Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives
Abstract
1. Introduction
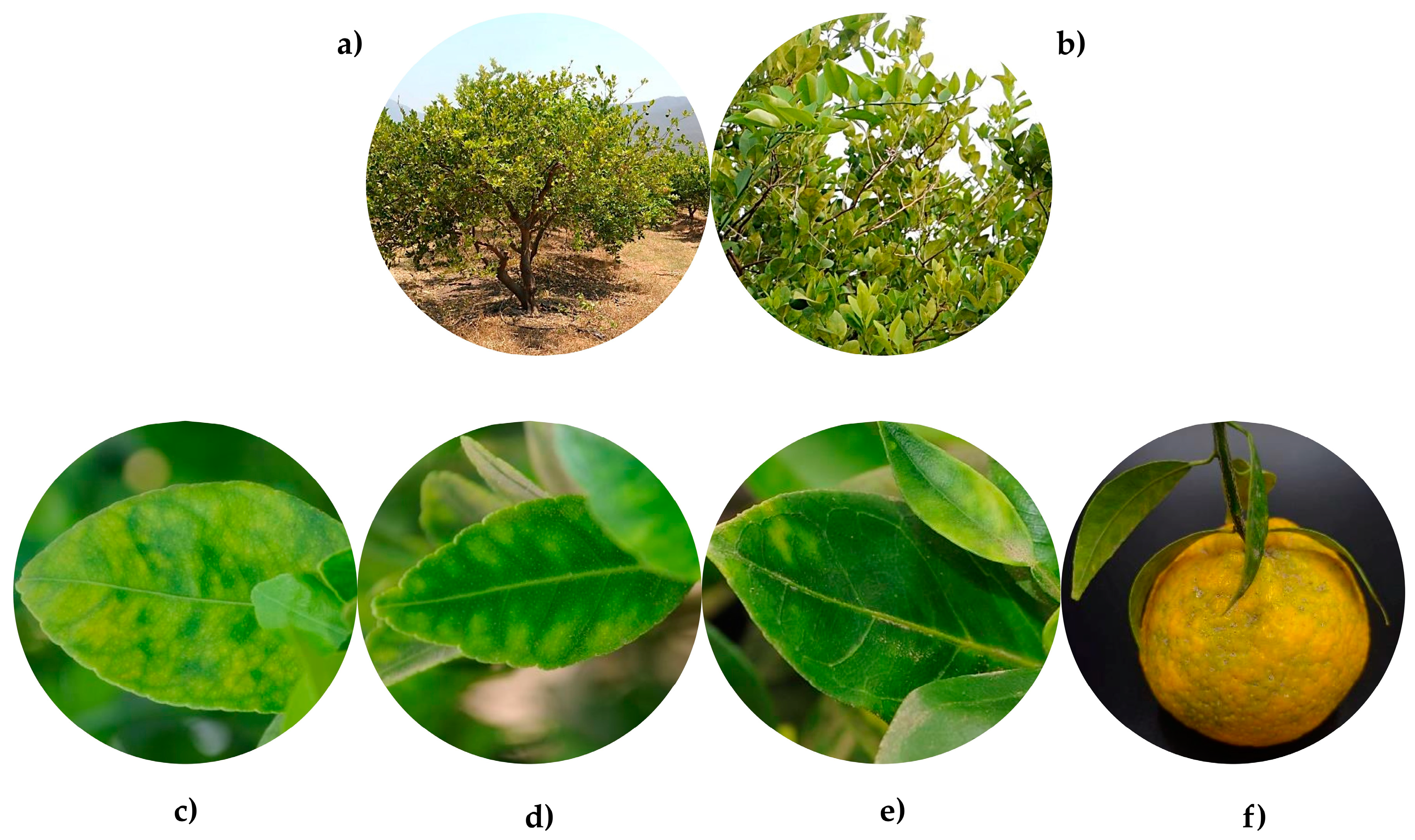
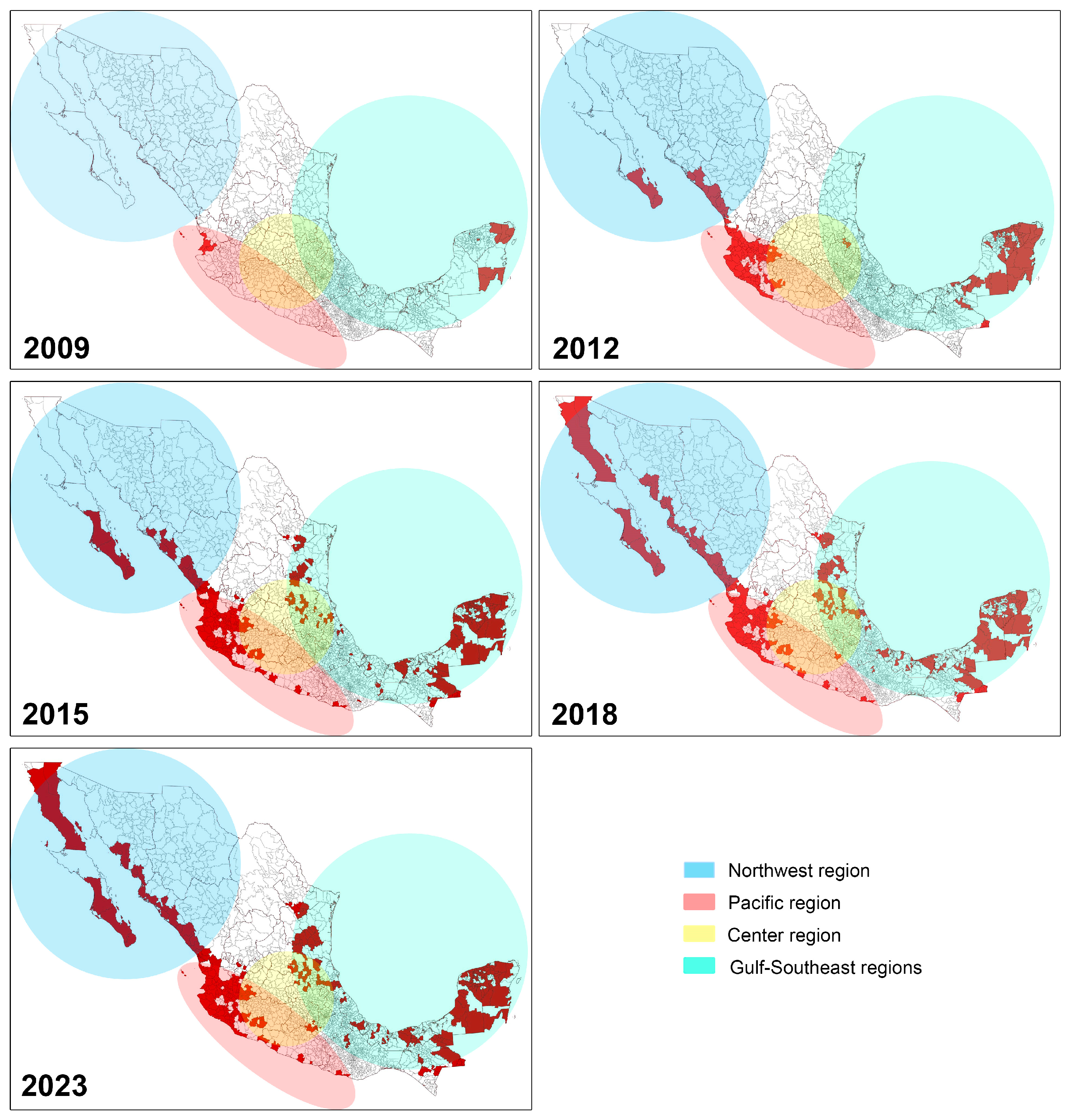
2. Epidemiology and Current Status of Huanglongbing in Mexico
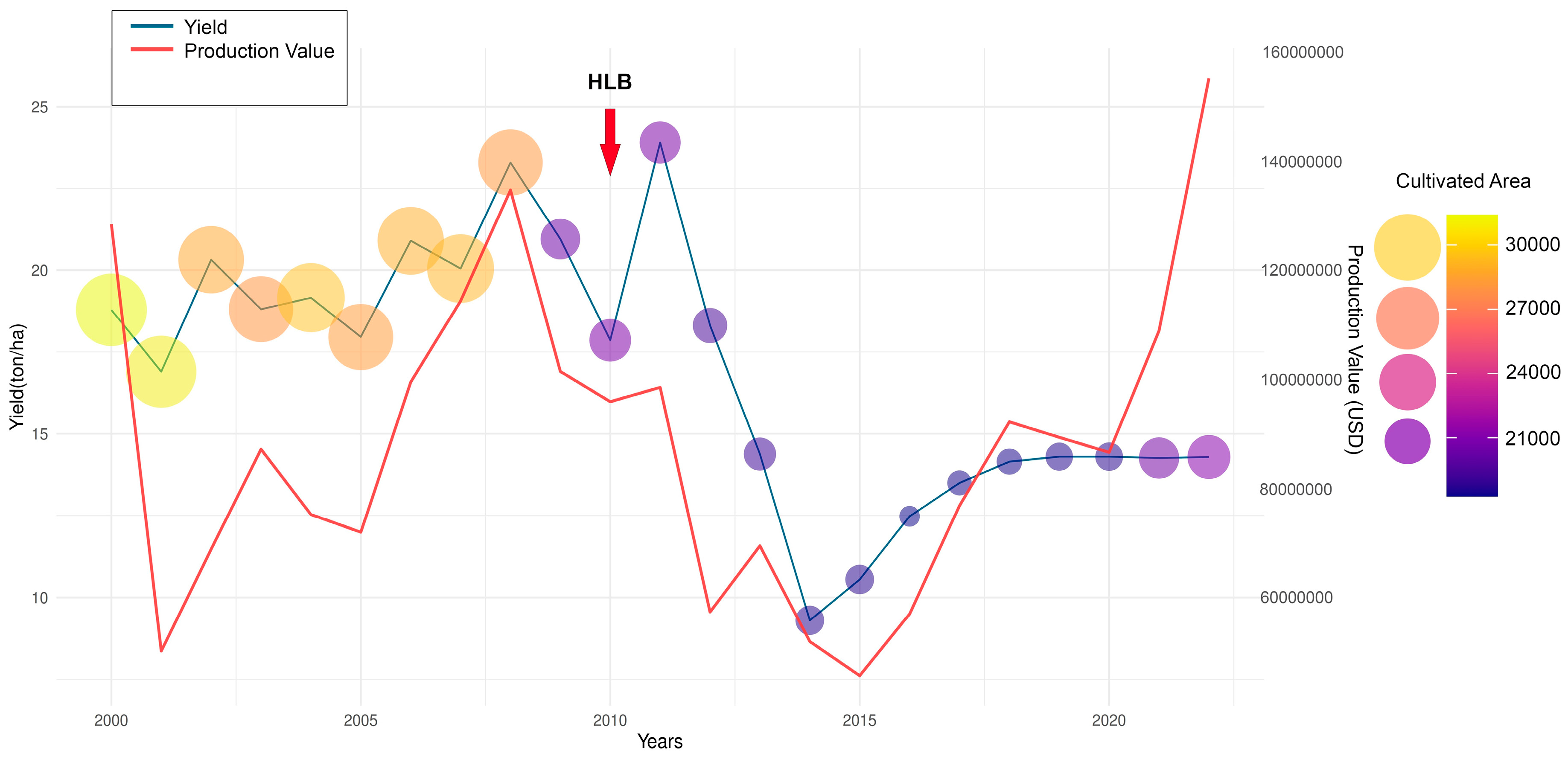
3. Economic, Social, and Employment Impact Caused by Huanglongbing

4. Control and Management Actions Implemented in Mexico
5. Future Perspectives and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT, Database 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 January 2024).
