Potential of Nettle Infusion to Protect Common Bean from Halo Blight Disease
Abstract
1. Introduction
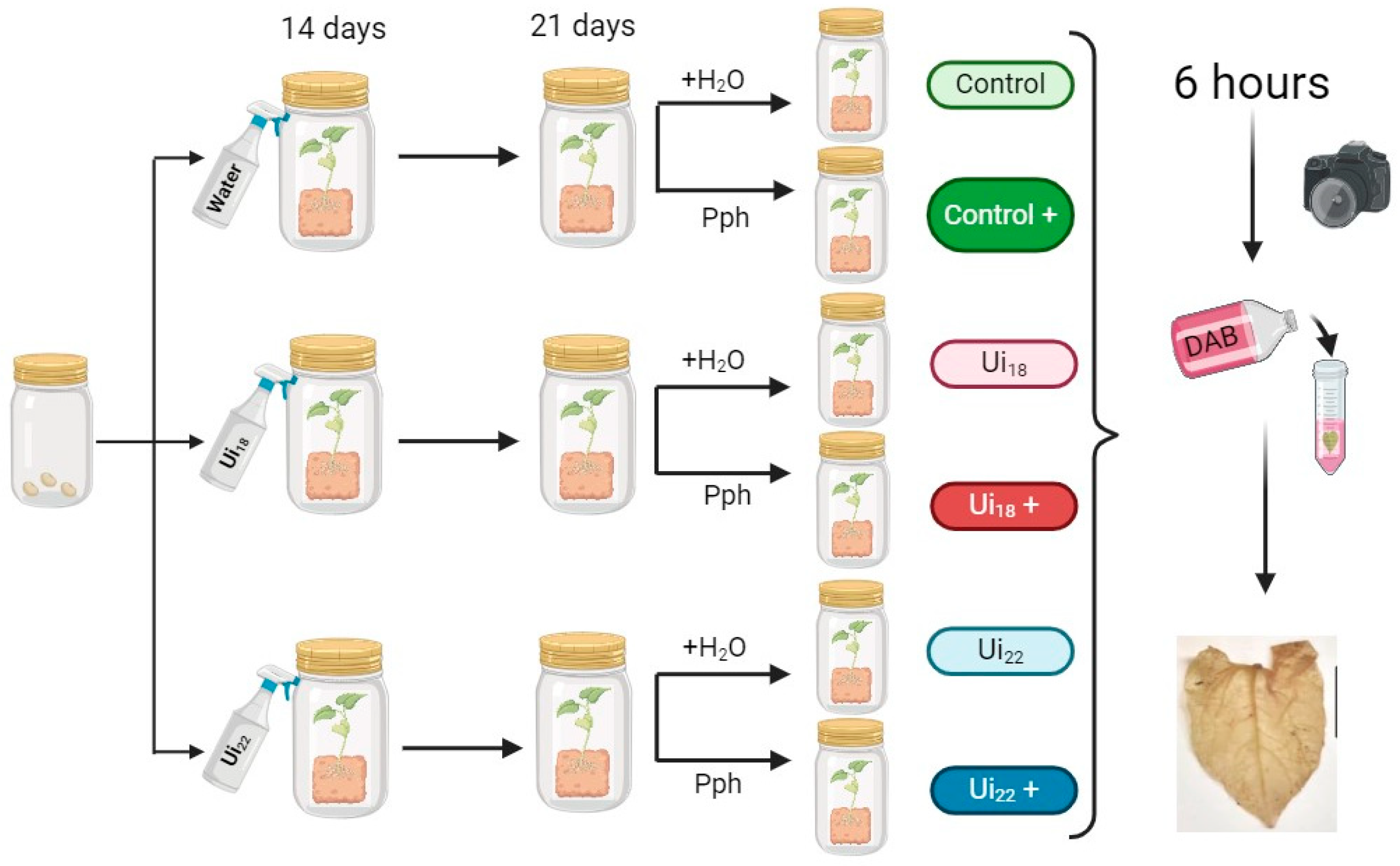
2. Materials and Methods
2.1. Nettle Sampling and Preparation of Nettle Infusions
2.2. Bioassays of Nettle Infusions against Pseudomonas syringae pv. Phaseolicola
2.3. Detection of ROS Released from Common Bean Leaf Discs in Response to flagellin22
2.4. Pph Inoculation of Plants and 3,3′-Diaminobendicin (DAB) Staining for Checking Oxidative Damage—Measurement of Non-Enzymatic Antioxidant Activity of the Nettle Infusions
2.5. Statistical Analysis and Data Representation
3. Results
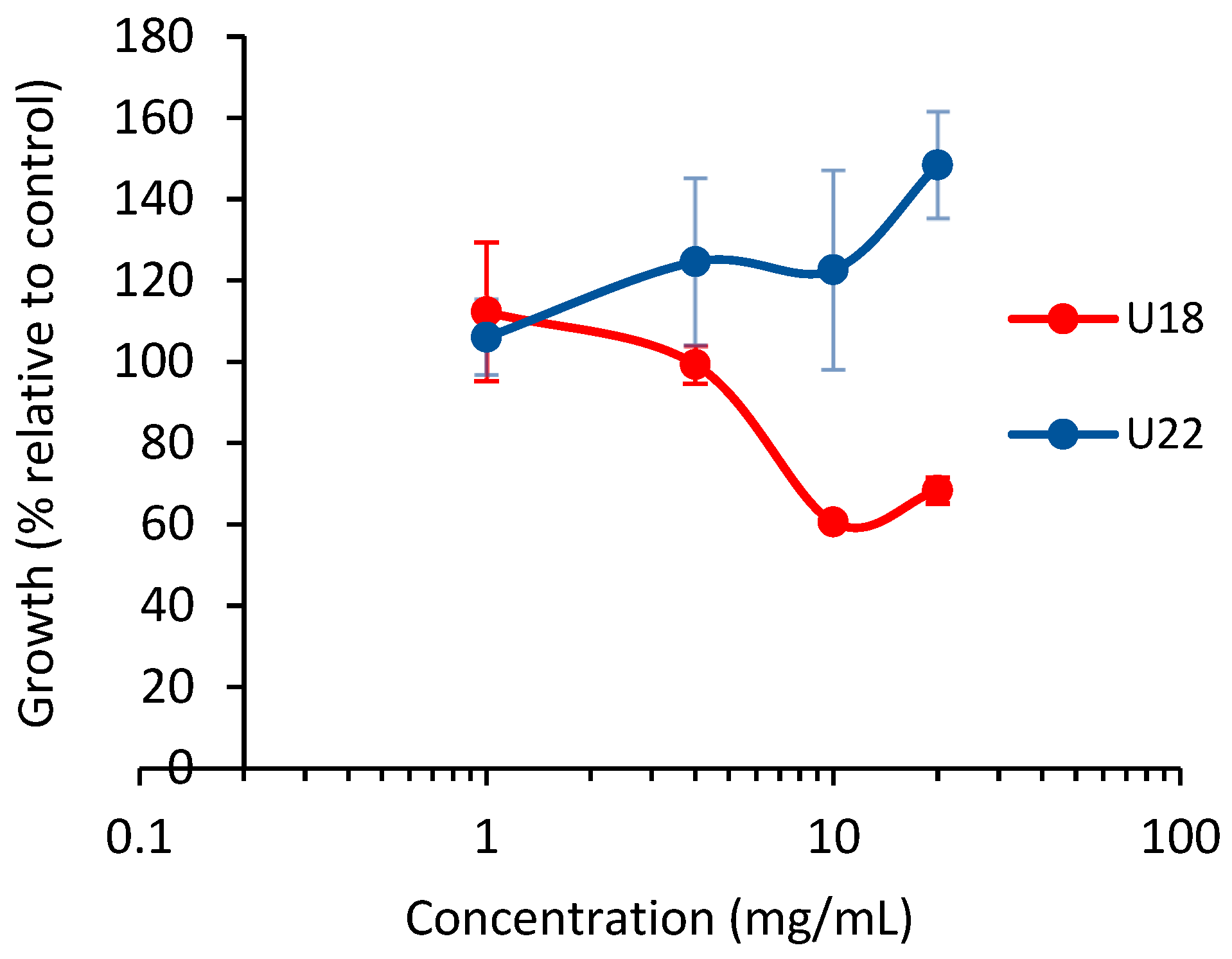
3.1. Effect of the Nettle Infusions on Pph In Vitro Growth
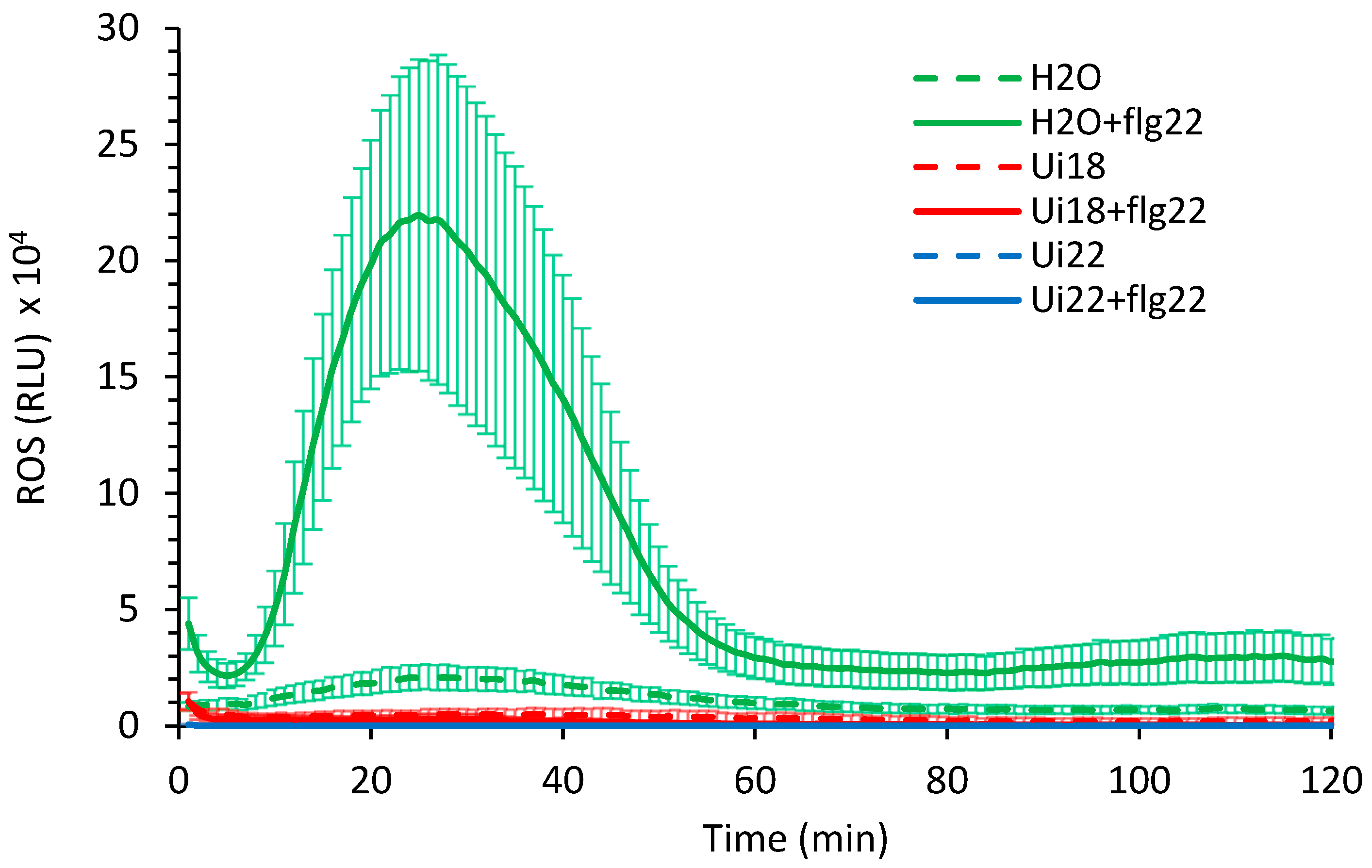
3.2. Nettle Infusion Putatively Neutralised the ROS Release Triggered by flg22 in Leaf Discs
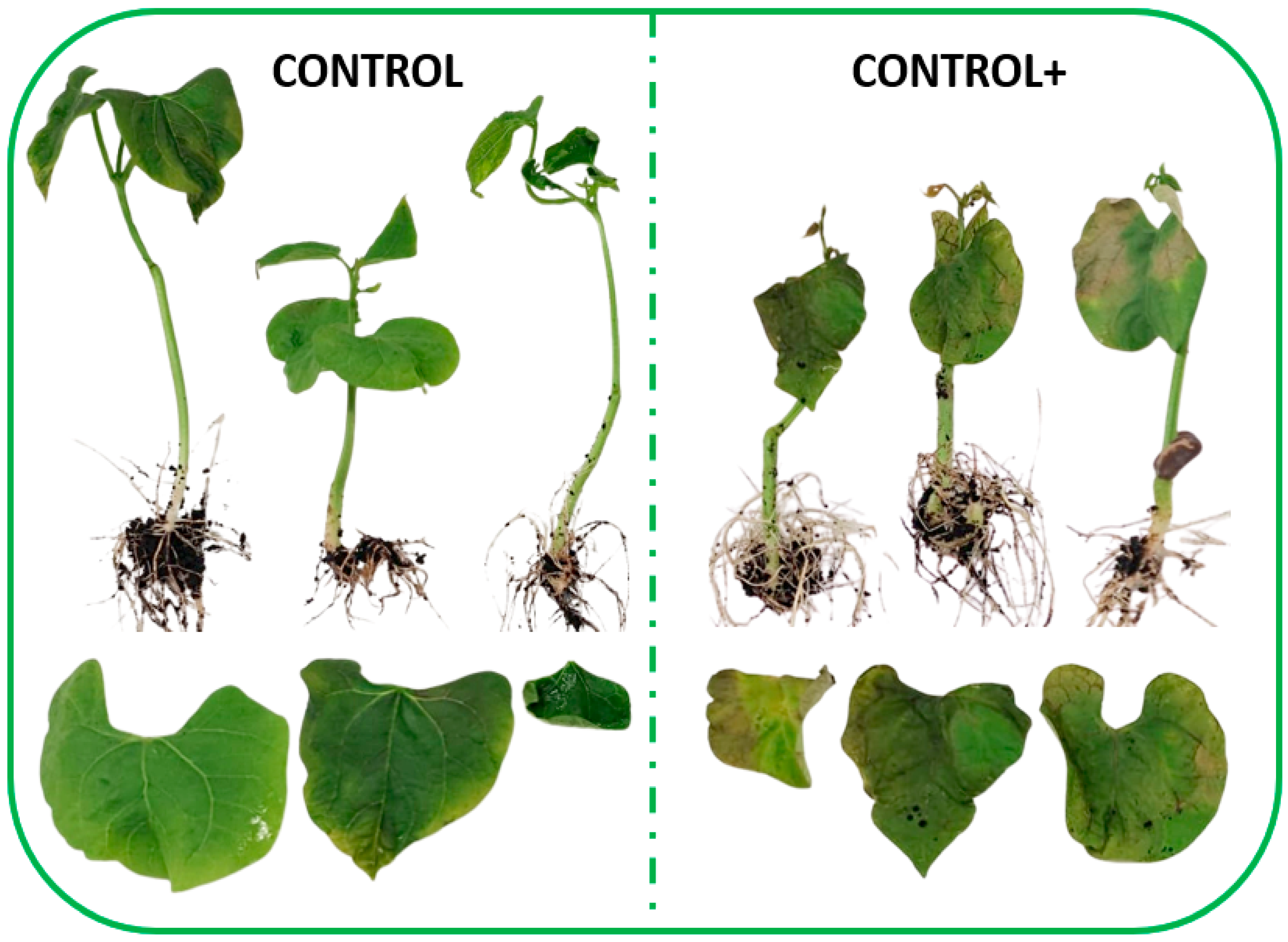
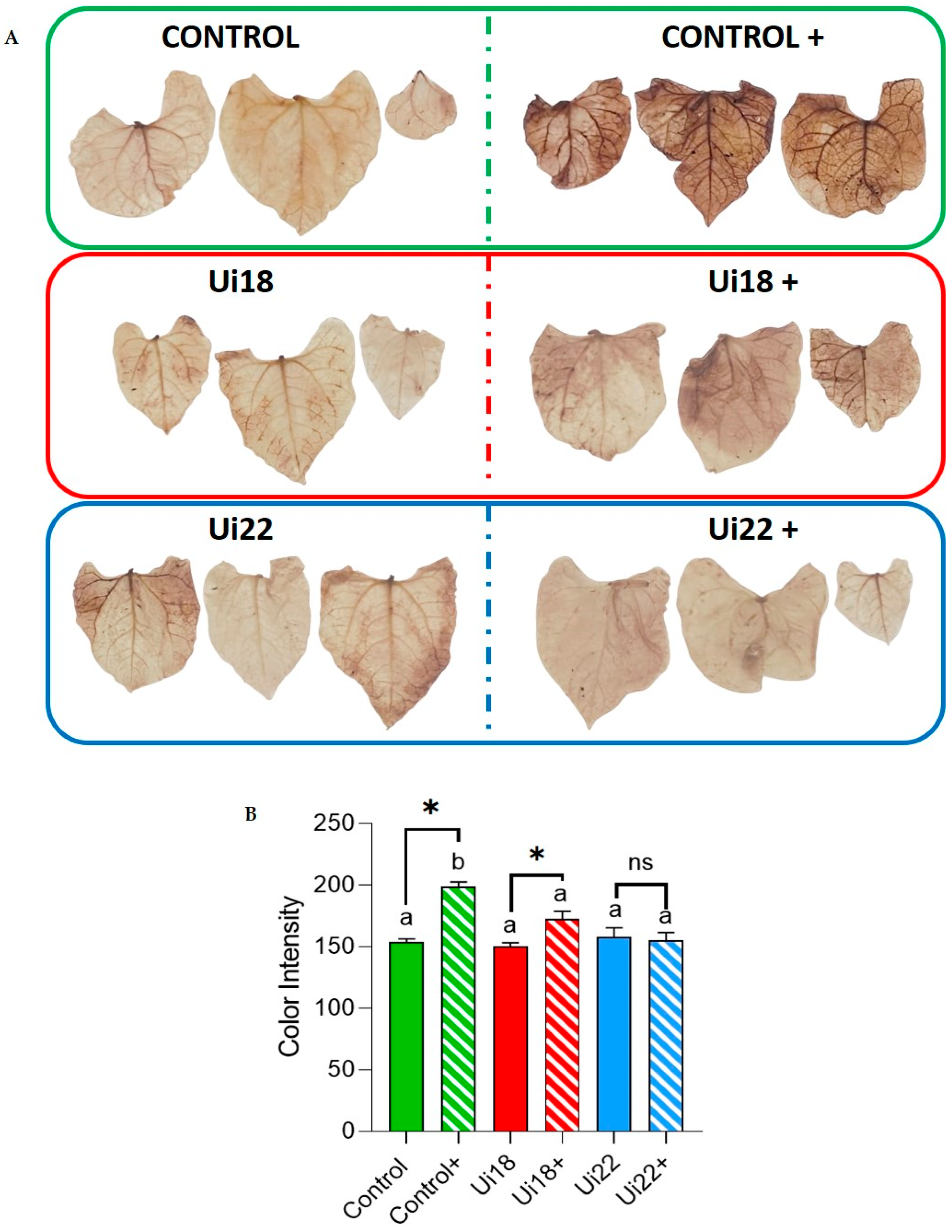
3.3. Nettle Infusion Reduced Oxidative Damage Caused by Pph Inoculation on Common Bean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrone, P.G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- De la Rubia, A.G.; De Castro, M.; Medina-Lozano, I.; García-Angulo, P. Using plant-based preparations to protect common bean against halo blight disease: The potential of nettle to trigger the immune system. Agronomy 2022, 12, 63. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The potential of stinging nettle (Urtica dioica L.) as a crop with multiple uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Das, N.; Singh, S.K.; Chellappan, D.K.; Dua, K. Stinging nettle (Urtica dioica L.): Nutritional composition, bioactive compounds, and food functional properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary plants with extraordinary properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.J. Antioxidant activity of Urtica dioica: An important property contributing to multiple biological activities. Antioxidants 2022, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Outcome of the consultation with Member Staters and EFSA on the basic substance applications for Urtica spp. For use in plant protection as insecticide, acaricide and fungicide. EFSA Support. Publ. 2016, 13, 1075E. [Google Scholar] [CrossRef]
- Merah, O.; Djazouli, Z.E.; Zebib, B. Aqueous extract of Algerian nettle (Urtica dioica L.) as possible alternative pathway to control some plant diseases. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 463–468. [Google Scholar] [CrossRef]
- Körpe, D.A.; İşerİ, Ö.D.; Sahin, F.I.; Cabi, E.; Haberal, M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. Int. J. Food Sci. Nutr. 2013, 64, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
- González, A.M.; Godoy, L.; Santalla, M. Dissection of Resistance Genes to Pseudomonas syringae pv. phaseolicola in UI3 Common Bean Cultivar. Int. J. Mol. Sci. 2017, 18, 2503. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.L.; Lovell, H.C.; Jackson, R.W.; Mansfield, J.W. Pseudomonas syringae pv. phaseolicola: From “has bean” to supermodel. Mol. Plant. Pathol. 2011, 12, 617–627. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia, A.G.; Centeno, M.L.; Moreno-González, V.; De Castro, M.; García-Angulo, P. Perception and first defense responses against Pseudomonas syringae pv. phaseolicola in Phaseolus vulgaris: Identification of Wall-Associated Kinase Receptors. Phytopathology 2021, 111, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia, A.G.; Mélida, H.; Centeno, M.L.; Encina, A.; García-Angulo, P. Immune priming triggers cell wall remodeling and increased resistance to halo blight disease in common bean. Plants 