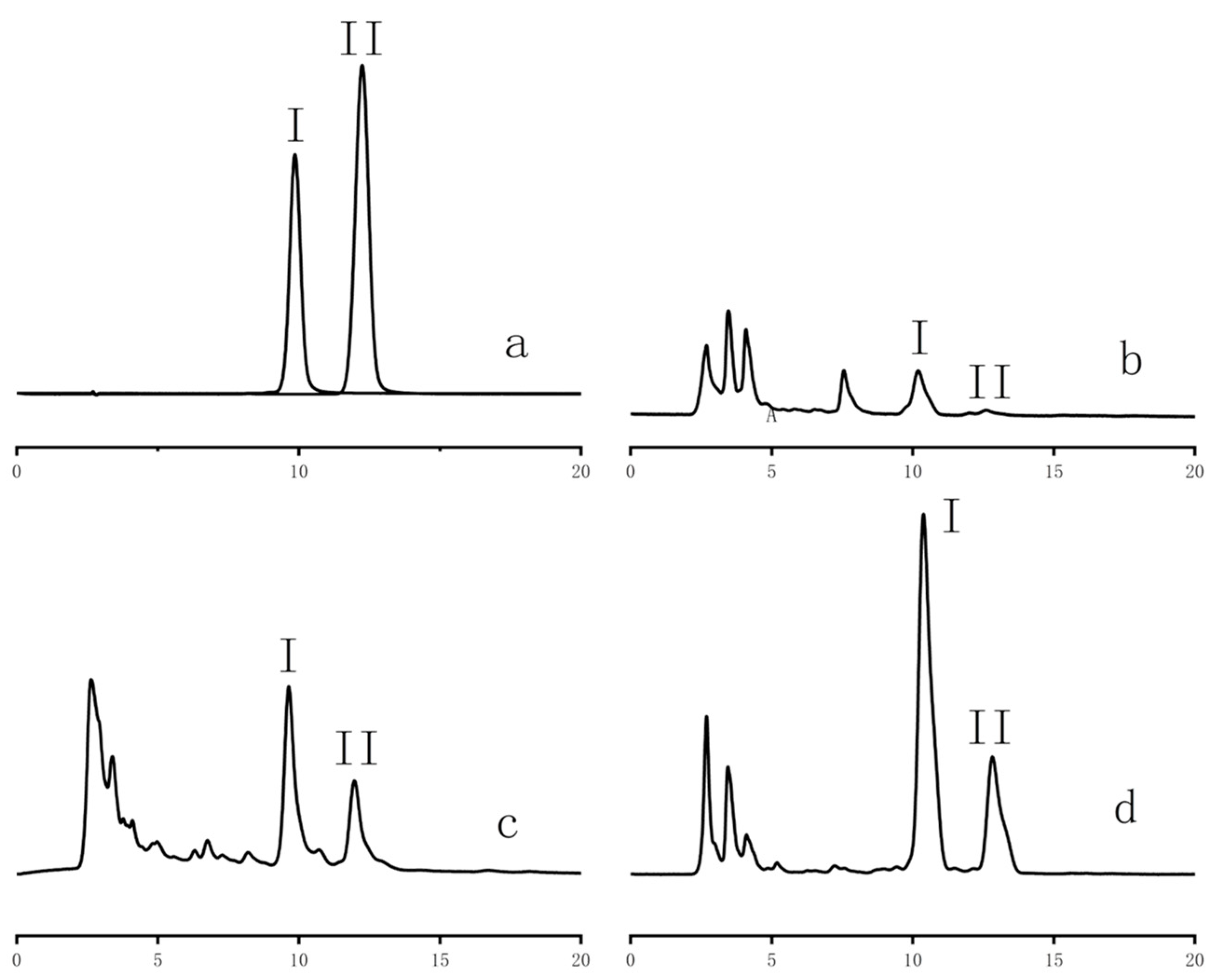
3.4. Determination of Ligustroflavone and Rhoifolin Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sena, S.; Kaur, H.; Kumar, V. Lycorine as a Lead Molecule in the Treatment of Cancer and Strategies for Its Biosynthesis Using the in Vitro Culture Technique. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The Antihypertensive Potential of Flavonoids from Chinese Herbal Medicine: A Review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Maan, G.; Sikdar, B.; Kumar, A.; Shukla, R.; Mishra, A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Testai, L. Flavonoids and Mitochondrial Pharmacology: A New Paradigm for Cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary Agents in Cancer Prevention: Flavonoids and Isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; Luca, A.D.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef]
- Zhou, Z.-G.; Li, D.-D.; Chen, Y.; Chen, X.; Man, R.-J. Discussion on the Structural Modification and Anti-Tumor Activity of Flavonoids. Curr. Top. Med. Chem. 2022, 22, 561–577. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic Potential of Flavonoids and Their Mechanism of Action against Microbial and Viral Infections—A Review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China: 2020 Edition (Part II), 11th ed.; China Medical Science and Technology Press: Beijing, China, 2020; p. 31. [Google Scholar]
- Yang, X.; Li, L.; Zhang, T.; Deng, J.; Lin, X.; Li, Y.; Xia, B.; Lin, L. GC-MS-Based Serum Metabolomic Investigations on the Ameliorative Effects of Polysaccharide from Turpiniae folium in Hyperlipidemia Rats. Oxidative Med. Cell Longev. 2021, 2021, 9180635. [Google Scholar] [CrossRef]
- Xiao, C.-R.; Tu, L.-F.; Zhang, R.-Z.; Liu, D.-P.; Luo, Y.-M. Research progress on chemical constituents and biological activities from Turpinia species. Zhongguo Zhong Yao Za Zhi 2019, 44, 1295–1304. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Yu, S.-C.; Jin, Y.; Lv, X.-W.; Zou, Y.-H.; Li, Y. Therapeutic Effects and Mechanisms of Total Flavonoids of Turpinia arguta Seen on Adjuvant Arthritis in Rats. J. Ethnopharmacolm 2008, 116, 167–172. [Google Scholar] [CrossRef]
- Ma, S.-G.; Yuan, S.-P.; Liu, Y.-B.; Qu, J.; Li, Y.; Wang, X.-J.; Wang, R.-B.; Xu, S.; Hou, Q.; Yu, S.-S. 3-Hydroxy-3-Methylglutaryl Flavone Glycosides from the Leaves of Turpinia arguta. Fitoterapia 2018, 124, 80–85. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Wang, W.; Zhao, Y. Development and Validation of an LC-ESI-MS/MS Method for Simultaneous Determination of Ligustroflavone and Rhoifolin in Rat Plasma and Its Application to a Pharmacokinetic Study. J. Chromatogr. Sci. 2017, 55, 267–274. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Wei, X. Megastigmans from Turpinia arguta. Chem. Nat. Compd. 2014, 50, 772–773. [Google Scholar] [CrossRef]
