An Updated Isotopic Database of Fertilizers Used in Intensive Organic Farming: A Case Study on Protein Hydrolyzed Derivatives and Chelated Nutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Instruments and Standards
2.3. Determination of 15N/14N Isotope Ratio by EA-IRMS
2.4. Statistical Analyses
3. Results and Discussion
3.1. Synthetic Fertilizers
3.2. Organic Fertilizers
3.2.1. Protein Hydrolyzed Fertilizers (PHFs)
3.2.2. Micronutrients
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willer, H.; Trávnícek, J.; Meier, C.; Schlatter, B. The World of Organic Agriculture. Statistics and Emerging Trends; IFOAM: Bonn, Germany, 2021; p. 340. [Google Scholar]
- Baron, J.; Jay-Russell, M.T. Assessment of Current Practices of Organic Farmers Regarding Biological Soil Amendments of Animal Origin in a Multi-Regional US Study. Food Prot. Trends 2018, 38, 347–362. [Google Scholar]
- Capuano, E.; Boerrigter-Eenling, R.; van der Veer, G.; van Ruth, S.M. Analytical Authentication of Organic Products: An Overview of Markers. J. Sci. Food Agric. 2013, 93, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.H.; Schjoerring, J.K.; Kelly, S.D.; Husted, S. Authentication of Organically Grown Plants–Advantages and Limitations of Atomic Spectroscopy for Multi-Element and Stable Isotope Analysis. TrAC Trends Anal. Chem. 2014, 59, 73–82. [Google Scholar] [CrossRef]
- Inácio, C.T.; Chalk, P.M.; Magalhães, A.M. Principles and Limitations of Stable Isotopes in Differentiating Organic and Conventional Foodstuffs: 1. Plant Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Chalk, P.M.; Magalhães, A.M.; Inácio, C.T. Towards an Understanding of the Dynamics of Compost N in the Soil-Plant-Atmosphere System Using 15N Tracer. Plant Soil 2013, 362, 373–388. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer Nitrogen Isotope Signatures. Isotopes Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.S.; Kelly, S.D.; Woolfe, M. Nitrogen Isotope Composition of Organically and Conventionally Grown Crops. J. Agric. Food Chem. 2007, 55, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Gentile, N.; Rossi, M.J.; Delémont, O.; Siegwolf, R.T.W. δ15N Measurement of Organic and Inorganic Substances by EA-IRMS: A Speciation-Dependent Procedure. Anal. Bioanal. Chem. 2013, 405, 159–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlesinger, W.H. On the Fate of Anthropogenic Nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Freyer, H.D.; Aly, A.I.M. Nitrogen-15 Variations in Fertilizer Nitrogen†. J. Environ. Qual. 1947; 3, 405–406. [Google Scholar] [CrossRef]
- Robinson, D. δ15N as an Integrator of the Nitrogen Cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Kwak, J.-H.; Lim, S.-S.; Park, H.-J.; Chang, S.X.; Lee, S.-M.; Arshad, M.A.; Yun, S.-I.; Kim, H.-Y. Synthetic Fertilizer and Livestock Manure Differently Affect δ15N in the Agricultural Landscape: A Review. Agric. Ecosyst. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, W.-J.; Lim, S.-S.; Kwak, J.-H.; Chang, S.X.; Kim, H.-Y.; Yoon, K.-S.; Ro, H.-M. Changes in Nitrogen Isotopic Compositions during Composting of Cattle Feedlot Manure: Effects of Bedding Material Type. Bioresour. Technol. 2008, 99, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Li, Y.; Moinet, G.Y.; Clough, T.J.; Whitehead, D. Organic Matter Contributions to Nitrous Oxide Emissions Following Nitrate Addition Are Not Proportional to Substrate-Induced Soil Carbon Priming. Sci. Total Environ. 2022, 851, 158274. [Google Scholar] [CrossRef] [PubMed]
- Saure, M.C. Why Calcium Deficiency Is Not the Cause of Blossom-End Rot in Tomato and Pepper Fruit—A Reappraisal. Sci. Hortic. 2014, 174, 151–154. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S. Content of Trace Elements in Soil Fertilized with Potassium and Nitrogen. Agriculture 2020, 10, 398. [Google Scholar] [CrossRef]
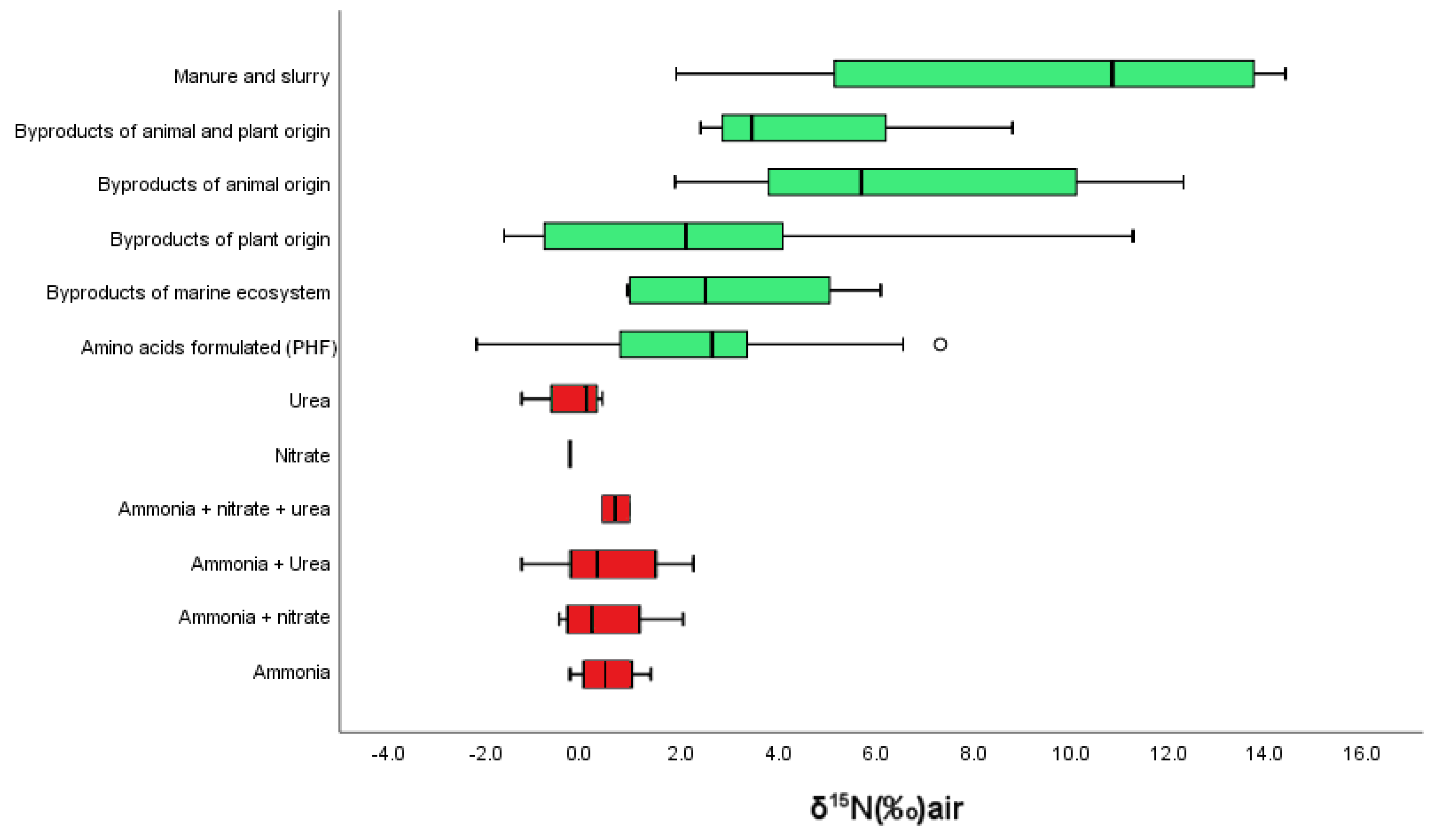

| Synthetic fertilizers δ15N (‰) | |||||||
| Composition | Origin | Mean | SD | Max | Min | n | |
| Ammonia | Air | 0.40 | 0.63 | 1.20 | −0.30 | 8 | |
| Ammonia + nitrate | Air | 0.35 | 0.88 | 1.80 | −0.50 | 16 | |