2021, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Gillmeister, M.; Ballert, S.; Raschke, A.; Geistlinger, J.; Kabrodt, K.; Baltruschat, H.; Deising, H.B.; Schellenberg, I. Polyphenols from rheum roots inhibit growth of fungal and oomycete phytopathogens and induce plant disease resistance. Plant Dis. 2019, 103, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant, and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Shabir, S.; Yousuf, S.; Singh, S.K.; Vamanu, E.; Singh, M.P. Ethnopharmacological effects of Urtica dioica, Matricaria chamomilla, and Murraya koenigii on rotenone-exposed D. melanogaster: An attenuation of cellular, biochemical, and organismal markers. Antioxidants 2022, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ghaima, K.K.; Hashim, N.M.; Ali, S.A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 2013, 3, 096–099. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Escudero, V.; Jordá, L.; Sopeña-Torres, S.; Mélida, H.; Miedes, E.; Muñoz-Barrios, A.; Swami, S.; Alexander, D.; McKee, L.S.; Sánchez-Vallet, A.; et al. Alteration of cell wall xylan acetylation triggers defense responses that counterbalance the immune deficiencies of plants impaired in the β-subunit of the heterotrimeric G-protein. Plant J. 2017, 92, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- De la Rubia, A.G.; Largo-Gosens, A.; Yusta, R.; Sepúlveda-Orellana, P.; Riveros, A.; Centeno, M.L.; Sanhueza, D.; Meneses, C.; Saez-Aguayo, S.; García-Angulo, P. A novel pectin methylesterase inhibitor, PMEI3, in common bean suggests a key role of pectin methylesterification in Pseudomonas resistance. J. Exp. Bot. 2024, 75, 364–390. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Nygaard Sorensen, J.; Thorup-Kristensen, K. Plant-based fertilizers for organic vegetable production. J. Plant Nut. Soil Sci. 2011, 174, 321–332. [Google Scholar] [CrossRef]
- Maričić, B.; Radman, S.; Romić, M.; Perković, J.; Major, N.; Urlić, B.; Palčić, I.; Ban, D.; Zorić, Z.; Ban, S.G. Stinging Nettle (Urtica dioica L.) as an aqueous plant-based extract fertilizer in green bean (Phaseolus vulgaris L.) sustainable agriculture. Sustainability 2021, 13, 4042. [Google Scholar] [CrossRef]
- Modarresi-Chahardehi, A.; Ibrahim, D.; Fariza-Sulaiman, S.; Mousavi, L. Screening antimicrobial activity of various extracts of Urtica dioica. Rev. Biol. Trop. 2012, 60, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.A.; Arif, E.D.; Ali, D.J. Antibacterial effect of nettle (Urtica dioica). Al-Qadisiyah J. Vet. Med. Sci. 2014, 13, 1–6. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef] [PubMed]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nettle leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Balpetek, K.D.; Gölkisik, C.; Aydin, S. An investigation of antibacterial and antioxidant activity of nettle (Urtica dioica L.), mint (Mentha piperita), thyme (Thyme serpyllum) and Chenopodium album L. plants from Yaylacik Plateau, Giresum, Turkey. Turk. JAF Sci. Technol. 2019, 7, 73–80. [Google Scholar] [CrossRef]
- Lou, S.N.; Lin, Y.S.; Hsu, Y.S.; Chiu, E.M.; Ho, C.T. Soluble and insoluble phenolic compounds and antioxidant activity of immature calamondin affected by solvents and heat treatment. Food Chem. 2014, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.A.; Silva, S.; Costa, E.M.; Saraiva, J.A.; Pintado, M. Effect of high hydrostatic pressure extraction on biological activities of stinging nettle extracts. Food Funct. 2020, 11, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic profile, antioxidant capacity and antimicrobial activity of nettle leaves extracts obtained by advanced extraction techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Rebaque, D.; Del Hierro, I.; López, G.; Bacete, L.; Vilaplana, F.; Dallabernardina, P.; Pfrengle, F.; Jordá, L.; Sánchez-Vallet, A.; Pérez, R.; et al. Cell wall-derived mixed-linked β-1, 3/1, 4-glucans trigger immune responses and disease resistance in plants. Plant J. 2021, 106, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.; Preston, G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 2012, 327, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ishiga, Y.; Ishiga, T.; Wangdi, T.; Mysore, K.S.; Uppalapati, S.R. NTRC and chloroplast-generated reactive oxygen species regulate Pseudomonas syringae pv. tomato disease development in tomato and Arabidopsis. MPMI 2012, 25, 294–306. [Google Scholar] [CrossRef]
- Nowogórska, A.; Patykowski, J. Selected reactive oxygen species and antioxidant enzymes in common bean after Pseudomonas syringae pv. phaseolicola and Botrytis cinerea infection. Acta Physiol. Plant. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development, and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. JFPP 2017, 41, e13203. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, M.; Cao, J.; Xiao, J.; Wang, Q. Effects of different pretreatments on flavonoids and antioxidant activity of Dryopteris erythrosora leave. PLoS ONE 2019, 14, e200174. [Google Scholar] [CrossRef] [PubMed]