- Satpute, A.D.; Fadli, A. Diseases of Citrus and Their Control. In Citrus Production: Technological Advancements and Adaptation to Changing Climate Hussain, 1st ed.; Khalid, S., Ali, M.F., Ahmed, M.A., Hasanuzzaman, M., Ahmad, S., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 239–266. [Google Scholar] [CrossRef]
- SIAP (Servicio de Información Agroalimentaria y Pesquera). Anuario Estadístico de la Producción Agrícola, Ciclo Agrícola. 2022. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 10 October 2023).
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria). Campaña de Protección Fitosanitaria Para Plagas de los Cítricos (Primer Informe Semestral 2023); SENASICA, SADER: Mexico City, Mexico, 2023. Available online: https://www.gob.mx/cms/uploads/attachment/file/838775/Primer_Informe_Semestral_CPC_2023.pdf (accessed on 23 April 2024).
- Barbieri, H.B.; Fernandes, L.S.; Pontes JG, D.M.; Pereira, A.K.; Fill, T.P. An Overview of the Most Threating Diseases That Affect Worldwide Citriculture: Main Features, Diagnose, and Current Control Strategies. Front. Nat. Prod. 2023, 2, 1045364. [Google Scholar] [CrossRef]
- Pérez-Zarate, L.A.; Villanueva-Jiménez, J.A.; Osorio-Acosta, F.; García-Pérez, E.; Flores-de la Rosa, F.R.; Martínez-Hernández, A. Mecanismos Involucrados en la Patogénesis de Candidatus Liberibacter asiaticus y Bases Moleculares de la Tolerancia en cítricos. Rev. Mex. Fitopatol. 2022, 40, 1–38. [Google Scholar] [CrossRef]
- Ghosh, D.; Kokane, S.; Savita, B.K.; Kumar, P.; Sharma, A.K.; Ozcan, A.; Kokane, A.; Santra, S. Huanglongbing Pandemic: Current Challenges and Emerging Management Strategies. Plants 2023, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Li, J.; Wang, N. Candidatus Liberibacter asiaticus: Virulence Traits and Control Strategies. Trop. Plant Pathol. 2020, 45, 285–297. [Google Scholar] [CrossRef]
- Wang, N. The Citrus Huanglongbing Crisis and Potential Solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Arce-Leal, Á.P.; Leyva-López, N.E.; Santos-Cervantes, M.E.; Rodríguez-Negrete, E.A.; Méndez-Lozano, J.; Manzanilla-Ramírez, M.Á.; Perea-Flores, M.J. Cambios en el Metabolismo de Carbohidratos Asociados a la Infección por Candidatus Liberibacter asiaticus en Limón Mexicano (Citrus aurantifolia). Agrociencia 2019, 53, 593–604. [Google Scholar]
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Rohrig, E.; Solís, D.; Thomas, M.H. Citrus Greening Disease (Huanglongbing) in Florida: Economic Impact, Management and the Potential for Biological Control. Agric. Res. 2016, 5, 109–118. [Google Scholar] [CrossRef]
- Wang, N. Candidatus Liberibacter Asiaticus (Citrus Greening). CABI Compendium. 2022. Available online: https://www.cabi.org/cpc/datasheet/16565 (accessed on 29 August 2023).
- Salcedo-Baca, D.; Hinojosa, R.A.; Mora-Aguilera, G.; Covarrubias-Gutiérrez, I.; De Paolis, F.; Mora-Flores, S.; Cíntora-González, C.L. Evaluación del Impacto Económico de Huanglongbing (HLB) en la Cadena Citrícola Mexicana; IICA, SAGARPA-SENASICA: Mexico City, Mexico, 2010; 146p, Available online: https://repositorio.iica.int/handle/11324/7271 (accessed on 10 June 2023).
- Trujillo-Arriaga, J.; Sánchez-Anguiano, H.M.; Robles-García, P.L. Situación Actual y Perspectivas del Huanglongbing y el Psílido Asiático de los Cítricos en México. In I Taller Internacional sobre Huanglongbing de los Cítricos (Candidatus Liberibacter spp.) y el psílido asiático de los cítricos (Diaphorina citri); Dirección General de Sanidad Vegetal: Hermosillo, México, 2008; Available online: https://swfrec.ifas.ufl.edu/hlb/database/pdf/13_Trujillo-Arriaga_08.pdf (accessed on 14 September 2023).
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Domínguez-Monge, S.; Acevedo-Sánchez, G.; Robles-García, P. Pérdidas en Producción Inducidas por Candidatus Liberibacter asiaticus en Limón Persa en Yucatán, México. Rev. Mex. Fitopatol. 2015, 33, 195–210. [Google Scholar]
- Hernández-Hernández, R.; Granados-Ramírez, G.R.; Mora-Aguilera, G.; Aguirre-Gómez, R.; León-García, I. Reconversión de Cultivos Como Resultado de la Presencia de Huanglongbing en Colima, México. Acta Univ. 2019, 29, 1–13. [Google Scholar] [CrossRef]
- Coletta-Filho, H.D.; Targon ML, P.N.; Takita, M.A.; De Negri, J.D.; Pompeu, J., Jr.; Machado, M.A.; do Amaral, A.M.; Muller, G.W. First Report of the Causal Agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 2004, 88, 1382. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A Destructive, Newly-Emerging, Century-old Disease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Luis, M.; Collazo, C.; Llauger, R.; Blanco, E.; Peña, I.; López, D.; González, C.; Casín, J.C.; Batista, L.; Kitajima, E.; et al. Occurrence of Citrus Huanglongbing in Cuba and Association of the Disease with Candidatus Liberibacter asiaticus. J. Plant Pathol. 2009, 91, 709–712. [Google Scholar]
- Salcedo-Baca, D.; González-Hernández, H.; Rodríguez-Leyva, E.; Vera-Villagrán, E.; Múzquiz-Fragoso, C.; Hurtado-Arellano, A. Evaluación de la Campaña Contra el HLB en 2008, 2009 y 2010; IICA, SAGARPA-SENASICA: Mexico City, Mexico, 2012; 128p, Available online: http://repositorio.iica.int/bitstream/handle/11324/6083/BVE17109295e.pdf?sequence=1&isAllowed=y (accessed on 14 September 2023).