- Liu, Z.L.; Li, L.; Tang, Y.Q.N.; Lin, L.M.; Xia, B.H. Chemical composition,antioxidant and anti-inflammatory activities of volatile oil from Turpiniae folium. Nat. Prod. Res. Dev. 2020, 34, 723–738. [Google Scholar] [CrossRef]
- Feng, R.; Ding, F.; Mi, X.-H.; Liu, S.-F.; Jiang, A.-L.; Liu, B.-H.; Lian, Y.; Shi, Q.; Wang, Y.-J.; Zhang, Y. Protective Effects of Ligustroflavone, an Active Compound from Ligustrum lucidum, on Diabetes-Induced Osteoporosis in Mice: A Potential Candidate as Calcium-Sensing Receptor Antagonist. Am. J. Chin. Med. 2019, 47, 457–476. [Google Scholar] [CrossRef]
- Kang, R.; Tian, W.; Cao, W.; Sun, Y.; Zhang, H.-N.; Feng, Y.-D.; Li, C.; Li, Z.-Z.; Li, X.-Q. Ligustroflavone Ameliorates CCl4-Induced Liver Fibrosis through down-Regulating the TGF-β/Smad Signaling Pathway. Chin. J. Nat. Med. 2021, 19, 170–180. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, G.; Sheng, C. Targeting Necroptosis in Anticancer Therapy: Mechanisms and Modulators. Acta Pharm. Sin. B 2020, 10, 1601–1618. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, F.; Zhang, W.; Feng, Z.; Yang, Y.; Mei, Z. Novel Insight into the Therapeutical Potential of Flavonoids from Traditional Chinese Medicine against Cerebral Ischemia/Reperfusion Injury. Front. Pharmacol. 2024, 15, 1352760. [Google Scholar] [CrossRef]
- Eldahshan, O.R. A Potent Antiproliferative Effect on Cancer Cell Lines. Br. J. Pharm. Res. 2013, 3, 46–53. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Liu, W.; Feng, F.; Xie, N. HPLC with Quadrupole TOF-MS and Chemometrics Analysis for the Characterization of Folium Turpiniae from Different Regions. J. Sep. Sci. 2013, 36, 2552–2561. [Google Scholar] [CrossRef]
- Yu, X.X.; Liu, Q.D.; Wu, J.W.; Liang, Z.K.; Zhao, M.Q.; Xu, X.J. Simultaneous Determination of Four Major Constituents in Citri Grandis Exocarpium by HPLC-DAD. Acta Chromatogr. 2016, 28, 129–143. [Google Scholar] [CrossRef]
- Guo, X.; Xia, Z.; Song, M.; Li, C.; Wang, J.; Kang, W. Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth. Open Chem. 2018, 16, 99–107. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, L.-J.; Li, Y.-M.; Zhang, Z.-M.; Lin, L.-M.; Xia, B.-H. Preparation and Characterization of Polysaccharides from Turpiniae folium and Its Antioxidative, Anti-Inflammatory Activities and Antiproliferative Effect on VSMCs. Chem. Biodivers. 2022, 19, e202200459. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zhou, G.P.; Yang, X.J.; Xie, E.L. HPLC Simultaneous Determination of Ligustroflavone and Rhoifolin in Shanxiangyuan Tablets. Chin. J. Pharm. Anal. 2009, 29, 912–914. [Google Scholar]
- Song, W.J.; Song, Q.L.; Zou, Z.H.; Chen, X.L.; Liu, L.X.; Tan, J.; Wei, L.M.; Xiong, P.W.; Tao, X.H.; Sun, R.P.; et al. Effects of Turpiniae folium Extract on Growth Performance, Serum Immune and Antioxidant Function and Intestinal Microflora of Wenchang Chickens. Chin. J. Anim. Nutr. 2022, 34, 4380–4393. [Google Scholar] [CrossRef]