| Ammonia + urea | Air | 0.47 | 1.15 | 2.00 | −1.20 | 12 | |
| Ammonia + nitrate + urea | Air | 0.47 | 0.29 | 0.80 | 0.30 | 6 | |
| Nitrate | Air | −0.30 | 0.00 | −0.30 | −0.30 | 4 | |
| Urea | Air | −0.23 | 0.68 | 0.30 | −1.20 | 8 | |
| Conventional organic fertilizers δ15N (‰) | |||||||
| Category | Type | Subtype | Mean | SD | Max | Min | n |
| Terrestrial | Animal | Blood | 5.24 | 1.56 | 7.00 | 3.40 | 5 |
| By-products | 7.97 | 4.32 | 12.31 | 1.88 | 7 | ||
| Horn | 4.65 | 4.33 | 5.40 | 3.90 | 2 | ||
| Manure and slurry | 11.21 | 3.14 | 14.40 | 5.15 | 8 | ||
| Plant | By-products | 2.71 | 3.83 | 11.27 | −1.63 | 23 | |
| Marine | Seaweed | 3.67 | 2.62 | 6.10 | 0.90 | 3 | |
| Animal | 2.30 | 0.57 | 2.70 | 1.90 | 2 | ||
| Organic fertilizers δ15N (‰) (declaration of nitrogen content not required) | |||||||
| PHF | 3.41 | 3.10 | 10.35 | −2.22 | 81 | ||
| Micronutrients | 1.09 | 3.12 | 6.79 | −2.34 | 20 | ||
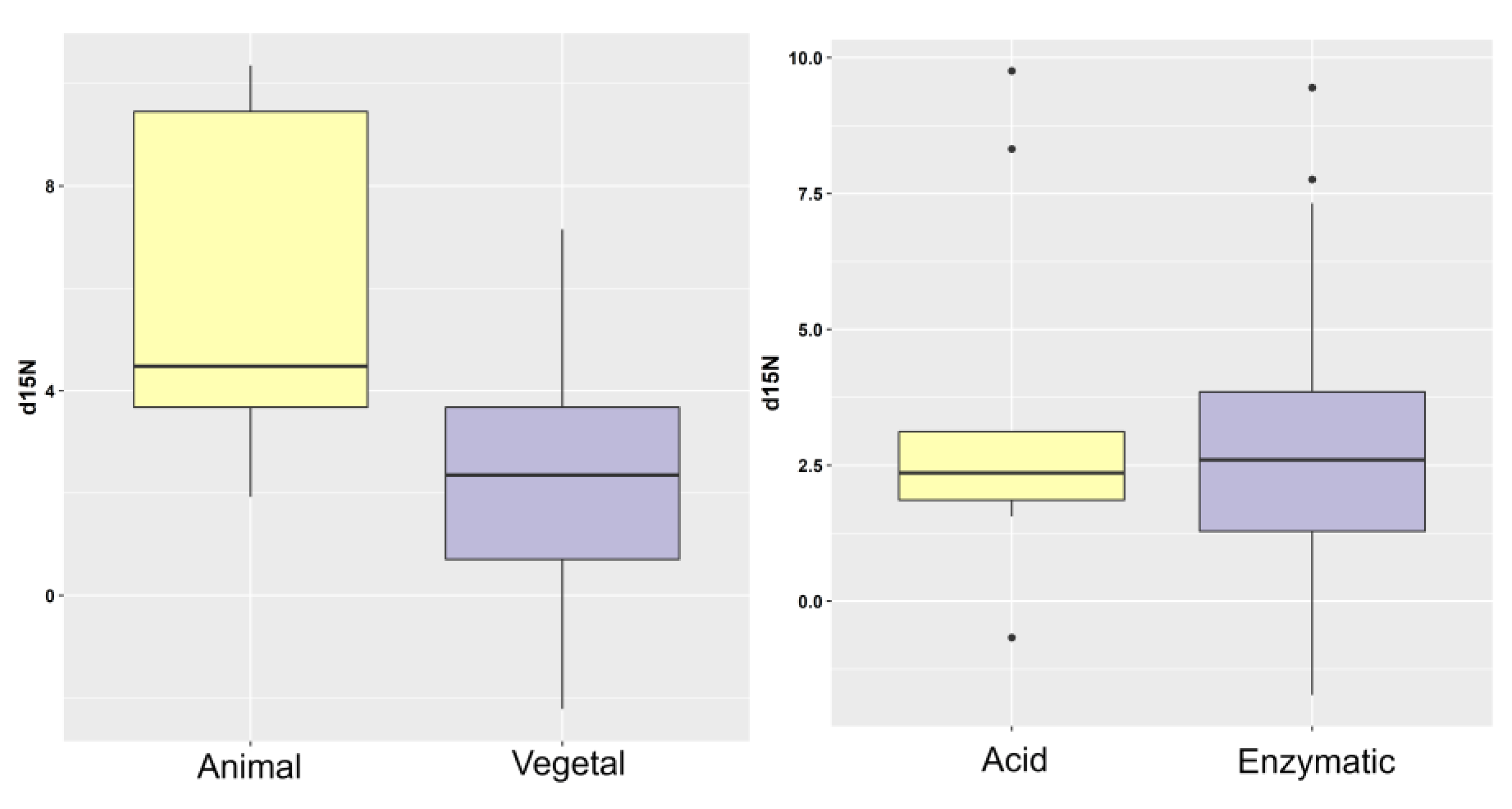
| Factor | PHF | δ15N Mean (‰) | δ15N SD (‰) | p-Value (ANOVA) | % N Mean (‰) | % N SD (‰) | p-Value (ANOVA) |
|---|---|---|---|---|---|---|---|
| Origin | Animal (N = 15) | 6.27 | 3.08 | <0.001 | 7.33 | 4.18 | <0.05 |
| Vegetal (N = 30) | 2.25 | 2.63 | 4.59 | 4.24 | |||
| Unknown (N = 36) | 3.18 | 2.78 | - | 6.65 | 4.73 | - | |
| Hydrolysis | Acid (N = 10) | 3.83 | 3.35 | ns | 8.13 | 7.00 | ns |
| Enzymatic (N = 51) | 2.89 | 2.42 | 5.58 | 4.23 | |||
| Unknown (N = 20) | 4.51 | 4.21 | - | 6.06 | 3.71 | - |
| Category | Declared Elements | δ15N | δ13C | %N |
|---|---|---|---|---|
| Chelated calcium | Ca, EDTA | −0.57 | −26.91 | 9.78 |
| Trace element | Fe, Mn, Zn, B, Cu, EDTA | −0.57 | −27.20 | 8.28 |
| Chelated calcium | Ca | 0.25 | −30.01 | 7.42 |
| Trace element | Zn, EDTA | −1.14 | −31.01 | 6.88 |
| Trace element | Bo | −2.34 | −30.25 | 4.99 |
| Organic complex | Ca | 1.11 | −30.41 | 4.89 |
| Trace element | Zn, Fe, B, Mo | 4.15 | −30.01 | 4.82 |
| Trace element | Zn, B, Mo | −1.87 | −26.78 | 3.43 |
| Calcium chloride | Ca, Mat.Org | 3.36 | −19.33 | 2.48 |