- Hernández-Fuentes, L.M.; López-Urías, M.A.; Gómez-Jaimes, R.; López-Arroyo, J.I.; Velázquez-Monreal, J.J.; Orozco-Santos, M. El Huanglongbing y su Vector Diaphorina Citri en Limón Persa en Nayarit: Recomendaciones para su Manejo; Libro Técnico NO. 3; INIFAP, Prometeo Editores, S.A. de C. V.: Nayarit, Mexico, 2014; 74p. [Google Scholar]
- Lara y Bretón, L.E.; González, R.M. A tres años del Huanglongbing y el Psílido Asiático de los Cítricos en México: Impactos en el Sistema Producto de Cítricos. In Huanglongbing y Psílido Asiático de los Cítricos: Un Acercamiento Metodológico Multidisciplinario, 1st ed.; Galindo-Mendoza, M.G., Contreras-Servín, C., Eds.; Universidad Autónoma de San Luis Potosí: San Luis Potosí, Mexico, 2014; pp. 78–100. [Google Scholar]
- SINAVEF (Sistema Nacional de Vigilancia Epidemiológica Fitosanitaria). Reporte Epidemiológico del Huanglongbing (HLB); SENASICA, SAGARPA, UASLP: San Luis Potosí, Mexico, 2010; 9p. Available online: http://langif.uaslp.mx/documentos/privada/BoletinesVarios/hlb/005.pdf (accessed on 24 September 2023).
- DOF (Diario Oficial de La Federación). NORMA Oficial Mexicana de Emergencia NOM-EM-047-FITO-2009, Por la que se establecen las acciones fitosanitarias para mitigar el riesgo de introducción y dispersión del Huanglongbing (HLB) de los cítricos (candidatus liberibacter spp.) en el territorio nacional; Secretaría de Gobernación: Ciudad Juárez, Mexico, 2009. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5097925&fecha=08/07/2009#gsc.tab=0 (accessed on 23 February 2024).
- Trujillo-Arriaga, J. Zona Bajo Control Fitosanitario el Estado de Colima, 16 de abril de 2010; Oficio: 02788, Circular No. 056; Dirección General de Sanidad Vegetal, SENASICA: Mexico City, Mexico, 2010. Available online: https://www.cofemersimir.gob.mx/expediente/9106/mir/35008/archivo/745812 (accessed on 20 September 2023).
- Robles-González, M.; Velázquez-Monreal, M.; Manzanilla-Ramírez, J.J.; Orozco-Santos, M.Á.; Medina-Urrutia, M.; López-Arroyo, V.M.; Flores-Virgen, R. Síntomas del Huanglongbing (HLB) en Árboles de Limón Mexicano (Citrus aurantifolia (Christm) Swingle) y su Dispersión en el Estado de Colima, México. Rev. Chapingo Ser. Hortic. 2013, 19, 15–31. [Google Scholar] [CrossRef]
- SINAVEF (Sistema Nacional de Vigilancia Epidemiológica Fitosanitaria). El HLB y Psílido Asiático de los Cítricos, Candidatus Liberibacter asiaticus y Diaphorina citri; Reporte Epidemiológico No. 022; SENASICA, SAGARPA, UASLP: San Luis Potosí, Mexico, 2012; 9p. Available online: http://langif.uaslp.mx/desarrollo/hlb2.0/AET/022.pdf (accessed on 20 September 2023).
- Zilch, R.J.F.; Ramírez, R.R.; Ramírez, M.C. Campaña contra Huanglongbing de los Cítricos; Informe Mensual No. 12; Dirección General de Sanidad Vegetal, SENASICA: Mexico City, Mexico, 2015; 12p. Available online: https://www.gob.mx/cms/uploads/attachment/file/231776/Diciembre.pdf (accessed on 20 September 2023).
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria). Vigilancia del Huanglongbing; SENASICA, SINAVEF: Mexico City, Mexico, 2018. Available online: https://prod.senasica.gob.mx/SIRVEF/HLBV2.aspx (accessed on 20 September 2023).
- Contreras-Servín, C.; Galindo-Mendoza, G.; Ibarra-Zapata, E. Condiciones Climáticas Asociados al Establecimiento y Dispersión de la Diaphorina citri y la Enfermedad (HLB) en México. Entomol. Mex. 2012, 11, 769–773. [Google Scholar]
- Mora-Aguilera, G.; Robles-García, P.; López-Arroyo, J.I.; Flores-Sánchez, J.; Acevedo-Sánchez, G.; Domínguez-Monge, S.; Gutiérrez-Espinosa, A.; Loeza-Kuk, E.; González-Gómez, R. Situación Actual y Perspectivas del Manejo del HLB de los Cítricos. Rev. Mex. Fitopatol. 2014, 32, 108–119. [Google Scholar]
- Mora-Aguilera, G. HLB-Huanglongbing, Ficha Técnica; SENASICA, SAGARPA: Mexico City, Mexico, 2011. Available online: https://www.gob.mx/cms/uploads/attachment/file/147557/Ficha_T_cnica_Candidatus_Liberibacter_spp.pdf (accessed on 20 September 2023).