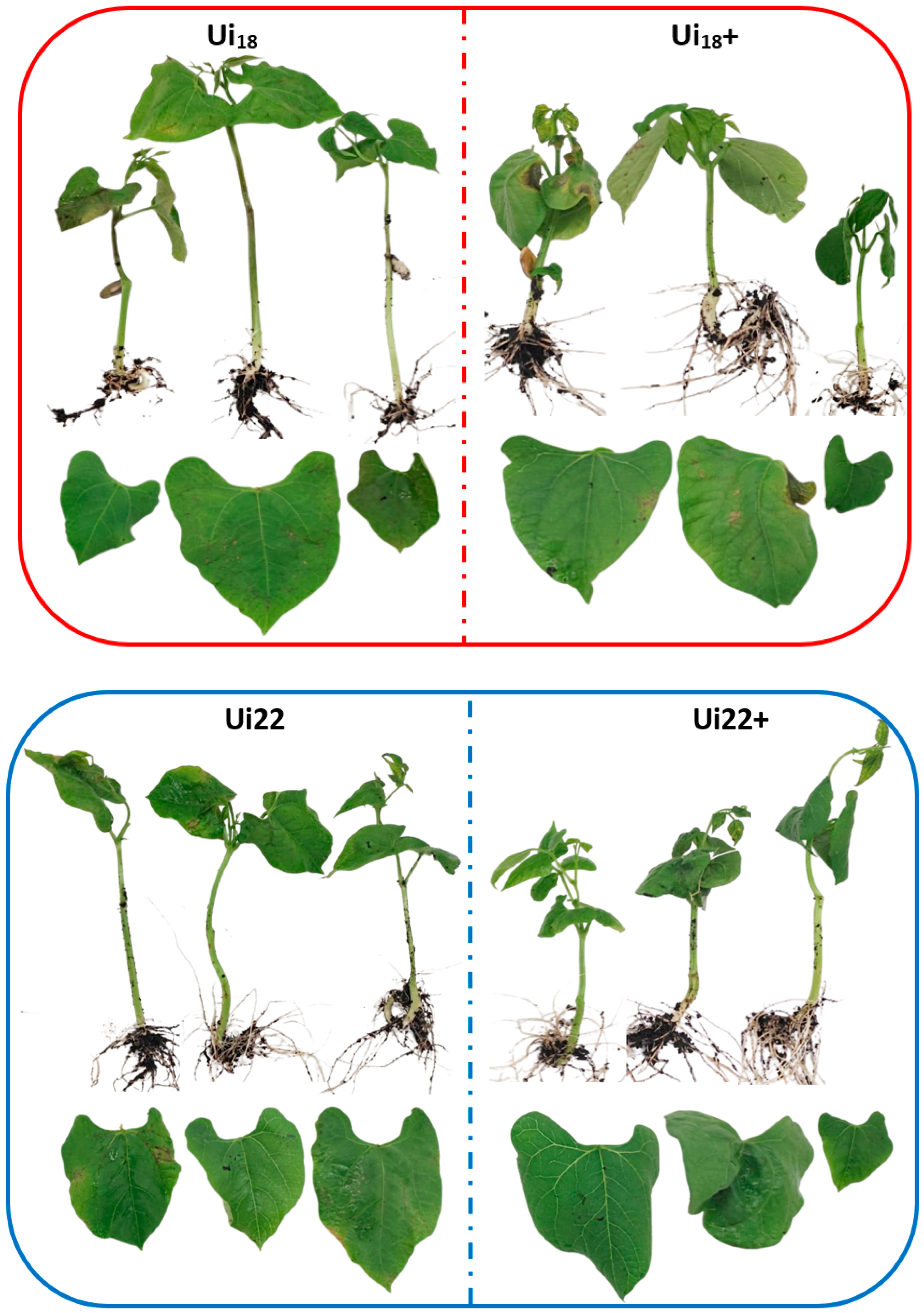
| Nettle Extract | 1 mg/mL | 10 mg/mL | 20 mg/mL |
|---|---|---|---|
| Ui18 | 0.12 ± 0.04 a | 1.06 ± 0.28 a | 1.88 ± 0.21 a |
| Ui22 | 0.23 ± 0.06 a | 1.75 ± 0.42 a | 3.03 ± 0.59 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerezo, C.; García-Angulo, P.; Largo-Gosens, A.; Centeno, M.L. Potential of Nettle Infusion to Protect Common Bean from Halo Blight Disease. Horticulturae 2024, 10, 536. https://doi.org/10.3390/horticulturae10060536
Cerezo C, García-Angulo P, Largo-Gosens A, Centeno ML. Potential of Nettle Infusion to Protect Common Bean from Halo Blight Disease. Horticulturae. 2024; 10(6):536. https://doi.org/10.3390/horticulturae10060536
Chicago/Turabian StyleCerezo, Carlota, Penélope García-Angulo, Asier Largo-Gosens, and María Luz Centeno. 2024. "Potential of Nettle Infusion to Protect Common Bean from Halo Blight Disease" Horticulturae 10, no. 6: 536. https://doi.org/10.3390/horticulturae10060536
APA StyleCerezo, C., García-Angulo, P., Largo-Gosens, A., & Centeno, M. L. (2024). Potential of Nettle Infusion to Protect Common Bean from Halo Blight Disease. Horticulturae, 10(6), 536. https://doi.org/10.3390/horticulturae10060536