- Tao, X.H.; Jiang, J.; Luo, H.W.; Li, D.; Luo, Y.S. Study on the Biological Characteristics and Container Seedling Raising Technology of Turpinia arguta Fresh Leaves. Jiang Sci. 2020, 38, 188–190. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Mukta, N.; Sreevalli, Y. Propagation Techniques, Evaluation and Improvement of the Biodiesel Plant, Pongamia pinnata (L.) Pierre—A Review. Ind. Crops Prod. 2010, 31, 1–12. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Gang, R.; Komakech, R.; Chung, Y.; Okello, D.; Kim, W.J.; Moon, B.C.; Yim, N.-H.; Kang, Y. In Vitro Propagation of Codonopsis pilosula (Franch.) Nannf. Using Apical Shoot Segments and Phytochemical Assessments of the Maternal and Regenerated Plants. BMC Plant Biol. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Shasmita; Behera, S.; Mishra, P.; Samal, M.; Mohapatra, D.; Monalisa, K.; Naik, S.K. Recent Advances in Tissue Culture and Secondary Metabolite Production in Hypericum perforatum L. Plant Cell Tissue Organ. Cult. 2023, 154, 13–28. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Shoot Multiplication from Mature Trees of Douglas-Fir (Pseudotsuga menziesii) and Sugar Pine (Pinus lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef]
- Lloyd, G.B.; McCown, B.H. Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia latifolia, by Use of Shoot-Tip Culture. Proc. Int. Plant Prop. 1980, 30, 421–427. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Establishment of Optimized in Vitro Disinfection Protocol of Pistacia vera L. Explants Mediated a Computational Approach: Multilayer Perceptron–Multi−objective Genetic Algorithm. BMC Plant Biol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Yan, H.; Mi, Y.; Li, Y.; Zang, H.; Guo, L.; Huo, J.; Man, Z.; Chen, Z.; Zhang, B.; Sang, M.; et al. First Report of Postharvest Fruit Rot Caused by Botrytis Cinerea on Blue Honeysuckle (Lonicera caerulea) Fruit in China. Plant Dis. 2024, 108, 527. [Google Scholar] [CrossRef]
- Zheng, W.; Yu, Z.; Huang, S.; Tang, L.; Chen, X.; Guo, T.; Li, Q.; Hsiang, T.; Wang, Y. Fruit Anthracnose on Cavendish Bananas Caused by Colletotrichum fructicola in Guangxi, China. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Sahu, P.K.; Tilgam, J.; Mishra, S.; Hamid, S.; Gupta, A.; K, J.; Verma, S.K.; Kharwar, R.N. Surface Sterilization for Isolation of Endophytes: Ensuring What (Not) to Grow. J. Basic. Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Ramanathan, S.; Sengodagounder, S.; Senniappan, C.; Shanmuganathan, R.; Brindhadevi, K.; Kaliannan, T. Optimizing the Sterilization Methods for Initiation of the Five Different Clones of the Eucalyptus hybrid Species. Biocatal. Agric. Biotechnol. 