- Bassanezi, R.B.; Lopes, S.A.; Júnior, J.B.; Spósito, M.B.; Yamamoto, P.T.; Miranda, M.P.; Teixeira, D.C.; Wulff, N.A. Epidemiologia do huanglongbing e suas Implicações para o Manejo da Doença. Citrus Res. Technol. 2010, 31, 11–23. [Google Scholar] [CrossRef]
- Esquivel-Chávez, F.; Valdovinos-Ponce, G.; Mora-Aguilera, G.; Gómez-Jaimes, R.; Velázquez-Monreal, J.J.; Manzanilla-Ramírez, M.Á.; Flores-Sánchez, L.J.; López-Arroyo, J.I. Análisis Histológico Foliar de Cítricos Agrios y Naranja Dulce con Síntomas Ocasionados por Candidatus Liberibacter asiaticus. Agrociencia 2012, 46, 769–782. [Google Scholar]
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Gutiérrez-Espinosa, M.A.; Velázquez-Monreal, J.J.; Domínguez-Monge, S.; Bassanezi, R.B.; Acevedo-Sánchez, G.; Robles-García, P. Diffusion Model for Describing the Regional Spread of Huanglongbing from First-Reported Outbreaks and Basing an area Wide Disease Management Strategy. Plant Dis. 2017, 101, 1119–1127. [Google Scholar] [CrossRef]
- Alves, M.N.; Lopes, S.A.; Raiol-Junior, L.L.; Wulff, N.A.; Girardi, E.A.; Ollitrault, P.; Peña, L. Resistance to “Candidatus Liberibacter asiaticus” the Huanglongbing Associated Bacterium, in Sexually and/or Graft-Compatible Citrus Relatives. Front. Plant Sci. 2021, 11, 617664. [Google Scholar] [CrossRef]
- Shimwela, M.M.; Schubert, T.S.; Albritton, M.; Halbert, S.E.; Jones, D.J.; Sun, X.; Roberts, P.D.; Singer, B.H.; Lee, W.S.; Jones, J.B.; et al. Regional Spatial-Temporal Spread of Citrus Huanglongbing is Affected by Rain in Florida. Phytopathology 2018, 108, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Pérez, F.J.; Flores-Sánchez, J.L.; Rodríguez-Mejía, L.; Márquez-Gómez, J.; Michereff, S.J.; Ancona, V.; Robles-Bermúdez, A.; Domínguez-Monge, S. Progress and Spatial Pattern of Huanglongbing in Persian Lime in Nayarit, México. Rev. Bio Cienc. 2018, 5, e351. [Google Scholar] [CrossRef]
- Mora-Aguilera, G.; Acevedo-Sánchez, G.; Guzmán-Hernández, E.; Flores-Colorado, E.; Martínez-Bustamente, V.; Coria-Contreras, J.; Robles-García, P.; López-Buenfil, A. El HLB de los Cítricos en México: Epidemiología, Manejo e Impactos Productivos. Rev. Ind. Agric. Tucumán 2019, 96, 1. [Google Scholar]
- Huang, J.; Alanís-Martínez, I.; Kumagai, L.; Dai, Z.; Zheng, Z.; Perez de León, A.A.; Chen, J.; Deng, X. Machine Learning and Analysis of Genomic Diversity of “Candidatus Liberibacter asiaticus” Strains from 20 Citrus Production States in Mexico. Front. Plant Sci. 2022, 13, 1052680. [Google Scholar] [CrossRef] [PubMed]
- Biblioteca de Publicaciones Oficiales del Gobierno de la República. Producción de Cítricos en México; Gobierno de Mexico: Mexico City, Mexico, 2022. Available online: https://www.gob.mx/publicaciones/articulos/produccion-de-citricos-en-mexico?idiom=es (accessed on 27 October 2023).
- Pérez-López, O.P.; Nava-Tablada, M.E. Evolución de la Citricultura Mexicana (1993–2018). El Caso del Municipio de Gutiérrez Zamora, Veracruz. Rev. Geogr. Agríc. 2021, 67, 9–25. [Google Scholar] [CrossRef]
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria). Huanglongbing Candidatus Liberibacter spp.; Ficha técnica No. 78; SENASICA, SADER: Ciudad de Mexico, Mexico, 2019. Available online: https://www.gob.mx/cms/uploads/attachment/file/463426/78._Ficha_t_cnica_Huanglongbing._Mayo_2019..pdf (accessed on 20 September 2023).