| Trace element | B, Ma, Mn | 9.02 | −26.06 | 1.97 |
| Calcium carbonate | Ca, Mg | 3.22 | −22.84 | 1.91 |
| Calcium carbonate | Ca, Mg | 2.76 | - | 1.78 |
| Trace element | Zn, B, Mo | −0.62 | −27.41 | 1.45 |
| Trace element + chelated calcium | Ca, Mg, Fe | 9.48 | −24.62 | 1.29 |
| Trace element | Zn, Fe, B Mo | −0.88 | −24.63 | 1.12 |
| Calcium chloride | Ca | 7.73 | −7.31 | 0.43 |
| Calcium sulfate | Ca, Mg | −0.21 | - | 0.10 |
| Calcium chloride | Ca | 6.79 | −3.34 | 0.09 |
| Calcium chloride | Ca | −0.88 | −17.65 | 0.04 |
| Trace element + calcium chloride | Zn, Ca | −0.19 | - | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Redondo, J.M.; Cuevas, F.J.; Montenegro, J.C.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M. An Updated Isotopic Database of Fertilizers Used in Intensive Organic Farming: A Case Study on Protein Hydrolyzed Derivatives and Chelated Nutrients. Horticulturae 2024, 10, 523. https://doi.org/10.3390/horticulturae10050523
Muñoz-Redondo JM, Cuevas FJ, Montenegro JC, Ordóñez-Díaz JL, Moreno-Rojas JM. An Updated Isotopic Database of Fertilizers Used in Intensive Organic Farming: A Case Study on Protein Hydrolyzed Derivatives and Chelated Nutrients. Horticulturae. 2024; 10(5):523. https://doi.org/10.3390/horticulturae10050523
Chicago/Turabian StyleMuñoz-Redondo, José Manuel, Francisco Julián Cuevas, José Carlos Montenegro, José Luis Ordóñez-Díaz, and José Manuel Moreno-Rojas. 2024. "An Updated Isotopic Database of Fertilizers Used in Intensive Organic Farming: A Case Study on Protein Hydrolyzed Derivatives and Chelated Nutrients" Horticulturae 10, no. 5: 523. https://doi.org/10.3390/horticulturae10050523
APA StyleMuñoz-Redondo, J. M., Cuevas, F. J., Montenegro, J. C., Ordóñez-Díaz, J. L., & Moreno-Rojas, J. M. (2024). An Updated Isotopic Database of Fertilizers Used in Intensive Organic Farming: A Case Study on Protein Hydrolyzed Derivatives and Chelated Nutrients. Horticulturae, 10(5), 523. https://doi.org/10.3390/horticulturae10050523