2019, 22, 101361. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Jiang, H.; Zhang, S.; Que, Q.; Chen, X.; Zhou, W. Efficient In Vitro Sterilization and Propagation from Stem Segment Explants of Cnidoscolus aconitifolius (Mill.) I.M. Johnst, a Multipurpose Woody Plant. Plants 2022, 11, 1937. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Aralbayeva, M.M.; Zemtsova, A.S.; Alexandrova, A.M.; Kazybayeva, S.Z.; Mikhailenko, N.V.; Kushnarenko, S.V.; Bettoni, J.C. In Vitro Collection for the Safe Storage of Grapevine Hybrids and Identification of the Presence of Plasmopara viticola Resistance Genes. Plants 2024, 13, 1089. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An Efficient Micropropagation Protocol for an Endangered Ornamental Tree Species (Magnolia sirindhorniae Noot. & Chalermglin) and Assessment of Genetic Uniformity through DNA Markers. Sci. Rep. 2019, 9, 9634. [Google Scholar] [CrossRef]
- An, Y.; Jiao, X.; Yang, S.; Wang, S.; Chen, N.; Huang, L.; Jiang, C.; Lu, M.; Zhang, J. Evaluation of Novel Promoters for Vascular Tissue-Specific Gene Expression in Populus. Plant Sci. 2024, 344, 112083. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Yang, M.; Zou, J.; Zheng, Y.; Li, D. EgMADS3 Directly Regulates EgLPAAT to Mediate Medium-Chain Fatty Acids (MCFA) Anabolism in the Mesocarp of Oil Palm. Plant Cell Rep. 2024, 43, 107. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Pop, V.C.; Hârța, M.; Andrecan, F.A.; Bunea, C.I. Investigation of Salinity Tolerance to Different Cultivars of Highbush Blueberry (Vaccinium corymbosum L.) Grown in Vitro. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13691. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Wei, X.-L.; Wang, X.; Wei, Y. Induction of Somatic Embryogenesis in Different Explants from Ormosia Henryi Prain. Plant Cell Tissue Organ. Cult. 2020, 142, 229–240. [Google Scholar] [CrossRef]
- Carlín, A.P.; Tafoya, F.; Alpuche Solís, A.G.; Pérez-Molphe-Balch, E. Effects of Different Culture Media and Conditions on Biomass Production of Hairy Root Cultures in Six Mexican Cactus Species. In Vitro Cell Dev. Biol.—Plant 2015, 51, 332–339. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin Promotes Plant Growth by Increasing Nitrogen Uptake and Assimilation under Nitrogen Deficient Condition in Winter Wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef]
- Rakesh, B.; Sudheer, W.N.; Nagella, P. Role of Polyamines in Plant Tissue Culture: An Overview. Plant Cell Tissue Organ. Cult. 2021, 145, 487–506. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Zakari, S.A.; Asad, M.-A.-U.; Han, Z.; Zhao, Q.; Cheng, F. Relationship of Nitrogen Deficiency-Induced Leaf Senescence with ROS Generation and ABA Concentration in Rice Flag Leaves. J. Plant Growth Regul. 2020, 39, 1503–1517. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Khan, M.A.; Ali, A.; Khan, L.; Khan, M.S.; Mashwani, Z.-R. Feasible Production of Biomass and Natural Antioxidants through Callus Cultures in Response to Varying Light Intensities in Olive (Olea europaea L.) Cult. Arbosana. J. Photochem. Photobiol. B 2019, 193, 140–147. [Google Scholar] [CrossRef]