- Ortiz-Isabel, B.E.; Manzo-Ramos, F. El Programa Arco Para el Control del Huanglongbing en Tecoman, Colima, y Axtla, San Luis Potosí, México. AGROProductividad 2019, 12, 25–31. [Google Scholar] [CrossRef]
- Robles-González, M.M.; Orozco-Santos, M.; Manzanilla-Ramírez, M.A.; Velázquez-Monreal, J.J.; Medina-Urrutia, V.M.; Sanches-Stuchi, E. Experiencias con Huanglonbing en Limón Mexicano en el Estado de Colima, México. Citrus Res. Technol. 2018, 39, e1039. [Google Scholar] [CrossRef]
- Hernández-Trujillo, J.M.; Botello-Triana, J. El Papel del Entorno en las Modificaciones de la Estructura Regional de la Producción de Limón y de Naranja en México. Anál. Econ. 2017, 32, 93–118. [Google Scholar]
- Villegas-Monter, A.; Mora-Aguilera, A. Avances de la Fruticultura en México. Rev. Bras. Frutic. 2011, 33, 179–186. [Google Scholar] [CrossRef][Green Version]
- Granados-Ramírez, R.; Hernández-Hernández, R.; León-García, I. Presencia del HLB en Colima, México. Alternativas de Adaptación. Rev. Inclusiones 2022, 9, 24–40. [Google Scholar]
- Lara y Bretón, L.E.; González, R.M. Vulnerabilidad a Plagas Debida a Factores Agroalimentarios y Socioeconómicos en Tipos de Citricultores de México. In Huanglongbing y Psílido Asiático de los Cítricos: Un Acercamiento Metodológico Mul-tidisciplinario, 1st ed.; Galindo-Mendoza, M.G., Contreras-Servín, C., Eds.; Universidad Autónoma de San Luis Potosí: San Luis Potosí, Mexico, 2014; pp. 165–185. [Google Scholar]
- Robles-González, M.M.; Orozco-Santos, M.; Manzanilla-Ramírez, M.Á.; Velázquez-Monreal, J.J.; Carrillo-Medrano, S.H. Efecto del HLB Sobre el Rendimiento de Limón Mexicano en Colima, México. Rev. Mex. Cienc. Agríc. 2017, 8, 1101–1111. [Google Scholar] [CrossRef][Green Version]
- Borja-Bravo, M.; Velez-Izquierdo, A.; Cuevas-Reyes, V.; Orozco-Santos, M. Rentabilidad y Competitividad del Limón Mexicano en un Ambiente Endémico de Huanglongbing bajo dos Manejos Tecnológicos. CienciaUAT 2021, 16, 102–115. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, S.; Domínguez-Monge, S.; Allende, R.; Mendoza García, J.; Pérez-Hernández, O.; Rodríguez-Quibrera, C.; Curti-Díaz, S.; Flores-Sanchez, J.; Sarmiento-Tejeda, R. Influencia de Huanglongbing en Pérdidas de Producción y Calidad de Frutos de Limón Persa en Veracruz. Supl. Rev. Mex. Fitopatol. 2022, 40, 5. [Google Scholar]
- Koh, J.; Morales-Contreras, B.E.; Guerra-Rosas, M.I.; Osorio-Hernández, E.; Culver, C.A.; Morales-Castro, J.; Wicker, L. Huanglongbing Disease and Quality of Pectin and Fruit Juice Extracted From Valencia Oranges. LWT-Food Sci. Technol. 2020, 131, 109692. [Google Scholar] [CrossRef]
- Galván-Vela, E.; Santos-González, G. Análisis de la Elasticidad del Precio y Ventaja Comparativa Revelada del Sector de Cítricos en México. Merc. Neg. 2019, 39, 87–104. [Google Scholar] [CrossRef]
- Zapata-Contreras YG, Z.; Osorio-Hernández, E.; Silva-Espinosa, J.H.; Delgado-Martínez, R.; Rodríguez-Herrera, R.; Álvarez-Ramos, R. Situación Actual, Impacto Económico y Control del Huanglongbing en Tamaulipas. Cienc. Lat. Rev. Cient. Multidiscip. 2022, 6, 4242–4259. [Google Scholar] [CrossRef]
- Hernández-Morales, L.M.; García-Pérez, E.; Cortés-Flores, J.I.; Villegas-Monter, Á.; Mora-Aguilera, J.A. Fertilización Integral en Árboles de Naranjo “Marrs”en Producción con Síntomas de Virus de la Tristeza de los Cítricos (VTC) Y Huanglongbing (HLB). Rev. Fitotec. Mex. 2021, 44, 59. [Google Scholar] [CrossRef]
- CONAHCYT (Consejo Nacional de Humanidades, Ciencias y Tecnologías). CONAHCYT Articula Esfuerzos para Afrontar Amarillamiento en Cítricos; Comunicado 387/2023; Gobierno de Mexico: Ciudad de Mexico, Mexico, 2023. Available online: https://conahcyt.mx/wp-content/uploads/comunicados/Conahcyt_387.pdf (accessed on 20 October 2023).
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria). Monitor Fitosanitario: Se Investigan Fitopatógenos Asociados con el Amarillamiento de los Cítricos en Veracruz; SENASICA, SADER: Ciudad de Mexico, Mexico, 2023. Available online: https://dj.senasica.gob.mx/Contenido/files/2023/junio/MonitorFitosanitario29062023_e46e2a68-5a59-47a4-b5e0-4933aa99137c.pdf (accessed on 20 October 2023).
- Valencia-Sandoval, K.; Duana-Avila, D. Los Cítricos en México: Análisis de Eficiencia Técnica. Anál. Econ. 2019, 34, 269–283. [Google Scholar] [CrossRef]
- Rinconada-Carbajal, F.; García-Fernández, F.; Serna-Hinojosa, J.A. Especialización y ventaja comparativa del sector citrícola en México: 1990–2018. Econ. Teor. Práct. 2022, 56, 155–174. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 10 June 2023).
- SENASICA (Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria). Protocolo Para Establecer Áreas Regionales de Control del Huanglongbing y el Psílido Asiático de los Cítricos (ARCOs); SENASICA, SADER: Mexico City, Mexico, 2012. Available online: http://publico.senasica.gob.mx/?doc=9364 (accessed on 20 September 2023).
- Cortez-Mondaca, E.; López-Arroyo, J.I.; Hernández-Fuentes, L.M.; Fú-Castillo, A.; Loera-Gallardo, J. Control Químico de Diaphorina citri Kuwayama en Cítricos Dulces, en México: Selección de Insecticidas y Épocas de Aplicación; Folleto Técnico No. 35; INIFAP, Editorial Panorama: Los Mochis, Sinaloa, Mexico, 2010; 23p, Available online: https://www.siafeson.com/sitios/simdia/docs/fichas_tecnicas/control_quimico_diapho.pdf (accessed on 20 September 2023).