- Shimotohno, A.; Aki, S.S.; Takahashi, N.; Umeda, M. Regulation of the Plant Cell Cycle in Response to Hormones and the Environment. Annu. Rev. Plant Biol. 2021, 72, 273–296. [Google Scholar] [CrossRef]
- Yoshida, T.; Fernie, A.R.; Shinozaki, K.; Takahashi, F. Long-Distance Stress and Developmental Signals Associated with Abscisic Acid Signaling in Environmental Responses. Plant J. 2021, 105, 477–488. [Google Scholar] [CrossRef]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different Regulatory Mechanisms of Plant Hormones in the Ripening of Climacteric and Non-Climacteric Fruits: A Review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, X.; Wang, J.; Zou, J.; Nie, W.-F. Decreased Low-Light Regulates Plant Morphogenesis through the Manipulation of Hormone Biosynthesis in Solanum lycopersicum. Environ. Exp. Bot. 2021, 185, 104409. [Google Scholar] [CrossRef]
- Hussain, S.; Nanda, S.; Zhang, J.; Rehmani, M.I.A.; Suleman, M.; Li, G.; Hou, H. Auxin and Cytokinin Interplay during Leaf Morphogenesis and Phyllotaxy. Plants 2021, 10, 1732. [Google Scholar] [CrossRef]
- Nie, W.-F.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral Light Quality Regulates the Morphogenesis, Architecture, and Flowering in Pepper. J. Photochem. Photobiol. B 2023, 241, 112673. [Google Scholar] [CrossRef]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as Central Regulators during Plant Growth and Stress Response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.-D.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, H.; Hou, H.; Ning, Y.; Mu, L.; Li, S. Establishment of a Highly Efficient In Vitro Regeneration System for Rhododendron aureum. Forests 2023, 14, 1335. [Google Scholar] [CrossRef]
- Lin, H.; Xu, J.; Wu, K.; Gong, C.; Jie, Y.; Yang, B.; Chen, J. An Efficient Method for the Propagation of Bougainvillea glabra ‘New River’ (Nyctaginaceae) from In Vitro Stem Segments. Forests 2024, 15, 519. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Wei, L.; Lin, K.; Xu, Z.-F. Genome-Wide Identification and Expression Analysis of Cytokinin Response Regulator (RR) Genes in the Woody Plant Jatropha curcas and Functional Analysis of JcRR12 in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 11388. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Udayabhanu, J.; Gu, X.; Wu, R.; Xin, S.; Chen, Q.; Zhang, Y.; Yang, X.; Peng, S.; Chen, J.; et al. Induction of Axillary Bud Swelling of Hevea brasiliensis to Regenerate Plants through Somatic Embryogenesis and Analysis of Genetic Stability. Plants 2023, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, H.; Zhang, D.; Ma, J. Ectopic Expression of the Apple Cytokinin Response Regulator MdRR9 Gene in Tomatoes Promotes Shoot Branching. Sci. Hortic. 2023, 321, 112228. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New Insights Into Tissue Culture Plant-Regeneration Mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Jiang, C.; Lu, M.-Z.; Zhang, J. Exogenous Hormones Supplementation Improve Adventitious Root Formation in Woody Plants. Front. Bioeng. Biotechnol. 2022, 10, 1009531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.; Zhang, H.; Li, S.; Li, Z. Establishment of a Highly Efficient In Vitro Propagation System of Diospyros lotus. Forests 2023, 14, 366. [Google Scholar] [CrossRef]
- Lizárraga, A.; Fraga, M.; Ascasíbar, J.; González, M.L. In Vitro Propagation and Recovery of Eight Apple and Two Pear Cultivars Held in a Germplasm Bank. Am. J. Plant Sci. 2017, 8, 2238–2254. [Google Scholar] [CrossRef]
- Oakes, A.D.; Pilkey, H.C.; Powell, W.A. Improving Ex Vitro Rooting and Acclimatization Techniques for Micropropagated American Chestnut1. J. Environ. Hortic. 2020, 38, 149–157. [Google Scholar] [CrossRef]
- Santos-Rufo, A.; Rodríguez-Solana, R.; Fernández-Recamales, M.Á.; Sayago-Gómez, A.; Weiland-Ardaiz, C.M. Comparative Analysis of Anatomical Characteristics and Phenolic Compounds of Two Highbush Blueberry (Vaccinium corymbosum L.) Cultivars with Different Rooting Ability of Semi-Hardwood Cuttings. Sci. Hortic. 2024, 324, 112591. [Google Scholar] [CrossRef]
- Qarachoboogh, A.F.; Alijanpour, A.; Hosseini, B.; Shafiei, A.B. Efficient and Reliable Propagation and Rooting of Foetid Juniper (Juniperus foetidissima Willd.), as an Endangered Plant under in Vitro Condition. In Vitro Cell Dev. Biol.—Plant 2022, 58, 399–406. [Google Scholar] [CrossRef]
- Upadhayay, P.K.; Kharal, S.; Shrestha, B. Effect of Indole-Butyric Acid (IBA) and Wounding on Rooting Ability and Vegetative Characteristics of Apple Rootstock Cuttings under Nepal Conditions. J. Agric. Sci. Pract. 2020, 5, 184–192. [Google Scholar] [CrossRef]
- Khan, N.; Hamid, F.; Ahmad, F.; Waheed, A. Optimization of IBA Concentration for Rapid Initiation of Roots and Ultimate Growth of Kiwi Seedlings and the Association between Root System Architecture and Seedlings Growth. Pak. J. Agric. Res. 2020, 33, 63–71. [Google Scholar] [CrossRef]