- Ruíz-Galván, I.; Bautista-Martínez, N.; Sánchez-Arroyo, H.; Valenzuela Escoboza, F.A. Control Químico de Diaphorina citri (Kuwayama) (Hemiptera: Liviidae) en Lima Persa. Acta Zool. Mex. 2015, 31, 41–47. [Google Scholar] [CrossRef][Green Version]
- Vázquez-García, M.; Velázquez-Monreal, J.; Medina-Urrutia, V.M.; de Jesús Cruz-Vargas, C.; Sandoval-Salazar, M.; Virgen-Calleros, G.; Torres-Morán, J.P. Insecticide Resistance in Adult Diaphorina citri Kuwayama from Lime Orchards in Central west Mexico. Southwest Entomol. 2013, 38, 579–596. [Google Scholar] [CrossRef]
- Miranda, M.P.; da Silva Scapin, M.; Vizoni, M.C.; Zanardi, O.Z.; Eduardo, W.I.; Volpe HX, L. Spray Volumes and Frequencies of Insecticide Applications for Suppressing Diaphorina citri Populations in Orchards. Crop Prot. 2021, 140, 105406. [Google Scholar] [CrossRef]
- Pardo, S.; Martínez, A.M.; Figueroa, J.I.; Chavarrieta, J.M.; Viñuela, E.; Rebollar-Alviter, Á.; Miranda, M.A.; Valle, J.; Pineda, S. Insecticide Resistance of Adults and Nymphs of Asian Citrus Psyllid Populations from Apatzingán Valley, Mexico. Pest Manag. Sci. 2018, 74, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zarate, L.A.; Osorio-Acosta, F.; Villanueva-Jiménez, J.A.; Ortega-Arenas, L.D.; Chiquito-Contreras, R.G. Factores que Inciden en el Control Químico de Diaphorina citri Kuwayama en Áreas Regionales de Control. Southwest Entomol. 2016, 41, 1037–1050. [Google Scholar] [CrossRef]
- García-Hernández, N.E.; Cardoso-Aguilar, L.; Zúñiga-Cruz, A.; Márquez-Pérez, F.J.; Quiroz-Ibáñez, I.F. Manual Operativo de la Campaña Contra Plagas de los Cítricos; SENASICA, SADER: Mexico City, Mexico, 2020; 71p. Available online: https://www.gob.mx/cms/uploads/attachment/file/614759/Manual_operativo_Plagas_de_los_C_tricos.pdf (accessed on 20 January 2024).
- Ramírez-Sánchez, A.K.; Rodríguez-Maciel, J.C.; Lagunes-Tejeda, A.; Bautista-Martínez, N.; Reyes MA, T.; Pardo-Melgarejo, S. Intraorchard Variation of Resistance to Imidacloprid in Diaphorina citri (Hemiptera: Liviidae) Adults. J. Entomol. Sci. 2023, 58, 266–276. [Google Scholar] [CrossRef]
- Mondaca, E.C.; Angulo NE, L.; Márquez, J.P.; Sánchez MA, A. Primer reporte de Enemigos Naturales y Parasitismo sobre Diaphorina citri Kuwayama en Sinaloa, México. Rev. Cient. UDO Agríc. 2011, 11, 97–103. [Google Scholar]
- Pérez-González, O.; Rodríguez-Guerra, R.; López-Arroyo, J.I.; Sandoval-Coronado, C.F.; Maldonado-Blanco, M.G. Radial Growth, Sporulation, and Virulence of Mexican Isolates of Hirsutella citriformis against Diaphorina citri. Southwest Entomol. 2015, 40, 111–120. [Google Scholar] [CrossRef]
- Pérez-González, O.; Gomez-Flores, R.; Tamez-Guerra, P. Insight Into Biological Control Potential of Hirsutella citriformis Against Asian Citrus Psyllid as a Vector of Citrus Huanglongbing Disease in America. J. Fungi. 2022, 8, 573. [Google Scholar] [CrossRef]
- Lezama-Gutiérrez, R.; Molina-Ochoa, J.; Chávez-Flores, O.; Angel-Sahagun, C.A.; Skoda, S.R.; Reyes-Martínez, G.; Barba-Reynoso, M.; Rebolledo-Domínguez, O.; Ruíz-Aguilar GM, L.; Foster, J.E. Use of the Entomopathogenic Fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to Control Diaphorina citri (Hemiptera: Psyllidae) in Persian Lime Under Field Conditions. Int. J. Trop. Insect Sci. 2012, 32, 39–44. [Google Scholar] [CrossRef]
- Mendoza-García, E.E.; Ortega-Arenas, L.D.; Serrato-Cruz, M.Á.; Villanueva-Jiménez, J.A.; López-Arroyo, J.I.; Pérez-Pacheco, R. Chemical composition, toxicity, and repellence of plant essential oils against Diaphorina citri (Hemiptera: Liviidae). Chil. J. Agric. Res. 2019, 79, 636–647. [Google Scholar] [CrossRef]
- Orozco-Santos, M.; Robles-González, M.; Hernández-Fuentes, L.M.; Velázquez-Monreal, J.J.; de Jesús Bermudez-Guzmán, M.; Manzanilla-Ramírez, M.; Manzo-Sánchez, G.; Nieto-Ángel, D. Uso de Aceites y Extractos Vegetales para el Control de Diaphorina citri Kuwayama en Lima Mexicana en el Trópico Seco de México. Southwest Entomol. 2016, 41, 1051–1066. [Google Scholar] [CrossRef]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; García-Ramos, J.C.; Bogdanchikova, N. Argovit™ Silver Nanoparticles to Fight Huanglongbing Disease in Mexican Limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Bassanezi, R.B.; Primiano, I.V.; Vescove, H.V. Effect of Enhanced Nutritional Programs and Exogenous Auxin Spraying on Huanglongbing Severity, Fruit Drop, Yield and Economic Profitability of Orange Orchards. Crop Prot. 2021, 145, 105609. [Google Scholar] [CrossRef]