- Wang, Y.; Khan, M.A.; Zhu, Z.; Hai, T.; Sang, Z.; Jia, Z.; Ma, L. Histological, Morpho-Physiological, and Biochemical Changes during Adventitious Rooting Induced by Exogenous Auxin in Magnolia wufengensis Cuttings. Forests 2022, 13, 925. [Google Scholar] [CrossRef]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of Tissue Cultured Plantlets: From Laboratory to Land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Hossain, M.M.; Sharma, M.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Acclimatization of in Vitro-Derived Dendrobium. Hortic. Plant J. 2017, 3, 110–124. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Mehta, S.R.; Manokari, M.; Priyadharshini, S.; Badhepuri, M.K.; Jogam, P.; Dey, A.; Rajput, B.S. Morpho-Anatomical and Physiological Changes of Indian Sandalwood (Santalum album L.) Plantlets in Ex Vitro Conditions to Support Successful Acclimatization for Plant Mass Production. Plant Cell Tissue Organ. Cult. 2021, 147, 423–435. [Google Scholar] [CrossRef]
- Leite, M.S.; Pinto, T.E.F.; Centofante, A.R.; Neto, A.R.; Silva, F.G.; Selari, P.J.R.G.; Martins, P.F. Acclimatization of Pouteria gardeneriana Radlk Micropropagated Plantlets: Role of in Vitro Rooting and Plant Growth–Promoting Bacteria. Curr. Plant Biol. 2021, 27, 100209. [Google Scholar] [CrossRef]
- Delgado-Paredes, G.E.; Vásquez-Díaz, C.; Esquerre-Ibañez, B.; Bazán-Sernaqué, P.; Rojas-Idrogo, C.; Delgado-Paredes, G.E.; Vásquez-Díaz, C.; Esquerre-Ibañez, B.; Bazán-Sernaqué, P.; Rojas-Idrogo, C. In Vitro Tissue Culture in Plants Propagation and Germplasm Conservation of Economically Important Species in Peru. Sci. Agropecu. 2021, 12, 337–349. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes. 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhan, H.; Qiao, Z.; Zheng, M.; Liu, W.; Feng, F.; Yan, F. Chemometric Analysis Based on HPLC Multi-Wavelength Fingerprints for Prediction of Antioxidant Components in Turpiniae folium. Chemom. Intell. Lab. Syst. 2016, 152, 54–61. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Jiang, M.; Wu, C.; Wang, G. An LC–MS-Based Metabolomic Approach Provides Insights into the Metabolite Profiles of Ginkgo biloba L. at Different Developmental Stages and in Various Organs. Food Res. Int. 2022, 159, 111644. [Google Scholar] [CrossRef]
- Nantitanon, W.; Yotsawimonwat, S.; Okonogi, S. Factors Influencing Antioxidant Activities and Total Phenolic Content of Guava Leaf Extract. LWT—Food Sci. Technol. 2010, 43, 1095–1103. [Google Scholar] [CrossRef]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the Consequence of Environmental Stress for Accumulation of Secondary Metabolites in Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction Processes with Several Solvents on Total Bioactive Compounds in Different Organs of Three Medicinal Plants. Molecules 2020, 25, 4672. [Google Scholar] [CrossRef]


| Treatments (Combination of 6-BA and NAA—mg/L) |
|---|
| 0.1 + 0.01 |
| 0.1 + 0.03 |
| 0.1 + 0.05 |
| 0.2 + 0.01 |
| 0.2 + 0.03 |
| 0.2 + 0.05 |
| 0.5 + 0.01 |
| 0.5 + 0.03 |
| 0.5 + 0.05 |
| Disinfection HgCl2 Time (min) | Contamination Rate (%) | Mortality Rate (%) | Survival Rate (%) |
|---|---|---|---|
| 4 | 68.01 ± 3.01 a | 7.97 ± 3.30 c | 24.02 ± 0.85 bc |
| 6 | 58.09 ± 4.82 b | 6.00 ± 0.21 c | 35.91 ± 4.99 a |
| 8 | 56.00 ± 2.95 bc | 16.05 ± 3.76 b | 27.94 ± 2.55 b |
| 10 | 51.96 ± 1.70 bc | 25.98 ± 3.06 a | 22.06 ± 3.89 bc |
| 12 | 50.00 ± 2.94 c | 30.51 ± 5.19 a | 20.47 ± 3.97 c |
| Medium Type | Proliferation Coefficient | Growth Situation |
|---|---|---|
| DCR | 1.65 ± 0.18 c | Poor growth and wilted sprout leaves |
| WPM | 2.27 ± 0.13 b | Poor growth, severe browning of callus, and dwarf plants |
| ½ MS | 1.42 ± 0.16 c | The leaves were curved, and the plant was short |
| MS | 3.12 ± 0.10 a | Plant growth was vigorous, the leaves were stretched, and the plantlets were tall and stout |
| Treatments (Combination of 6-BA and NAA—mg/L) | Proliferation Coefficient |
|---|---|
| 0.1 + 0.01 | 1.33 ± 0.13 d |
| 0.1 + 0.03 | 2.08 ± 0.14 c |
| 0.1 + 0.05 | 1.93 ± 0.23 c |
| 0.2 + 0.01 | 1.90 ± 0.19 c |
| 0.2 + 0.03 | 3.13 ± 0.10 a |
| 0.2 + 0.05 | 2.57 ± 0.08 b |
| 0.5 + 0.01 | 2.77 ± 0.29 b |
| 0.5 + 0.03 | 3.12 ± 0.06 a |
| 0.5 + 0.05 | 2.60 ± 0.17 b |
| Source of Variation (Factor) | Sum of Squares (SS) | Degrees of Freedom (df) | Mean Square (MS) | F |
|---|---|---|---|---|
| 6-BA | 5.22 | 2 | 2.61 | 90.446 * |
| NAA | 2.725 | 2 | 1.363 | 47.217 * |
| 6-BA × NAA | 0.924 | 4 | 0.231 | 8.002 * |
| error | 0.519 | 18 | 0.029 | |
| total | 162.518 | 27 |
| IBA (mg/L) | Rooting Rate (%) | Number of Roots |
|---|---|---|
| 0.5 | 22.41 ± 2.51 e | 1.75 ± 0.10 d |
| 1.0 | 35.61 ± 1.06 d | 2.44 ± 0.16 c |
| 1.5 | 40.60 ± 3.28 d | 3.26 ± 0.14 b |
| 2.0 | 56.67 ± 2.89 b | 3.67 ± 0.20 a |
| 2.5 | 66.60 ± 1.86 a | 3.50 ± 0.16 ab |
| 3.0 | 50.79 ± 3.25 c | 3.34 ± 0.10 b |
| Growth Period (Months) | Ligustroflavone (%) | Rhoifolin (%) |
|---|---|---|
| 0 | 0.172 ± 0.026 a | 0.030 ± 0.039 a |
| 3 | 0.237 ± 0.019 b | 0.118 ± 0.035 b |
| 5 | 0.469 ± 0.018 c | 0.188 ± 0.013 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Cai, J.; Hu, X.; Wang, L.; Cheng, Q.; Tao, X. Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content. Horticulturae 2024, 10, 587. https://doi.org/10.3390/horticulturae10060587
Hu J, Cai J, Hu X, Wang L, Cheng Q, Tao X. Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content. Horticulturae. 2024; 10(6):587. https://doi.org/10.3390/horticulturae10060587
Chicago/Turabian StyleHu, Jiangmei, Junhuo Cai, Xinrui Hu, Lijun Wang, Qiangqiang Cheng, and Xiuhua Tao. 2024. "Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content" Horticulturae 10, no. 6: 587. https://doi.org/10.3390/horticulturae10060587
APA StyleHu, J., Cai, J., Hu, X., Wang, L., Cheng, Q., & Tao, X. (2024). Efficient In Vitro Propagation of Turpinia arguta and Quantitative Analysis of Its Ligustroflavone and Rhoifolin Content. Horticulturae, 10(6), 587. https://doi.org/10.3390/horticulturae10060587