- Orozco-Santos, M.; Preciado JC, G.; Velázquez-Monreal, J.J.; Hernández-Fuentes, L.M.; Robles-González, M.M.; Manzanilla-Ramírez, M.Á.; Manzo-Sánchez, G. Uso de Acolchados Plásticos para Reducir Diaphorinia citri-Huanglongbing e Incrementar el Rendimiento de Lima Mexicana en el Trópico Seco de México. Southwest Entomol. 2022, 47, 927–934. [Google Scholar] [CrossRef]
- Arce-Leal, Á.P.; Bautista, R.; Rodríguez-Negrete, E.A.; Manzanilla-Ramírez, M.Á.; Velázquez-Monreal, J.J.; Mén-dez-Lozano, J.; Bejarano, E.R.; Castillo, A.G.; Claros, M.G.; Leyva-López, N.E. de novo Assembly and Functional Annotation of Citrus aurantifolia Transcriptome from Candidatus Liberibacter asiaticus Infected and non-Infected Trees. Data Br. 2020, 29, 105198. [Google Scholar] [CrossRef]
- Estrella-Maldonado, H.; González-Cruz, C.; Matilde-Hernández, C.; Adame-García, J.; Santamaría, J.M.; Santillán-Mendoza, R.; Flores-de la Rosa, F.R. Insights into the Molecular Basis of Huanglongbing Tolerance in Persian Lime (Citrus latifolia Tan.) through a Transcriptomic Approach. Int. J. Mol. Sci. 2023, 24, 7497. [Google Scholar] [CrossRef]
- Arce-Leal, A.P.; Bautista, R.; Rodríguez-Negrete, E.A.; Manzanilla-Ramírez, M.A.; Velázquez-Monreal, J.J.; Santos-Cervantes, M.E.; Méndez-Lozano, J.; Beuzón, C.R.; Bejarano, E.R.; Castillo, A.G.; et al. Gene Expression Profile of Mexican Lime (Citrus aurantifolia) Trees in Response to Huanglongbing Disease Caused by Candidatus Liberibacter asiaticus. Microorganisms 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez-Orozco, A.M.; Arce-Leal, Á.P.; Montes RA, C.; Santos-Cervantes, M.E.; Cruz-Mendívil, A.; Méndez-Lozano, J.; Castillo, A.G.; Rodríguez-Negrete, E.A.; Leyva-López, N.E. Differential Expression of miRNAs Involved in Response to Candidatus Liberibacter asiaticus Infection in Mexican Lime at Early and Late Stages of Huanglongbing Disease. Plants 2023, 12, 1039. [Google Scholar] [CrossRef]
- Flores de la Rosa, F.D.; González-Cruz, C.; Adame-García, J.; Chan-León, A.C.; Santillán-Mendoza, R.; Santamaría, J.M.; Estrella-Maldonado, H. Transcriptome-wide Identification of CDR Family in Citrus latifolia and its Expression During HLB Disease. Trop. Plant Biol. 2023, 16, 32–40. [Google Scholar] [CrossRef]
- Rueda-Silva, J.C.; González-Campos, L.I.; Durán-Armenta, L.F.; Karam-Coppola, A.; Antonio-Pérez, A.; Ordoñez-Rodríguez, J.; Torres-Huerta, A.L. Novel Bacterial Plasmid Produces Small Interfering RNAs (siRNAs) that Induce Effective Gene Silencing in the Asian Citrus psyllid Diaphorina citri. Electron. J. Biotechnol. 2023, 64, 59–68. [Google Scholar] [CrossRef]
- Gómez-Flores, W.; Garza-Saldaña, J.J.; Varela-Fuentes, S.E. A Huanglongbing Detection Method for Orange Trees Based on Deep Neural Networks and Transfer Learning. IEEE Access 2022, 10, 116686–116696. [Google Scholar] [CrossRef]
- Lv, Y.; Zhong, Y.; Jiang, B.; Yan, H.; Ren, S.; Cheng, C. microRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus. Int. J. Mol. Sci. 2023, 24, 5737. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Li, X.; Duan, S.; Xing, Q.; Müller-Xing, R. Citrus Threat Huanglongbing (HLB)-Could the Rootstock Provide the cure? Front. Plant Sci. 2024, 15, 1330846. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aguilar, O.; López-Collado, J.; Soto-Estrada, A.; de la Cruz Vargas-Mendoza, M.; García-Avila, C. Future Spatial Distribution of Diaphorina citri in Mexico Under Climate Change Models. Ecol. Complex 2023, 53, 101041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar-Luna, H.; Santos-Cervantes, M.E.; Rodríguez-Negrete, E.A.; Méndez-Lozano, J.; Leyva-López, N.E. Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives. Horticulturae 2024, 10, 481. https://doi.org/10.3390/horticulturae10050481
Villar-Luna H, Santos-Cervantes ME, Rodríguez-Negrete EA, Méndez-Lozano J, Leyva-López NE. Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives. Horticulturae. 2024; 10(5):481. https://doi.org/10.3390/horticulturae10050481
Chicago/Turabian StyleVillar-Luna, Hernán, María Elena Santos-Cervantes, Edgar Antonio Rodríguez-Negrete, Jesús Méndez-Lozano, and Norma Elena Leyva-López. 2024. "Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives" Horticulturae 10, no. 5: 481. https://doi.org/10.3390/horticulturae10050481
APA StyleVillar-Luna, H., Santos-Cervantes, M. E., Rodríguez-Negrete, E. A., Méndez-Lozano, J., & Leyva-López, N. E. (2024). Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives. Horticulturae, 10(5), 481. https://doi.org/10.3390/horticulturae10050481





